Abstract
Cancer immunotherapy was deemed the medical breakthrough of 2013, in part because it can induce a rapid, durable, self-propagating and adaptable immune response. Specifically in prostate cancer, immunotherapy has emerged as a viable and attractive treatment strategy. To date, therapeutic cancer vaccines and immune checkpoint inhibitors are the two classes of immunotherapy that have demonstrated improvements in overall survival in patients with advanced tumors. The 2010 Food and Drug Administration approval of sipuleucel-T for asymptomatic or minimally symptomatic metastatic prostate cancer set the stage for ongoing phase III trials with the cancer vaccine PSA-TRICOM and the immune checkpoint inhibitor ipilimumab. A class effect of these approved immune-based therapies is a benefit in overall survival without short-term changes in disease progression, apparently due to modulation of tumor growth rate kinetics, in which the activated immune system exerts constant immunologic pressure that slows net tumor growth. A growing body of evidence suggests that the ideal population for clinical trials of cancer vaccines as monotherapy is patients with lower tumor volume and less aggressive disease. Combination strategies include immunotherapy with standard therapies or with other immunotherapies. Here we review emerging data on immunotherapy for patients with prostate cancer.
Keywords: antigen cascade, antigen spreading, combination therapy, immune checkpoint inhibitor, immune intensification, immunotherapy
Introduction
Interest in immunotherapy for prostate cancer has intensified in recent years. The goal of immunotherapy is to harness the immune system’s ability to recognize and destroy tumor cells. Prostate cancer is amenable to immunotherapeutic approaches for several reasons [Gulley and Drake, 2011]. First, early detection and the generally indolent course of prostate cancer allow sufficient time to generate immune responses that may take weeks or months to mount. Next, because the prostate is a nonessential organ, eradication of residual normal prostate tissue as a result of an immune response has no clinical sequelae. Finally, and perhaps most importantly, prostate cancer cells express several tumor-associated antigens (TAAs) such as prostate-specific antigen (PSA), prostatic acid phosphatase (PAP) and prostate-specific membrane antigen. These TAAs are ideal targets for activated immune cells [Bostwick et al. 1998; Goldfarb et al. 1986; Wang et al. 1979].
At center stage for immunotherapy of prostate cancer are therapeutic cancer vaccines and immune checkpoint inhibitors. Therapeutic cancer vaccines, which are associated with minimal toxicity, are designed to stimulate immune cells to target specific TAAs that are overexpressed on cancer cells. Antigen-presenting cells (APCs) present antigens to the immune system via major histocompatibility complex (MHC) molecules, which bind to appropriate T-cell receptors (TCRs). Activated T cells travel to the tumor, which they recognize by way of the TAAs presented in the context of the MHC, leading to T cell-mediated killing of tumor cells, known as immunogenic cell death. Unlike standard cancer treatment effects, immunotherapeutic effects may persist well beyond tumor cell death. Over time, the immune system may broaden its response to target multiple TAAs not included in the initial vaccine construct. As T cells lyse tumor cells, additional TAAs may be taken up by APCs and presented to immune cells as potential new targets. This expanded T-cell response, known as antigen spreading or antigen cascade, may become more clinically relevant over time [Gulley, 2013].
Immune checkpoint inhibitors interfere with the immune system’s autoregulatory mechanisms, allowing for an expanded T-cell response and greater antitumor effects [Krummel and Allison, 1995]. Ipilimumab, a fully human monoclonal antibody, inhibits negative signals sent to T cells through the cell-surface molecule cytotoxic T lymphocyte antigen-4 (CTLA-4), thus blocking a negative checkpoint and removing a physiologic brake on the immune system. This first-in-class immune checkpoint inhibitor was approved by the US Food and Drug Administration (FDA) for the treatment of metastatic melanoma, based on overall benefit seen in clinical trials. Ipilimumab has been evaluated in late-stage clinical trials in patients with metastatic castration-resistant prostate cancer (mCRPC) [Hodi et al. 2010].
Sipuleucel-T (Provenge®)
Sipuleucel-T is an FDA-approved autologous dendritic cell vaccine designed to target PAP. It is currently used to treat minimally symptomatic or asymptomatic mCRPC. A patient’s peripheral blood mononuclear cells are harvested and shipped to a central processing facility where APCs are enriched by density centrifugation and pulsed with PA2024, a fusion protein consisting of PAP linked to the immunomodulatory cytokine granulocyte-macrophage colony-stimulating factor (GM-CSF) [Patel and Kockler, 2008; Rini, 2002]. The resulting product must meet a minimum threshold of CD54 expression, a marker of APC activation, before it can be released for use. The vaccine is then infused into the patient three times at biweekly intervals. A pair of small phase III trials of sipuleucel-T showed no improvement in time to progression (TTP), the primary endpoint, but did show a consistent benefit in overall survival (OS). These results led to the larger phase III registration trial known as IMPACT (n = 512), which was designed with OS rather than TTP as the primary endpoint. At a median follow up of 34 months, patients treated with sipuleucel-T showed significantly improved OS compared with placebo [25.8 versus 21.7 months; hazard ratio (HR) 0.78; 95% confidence interval (CI 0.61–0.98) [Kantoff et al. 2010a]. As in the earlier trials, there was no significant change in time to radiographic or PSA progression, and there were few sustained declines in PSA >50%.
Since the FDA approval of sipuleucel-T, further studies have shed light on the types of patients who may derive the most benefit from immunotherapy. A retrospective analysis of the IMACT trial found that patients in the lowest quartile of PSA values received the greatest benefit from the vaccine, with a 13-month improvement in OS (41.3 months with sipuleucel-T versus 28.3 months with placebo; HR 0.51; 95% CI 0.35–0.85). In contrast, patients in the highest baseline PSA quartile had a median OS of 18.4 versus 15.6 months for placebo (HR 0.84; 95% CI 0.55–1.29), an improvement of only 2.8 months [Schellhammer et al. 2013]. Recent retrospective data also suggest evidence of antigen cascade with sipuleucel-T [Drake et al. 2014a], as previously described with the prostate cancer vaccine PROSTVAC-VF [Gulley et al. 2014]. With sipuleucel-T, the target antigen is PAP, while immunoglobulin G (IgG) antibodies to PAP serve as evidence of adaptive immune response. While no anti-PAP IgG antibodies were induced in the placebo group, patients in the treatment group showed evidence of induction that was statistically significant for this and a number of other antigens as well. This retrospective analysis revealed that OS was greater in patients receiving sipuleucel-T who had IgG responses to >2 secondary antigens (antigen spreading) compared with patients who had no such response (Cox model, p 6 0.01, HR 6 0.4) [Drake et al. 2014a]. In all three trials, sipuleucel-T was well-tolerated, with minimal toxicities (headache, transient fever, and flu-like symptoms were most frequently reported).
Trials investigating optimal sequencing of sipuleucel-T and androgen-deprivation therapy (ADT), as well as the use of concurrent and sequential abiraterone, are ongoing. In order to evaluate the impact of concurrent abiraterone on product characteristics, an ongoing phase II trial is examining sipuleucel-T with concurrent or sequential abiraterone plus prednisone. Preliminary results have found no significant differences in median cumulative CD54 upregulation and CD54+ count between arms A and B. Increased CD54 upregulation with the second and third sipuleucel-T vaccinations indicated a prime-boost effect in both arms. These data suggest that sipuleucel-T can be manufactured during treatment with abiraterone and prednisone while maintaining product potency and a prime-boost effect similar to sipuleucel-T alone [Small et al. 2013]. Additionally, preliminary data from the long-term phase II STAND study were recently presented at the European Association of Urology (EAU) Annual Congress [Antonarakis et al. 2014]. The STAND study is a randomized phase II trial with two treatment arms: one completes treatment with sipuleucel-T 2 weeks before the start of ADT; the second begins sipuleucel-T treatment 3 months after the start of ADT. Preliminary results suggest enhanced cellular immune response when sipuleucel-T is given after ADT. These responses were robust and persisted for at least 12 months in both patient groups [Antonarakis et al. 2014].
PSA-TRICOM (PROSTVAC-VF)
PSA-TRICOM is a poxviral vector-based vaccine consisting of a priming dose of recombinant vaccinia followed by five or six boosts with recombinant fowlpox [Madan et al. 2009; Longo, 2010]. Both the vaccinia and fowlpox vectors are engineered to express PSA and three costimulatory molecules (TRICOM) designed to enhance the immune response. PSA-TRICOM is an off-the-shelf vaccine that can be cost-effectively produced in large quantities. Frozen doses are thawed and injected into patients, making PSA-TRICOM a logistically simple yet immunologically advanced vaccine. Several early trials demonstrated that the prime-boost regimen was well tolerated, with toxicities consisting mainly of transient flu-like symptoms and injection-site reactions [Arlen et al. 2007; Eder et al. 2000; Marshall et al. 2000; Gulley et al. 2002]. As in trials of sipuleucel-T, a randomized double-blind placebo-controlled phase II trial of PSA-TRICOM in men with mCRPC showed no difference in TTP [Kantoff et al. 2010b]. However, a mature follow up showed that PSA-TRICOM conferred significantly improved OS (25.1 versus 16.6 months; HR 0.56; 95% CI 0.37–0.85), with a 3-year survival of 30% versus 17% for placebo [Kantoff et al. 2010b]. TAA-specific responses were also observed by ELIPSOT in patients treated with PSA-TRICOM. Specifically, ELISPOT analyses of patients’ T-cell responses to the PSA epitope in the vaccine demonstrated a trend toward a difference in OS for patients with a >6-fold postvaccination ELISPOT response to PSA versus patients with a <6-fold postvaccination ELISPOT response to PSA (p = 0.055) [Gulley et al. 2010].
An international phase III trial is currently open and accruing 1200 patients with asymptomatic or minimally symptomatic mCRPC, with OS as the primary endpoint [ClinicalTrials.gov identifier: NCT01322490]. Patients are randomized to receive PSA-TRICOM with adjuvant GM-CSF, PSA-TRICOM with placebo GM-GSF, or wildtype fowlpox with placebo GM-CSF. This trial is expected to complete accrual in 2014.
GVAX
GVAX vaccine is based on a platform of irradiated hormone-sensitive (LNCaP) and hormone-resistant (PC-3) prostate cancer cell lines genetically modified to secrete GM-CSF. Two phase III trials of this allogeneic cell-based vaccine have had disappointing results [Cell Genesys, 2008a, 2008b]. VITAL-1 randomized asymptomatic mCRPC patients to receive vaccine or docetaxel-prednisone, but was terminated early when estimates showed a <30% chance that the trial would meet its primary endpoint. Preliminary data suggested an HR of 1.01, indicating no improvement in OS compared with docetaxel, even though GVAX was associated with fewer serious adverse events (4.2 versus 16.9 %). GVAX is currently being tested in the neoadjuvant setting in a phase II trial in which patients with medium- to high-risk disease are randomized to hormonal therapy alone versus GVAX followed by hormonal therapy. All patients then undergo radical prostatectomy. The primary endpoint of this ongoing trial is to determine intraprostatic CD8+ T-cell infiltration [ClinicalTrials.gov identifier: NCT01696877].
Listeria-based vaccines
A live-attenuated recombinant Listeria monocytogenes (Lm)-based PSA vaccine is currently in development for use in prostate cancer. Listeria is an intracellular pathogen that is actively phagocytosed by APCs. It replicates in the cytosol after escaping from the phagosome, through expression of its virulence factors [Barry et al. 1992; Tilney and Portnoy, 1989]. By processing antigens through the MHC I pathway, Lm can generate CD8+ immune responses, making it an ideal vector for cancer vaccines. Novel technology allows attenuated Listeria to be given safely to humans, providing an expanded therapeutic window [Brockstedt and Dubensky, 2008]. Listeria-based vaccines have been employed in several malignancies, including pancreatic cancer and mesothelioma [Clinical trial identifier for NCT01675765; Le et al. 2013].
Therapeutic cancer vaccines have the ability to induce an immune response, which in turn can lead to killing of tumor cells. Patients with no underlying antitumor immune response may still benefit from a therapeutic vaccine. On the other hand, immune checkpoint inhibitors like ipilimumab and anti-PD-1/PDL-1 require an underlying immune response to be clinically active. In that scenario, immune checkpoint inhibitors can unleash underlying, but previously ineffective, immune responses.
Ipilimumab
Ipilimumab, a fully human anti-CTLA-4 monoclonal antibody, is a first-in-class immune checkpoint inhibitor. CTLA-4, the most extensively studied immune checkpoint blockade, is expressed on cytotoxic T lymphocytes (CTLs) after activation by APCs. The CTLA-4 receptor on CTLs is a negative regulator of T-cell activation that outcompetes CD28 for binding to B7 on APCs. In contrast to CD28/B7 binding, which acts as a costimulatory signal, the binding of CTLA-4 by ipilimumab removes the physiologic brake, augmenting the immune response by blocking the interaction of CTLA and B7 [Krummel and Allison, 1995].
Ipilimumab was FDA-approved in 2011 for the treatment of unresectable or metastatic melanoma. The approval was based on a randomized (3:1:1) double-blind clinical trial that demonstrated an OS benefit in patients receiving ipilimumab. Phase III trials of ipilimumab are ongoing in both chemotherapy-naïve and chemotherapy-refractory mCRPC, based on the results of early-phase studies [Slovin et al. 2013]. The first trial randomizes chemotherapy-naïve patients to ipilimumab versus placebo [ClinicalTrials.gov identifier: NCT01057810]. The second compares limited radiation plus ipilimumab to limited radiation plus placebo in patients previously treated with chemotherapy [ClinicalTrials.gov identifier: NCT00861614]. As presented at the European Cancer Congress, results of this trial showed a median OS favoring ipilimumab over placebo (11.2 versus 10 months; HR 0.85, 95% CI 0.72–1.00), though statistical significance was not achieved (p = 0.053). Interestingly, median progression-free survival (PFS) also favored ipilimumab over placebo (HR 0.70, 95% CI 0.61–0.82), as did PSA declines of ≥50% in evaluable patients (13.1% versus. 5.3%) [Gerritsen et al. 2013]. Results of subset analyses of OS were recently reported at the 2014 American Society of Clinical Oncology (ASCO) Genitourinary Cancers Symposium [Drake et al. 2014b]. In this retrospective analysis, patients with visceral metastases or higher disease burden had a shorter OS and did not appear to have a treatment effect with ipilimumab (HR 1.644, 95% CI 1.157–2.336). These results support the concept that immunotherapy given earlier in the course of disease may produce better outcomes and suggests the need for further investigation of ipilimumab in mCRPC patients prechemotherapy.
Growth rate kinetics and antigen cascade
There are several key distinctions between conventional and immune-based therapies for cancer [Gulley et al. 2013]. Response to immunotherapy is best described as an iterative process, in which the immune response improves and broadens over time. Following administration of therapeutic vaccines and other immunotherapies, the combination of immunogenic tumor targeting often results in a delayed response, and in some cases tumors actually progress before they regress [Harty and Badovinac, 2008]. While the initial immune response to a single TAA is quite brisk, the activated immune response may lead to the development of long-lived memory cells that can sustain clinical benefit beyond the period of treatment [Schlom et al. 2007]. Over time, the immune response may broaden to target TAAs not found in the initial vaccine construct. This antigen cascade is an iterative process that may persist long-term and become more clinically relevant over time [Gulley, 2013]. This delayed yet prolonged response leads to a different kinetic profile of clinical response following immunotherapy compared with conventional treatments.
In an analysis of clinical trials conducted in prostate cancer at the US National Cancer Institute (NCI) over the last decade, investigators evaluated tumor burden as measured by PSA [Stein et al. 2011]. They found a reliable decrease in tumor burden in a majority of patients treated with the most aggressive chemotherapy regimen. However, when disease invariably began to progress, it appeared that the tumor growth rate, as measured by PSA, rose to prechemotherapy levels, allowing for easy predictions of survival [Madan et al. 2011]. Therapeutic cancer vaccines, on the other hand, have a very different kinetic profile. In the same retrospective analysis of NCI trials, the majority of patients receiving immunotherapy had no significant decrease in tumor burden as measured by PSA. This may have been due in part to antigen cascade, where over time the dynamic, iterative immune response becomes broader and more clinically relevant. Indeed, patients lived much longer than predicted based on this model, suggesting an eventual slowing of the tumor growth rate. This change in growth rate kinetics may partially explain why clinical trials with immunotherapy see improvements in OS without an advantage in PFS [Madan et al. 2010]. If this model is correct, it suggests that rational combinations of standard-of-care therapies with immunotherapy could control disease long enough to generate a clinically significant immune response that could slow the tumor growth rate. The net result could be an improvement in PFS compared with standard-of-care alone, something not routinely seen in studies of immunotherapy alone. This could accelerate proof-of-concept studies and potentially shorten the development cycle of new immunotherapeutic agents.
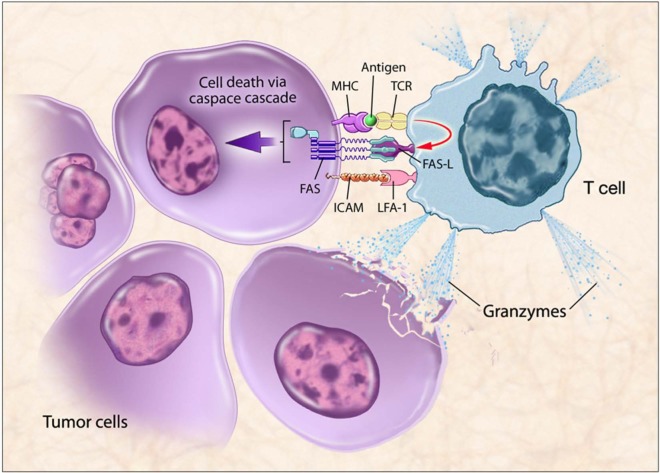
To be effective, immunotherapies must generate an antitumor immune response. TAA-specific lymphocytes must travel to the tumor, recognize it, and remain functional within the tumor microenvironment. They can only do this when TCRs recognize antigen peptides bound to appropriate molecules of the MHC complex on cancer cells. When T cells specific for that MHC-antigen complex become activated, they upregulate Fas ligand, bind to Fas on the tumor cell, and initiate a downstream caspase cascade, leading to tumor cell apoptosis. Activated T cells can also release granzymes that can kill the target cell and surrounding tumor cells. Thus, expression of MHC and TAAs, as well as Fas, on the tumor cell enables T-cell recognition and killing of tumor cells (Figure 1).
Figure 1.
T-cell mediated killing of tumor cells.
Therapeutic cancer vaccines are designed to stimulate immune cells to target specific tumor-associated antigens (TAAs) overexpressed on cancer cells. Antigen-presenting cells (APCs) present antigens to the immune system via major histocompatibility complex molecules, which bind to T-cell receptors (TCRs). Activated T-cells travel to the tumor, recognized by the TAAs presented in the context of the major histocompatibility complex (MHC), leading to T cell-mediated killing of tumor cells by immunogenic cell death. When T cells specific for that MHC-antigen complex become activated, they upregulate Fas ligand, bind to Fas on the tumor cell, and initiate a downstream caspase cascade, leading to tumor cell apoptosis. Activated T cells can also release granzymes that can kill the target cell and surrounding tumor cells. Thus, expression of MHC and TAAs, as well as Fas, on the tumor cell enables T-cell recognition and killing of tumor cells.
FAS-L, Fas-ligand; ICAM, intracellular adhesion molecule; LFA-1, lymphocyte function-associated antigen 1.
Combination therapy (Table 1)
Table 1.
Selected combination immunotherapy trials for patients with prostate cancer.
| Phase | Agent | NCT number | Study design | Primary endpoint | Expected completion date |
|---|---|---|---|---|---|
| II | Sipuleucel-T/ ADT | NCT01431391 | Patients with nonmetastatic prostate cancer randomized to receive sipuleucel-T before or after ADT | Immune response | August 2014 |
| II | Sipuleucel-T/ Abiraterone | NCT01487863 | Patients with metastatic CRPC randomized to receive sipuleucel-T plus abiraterone and prednisone, administered either sequentially or concurrently | Immune response (including PAP-specific T-cell response); safety | July 2015 |
| II | Sipuleucel-T/ Enzalutamide | NCT01981122 | Patients with metastatic CRPC randomized to receive sipuleucel-T plus enzalutamide, administered either sequentially or concurrently | Immune response | September 2015 |
| II | Flutamide ±PSA-TRICOM/ | NCT00450463 | Patients with nonmetastatic CRPC randomized to receive flutamide with or without PSA-TRICOM | Time to treatment failure | April 2014 |
| II | Enzalutamide ±PSA-TRICOM | NCT01875250 | Patients with nonmetastatic castration-sensitive prostate cancer randomized to receive enzalutamide for 3 months with or without PSA-TRICOM | Decrease in tumor regrowth rate | June 2016 |
| II | Enzalutamide ±PSA-TRICOM | NCT01867333 | Patients with metastatic CRPC randomized to receive enzalutamide for 3 months with or without PSA-TRICOM | Increase in time to progression | June 2016 |
| II | Prostvac versus. Nilutamide | NCT00020254 | Patients with nonmetastatic castration-sensitive prostate cancer randomized to receive nilutamide or Prostvac | Time to progression | Completed |
| II | PSA-TRICOM/ ADT | NCT00108732 | Patients with PSA progression after local therapy randomized to receive PSA-TRICOM followed by ADT | Biochemical PSA progression | Completed |
| II | Prostvac ± Docetaxel | NCT00045227 | Patients with metastatic CRPC randomized to receive Prostvac with or without concurrent docetaxel | Immune response | Completed |
| III | Docetaxel/ GVAX | NCT00089856 | Patients with metastatic CRPC randomized to receive docetaxel or GVAX | Overall survival | Terminated |
| III | Ipilimumab/ XRT | NCT00861614 | Patients with metastatic CRPC post chemo randomized to ipilimumab/XRT compared with placebo/XRT | Overall survival | Completed |
| II | 153Sm-EDTMP (Quadramet)/ PSA-TRICOM | NCT00450619 | Patients with metastatic CRPC randomized to 153Sm-EDTMP with or without PSA-TRICOM | Progression-free survival at 4 months | Completed |
| I | Ipilimumab/ GVAX | NCT01510288 | Patients with metastatic CRPC treated with GVAX and escalating doses of ipilimumab | Safety | Completed |
| I | Ipilimumab/ PSA-TRICOM | NCT00113984 | Patients with metastatic CRPC treated with PSA-TRICOM and escalating doses of ipilimumab | Safety | Completed |
| Pilot | Sipuleucel-T/ Anti-PD1 antibody | NCT01420965 | Patients with metastatic CRPC randomized to sipuleucel-T with or without anti-PD1 and cyclophosphamide | Feasibility and immune response | December 2017 |
ADT, androgen-deprivation therapy; CRPC, castration-resistant prostate cancer; PAP, prostatic acid phosphatase; PSA, prostate-specific antigen; XRT, radiotherapy.
Immunogenic modulation
Many standard antitumor therapies can modulate tumor phenotype in a way that facilitates immune recognition and killing, a phenomenon called immunogenic modulation. A growing body of clinical evidence suggests that various modalities, such as radiation and hormonal therapy, play a role in upregulating the antitumor immune response [Hodge et al. 2013]. The killing of tumor cells can lead to immunogenic cell death, in which cells are taken up by APCs and presented to the immune system, a concept that has been fully reviewed elsewhere [Kroemer et al. 2013]. However, for the patient, the cells that survive this process are more clinically relevant. Preclinical models show that low-dose radiation upregulates MHC class I, adhesion molecules and Fas, as well as novel pools of peptides [Chakraborty et al. 2008]. Data from a small randomized phase II study involving radiation therapy suggest a potential impact on tumor that may be immunologically relevant [Gulley et al. 2005; Hodge et al. 2008]. This facilitates a broader immune response, or antigen spreading, which may be more clinically relevant than direct tumor cell lysis.
153Sm-EDTMP is a therapeutic agent consisting of radioactive samarium (153Sm) and ethylenediamine tetra(methylene phosphonic acid) (EDTMP), a phosphonic acid with chelating properties. With a structure similar to phosphorus, 153Sm-EDTMP preferentially binds to osteoblastic metastatic tumor deposits in bone. 153Sm-EDTMP is currently FDA-approved for palliation of bone metastases in multiple malignancies. In vitro studies have shown that 153Sm-EDTMP upregulates TAAs, MHC class I and Fas, making it easier for the immune system to recognize and kill tumor cells [Chakraborty et al. 2003, 2004]. In preclinical studies, LNCaP cells incubated with 153Sm-EDTMP were more susceptible to T-cell killing. In a small randomized phase II study, patients with CRPC metastatic to bone who had prior docetaxel were randomized to receive 153Sm-EDTMP with or without vaccine [ClinicalTrials.gov identifier: NCT00450619]. In this proof-of-concept study, PFS, the primary endpoint, was about double in the combination arm as the control, and combination therapy was associated with a favorable PSA response [Heery et al. 2013].
There is also a convincing biologic rationale for combining ADT with immunotherapy. Preclinical data show that ADT increases thymic size; data from patients suggest that ADT promotes new activity within the thymus and significantly increases thymic immigrants [Aragon-Ching et al. 2007]. In the neoadjuvant setting, patients treated with ADT prior to radical prostatectomy showed an increase in CD3+ cells within the prostate [Mercader et al. 2001]. Finally, in murine models ADT has been shown to break tolerance to prostate-associated antigens [Drake et al. 2005].
Preclinical studies involving enzalutamide, a novel androgen receptor antagonist, have enhanced the rationale for combination therapy. In murine models, treatment with enzalutamide increased thymic weight and serum levels were similar to what is clinically relevant [Ardiani et al. 2013]. New naïve T cells were produced, as measured indirectly by TCR excision circles, suggesting increased thymic function. Even more intriguing is the observation that enzalutamide appears to mediate immunogenic modulation. TRAMP-C2 prostate cells incubated with enzalutamide had a five-fold increase in MHC class I expression, and levels of Fas on the surface of tumor cells nearly doubled, enhancing tumor-cell recognition and killing [Ardiani et al. 2013]. Studies in TRAMP models evaluating treatment with vaccine, enzalutamide or the combination of both found no activity with either agent as monotherapy. On the other hand, mice treated with the combined agents had a substantial increase in survival (27 versus 10 weeks).
In a recently published case report [Graff et al. 2013], a patient with mCRPC initially treated with enzalutamide had a good response but eventually progressed biochemically. He was then treated with sipuleucel-T and achieved an initial stabilization followed by a sustained complete biochemical response. A randomized phase II trial is currently enrolling patients with nonmetastatic CRPC to receive a 3-month course of enzalutamide with or without PSA-TRICOM [ClinicalTrials.gov identifier: NCT01875250]. The primary endpoint of this trial is to determine the vaccine’s effects on PSA growth kinetics after enzalutamide is discontinued. This proof-of-concept study will help define the benefits of combining ADT with immunotherapy. A similar study (enzalutamide until progression versus enzalutamide with PSA-TRICOM) is being conducted in chemotherapy-naïve patients with mCRPC [ClinicalTrials.gov identifier: NCT01867333].
Immunogenic intensification
One of the most compelling areas of study in immunotherapy is the effects of combining immunotherapeutic approaches, a process known as immunogenic intensification. Novel approaches such as combining vaccine with immune checkpoint inhibitors highlight the potential of immunogenic intensification [Tarassoff et al. 2006], whereby combining immunotherapies could potentially generate a greater immune response and enhance tumor cell killing. Previous clinical studies have shown evidence of enhanced clinical outcomes without increased toxicity. For example, combining ipilimumab, an anti-CTLA-4 antibody, and PSA-TRICOM provides direct T-cell activation while removing physiologic brakes on the immune system, allowing for a more robust CTL response. Activated T cells upregulate CTLA-4, which sends a negative regulatory signal to T cells, a signal that is blocked by anti-CTLA-4 antibody. Anti-CTLA-4 may also increase T-cell avidity [Hodge et al. 2005; Simpson et al. 2013]. Regulatory T cells (Tregs) constitutively express CTLA-4. Simpson and colleagues recently demonstrated that anti-CTLA-4 antibody can selectively deplete Tregs within the tumor [Simpson et al. 2013].
A phase I study treated 30 patients with docetaxel-refractory or chemotherapy-naïve mCRPC with a fixed dose of PSA-TRICOM in conjunction with escalating doses of ipilimumab given at monthly intervals. Of the 24 patients who were chemotherapy-naïve, 14 (58%) had PSA declines, 6 of which (25%) were >50%. The median OS of patients treated with this combination was 34.4 months with a median Halabi predicted survival of 17.2 months. In a like-sized trial at the same institution of a parallel patient population, patients were treated with the PSA-TRICOM vaccine alone. In this trial, the median Halabi predicted survival was 17.2 months with an OS of 26.3 months. [Gulley et al. 2010; Madan et al. 2012]. These hypothesis-generating data suggest that intensification of immune-based therapies by combining vaccine with immunotherapies may improve clinical outcomes. A recently published analysis of immune correlates demonstrated trends toward associations for longer OS with specific immune subsets before immunotherapy. There were also trends for longer OS favoring a lower Halabi score, a longer PSA doubling time, and a higher baseline hemoglobin level [Jochems et al. 2014].
Based on preclinical models suggesting synergy between ipilimumab and GVAX [Hurwitz et al. 2000], a phase I dose escalation trial enrolled chemotherapy-naïve patients with mCRPC and treated them with GVAX and concurrent intravenous ipilimumab for 24 weeks. PSA declines of >50% were observed in 25% of men, with a median OS of 29.2 months [van den Eertwegh et al. 2012]. Approximately 29% of patients experienced grade 3 immune-related adverse events (5 mg/kg led to a dose-limiting toxicity in one patient). Exploratory T-cell monitoring revealed prolonged OS for patients with high pretreatment frequencies of CD4 + CTLA-4+, CD4 + PD-1+, or differentiated CD8+ T cells, or low pretreatment frequencies of CD4+ cells. Unsupervised clustering of these immune biomarkers revealed cancer-related expression of CTLA-4 in CD4+ T cells to be a dominant predictor of survival after GVAX/ipilimumab treatment, highlighting the significance of biomarkers and patient selection in immunotherapy [Santegoets et al. 2013]. Early safety and efficacy studies of ipilimumab plus vaccine have given way to trials of combination therapy with immune checkpoint inhibitors. These trials, in turn, provide a rationale for combining prostate cancer vaccines with monoclonal antibodies that target immune checkpoint inhibitors including programmed cell death protein 1 (PD-1) or its ligand (PD-L1) [Brahmer et al. 2010; Brahmer, 2012; Topalian et al. 2012].
Conclusion
Recent FDA approval of multiple agents with proven benefit in prostate cancer has laid the groundwork for studies of rational sequential or combination regimens (Table 2). The lack of additive toxicities and the possibility of immunogenic modulation make the combination of traditional modalities with immunotherapy, as well as the combination of vaccine with immune checkpoint inhibitors, very compelling. Our growing understanding of how best to employ immunotherapy, along with mounting enthusiasm for this approach, make it reasonable to contemplate major advances in the treatment of prostate cancer in the near future as combination and sequential therapies continue to emerge.
Table 2.
Completed phase III trials: immunotherapy and therapeutic cancer vaccines in prostate cancer.
| Agent | Primary endpoint | Comments |
|---|---|---|
| Sipuleucel-T (two identically designed, randomized, double-blind, placebo-controlled trials) D9901, D9902A | Time to disease progression | Improved OS, no improvement in TTPIntegrated analysis (n = 225)Treatment group with 33% reduction in risk of death (HR 1.50; 95% CI 1.10–2.05; p = 0.011) |
| Sipuleucel-T (IMPACT) | OS | Improved OS compared with placebo (n = 512): 25.8 versus 21.7 months; HR 0.78; 95% CI, 0.61–0.98Led to FDA approval in 2010 |
| GVAX (VITAL-1)GVAX versus docetaxel-prednisone | OS | GVAX compared with docetaxel (HR 1.01) – trial stopped early after futility analysis |
| GVAX (VITAL-2)Docetaxel versus docetaxel-GVAX | OS | Apparent worse outcome with vaccine compared to docetaxel – trial halted |
| IpilimumabDouble-blind trial ipilimumab versus placebo | OS | No improvement in OS (HR 0.85; 95% CI 0.72–1.00; p = 0.053); however, improved OS in subgroup with less advanced diseaseApparent improvement in PFS |
CI, confidence interval; FDA, Food and Drug Administration; HR, hazard ratio; OS, overall survival; PFS, progression-free survival; TTP, time to progression.
Footnotes
Funding: The authors acknowledge the Intramural Research Program of the NIH, National Cancer Institute, Center for Cancer Research for their support of this study.
Conflict of interest statement: The authors declare no conflicts of interest in preparing this article.
Contributor Information
B. Harpreet Singh, Genitourinary Malignancies Branch, Center for Cancer Research, National Cancer Institute, National Institutes of Health, Bethesda, MD, USA.
James L. Gulley, Chief, Genitourinary Malignancies Branch, Center for Cancer Research, National Cancer Institute, National Institutes of Health, 10 Center Drive, 12N226, Bethesda, MD 20892, USA
References
- CRS-207 Cancer Vaccine in Combination With Chemotherapy as Front-line Treatment for Malignant Pleural Mesothelioma. Available at: http://clinicaltrials.gov/show/NCT01675765
- Antonarakis E., Kibel A., Adams G., Karsh L., Elfiky A., Shore N., et al. (2014) A randomized phase 2 study evaluating the optimal sequencing of sipuleucel-T and androgen deprivation therapy in biochemically-recurrent prostate cancer: Immune results with a focus on humoral responses. In: Proceedings of the European Association of Andrology Annual Congress, Stockholm, 11–15 April, poster 980 [Google Scholar]
- Aragon-Ching J., Williams K., Gulley J. (2007) Impact of androgen-deprivation therapy on the immune system: implications for combination therapy of prostate cancer. Front Biosci 12: 4957–4971 [DOI] [PubMed] [Google Scholar]
- Ardiani A., Farsaci B., Rogers C., Protter A., Guo Z., King T., et al. (2013) Combination therapy with a second-generation androgen receptor antagonist and a metastasis vaccine improves survival in a spontaneous prostate cancer model. Clin Cancer Res 19: 6205–6218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arlen P., Skarupa L., Pazdur M., Seetharam M., Tsang K., Grosenbach D., et al. (2007) Clinical safety of a viral vector based prostate cancer vaccine strategy. J Urol 178: 1515–1520 [DOI] [PubMed] [Google Scholar]
- Barry R., Bouwer H., Portnoy D., Hinrichs D. (1992) Pathogenicity and immunogenicity of Listeria monocytogenes small-plaque mutants defective for intracellular growth and cell-to-cell spread. Infect Immun 60: 1625–1632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bostwick D., Pacelli A., Blute M., Roche P., Murphy G. (1998) Prostate specific membrane antigen expression in prostatic intraepithelial neoplasia and adenocarcinoma: a study of 184 cases. Cancer 82: 2256–2261 [DOI] [PubMed] [Google Scholar]
- Brahmer J. (2012) PD-1-targeted immunotherapy: recent clinical findings. Clin Adv Hematol Oncol 10: 674–675 [PubMed] [Google Scholar]
- Brahmer J., Drake C., Wollner I., Powderly J., Picus J., Sharfman W., et al. (2010) Phase I study of single-agent anti-programmed death-1 (MDX-1106) in refractory solid tumors: safety, clinical activity, pharmacodynamics, and immunologic correlates. J Clin Oncol 28: 3167–3175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brockstedt D., Dubensky T. (2008) Promises and challenges for the development of Listeria monocytogenes-based immunotherapies. Exp Rev Vaccines 7: 1069–1084 [DOI] [PubMed] [Google Scholar]
- Cell Genesys (2008a) Cell Genesys Announces Termination of VITAL-1 Phase 3 Trial of GVAX Immunotherapy for Prostate Cancer; available at http://www.drugs.com/news/cell-genesys-announces-termination-vital-1-phase-3-trial-gvax-immunotherapy-prostate-cancer-14159.html (accessed 28 May 2014).
- Cell Genesys (2008b) Cell Genesys Halts VITAL-2 GVAX Trial in Advanced Prostate Cancer; available at http://www.businesswire.com/news/home/20080827005471/en/Cell-Genesys-Halts-VITAL-2-GVAX-Trial-Advanced-.UxX-rY7iMqY (accessed 28 May 2014).
- Chakraborty M., Abrams S., Camphausen K., Liu K., Scott T., Coleman C., et al. (2003) Irradiation of tumor cells up-regulates Fas and enhances CTL lytic activity and CTL adoptive immunotherapy. J Immunol 170: 6338–6347 [DOI] [PubMed] [Google Scholar]
- Chakraborty M., Abrams S., Coleman C., Camphausen K., Schlom J., Hodge J. (2004) External beam radiation of tumors alters phenotype of tumor cells to render them susceptible to vaccine-mediated T-cell killing. Cancer Res 64: 4328–4337 [DOI] [PubMed] [Google Scholar]
- Chakraborty M., Wansley E., Carrasquillo J., Yu S., Paik C., Camphausen K., et al. (2008) The use of chelated radionuclide (samarium–153-ethylenediaminetetramethylenephosphonate) to modulate phenotype of tumor cells and enhance T cell-mediated killing. Clin Cancer Res 14: 4241–4249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drake C., Doody A., Mihalyo M., Huang C., Kelleher E., Ravi S., et al. (2005) Androgen ablation mitigates tolerance to a prostate/prostate cancer-restricted antigen. Cancer Cell 7: 239–249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drake C., Fan L., Thakurta D., Stewart F., Kantoff P., Small E., et al. (2014a) Antigen spread and survival with sipuleucel-T in patients with advanced prostate cancer. J Clin Oncol 32(Suppl. 4): abstract 88 [Google Scholar]
- Drake C., Kwon E., Fizazi K., Bossi A., van den Eertwegh A., Logothetis C., et al. (2014b) Results of subset analyses on overall survival (OS) from study CA184–043: Ipilimumab (Ipi) versus placebo (Pbo) in post-docetaxel metastatic castration-resistant prostate cancer (mCRPC). J Clin Oncol 32(Suppl. 4): abstract 2 [Google Scholar]
- Eder J., Kantoff P., Roper K., Xu G., Bubley G., Boyden J., et al. (2000) A phase I trial of a recombinant vaccinia virus expressing prostate-specific antigen in advanced prostate cancer. Clin Cancer Res 6: 1632–1638 [PubMed] [Google Scholar]
- Gerritsen W., Fizazi A., Drake C. (2013) A randomized, multicenter, double-blind phase 3 trial comparing overall survival (OS) in patients (pts) with post-docetaxel castration resistant prostate cancer (CRPC) and bone metastases treated with ipilimumab (ipi) versus placebo (pbo), each following single-dose radiotherapy (RT). In: Proceedings of the European Cancer Congress, Amsterdam, 27 September to 1 October, abstract 2850 [Google Scholar]
- Goldfarb D., Stein B., Shamszadeh M., Petersen R. (1986) Age-related changes in tissue levels of prostatic acid phosphatase and prostate specific antigen. J Urol 136: 1266–1269 [DOI] [PubMed] [Google Scholar]
- Graff J., Drake C., Beer T. (2013) Complete biochemical (prostate-specific antigen) response to sipuleucel-T with enzalutamide in castration-resistant prostate cancer: a case report with implications for future research. Urology 81: 381–383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulley J. (2013) Therapeutic vaccines: the ultimate personalized therapy? Hum Vaccin Immunother 9: 219–221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulley J., Arlen P., Bastian A., Morin S., Marte J., Beetham P., et al. (2005) Combining a recombinant cancer vaccine with standard definitive radiotherapy in patients with localized prostate cancer. Clin Cancer Res 11: 3353. [DOI] [PubMed] [Google Scholar]
- Gulley J., Arlen P., Madan R., Tsang K., Pazdur M., Skarupa L., et al. (2010) Immunologic and prognostic factors associated with overall survival employing a poxviral-based PSA vaccine in metastatic castrate-resistant prostate cancer. Cancer Immunol Immunother 59: 663–674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulley J., Chen A., Dahut W., Arlen P., Bastian A., Steinberg S., et al. (2002) Phase I study of a vaccine using recombinant vaccinia virus expressing PSA (rV-PSA) in patients with metastatic androgen-independent prostate cancer. Prostate 53: 109–117 [DOI] [PubMed] [Google Scholar]
- Gulley J., Drake C. (2011) Immunotherapy for prostate cancer: recent advances, lessons learned, and areas for further research. Clin Cancer Res 17: 3884–3891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulley J., Madan R., Heery C. (2013) Therapeutic vaccines and immunotherapy in castration-resistant prostate cancer. Am Soc Clin Oncol Educ Book: 166–170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulley J., Madan R., Tsang K., Jochems C., Marte J., Farsaci B., et al. (2014) Immune impact induced by PROSTVAC (PSA-TRICOM), a therapeutic vaccine for prostate cancer. Cancer Immunol Res 2: 133–141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harty J., Badovinac V. (2008) Shaping and reshaping CD8+ T-cell memory. Nat Rev Immunol 8: 107–119 [DOI] [PubMed] [Google Scholar]
- Heery C., Madan R., Bilusic M., Kim J., Singh N., Rauckhorst M., et al. (2013) A phase II randomized clinical trial of samarium-153 EDTMP (Sm-153) with or without PSA-tricom vaccine in metastatic castration-resistant prostate cancer (mCRPC) after docetaxel. J Clin Oncol 31(Suppl. 6): abstract 102 [Google Scholar]
- Hodge J., Chakraborty M., Kudo-Saito C., Garnett C., Schlom J. (2005) Multiple costimulatory modalities enhance CTL avidity. J Immunol 174: 5994–6004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodge J., Garnett C., Farsaci B., Palena C., Tsang K., Ferrone S., et al. (2013) Chemotherapy-induced immunogenic modulation of tumor cells enhances killing by cytotoxic T lymphocytes and is distinct from immunogenic cell death. Int J Cancer 133: 624–636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodge J., Guha C., Neefjes J., Gulley J. (2008) Synergizing radiation therapy and immunotherapy for curing incurable cancers. Opportunities and challenges. Oncology 22: 1064–1070; discussion 1075,, 1080,–1061, 1084 [PMC free article] [PubMed] [Google Scholar]
- Hodi F., O’Day S., McDermott D., Weber R., Sosman J., Haanen J., et al. (2010) Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med 363: 711–723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurwitz A., Foster B., Kwon E., Truong T., Choi E., Greenberg N., et al. (2000) Combination immunotherapy of primary prostate cancer in a transgenic mouse model using CTLA-4 blockade. Cancer Res 60: 2444–2448 [PubMed] [Google Scholar]
- Jochems C., Tucker J., Tsang K., Dahut W., Liewehr D., et al. (2014) A combination trial of vaccine plus ipilimumab in metastatic castration-resistant prostate cancer patients: immune correlates. Cancer Immunol Immunother 63: 407–418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kantoff P., Higano C., Shore N., Berger E., Small E., Penson D., et al. (2010a) Sipuleucel-T immunotherapy for castration-resistant prostate cancer. N Engl J Med 363: 411–422 [DOI] [PubMed] [Google Scholar]
- Kantoff P., Schuetz T., Blumenstein B., Glode L., Bilhartz D., Wyand M., et al. (2010b) Overall survival analysis of a phase II randomized controlled trial of a Poxviral-based PSA-targeted immunotherapy in metastatic castration-resistant prostate cancer. J Clin Oncol 28: 1099–1105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroemer G., Galluzzi L., Kepp O., Zitvogel L. (2013) Immunogenic cell death in cancer therapy. Annu Rev Immunol 31: 51–72 [DOI] [PubMed] [Google Scholar]
- Krummel M., Allison J. (1995) CD28 and CTLA-4 have opposing effects on the response of T cells to stimulation. J Exp Med 182: 459–465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le D., Wang-Gillam A., Picozzi V., Greten T., Crocenzi T., Springett G., et al. (2013) Interim safety and efficacy analysis of a phase II, randomized study of GVAX pancreas and CRS-207 immunotherapy in patients with metastatic pancreatic cancer. J Clin Oncol 31(Suppl.): abstract 4040 [Google Scholar]
- Longo D. (2010) New therapies for castration-resistant prostate cancer. N Engl J Med 363: 479–481 [DOI] [PubMed] [Google Scholar]
- Madan R., Aragon-Ching J., Gulley J., Dahut W. (2011) From clinical trials to clinical practice: therapeutic cancer vaccines for the treatment of prostate cancer. Expert Rev Vaccines 10: 743–753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madan R., Arlen P., Mohebtash M., Hodge J., Gulley J. (2009) Prostvac-VF: a vector-based vaccine targeting PSA in prostate cancer. Exp Opin Investig Drugs 18: 1001–1011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madan R., Gulley J., Fojo T., Dahut W. (2010) Therapeutic cancer vaccines in prostate cancer: the paradox of improved survival without changes in time to progression. Oncologist 15: 969–975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madan R., Mohebtash M., Arlen P., Vergati M., Rauckhorst M., Steinberg S., et al. (2012) Ipilimumab and a poxviral vaccine targeting prostate-specific antigen in metastatic castration-resistant prostate cancer: a phase 1 dose-escalation trial. Lancet Oncol 13: 501–508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall J., Hoyer R., Toomey M., Faraguna K., Chang P., Richmond E., et al. (2000) Phase I study in advanced cancer patients of a diversified prime-and-boost vaccination protocol using recombinant vaccinia virus and recombinant nonreplicating avipox virus to elicit anti-carcinoembryonic antigen immune responses. J Clin Oncol 18: 3964–3973 [DOI] [PubMed] [Google Scholar]
- Mercader M., Bodner B., Moser M., Kwon P., Park E., Manecke R., et al. (2001) T cell infiltration of the prostate induced by androgen withdrawal in patients with prostate cancer. Proc Natl Acad Sci U S A 98: 14565–14570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel P., Kockler D. (2008) Sipuleucel-T: a vaccine for metastatic, asymptomatic, androgen-independent prostate cancer. Ann Pharmacother 42: 91–98 [DOI] [PubMed] [Google Scholar]
- Rini B. (2002) Technology evaluation: APC-8015, Dendreon. Curr Opin Mol Ther 4: 76–79 [PubMed] [Google Scholar]
- Santegoets S., Stam A., Lougheed S., Gall H., Scholten P., Reijm M., et al. (2013) T cell profiling reveals high CD4+CTLA-4 + T cell frequency as dominant predictor for survival after prostate GVAX/ipilimumab treatment. Cancer Immunol Immunother 62: 245–256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schellhammer P., Chodak G., Whitmore J., Sims R., Frohlich M., Kantoff P. (2013) Lower baseline prostate-specific antigen is associated with a greater overall survival benefit from sipuleucel-T in the Immunotherapy for Prostate Adenocarcinoma Treatment (IMPACT) trial. Urology 81: 1297–1302 [DOI] [PubMed] [Google Scholar]
- Schlom J., Arlen P., Gulley J. (2007) Cancer vaccines: moving beyond current paradigms. Clin Cancer Res 13: 3776–3782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson T., Li F., Montalvo-Ortiz W., Sepulveda M., Bergerhoff K., Arce F., et al. (2013) Fc-dependent depletion of tumor-infiltrating regulatory T cells co-defines the efficacy of anti-CTLA-4 therapy against melanoma. J Exp Med 210: 1695–1710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slovin S., Higano C., Hamid O., Tejwani S., Harzstark A., Alumkal J., et al. (2013) Ipilimumab alone or in combination with radiotherapy in metastatic castration-resistant prostate cancer: results from an open-label, multicenter phase I/II study. Ann Oncol 24: 1813–1821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Small E., Lance R., Gardner T., Karsh L., Stubbs A., McCoy C., et al. (2013) A randomized phase II, open-label study of sipuleucel-T with concurrent or sequential abiraterone acetate (AA) in metastatic castrate-resistant prostate cancer (mCRPC). J Clin Oncol 31(Suppl. 6): abstract 114 [Google Scholar]
- Stein W., Gulley J., Schlom J., Madan R., Dahut W., Figg W., et al. (2011) Tumor regression and growth rates determined in five intramural NCI prostate cancer trials: the growth rate constant as an indicator of therapeutic efficacy. Clin Cancer Res 17: 907–917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarassoff C., Arlen P., Gulley J. (2006) Therapeutic vaccines for prostate cancer. Oncologist 11: 451–462 [DOI] [PubMed] [Google Scholar]
- Tilney L., Portnoy D. (1989) Actin filaments and the growth, movement, and spread of the intracellular bacterial parasite, Listeria monocytogenes. J Cell Biol 109: 1597–1608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Topalian S., Hodi F., Brahmer J., Gettinger S., Smith D., McDermott D., et al. (2012) Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med 366: 2443–2454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van den Eertwegh A., Versluis J., van den Berg H., Santegoets S., van Moorselaar R., van der Sluis T., et al. (2012) Combined immunotherapy with granulocyte-macrophage colony-stimulating factor-transduced allogeneic prostate cancer cells and ipilimumab in patients with metastatic castration-resistant prostate cancer: a phase 1 dose-escalation trial. Lancet Oncol 13: 509–517 [DOI] [PubMed] [Google Scholar]
- Wang M., Valenzuela L., Murphy G., Chu T. (1979) Purification of a human prostate specific antigen. Invest Urol 17: 159–163 [PubMed] [Google Scholar]