Abstract
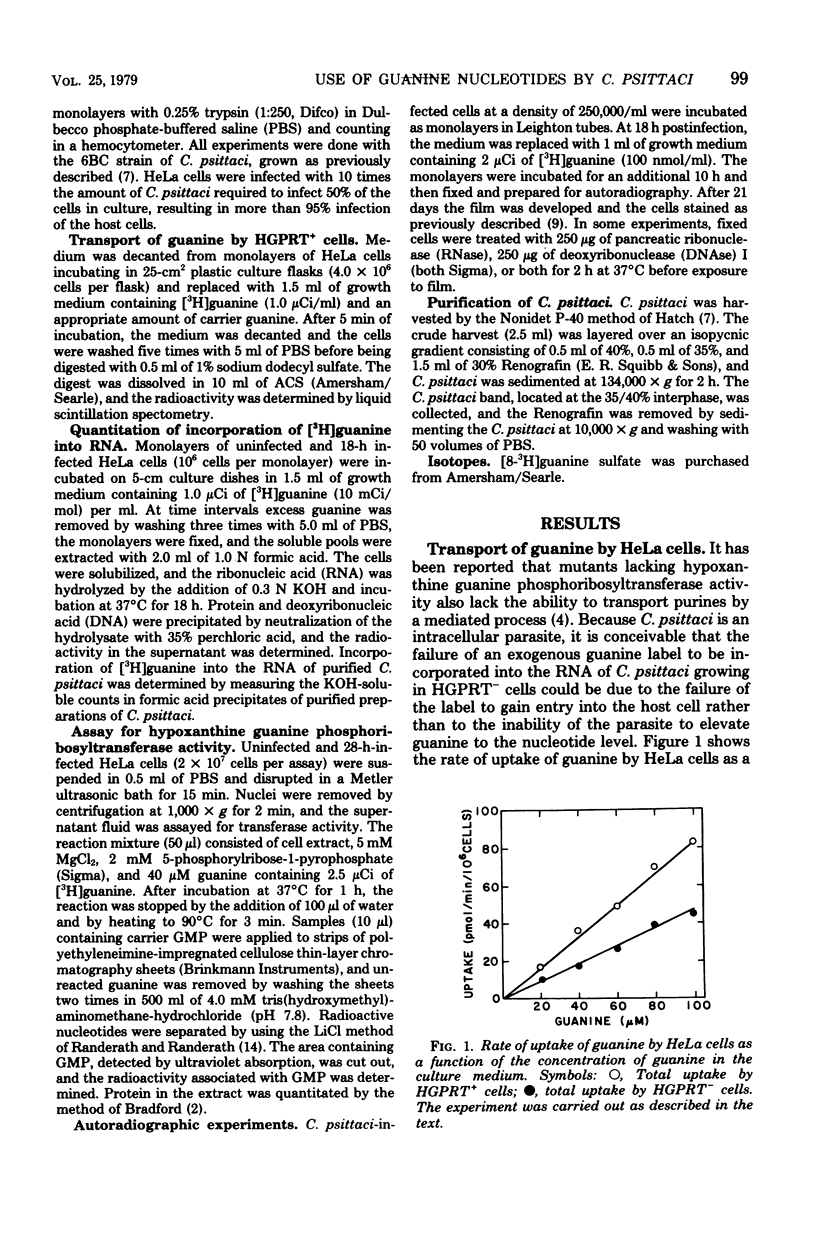
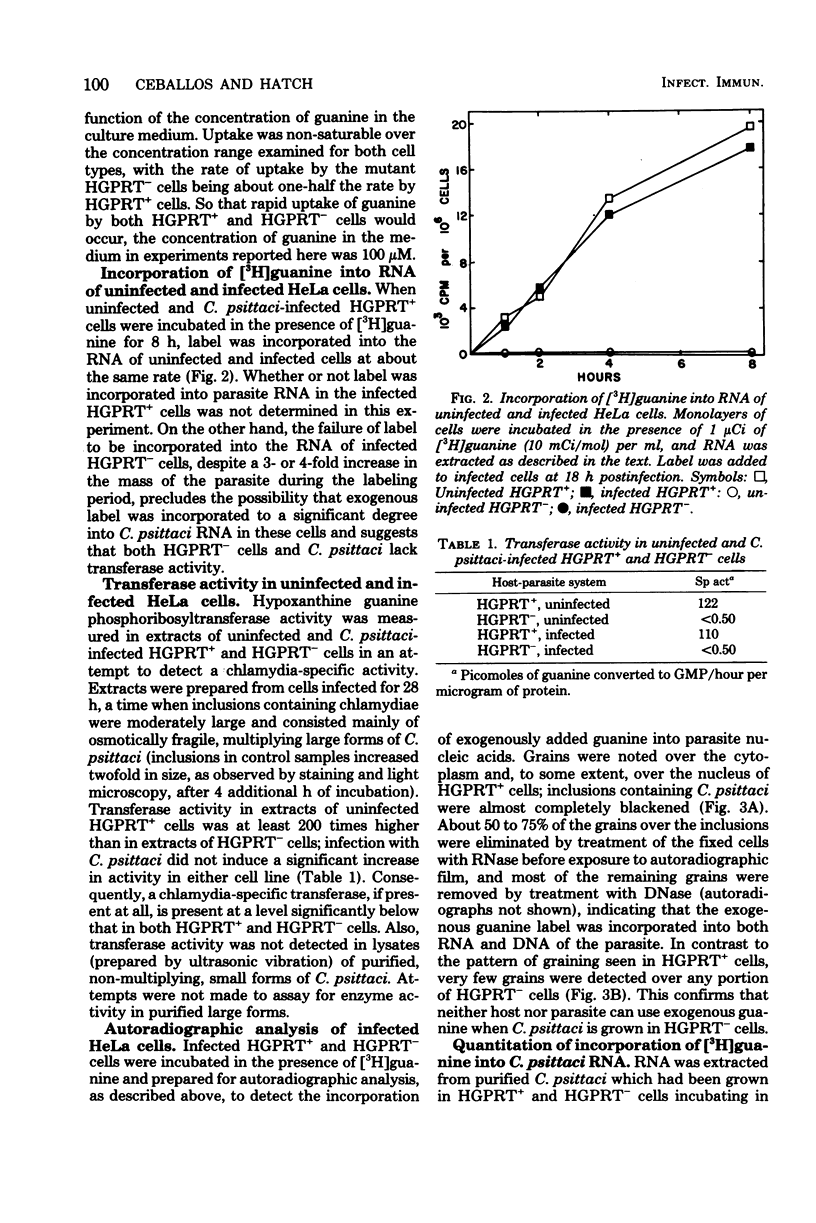
Exogenous guanine was found to be incorporated into the nucleic acids of Chlamydia psittaci when the parasite was grown in HeLa cells containing hypoxanthine guanine phosphoribosyltransferase (EC 2.4.2.8) activity but not when the parasite was grown in transferase-deficient HeLa cells. No evidence for a chlamydia-specific transferase activity was found in either transferase-containing or transferase-deficient infected HeLa cells. It is concluded that C. psittaci is incapable of metabolizing guanine, but that the parasite can use host-generated guanine nucleotides as precursors for nucleic acid synthesis.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BOVARNICK M. R. Phosphorylation accompanying the oxidation of glutamate by the Madrid E strain of typhus rickettsiae. J Biol Chem. 1956 May;220(1):353–361. [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Christian R. G., Paretsky D. Synthesis of ribonucleotides and their participation in ribonucleic acid synthesis by Coxiella burnetii. J Bacteriol. 1977 Dec;132(3):841–846. doi: 10.1128/jb.132.3.841-846.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein J., Littlefield J. W. Hypoxanthine transport in normal and hypoxanthine guanine phosphoribosyltransferase (HGPRT) deficient diploid human lymphoblasts. Exp Cell Res. 1977 May;106(2):247–251. doi: 10.1016/0014-4827(77)90169-0. [DOI] [PubMed] [Google Scholar]
- Gill S. D., Stewart R. B. Effect of metabolic inhibitors on the production of Chlamydia psittaci by infected L cells. Can J Microbiol. 1970 Nov;16(11):1079–1085. doi: 10.1139/m70-182. [DOI] [PubMed] [Google Scholar]
- Gill S. D., Stewart R. B. Glucose requirements of L cells infected with Chlamydia psittaci. Can J Microbiol. 1970 Oct;16(10):997–1001. doi: 10.1139/m70-169. [DOI] [PubMed] [Google Scholar]
- Hatch T. P. Competition between Chlamydia psittaci and L cells for host isoleucine pools: a limiting factor in chlamydial multiplication. Infect Immun. 1975 Jul;12(1):211–220. doi: 10.1128/iai.12.1.211-220.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatch T. P. Utilization of L-cell nucleoside triphosphates by Chlamydia psittaci for ribonucleic acid synthesis. J Bacteriol. 1975 May;122(2):393–400. doi: 10.1128/jb.122.2.393-400.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatch T. P. Utilization of exogenous thymidine by Chlamydia psittaci growing in the thymidine kinase-containing and thymidine kinase-deficient L cells. J Bacteriol. 1976 Feb;125(2):706–712. doi: 10.1128/jb.125.2.706-712.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin H. S. Inhibition of thymidine kinase activity and deoxyribonucleic acid synthesis in L cells infected with the meningopneumonitis agent. J Bacteriol. 1968 Dec;96(6):2054–2065. doi: 10.1128/jb.96.6.2054-2065.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MALLAVIA L., PARETSKY D. STUDIES ON THE PHYSIOLOGY OF RICKETTSIAE. V. METABOLISM OF CARBAMYL PHOSPHATE BY COXIELLA BURNETII. J Bacteriol. 1963 Aug;86:232–238. doi: 10.1128/jb.86.2.232-238.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moulder J. W. Glucose Metabolism of L Cells Before and After Infection with Chlamydia psittaci. J Bacteriol. 1970 Dec;104(3):1189–1196. doi: 10.1128/jb.104.3.1189-1196.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfefferkorn E. R., Pfefferkorn L. C. Toxoplasma gondii: specific labeling of nucleic acids of intracellular parasites in Lesch-Nyhan cells. Exp Parasitol. 1977 Feb;41(1):95–104. doi: 10.1016/0014-4894(77)90134-5. [DOI] [PubMed] [Google Scholar]
- RANDERATH E., RANDERATH K. RESOLUTION OF COMPLEX NUCLEOTIDE MIXTURES BY TWO-DIMENSIONAL ANION-EXCHANGE THIN-LAYER CHROMATOGRAPHY. J Chromatogr. 1964 Oct;16:126–129. doi: 10.1016/s0021-9673(01)82446-8. [DOI] [PubMed] [Google Scholar]
- Rittenberg S. C., Langley D. Utilization of nucleoside monophosphates per Se for intraperiplasmic growth of Bdellovibrio bacteriovorus. J Bacteriol. 1975 Mar;121(3):1137–1144. doi: 10.1128/jb.121.3.1137-1144.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trager W. Some aspects of intracellular parasitism. Science. 1974 Jan 25;183(4122):269–273. doi: 10.1126/science.183.4122.269. [DOI] [PubMed] [Google Scholar]
- Tribby I. I., Moulder J. W. Availability of bases and nucleosides as precursors of nucleic acids in L cells and in the agent of meningopneumonitis. J Bacteriol. 1966 Jun;91(6):2362–2367. doi: 10.1128/jb.91.6.2362-2367.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weidner E., Trager W. Adenosine triphosphate in the extracellular survival of an intracellular parasite (Nosema michaelis, Microsporidia). J Cell Biol. 1973 May;57(2):586–591. doi: 10.1083/jcb.57.2.586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss E. Adenosine Triphosphate and Other Requirements for the Utilization of Glucose by Agents of the Psittacosis-Trachoma Group. J Bacteriol. 1965 Jul;90(1):243–253. doi: 10.1128/jb.90.1.243-253.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss E. Transaminase activity and other enzymatic reactions involving pyruvate and glutamate in Chlamydia (psittacosis-trachoma group). J Bacteriol. 1967 Jan;93(1):177–184. doi: 10.1128/jb.93.1.177-184.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss E., Wilson N. N. Role of exogenous adenosine triphosphate in catabolic and synthetic activities of Chlamydia psittaci. J Bacteriol. 1969 Feb;97(2):719–724. doi: 10.1128/jb.97.2.719-724.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams J. C., Peterson J. C. Enzymatic activities leading to pyrimidine nucleotide biosynthesis from cell-free extracts of Rickettsia typhi. Infect Immun. 1976 Aug;14(2):439–448. doi: 10.1128/iai.14.2.439-448.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams J. C., Weiss E. Energy metabolism of Rickettsia typhi: pools of adenine nucleotides and energy charge in the presence and absence of glutamate. J Bacteriol. 1978 Jun;134(3):884–892. doi: 10.1128/jb.134.3.884-892.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkler H. H. Rickettsial permeability. An ADP-ATP transport system. J Biol Chem. 1976 Jan 25;251(2):389–396. [PubMed] [Google Scholar]



