Abstract
Objectives:
The objective was to conduct a US multicenter, retrospective medical record study examining the effectiveness, safety, and patterns of use of valrubicin for treatment of nonmuscle-invasive bladder cancer (NMIBC) by clinicians since the 2009 reintroduction of valrubicin.
Methods:
Patients ≥ 18 years with NMIBC who received had one or more instillations of valrubicin (October 2009– September 2011) were eligible. The primary endpoint was event-free survival (EFS). Safety and tolerability were also assessed.
Results:
The medical records of 113 patients met the inclusion criteria; 100 patients (88.5%) completed valrubicin treatment. The median age was 75 years (range 42–95 years). The median NMIBC duration was 31 months since diagnosis: 51.3% (58/113) had carcinoma in situ (CIS) alone, and 31.9% (36/113) had unspecified NMIBC. Most patients, 94.7% (107/113), had more than three valrubicin instillations and 70.8% (80/113) completed a full course. The EFS rate (95% confidence interval) was 51.6% (40.9–61.3%), 30.4% (20.4–41.1%), and 16.4% (7.9–27.5%) at 3, 6, and 12 months, respectively. Median time to an event was 3.5 (2.5–4.0) months after the first valrubicin instillation. Local adverse reactions (LARs) were experienced by 49.6% (56/113) of patients; most LARs were mild (93.6%). The most frequent LARs were hematuria, pollakiuria, micturition urgency, bladder spasm, and dysuria. In total, 4.4% (5/113) of patients discontinued valrubicin because of adverse events or LARs.
Conclusions:
Data from the present retrospective study are consistent with previous prospective clinical trials that demonstrated valrubicin effectiveness and tolerability for select patients with CIS, before considering cystectomy. Additional prospective studies are warranted to evaluate valrubicin safety and efficacy in the broader patient population with NMIBC.
Keywords: bladder cancer, chemotherapy, cystectomy, disease progression, recurrence
Introduction
The recommended first-line treatment for high-risk nonmuscle-invasive bladder cancer (NMIBC), following transurethral resection of the bladder tumor (TURBT), is an induction course plus maintenance with intravesical (IVe) bacillus Calmette–Guérin (BCG) [Brausi et al. 2011; Hall et al. 2007; Montie et al. 2011]. However, approximately 33–39% of patients with NMIBC experience tumor recurrence after this treatment [Bohle et al. 2003; Pansadoro et al. 2002]. Radical cystectomy is recommended for most high-risk patients upon recurrence [Brausi et al. 2011; Hall et al. 2007; Montie et al. 2011], but may significantly decrease patient quality of life [Gilbert et al. 2007]. Salvage IVe therapy for patients who fail two courses of BCG with/without maintenance remains an area of active investigation, particularly in patients who are not candidates for cystectomy.
Valrubicin was approved in the USA in 1998, based on data from a pivotal phase III study [Steinberg et al. 2000], for use in patients with BCG-refractory carcinoma in situ (CIS) when cystectomy is not an option because of morbidity and mortality [Montie et al. 2011; Endo Pharmaceuticals, 2011]. Several other trials have provided supportive evidence of safety and effectiveness [Blum et al. 1981; Dinney et al. 2013; Greenberg et al. 1997; Ignatoff et al. 2009; Patterson et al. 2000]. A post-hoc analysis from the pivotal trial showed that the effectiveness of valrubicin was similar regardless of patient age, sex, smoking status, duration of bladder cancer, baseline urinary symptoms, and history of prior NMIBC therapies [Steinberg et al. 2011].
Valrubicin was voluntarily removed from the market in 2002 because of manufacturing issues, but remained on the ‘short list’ of the Food and Drug Administration Center for Drug Evaluation and Research Drug Shortage Program. No data from postapproval studies of valrubicin have been published since its reintroduction to the market in 2009. This medical record review examines real-world effectiveness, safety, and patterns of use of valrubicin for treatment of NMIBC since its reintroduction.
Methods
Study design
This US, multicenter, retrospective, real-world study (NCT01304173) examined patient medical records abstracted from 30 March 2011 to 13 September 2011. Sites were selected based on the volume of NMIBC patients treated with valrubicin since 2009. The objective was to examine medical records from about 200 patients; no formal sample size was estimated. The study followed the Declaration of Helsinki and complied with US and local regulations for patient privacy. Institutional review board (IRB) or independent ethics committee (IEC) approvals were obtained for each site. Waivers of the requirement for patient consent were sought from the IRBs/IECs because of the noninterventional nature of the study; if a waiver was not approved, patients provided written informed consent.
Patient inclusion and exclusion criteria
Patients were aged ≥ 18 years, had a documented diagnosis of NMIBC, had received one or more instillations of valrubicin between October 2009 and September 2011, and had completed valrubicin therapy or were no longer receiving therapy when the study started. Patients were excluded if they had received non-IVe administration of valrubicin.
Medical record abstraction
After a site qualification telephone interview, trained medical abstracters (not employed by the sponsor, Endo Pharmaceuticals Inc.) conducted an on-site visit and collected data from patient medical records into an electronic case report form. Investigators provided missing records to medical abstracters. Site characteristics and patient baseline history (e.g. demographics, bladder cancer history, disease characteristics, previous therapies/surgeries related to bladder cancer, etc.) were collected. A full course of IVe therapies and valrubicin was defined as six weekly instillations; however, some patient medical records showed that fewer or more than six instillations were received. A separation in time of more than several weeks after multiple weekly instillations defined a new course of therapy.
Assessments
The effectiveness of valrubicin was assessed based on events (i.e. recurrence, progression, and death from any cause) during or after treatment. Recurrence was defined as evidence of any bladder cancer according to biopsy, cytology (two or more separate occasions, only if biopsy data were lacking), or both. Progression was defined as biopsy confirmation of muscle-invasive bladder cancer (MIBC; stage T2 or higher). Worsening was defined as recurrence, progression, change of antibladder cancer therapy, cystectomy, or death (any cause).
The tolerability and safety of valrubicin were assessed based on data recorded during treatment and for 30 days after ending valrubicin. Safety data were focused on specific events because the retrospective medical record study design precluded uniform adverse event (AE) reporting. Events of interest included local adverse reactions (LARs) (i.e. local bladder AEs described in the prescribing information [Endo Pharmaceuticals, 2011], which were ‘probably’ or ‘possibly’ related to valrubicin or of unknown cause), the use of concomitant medications to treat LARs, and serious AEs (SAEs), resulting in death, hospitalization, substantial disability, a congenital anomaly, or were life threatening).
Statistical analysis
The primary effectiveness endpoint was event-free survival (EFS), and it was censored at the last effectiveness assessment in patients who were lost to follow up before an event occurred. Secondary effectiveness endpoints were 6- and 12-month event-free rates, and 3- and 6-month disease-free rates (i.e. the number of days from first instillation of valrubicin to recurrence or the first occurrence of progression). For patients with missing data, disease-free survival (DFS) was censored at the last effectiveness assessment before the start of other antibladder cancer therapy. Death was not considered an event in the DFS analysis; if a death occurred, the last effectiveness assessment before the death was used. Time to disease progression, that is, progression-free survival (PFS) and worsening-free survival (WFS), were censored at the last effectiveness assessment in patients who had no event during the study or the last assessment before starting another antibladder cancer therapy. Time to cystectomy was censored at the last visit or the start of new bladder cancer treatment if cystectomy was not performed at the end of the study. Effectiveness endpoints were calculated from the first instillation of valrubicin to the earliest event that occurred ≥ 42 days afterwards to reflect the nature of a valrubicin course.
Effectiveness endpoints were analyzed using Kaplan–Meier methods to yield estimated rates, median values, and 95% confidence intervals (CIs). Baseline characteristics, valrubicin exposure, concomitant medication use, and safety were summarized using descriptive statistics. Statistical analyses were performed using SAS® version 9.2 (SAS Institute Inc, Cary, NC, USA).
Results
Medical practice characteristics and patient disposition
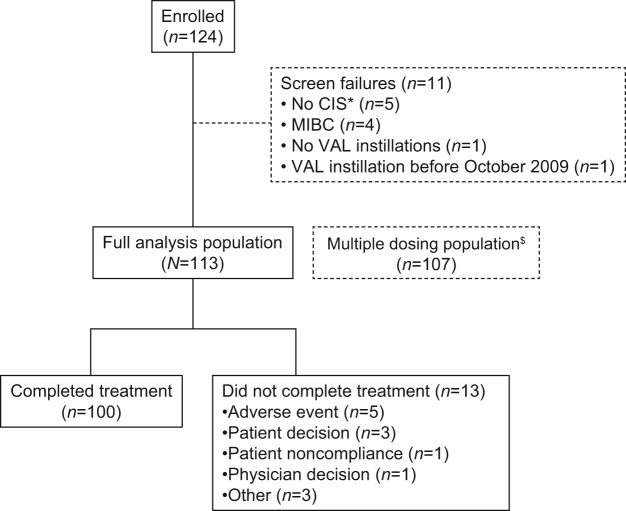
Practice characteristics are shown in Table 1; most sites had two or more physicians (84.0%) and were nonacademic (92.0%). Medical records of 124 patients were examined (Figure 1); records from 11 patients were excluded because the patients failed screening (MIBC, n = 4; no NMIBC, n = 5; no instillations, n = 1; valrubicin received before October 2009, n = 1), leaving 113 patients for record abstraction. A total of 100 patients (88.5%) completed valrubicin treatment; 11.5% (13/113) did not complete treatment because of AEs (4.4% [5/113]), patient decision (2.7% [3/113]), physician decision (0.9% [1/113]), patient noncompliance (0.9% [1/113]), or other reasons (2.7% [3/113]).
Table 1.
Study site characteristics.
| Characteristic | Sites, n (%) (N = 25) |
|---|---|
| Practice size | |
| Large group (more than seven physicians) | 9 (36.0) |
| Small group (2–7 physicians) | 12 (48.0) |
| Solo (one physician) | 4 (16.0) |
| Practice type, n (%) | |
| Academic center | 2 (8.0) |
| Nonacademic | 23 (92.0) |
| Geographic region, n (%) | |
| Northeast | 11 (44.0) |
| South | 9 (36.0) |
| West | 3 (12.0) |
| Midwest | 2 (8.0) |
Figure 1.
Patient disposition. *One of these patients was later enrolled, under a different patient identification number, after a protocol amendment allowed entry of patients with any type of NMIBC. $The multiple-dosing population (received four or more instillations) is not further described because results were similar to those of the full-analysis population.
CIS, carcinoma in situ; MIBC, muscle-invasive bladder cancer; NMIBC, nonmuscle-invasive bladder cancer; VAL, valrubicin.
Demographic and baseline characteristics
The median age was 75 years (range 42–95 years); 81.4% (92/113) of patients were aged ≥ 65 years; 65.5% (74/113) of patients were aged 70–89 years (Table 2). Most patients were White men. The median NMIBC duration was 31 months, and at the time of valrubicin treatment, 51.3% (58/113) had CIS alone, 31.9% (36/113) had unspecified NMIBC, and the rest had both CIS and papillary tumors (10.6% [12/113]), or papillary tumors alone (6.2% [7/113]). Patients received extensive medical and surgical therapy for NMIBC before receiving valrubicin (Table 2). Immunotherapy was the most common prior medical treatment, followed by IVe chemotherapy. TURBT was the most common prior surgical treatment.
Table 2.
Demographic and baseline disease characteristics of the study population.
| Characteristic | Patients (N = 113) |
|---|---|
| Age (years) | |
| Median (range) | 75 (42–95) |
| Mean (SD) | 73.7 (9.8) |
| < 65, n (%) | 21 (18.6) |
| ≥ 65, n (%) | 92 (81.4) |
| 65–69, n (%) | 17 (15.0) |
| 70–79, n (%) | 39 (34.5) |
| 80–89, n (%) | 35 (31.0) |
| ≥ 90, n (%) | 1 (0.9) |
| Sex, n (%) | |
| Men | 91 (80.5) |
| Race, n (%) | |
| White | 91 (80.5) |
| Black | 7 (6.2) |
| Asian | 1 (0.9) |
| Other | 7 (6.2) |
| Missing | 7 (6.2) |
| Ethnicity, n (%) | |
| Hispanic | 4 (3.5) |
| Non-Hispanic | 81 (71.7) |
| Unknown | 28 (24.8) |
| NMIBC tumor types, n (%) | |
| CIS alone | 58 (51.3) |
| NMIBC, unspecified | 36 (31.9) |
| CIS and papillary | 12 (10.6) |
| Papillary alone | 7 (6.2) |
| Time since diagnosis of bladder cancer (months) | |
| Median (range) | 31 (1–140) |
| Mean (SD) | 41 (30) |
| Prior treatment | |
| Intravesical immunotherapy (no. courses)$ | |
| Median (range)‡ | 2 (0–20) |
| Mean (SD) ‡ | 3.5 (4.3) |
| Intravesical chemotherapy (no. courses) | |
| Median (range) ‡ | 0 (0–15) |
| Mean (SD) ‡ | 0.7 (1.8) |
| Transurethral resection of the bladder tumor (no. procedures) | |
| Median (range) | 2 (0–13) |
| Mean (SD) | 2.5 (2.4) |
| Fulguration (no. procedures) | |
| Median (range) | 0 (0–15) |
| Mean (SD) | 1.1 (2.0) |
At the time of the first valrubicin instillation. $Includes bacillus Calmette–Guérin and unspecified immunotherapy. ‡Courses could have included up to six weekly instillations, but patient medical records did include cases in which fewer instillations or maintenance doses were received. In the review of patient medical records, courses were defined as a separation in time of more than several weeks after multiple weekly instillations.
CIS, carcinoma in situ; NMIBC, nonmuscle-invasive bladder cancer; SD, standard deviation.
Patient histories included a wide range of comorbid conditions (Table 3), some of which were potential contraindications for cystectomy or other surgery, for example, ischemic cardiomyopathy (2.7%; 3/113), aortic aneurysm (3.5%; 4/113), and chronic obstructive pulmonary disease/emphysema (13.3%; 15/113).
Table 3.
Comorbid conditions in 5% or more of the study population.
| Comorbidity, n (%) | Patients (N = 113) |
|---|---|
| Hypertension or blood pressure increased | 71 (62.8) |
| Diabetes mellitus | 31 (27.4) |
| Benign prostatic hyperplasia or prostatomegaly | 26 (23.0) |
| Hypercholesterolemia or blood cholesterol increased | 26 (23.0) |
| Arthritis, osteoarthritis, or spinal osteoarthritis | 23 (20.4) |
| Coronary artery disease | 23 (20.4) |
| Hyperlipidemia or dyslipidemia | 21 (18.6) |
| Hematuria | 16 (14.2) |
| Chronic obstructive pulmonary disease or emphysema | 15 (13.3) |
| Depression | 12 (10.6) |
| Gastroesophageal reflux disease | 12 (10.6) |
| Nocturia | 12 (10.6) |
| Drug hypersensitivity | 11 (9.7) |
| Urinary tract infection | 11 (9.7) |
| Atrial fibrillation | 10 (8.8) |
| Erectile dysfunction | 10 (8.8) |
| Hypothyroidism, hyperthyroidism, or other thyroid disorder | 10 (8.8) |
| Prostate cancer | 9 (8.0) |
| Renal cyst | 8 (7.1) |
| Peripheral vascular disorder | 7 (6.2) |
| Glaucoma | 6 (5.3) |
| Gout | 6 (5.3) |
| Micturition urgency | 6 (5.3) |
| Pollakiuria | 6 (5.3) |
Valrubicin treatment history
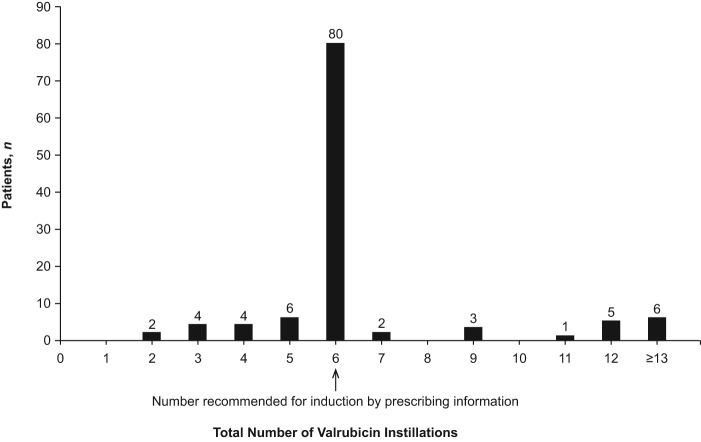
From 4 October 2009 to 1 September 2011, patients received a median of six instillations (range 2–18; mean [standard deviation], 6.6 [2.6]), as recommended by the product label, with a median treatment duration of 38 days (Figure 2). Most patients (94.7% [107/113]) had more than three instillations and 70.8% (80/113) completed a full course.
Figure 2.
Number of valrubicin instillations per patient.
Effectiveness of valrubicin
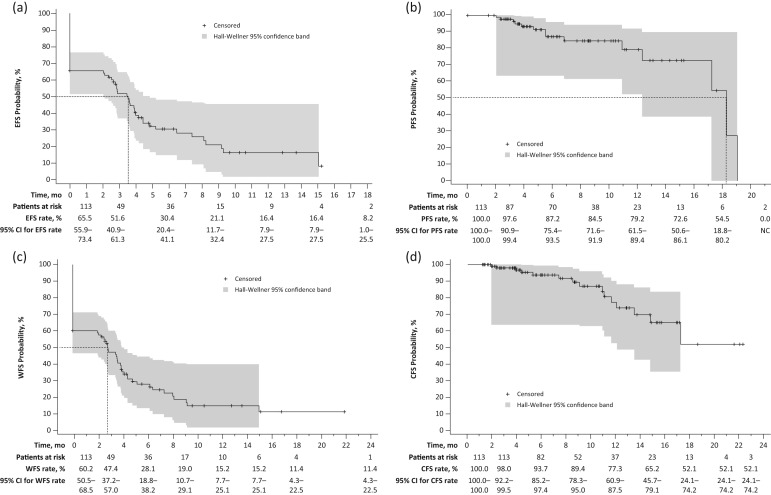
The EFS rate was 51.6% (95% CI, 40.9–61.3%), 30.4% (20.4–41.1%), and 16.4% (7.9–27.5%) at 3, 6, and 12 months, respectively (Figure 3a). Median time to an event was 3.5 (2.5–4.0) months after the first valrubicin instillation; 61.9% (70/113) of patients experienced an event. As no deaths were observed as an event, results for EFS were identical to DFS.
Figure 3.
Kaplan–Meier survival analyses in patients treated with valrubicin for (a) EFS*; (b) PFS$; (c) WFS‡; (d) CFS§.
*An event was defined as recurrence, progression, or death from any cause. Dashed lines depict the median EFS time (3.5 months). $Disease progression is defined as progression to muscle-invasive disease (stage T2 or higher) confirmed by biopsy, and PFS time was defined as the number of days from the date of the first valrubicin dose to the date of progression recorded on the effectiveness outcomes form. Dashed lines depict the median PFS time (18.2 months). PFS 95% CIs were NC at 21 months. ‡Defined as recurrence, progression, cystectomy, change of antibladder cancer therapy, or death from any cause. §Time to cystectomy was the number of days between the first dose of valrubicin and cystectomy. For patients who did not undergo cystectomy, the censoring date was the start of other antibladder cancer therapy or, for those who did start another antibladder cancer therapy, the last visit. Median CFS could not be calculated.
CFS, cystectomy-free survival; CI, confidence interval; EFS, event-free survival; NC, not calculable; PFS, progression-free survival; WFS, worsening-free survival.
The PFS rate was 97.6% (90.9–99.4%) and 87.2% (75.4–93.5%) at 3 and 6 months, respectively. The median time to progression was 18.2 (17.2–19.0) months (Figure 3b); 12.4% (14/113) of patients experienced progression. The WFS rate was 47.4% (37.2–57.0%) and 28.1% (18.8–38.2%) at 3 and 6 months. The median time to worsening was 2.9 (2.0–3.9) months; 68.1% (77/113) of patients experienced worsening (Figure 3c). Cystectomy-free survival (CFS) was 98.0% (92.2–99.5%) and 93.7% (85.2–97.4%) at 3 and 6 months (Figure 3d). Median time to cystectomy was not reached because the CFS rate did not decline to 50% during the period with available follow-up data; 13.3% (15/113) patients underwent cystectomy after valrubicin.
Tolerability and safety of valrubicin
More than 70% of patients received a full course of valrubicin (Figure 2); a few patients (4.4% [5/113]) discontinued valrubicin because of AEs or LARs (Table 4). Half of patients experienced a LAR (49.6% [56/113]), most of which were mild (93.6%). The most frequent LARs were hematuria, pollakiuria, micturition urgency, bladder spasm, and dysuria. All LARs classified as instillation-site pain and bladder pain were mild.
Table 4.
Safety of valrubicin treatment.
| Safety parameter | Patients, n (%) (N = 113) |
|
|---|---|---|
| Experienced LAR or SAE* | Discontinued valrubicin because of LAR or SAE* | |
| LAR$ | ||
| Any | 56 (49.6) | 4 (3.5) |
| Hematuria | 20 (17.7) | 2 (1.8) |
| Pollakiuria | 20 (17.7) | 0 |
| Micturition urgency | 18 (15.9) | 0 |
| Bladder spasm | 16 (14.2) | 0 |
| Dysuria | 15 (13.3) | 0 |
| Instillation-site pain | 8 (7.1) | 0 |
| Cystitis | 7 (6.2) | 2 (1.8) |
| Nocturia | 5 (4.4) | 0 |
| Urinary incontinence | 2 (1.8) | 0 |
| Bladder pain | 1 (0.9) | 0 |
| SAE | ||
| Any | 7 (6.2) | 2 (1.8) |
| Pneumonia‡ | 3 (2.7) | 1 (0.9)‡ |
| Hematuria | 1 (0.9)‡ | 1 (0.9)§ |
| Back pain | 1 (0.9) | 0 |
| Device-related infection | 1 (0.9) | 0 |
| Failure to thrive‡ | 1 (0.9) | 0 |
| Gastric ulcer | 1 (0.9) | 0 |
| Hydronephrosis | 1 (0.9) | 0 |
| Renal failure | 1 (0.9) | 0 |
| Ureteric calculus | 1 (0.9) | 0 |
Each patient may have experienced one or more LAR or SAE. $Any adverse event that was ‘probably’ or ‘possibly’ related to valrubicin or of unknown causality. ‡An 87-year-old male patient had a reported SAE of ‘failure to thrive’ and pneumonia, from which he was hospitalized, returned home, and was recovering. The patient had a previous history of renal and rectal cancer (in remission) as well as carcinoma in situ of the bladder (current). Valrubicin treatment was discontinued when the patient had pneumonia (SAE). The patient had metastatic disease and died 1.8 months after starting valrubicin treatment (and 35 days after receiving the last valrubicin instillation). Deaths occurring more than 30 days after the last instillation of valrubicin were not included in the safety analysis per the study protocol. §This event of hematuria was counted as both an LAR and an SAE.
LAR, local adverse reaction; SAE, serious adverse event.
Commonly prescribed concomitant medications included antibiotics, antimuscarinics, analgesics, local anesthetics, and benzodiazepines. A total of 55 patients (48.7%) were treated with one or more concomitant medication to manage an LAR; the most common were urinary antispasmodics (21.2% [24/113]), most often oxybutynin immediate release and solifenacin (6.2% [7/113] each); fluoroquinolone antibiotics (14.2% [16/113]), most often ciprofloxacin (12.4% [14/113]); other urologic agents (14.2% [16/113]), most often phenazopyridine (7.1% [8/113]) and lidocaine (6.2% [7/113]).
Seven patients (6.2%) experienced 11 SAEs, all rated as mild. Pneumonia (2.7% [3/113]) was the only SAE that occurred in more than one patient. Two SAEs resulted in valrubicin discontinuation: one case of pneumonia occurred in a man who was later diagnosed with metastatic disease in the chest (see below), and one case of hematuria (also counted as an LAR) occurred in a man with a history of recurrent cystitis, hematuria, and dysuria. No SAEs resulted in death or were life threatening. Two deaths were reported; however, because they occurred more than 30 days after the last valrubicin instillation, the deaths were not included in the safety analysis. An 87-year-old man died of metastatic cancer 1.8 months after starting valrubicin (35 days after receiving the fourth instillation). Another patient (aged 86 years) died of unknown causes 12.6 months after starting valrubicin.
Discussion
The results of this first study on the clinical, real-world use of valrubicin since its reintroduction to the market in 2009 are consistent with data from previous, prospective studies demonstrating the effectiveness and safety for treating NMIBC, including CIS, in patients who are not undergoing immediate cystectomy [Dinney et al. 2013; Greenberg et al. 1997; Ignatoff et al. 2009]. As expected in an NMIBC population, the patient population was heavily pretreated, of advanced age, and had significant comorbid burden, including some conditions that were contraindications to surgery. In this group of patients, valrubicin was associated with delayed recurrence and progression, and the cystectomy rate was low. Valrubicin was generally well tolerated and most patients received a full course. LARs were common, but manageable, and rarely led to valrubicin discontinuation. SAEs were infrequent and not life threatening.
Treatment with valrubicin in clinical practice appeared to have beneficial effects in this population of patients who had previously received many bladder cancer treatments; the median time to an event was 3.5 months and time to progression was 18.2 months. Although few patients had more than 6 months of follow up after beginning valrubicin treatment, at least some of the patients experienced a long-term response.
The 6-month EFS rate (30%) in this medical record study was higher than the complete response rate in the pivotal phase III study (18% at 6 months) [Endo Pharmaceuticals, 2011], and a phase II–III study (18% at 6 months) [Dinney et al. 2013], and is noteworthy considering the multiple previous therapies used in this population. However, these results should be interpreted cautiously because the definition of recurrence differed between this retrospective study and the two previous prospective studies. In this study, disease free was defined as no evidence of bladder cancer from either cytology or biopsy, whereas complete response in the pivotal and phase II–III studies was more stringently defined as the absence of bladder cancer based on both cytology and biopsy [Dinney et al. 2013; Steinberg et al. 2000]. Fewer patients in this study (13% [15/113]) had a cystectomy after valrubicin, than in the pivotal and phase II–III studies (56% [44/79] and 25% [20/80], respectively). However, this real-world study’s retrospective design (with few protocol limitations) limits comparisons with the previous prospective clinical trials, which had rigidly defined protocols. Other possible reasons for the differences between the current study and previous studies include the degree of patient pretreatment as well as tumor types allowed in the study (current study: CIS or other NMIBC; pivotal trial: CIS or CIS + papillary; phase II–III study: 98% [78/80] had CIS or CIS + papillary). Finally, changes in clinical practice from the late 1990s to 2009–2011 may account for some of the noted differences between this study and the prospective registration trials.
Valrubicin administration adhered closely to label instructions and was well tolerated. The incidence of hematuria in this study was 18%, even though 14% of patients had a medical history of hematuria at baseline. In comparison, hematuria occurred during valrubicin treatment in 17% [Steinberg et al. 2000] and 26% [Dinney et al. 2013] of patients in the pivotal and phase II–III studies, respectively. Hematuria with valrubicin appears lower than that usually observed with BCG (26–38% per prescribing information) [Organon Teknika, 2009]. Few patients (around 4%) had to stop valrubicin because of side effects.
The acceptable tolerability of valrubicin is reassuring in this advanced-age population with comorbidities. Advanced-age populations exhibit substantial risks of mortality and complications with cystectomy [Bostrom et al. 2009; Liberman et al. 2011; Svatek et al. 2010]. One study found that postoperative mortality after cystectomy was greater in patients aged 70–79 years (odds ratio [OR], 2.85; p < 0.001) and 80–89 years (OR, 5.03; p < 0.001) than in patients younger than 70 years [Liberman et al. 2011]. Another study found that after cystectomy, older age was associated with longer median hospitalization stays (patients aged ≤ 70 years [9 days]; 71–80 years [10 days]; ≥ 81 years [12 days]; p < 0.001), and more frequent complications (e.g. postoperative ileus, p = 0.04; new-onset cardiac arrhythmia, p = 0.02) [Svatek et al. 2010].
This study had several limitations. The retrospective design was not intended to rigorously prove effectiveness, which was prospectively established in the trials for product approval [Steinberg et al. 2000]. This study design also might have selection/information biases, confounding variables, potential medical record inaccuracies, and uncontrolled heterogeneity in procedures among different sites (physicians using their standard-of-care practices) [Hess, 2004]. The population in this study included those with CIS, as follows the prescribing information; other forms of NMIBC were also treated in this study, which is outside the product labeling for valrubicin [Endo Pharmaceuticals, 2011]. In addition, this study included a small number of patients, and the number of patients at risk at 6 months was very small. Despite being an uncontrolled study, the patient population comprised a fairly homogeneous group of mostly White men of advanced age, which should be representative of clinical practice. However, the use of valrubicin at study sites might not be directly representative of use at the broader spectrum of cancer centers.
Conclusion
For select patients with CIS, valrubicin was previously shown to be an effective, well-tolerated option before considering cystectomy, which may have a negative impact on patient quality of life, morbidity, and mortality [Clark et al. 2005; Dinney et al. 2013; Greenberg et al. 1997; Ignatoff et al. 2009]. Medical records data from the current study provide a good description of the impact of interventions in actual clinical practice, are also relevant for decision making, and complement data derived within the artificial constructs of a clinical trial. However, additional prospective clinical studies are needed to evaluate the efficacy and safety of valrubicin in patients with NMIBC.
Acknowledgments
All authors were responsible for the preparation, review, and final approval of the manuscript before submission. All coauthors contributed scientifically to the manuscript, but the first author exercised editorial control with final responsibility for content decisions and conclusions. MSC and SSC are consultants for Endo Pharmaceuticals Inc. MSC was the principal investigator and RFT and SSC were investigators in this medical record clinical study for Endo Pharmaceuticals Inc. Editorial assistance, including medical writing, was provided by Michael J. Theisen, PhD and Kristine W. Schuler, MS of Complete Healthcare Communications, Inc.
Footnotes
Funding: Funding for this study was provided by Endo Pharmaceuticals Inc. (Malvern, PA, USA). The data extraction and initial analysis were conducted by Outcome Sciences, Inc. (Cambridge, MA, USA), which was funded by Endo Pharmaceuticals Inc. Editorial assistance was funded by Endo Pharmaceuticals Inc.
Conflict of interest statement: MSC is a consultant and participated as a principal investigator in one clinical trial for Endo Pharmaceuticals Inc., is a consultant and investigator for Spectrum Pharmaceuticals, Inc., and is a consultant for US HIFU-High Intensity Focused Ultrasound. SSC was an investigator and is a consultant for Endo Pharmaceuticals Inc., Allergan, and Biopredictive Science. RFT was an investigator in one clinical trial for Endo Pharmaceuticals Inc. All other authors are employed or were formerly employed by Endo Pharmaceuticals Inc.
Contributor Information
Michael S. Cookson, Department of Urologic Surgery, Vanderbilt University Medical Center, MCN A-1302, Nashville, TN 37027, USA
Sam S. Chang, Vanderbilt University Medical Center, Nashville, TN, USA
Christine Lihou, Formerly of Endo Pharmaceuticals Inc., Malvern, PA, USA.
Thomas Li, Endo Pharmaceuticals Inc., Malvern, PA, USA.
Samira Q. Harper, Formerly of Endo Pharmaceuticals Inc., Malvern, PA, USA
Zhihui Lang, Formerly of Endo Pharmaceuticals Inc., Malvern, PA, USA.
Ronald F. Tutrone, Chesapeake Urology Research Associates, Towson, MD, USA
References
- Blum R., Garnick M., Israel M., Panellos G., Henderson I., Frei E., III (1981) Preclinical rationale and phase I clinical trial of the adriamycin analog, AD 32. Recent Results Cancer Res 76: 7–15 [DOI] [PubMed] [Google Scholar]
- Bohle A., Jocham D., Bock P. (2003) Intravesical bacillus Calmette-Guérin versus mitomycin C for superficial bladder cancer: a formal meta-analysis of comparative studies on recurrence and toxicity. J Urol 169: 90–95 [DOI] [PubMed] [Google Scholar]
- Bostrom P., Kossi J., Laato M., Nurmi M. (2009) Risk factors for mortality and morbidity related to radical cystectomy. BJU Int 103: 191–196 [DOI] [PubMed] [Google Scholar]
- Brausi M., Witjes J., Lamm D., Persad R., Palou J., Colombel M., et al. (2011) A review of current guidelines and best practice recommendations for the management of nonmuscle invasive bladder cancer by the international bladder cancer group. J Urol 186: 2158–2167 [DOI] [PubMed] [Google Scholar]
- Clark P., Stein J., Groshen S., Cai J., Miranda G., Lieskovsky G., et al. (2005) Radical cystectomy in the elderly: comparison of clincal outcomes between younger and older patients. Cancer 104: 36–43 [DOI] [PubMed] [Google Scholar]
- Dinney C., Greenberg R., Steinberg G. (2013) Intravesical valrubicin in patients with bladder carcinoma in situ and contraindication to or failure after bacillus Calmette-Guérin. Urol Oncol 31: 1635–1642 [DOI] [PubMed] [Google Scholar]
- Endo Pharmaceuticals (2011) Valstar® (valrubicin). Full prescribing information. Chadds Ford, PA: Endo Pharmaceuticals Inc [Google Scholar]
- Gilbert S., Wood D., Dunn R., Weizer A., Lee C., Montie J., et al. (2007) Measuring health-related quality of life outcomes in bladder cancer patients using the Bladder Cancer Index (BCI). Cancer 109: 1756–1762 [DOI] [PubMed] [Google Scholar]
- Greenberg R., Bahnson R., Wood D., Childs S., Bellingham C., Edson M., et al. (1997) Initial report on intravesical administration of N-trifluoroacetyladriamycin-14-valerate (AD 32) to patients with refractory superficial transitional cell carcinoma of the urinary bladder. Urology 49: 471–475 [DOI] [PubMed] [Google Scholar]
- Hall M., Chang S., Dalbagni G., Pruthi R., Seigne J., Skinner E., et al. (2007) Guideline for the management of nonmuscle invasive bladder cancer (stages Ta, T1, and Tis): 2007 update. J Urol 178: 2314–2330 [DOI] [PubMed] [Google Scholar]
- Hess D. (2004) Retrospective studies and chart reviews. Respir Care 49: 1171–1174 [PubMed] [Google Scholar]
- Ignatoff J., Chen Y., Greenberg R., Pow-Sang J., Messing E., Wilding G. (2009) Phase II study of intravesical therapy with AD32 in patients with papillary urothelial carcinoma or carcinoma in situ (CIS) refractory to prior therapy with bacillus Calmette-Guérin (E3897): a trial of the Eastern Cooperative Oncology Group. Urol Oncol 27: 496–501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liberman D., Lughezzani G., Sun M., Alasker A., Thuret R., Abdollah F., et al. (2011) Perioperative mortality is significantly greater in septuagenarian and octogenarian patients treated with radical cystectomy for urothelial carcinoma of the bladder. Urology 77: 660–666 [DOI] [PubMed] [Google Scholar]
- Montie J., Clark P., Agerwal N., Eisenberger M., El-Galley R., Greenberg R., et al. (2011) NCCN Clinical Practice Guidelines in Oncology (NCCN GuidelinesTM): Bladder Cancer, version 2.2011. Fort Washington, PA: National Comprehensive Cancer Network [Google Scholar]
- Organon Teknika (2009) TICE® BCG (BCG live [for intravesical use]). Full prescribing information. Durham, NC: Organon Teknika Corporation LLC.0 [Google Scholar]
- Pansadoro V., Emiliozzi P., De Paula F., Scarpone P., Pansadoro A., Sternberg C. (2002) Long-term follow-up of G3T1 transitional cell carcinoma of the bladder treated with intravesical bacille Calmette-Guérin: 18-year experience. Urology 59: 227–231 [DOI] [PubMed] [Google Scholar]
- Patterson A., Greenberg R., Weems L., Bahnson R., Wajsman Z., Israel M., et al. (2000) Pilot study of the tolerability and toxicity of intravesical valrubicin immediately after transurethral resection of superficial bladder cancer. Urology 56: 232–235 [DOI] [PubMed] [Google Scholar]
- Steinberg G., Bahnson R., Brosman S., Middleton R., Wajsman Z., Wehle M. (2000) Efficacy and safety of valrubicin for the treatment of bacillus Calmette-Guérin refractory carcinoma in situ of the bladder. The Valrubicin Study Group. J Urol 163: 761–767 [PubMed] [Google Scholar]
- Steinberg G., Smith N., Ryder K., Strangman N., Slater S. (2011) Factors affecting valrubicin response in patients with bacillus Calmette-Guérin-refractory bladder carcinoma in situ. Postgrad Med 123: 28–34 [DOI] [PubMed] [Google Scholar]
- Svatek R., Fisher M., Matin S., Kamat A., Grossman H., Nogueras-Gonzalez G., et al. (2010) Risk factor analysis in a contemporary cystectomy cohort using standardized reporting methodology and adverse event criteria. J Urol 183: 929–934 [DOI] [PubMed] [Google Scholar]