A 2 + 1 seven-valent pneumococcal conjugate vaccine schedule is effective against vaccine-serotype invasive pneumococcal disease (IPD) in HIV-uninfected children and HIV-exposed but -uninfected children and against all-serotype multidrug-resistant IPD in HIV-uninfected children.
Keywords: children, HIV, pneumococcus, pneumococcal conjugate vaccine, South Africa
Abstract
Background. South Africa introduced 7-valent pneumococcal conjugate vaccine (PCV7) in April 2009 using a 2 + 1 schedule (6 and 14 weeks and 9 months). We estimated the effectiveness of ≥2 PCV7 doses against invasive pneumococcal disease (IPD) in human immunodeficiency virus (HIV)–infected and -uninfected children.
Methods. IPD (pneumococcus identified from a normally sterile site) cases were identified through national laboratory-based surveillance. Specimens were serotyped by Quellung or polymerase chain reaction. Four controls, matched for age, HIV status, and hospital were sought for each case. Using conditional logistic regression, we calculated vaccine effectiveness (VE) as 1 minus the adjusted odds ratio for vaccination.
Results. From March 2010 through November 2012, we enrolled 187 HIV-uninfected (48 [26%] vaccine serotype) and 109 HIV-infected (43 [39%] vaccine serotype) cases and 752 HIV-uninfected and 347 HIV-infected controls aged ≥16 weeks. Effectiveness of ≥2 PCV7 doses against vaccine-serotype IPD was 74% (95% confidence interval [CI], 25%–91%) among HIV-uninfected and −12% (95% CI, −449% to 77%) among HIV-infected children. Effectiveness of ≥3 doses against vaccine-serotype IPD was 90% (95% CI, 14%–99%) among HIV-uninfected and 57% (95% CI, −371% to 96%) among HIV-infected children. Among HIV-exposed but -uninfected children, effectiveness of ≥2 doses was 92% (95% CI, 47%–99%) against vaccine-serotype IPD. Effectiveness of ≥2 doses against all-serotype multidrug-resistant IPD was 96% (95% CI, 62%–100%) among HIV-uninfected children.
Conclusions. A 2 + 1 PCV7 schedule was effective in preventing vaccine-serotype IPD in HIV-uninfected and HIV-exposed, uninfected children. This finding supports the World Health Organization recommendation for this schedule as an alternative to a 3-dose primary series among HIV-uninfected individuals.
The pneumococcal polysaccharide-protein conjugate vaccine (PCV) is recommended for use globally, particularly in developing countries with a high childhood mortality [1]. A clinical trial in South Africa of a 9-valent PCV (PCV9) administered at 6, 10, and 14 weeks of age (ie, 3 + 0 schedule, 3-dose primary series and no booster dose) demonstrated efficacy of 83% (95% confidence interval [CI], 39%–97%) in HIV-uninfected children and 65% (95% CI, 24%–86%) in HIV-infected children against vaccine-serotype (VT) invasive pneumococcal disease (IPD) [2]. The 7-valent PCV (PCV7), administered in 3 + 1 or 2 + 1 schedules, has been shown to be highly effective against IPD in developed countries [3–10].
South Africa introduced PCV7 into the Expanded Program on Immunization (EPI) in April 2009 [11]. A novel, accelerated 2 + 1 schedule (6 weeks, 14 weeks, and early booster at 9 months), with no catch-up, was used [11]. This schedule was based on evidence of sufficient immunogenicity with 2 primary doses, cost savings afforded by a 2- rather than 3-dose primary series, data indicating waning efficacy without a booster dose in HIV-infected children (approximately 4% of South African children <5 years in 2009), and the need to deliver the primary and the booster doses at the youngest possible ages [12–14]. The 13-valent PCV (PCV13) replaced PCV7 in June 2011.
There are no published studies evaluating the effectiveness of routine PCV use on disease in Africa. Additionally, the effectiveness of the accelerated 2 + 1 schedule is unknown. Our primary objectives were to determine the effectiveness of ≥2 doses of routinely administered PCV7 against VT IPD and all-serotype IPD among HIV-uninfected and HIV-infected children. In addition, we evaluated whether HIV exposure altered vaccine effectiveness (VE), because the increasing availability of interventions for prevention of mother-to-child transmission (PMTCT) of HIV in high HIV-prevalence settings has led to increasing numbers of HIV-exposed but -uninfected children; however, there are no published data on PCV efficacy or effectiveness in this group [15, 16].
METHODS
Ethics
The study protocol was approved by institutional review boards at the University of the Witwatersrand, the surveillance sites, the Centers for Disease Control and Prevention, and the Johns Hopkins Bloomberg School of Public Health.
Study Population and Study Design
We conducted a matched case-control study. Cases were defined as an episode of illness in an individual with identification of Streptococcus pneumoniae from normally sterile-site specimens (eg, cerebrospinal fluid [CSF], blood, pleural fluid, joint fluid) at 24 sentinel surveillance hospitals. Eligible cases and controls were aged ≥8 weeks at the time of specimen collection or admission, eligible to receive at least 1 dose of PCV through the EPI, and resident in South Africa from 6 weeks of age.
Pneumococcal isolates were serotyped by Quellung using specific antisera, including serotypes 6A, 6B, 6C, and 6D (Statens Serum Institut, Copenhagen, Denmark). VTs were serotypes included in PCV7 (4, 6B, 9V, 14, 18C, 19F, 23F). Serotype 6A was deemed vaccine-related due to cross-protection with PCV7 [8]. All other serotypes were designated as nonvaccine types. Streptococcus pneumoniae identification and susceptibility testing was based on standardized methodologies [17]. Multidrug resistance was defined as nonsusceptibility to ≥3 different antibiotic classes [18]. Specimen source was defined as CSF, blood culture, and other (eg, pleural fluid, joint fluid). Clinical syndrome was defined hierarchically as follows: meningitis, bacteremic pneumonia, bacteremia without focus (clinical signs consistent with sepsis but no clinical pneumonia or meningitis, or other focal infection), and other.
We aimed to enroll at least 4 controls per case, matching to the case by date of birth, hospital, and HIV status. Children admitted to or attending outpatient departments at the same hospital as the case were eligible. Children were excluded as potential controls if they had IPD, pneumonia, or another nondiarrheal vaccine-preventable disease. We enrolled HIV-infected controls from HIV clinics if the clinic did not have a policy of active review of vaccination status or offer vaccination. Exclusion criteria for cases and controls included absence of verified HIV status, previous enrollment as a case, enrollment of a twin, and reporting receiving any dose of PCV13 before the case specimen date. For controls with febrile seizures, clinical investigations were performed as indicated by the attending physician and these cases were reviewed by a study medical officer to exclude possible meningitis, otitis, or pneumonia.
Data Collection
Data were collected through standardized interviews of guardians and patient records review. Data from 1 month preceding the date of pneumococcal specimen collection (the reference period) were collected from each case and their matched controls. Children with a history of being HIV infected were included as HIV infected. HIV testing is recommended for all hospitalized children with unknown HIV status in South Africa and was performed by enzyme-linked immunosorbent assay (ELISA) with confirmation by ELISA on a second specimen for children ≥18 months of age, and qualitative HIV DNA polymerase chain reaction testing for children <18 months of age. Documented maternal HIV status data was sought for all children from antenatal records or recent testing. CD4+ lymphocyte counts were determined at clinician discretion by flow cytometry [19]. Children were classified as having severe immunosuppression based on CD4+ percentage of total lymphocyte cell count [20]. Children were classified as HIV exposed but uninfected if they had a documented HIV-negative status but positive maternal HIV status. Children with weight-for-age z scores in the reference period <−2 using the 2009 World Health Organization (WHO) child growth standards (adjusting for prematurity for those born before 37 weeks' gestation) and those with nutritional edema were classified as being malnourished [21]. Written documentation of immunization history was sought for all cases and controls, from patient-held immunization records and vaccination records at health facilities, as relevant. Patients giving a history of not receiving any vaccines were recorded as unvaccinated.
Sample Size
We assumed VE against all-serotype IPD of 40% in HIV-uninfected and 55% in HIV-infected children and against PCV7 serotypes of 85% in HIV-uninfected and 65% in HIV-infected children [2]. We assumed a case-control PCV7 vaccination correlation of 0.2 [22]. Assuming vaccine coverage of 60% with a 4:1 match of controls to cases at a significance level (α) of .05 and a power of 0.80, we needed to enroll 171 HIV-uninfected cases (13 vaccine serotype) and 70 HIV-infected cases (42 vaccine serotype).
Statistical Analysis
We used surveillance data to compare the characteristics of enrolled and non enrolled IPD case patients. PCV doses were counted only if received ≥14 days before the specimen collection date. The matched odds ratio of vaccination (vs no vaccination), controlling for confounders, was estimated using conditional logistic regression. We evaluated each individual potential confounder to identify those that altered the odds ratio of PCV vaccination by >10% irrespective of statistical significance; these were further evaluated in multivariable models [23]. We did not group related confounders. We included a single set of confounders for HIV-uninfected children and a second set for HIV-infected children for all adjusted VE analyses to ease comparisons of VE estimates within each group. VE was calculated as 1 minus the adjusted matched odds ratio ×100%. P values <.05 were considered statistically significant. VE in subgroups for which cases and controls were not matched (eg, HIV exposure) was evaluated by inclusion of an interaction term in the multivariable model. For the primary objective (to assess effectiveness of ≥2 doses of PCV7) we included all children aged ≥16 weeks (old enough to receive the 14-week dose plus 2 weeks for an immune response) in the analysis. To assess the effectiveness of ≥3 doses of PCV7, we included children aged ≥41 weeks. Children aged 8–15 weeks contributed to the analysis of the effectiveness of a single PCV dose. Additional details of case and control enrollment, laboratory methods, and statistical analysis are provided in the Supplementary Data.
RESULTS
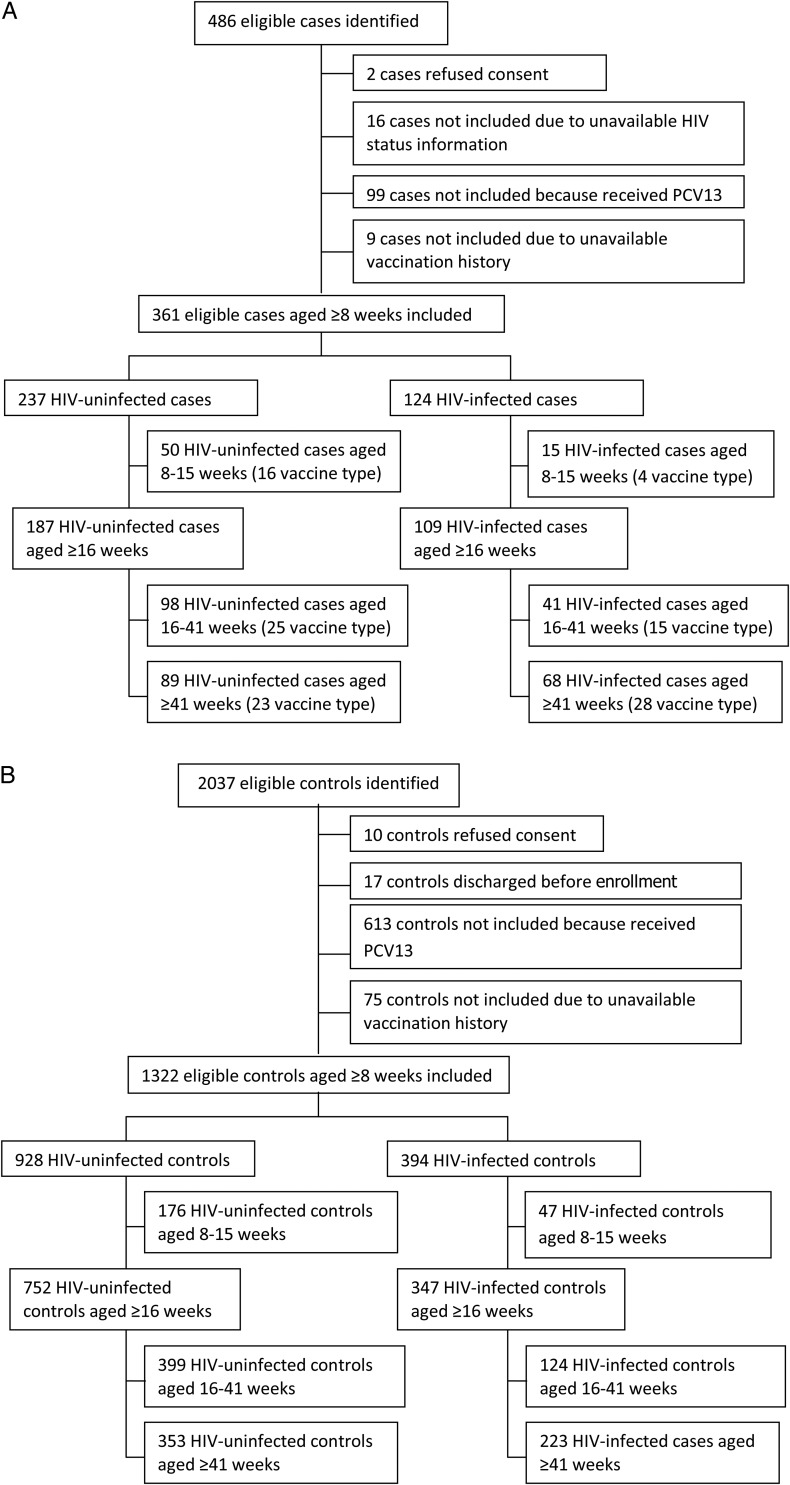
From March 2010 through November 2012, we identified 486 eligible children with IPD, of whom 126 were excluded (Figure 1A). We included 361 case patients aged ≥8 weeks; 237 (66%) were HIV uninfected. For the main analysis of the effectiveness of ≥2 doses, we included 296 children aged ≥16 weeks (187 [63%] HIV uninfected). The median age of all enrolled case patients was 43 weeks (interquartile range [IQR], 17–112), 51% (184/361) were male, 97% (351/361) were hospitalized, and the commonest clinical syndrome was bacteremic pneumonia (182/361 [50%]), followed by meningitis (121/361 [34%]), bacteremia without focus (44/361 [12%]), and other (14/361 [4%]). Cases included did not differ statistically from nonenrolled cases with regard to HIV infection status, sex, race, or case-fatality ratio (data not shown) but did differ with regard to specimen type and province (Supplementary Data).
Figure 1.
Flowchart of patients enrolled in the study. A, Cases. B, Controls. Abbreviations: HIV, human immunodeficiency virus; PCV13, 13-valent pneumococcal vaccine.
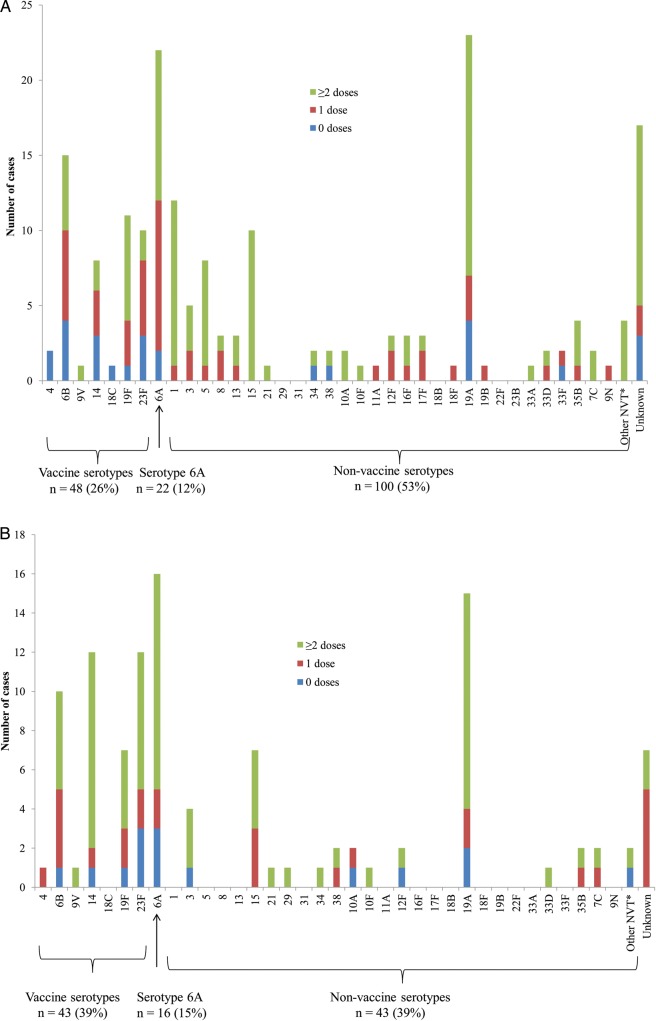
Among HIV-uninfected cases aged ≥16 weeks, 26% (48/187) had VT disease and 35% of these (17/48) had received ≥2 doses of PCV (Figure 2). An additional 12% (22/187) of disease was due to serotype 6A. Of available isolates from HIV-uninfected children ≥16 weeks, 49% (79/161) were nonsusceptible to penicillin and 16% (25/161) were multidrug resistant (MDR). Among HIV-infected cases aged ≥16 weeks, 39% (43/109) had VT disease and 63% (27/43) had received ≥2 doses of PCV7 (Figure 2). An additional 15% (16/109) of disease was due to serotype 6A. Among all isolates from HIV-infected children (≥16 weeks), 67% (68/101) were nonsusceptible to penicillin and 30% (32/101) were MDR. Among all cases, 67% (96/144) of penicillin-nonsusceptible and 85% (46/54) of MDR isolates with available serotyping data were VT or serotype 6A.
Figure 2.
Number of cases included in the analysis (aged ≥16 weeks) by serotype and vaccination status. A, Human immunodeficiency virus (HIV)–uninfected patients (n = 187). B, HIV-infected patients (n = 109). *Confirmed to be a nonvaccine type on polymerase chain reaction (PCR). Unknown serotypes occurred either because an isolate was not available or because only serogroup(s) could be determined using PCR. Abbreviation: NVT, nonvaccine type.
We identified 2037 eligible age-matched children as potential controls, of whom 715 were excluded (Figure 1B). The median number of controls per case was 4 for HIV-uninfected and 3 for HIV-infected children. The median interval between case specimen collection and control enrollment was 30 days (IQR, 4–144) for HIV-uninfected and 84 days (IQR, 9–276) for HIV-infected controls. Among HIV-uninfected controls aged ≥8 weeks (n = 928), 389 (42%) had a diagnosis of diarrhea, 133 (14%) had a surgical diagnosis (including burns), 87 (9%) had diarrhea and malnutrition, 74 (8%) had malnutrition alone, 68 (7%) had febrile seizures, and 177 (19%) had another diagnosis (Supplementary Data). Among HIV-infected controls aged ≥8 weeks (n = 394), 176 (45%) were enrolled during an HIV-clinic visit, 66 (17%) had diarrhea and malnutrition, 64 (16%) had malnutrition alone, 60 (15%) had diarrhea alone, and 28 (7%) had another diagnosis. HIV-uninfected and -infected controls aged ≥16 weeks were similar to cases in age and sex distribution but differed for other characteristics (Table 1).
Table 1.
Characteristics of HIV-Uninfected and -Infected Cases and Controls Aged ≥16 Weeks, South African Invasive Pneumococcal Disease Case-Control Study of 7-Valent Pneumococcal Conjugate Vaccine Effectiveness
| Characteristic | HIV-Uninfected |
HIV-Infected |
||||
|---|---|---|---|---|---|---|
| Cases (n = 187) | Controls (n = 752) | P Valuea | Cases (n = 109) | Controls (n = 347) | P Valuea | |
| Demographics | ||||||
| Age, wk, median (IQR) | 39 (18–107) | 38 (16–106) | .596 | 52 (18–123) | 54 (20–115) | .440 |
| Male | 94/187 (50) | 440/752 (59) | .070 | 57/109 (52) | 178/347 (51) | .739 |
| Not black race | 19/187 (10) | 129/751 (17) | .018 | 4/109 (4) | 19/347 (5) | .316 |
| Risk factors | ||||||
| Malnutritionb | 71/184 (39) | 207/669 (31) | .027 | 70/105 (67) | 107/288 (37) | <.001 |
| Low birth weightc | 40/180 (22) | 149/738 (20) | .351 | 19/107 (18) | 71/340 (21) | .493 |
| Pretermd | 36/173 (21) | 98/707 (14) | .074 | 12/100 (12) | 38/310 (12) | .945 |
| Underlying conditions (not HIV)e | 37/187 (20) | 105/752 (14) | .136 | 18/109 (17) | 41/347 (12) | .087 |
| Smoking exposure | 43/183 (24) | 180/752 (24) | .838 | 26/108 (24) | 68/346 (20) | .387 |
| Day care attendance | 44/183 (24) | 129/751 (17) | .025 | 14/108 (13) | 37/347 (11) | .490 |
| No. of children aged <5 y in household | ||||||
| 0 | 87/181 (48) | 447/751 (60) | .018 | 62/108 (57 | 232/344 (67) | .396 |
| 1–2 | 84/181 (46) | 580/751 (37) | 42/108 (39) | 101/344 (29) | ||
| ≥3 | 10/181 (6) | 24/751 (3) | 4/108 (4) | 11/344 (3) | ||
| Wood fire in home | 15/184 (8) | 43/752 (6) | .098 | 7/108 (6) | 18/347 (5) | .688 |
| Previous hospital admission (in past 12 mo) | 55/185 (30) | 145/752 (19) | .001 | 49/109 (45) | 122/346 (35) | .026 |
| Breastfed in reference periodf | 73/185 (39) | 255/751 (34) | .136 | 30/108 (28) | 45/346 (13) | <.001 |
| Socioeconomic factors | ||||||
| Residence in an informal dwelling | 49/185 (26) | 220/752 (29) | .845 | 33/109 (30) | 107/347 (31) | .973 |
| Crowding | ||||||
| ≤2 people/room | 78/181 (43) | 356/752 (47) | .185 | 53/108 (49) | 153/346 (44) | .595 |
| 3–4 people/room | 72/181 (40) | 308/752 (41) | 42/108 (39) | 141/346 (41) | ||
| 5–30 people/room | 31/181 (17) | 86/752 (11) | 13/108 (12) | 52/346 (15) | ||
| Maternal education | ||||||
| No secondary | 31/181 (17) | 100/750 (13) | .013 | 21/108 (19) | 73/346 (21) | .119 |
| Some secondary | 108/181 (60) | 407/750 (54) | 56/108 (52) | 200/346 (58) | ||
| Completed secondary | 42/181 (23) | 243/750 (33) | 31/108 (29) | 73/346 (21) | ||
| Has a car | 18/187 (10) | 142/752 (19) | .004 | 19/109 (17) | 41/346 (12) | .222 |
| HIV-related factors | ||||||
| HIV exposed | 79/181 (44) | 217/725 (30) | .001 | |||
| HIV clinic attendance | 22/103 (21) | 195/336 (58) | <.001 | |||
| HIV stage | ||||||
| 1 | 8/104 (8) | 51/329 (16) | .002 | |||
| 2 | 3/104 (3) | 17/329 (5) | ||||
| 3 | 38/104 (37) | 146/329 (44) | ||||
| 4 | 55/104 (53) | 115/329 (35) | ||||
| Receiving HAART | 28/106 (26) | 178/339 (53) | <.001 | |||
| Severe immunosuppression | 41/54 (76) | 113/205 (55) | <.001 | |||
| Receiving trimethoprim-sulfamethoxazole prophylaxis | 10/182 (5) | 25/661 (3) | .214 | 51/108 (47) | 219/344 (64) | .025 |
| Current tuberculosis treatment | 1/183 (1) | 9/661 (1) | .469 | 22/108 (20) | 45/340 (13) | .039 |
| Vaccines | ||||||
| Hepatitis B at 16 wk | 140/187 (75) | 595/752 (79) | .322 | 81/109 (74) | 292/347 (84) | .071 |
| DTP vaccine at 16 wk | 106/187 (57) | 504/752 (67) | .013 | 67/109 (61) | 264/347 (76) | .011 |
| PCV7 ≥2 doses | 110/187 (60) | 509/752 (67) | .109 | 68/109 (62) | 246/347 (71) | .466 |
| PCV7 ≥3 doses | 30/187 (16) | 165/752 (22) | .049 | 26/109 (24) | 85/347 (25) | .438 |
| Age of receipt of PCV7 doses, wk, median (IQR) | ||||||
| Dose 1 | 6 (5–17) | 6 (5–17) | .265 | 6 (5–17) | 6 (5–22) | .321 |
| Dose 2 | 15 (13–39) | 15 (13–31) | .739 | 16 (13–43) | 16 (13–39) | 1.000 |
| Dose 3 | 40 (20–51) | 40 (25–48) | .785 | 40 (38–62) | 40 (38–52) | .597 |
Abbreviations: DTP, diphtheria, tetanus, pertussis; HAART, highly active antiretroviral therapy; HIV, human immunodeficiency virus; IQR, interquartile range; PCV7, 7-valent pneumococcal conjugate vaccine.
a Matched.
b Weight <80% of expected for age adjusted for prematurity or edema.
c <2500 g.
d Less than 37 completed weeks.
e Asplenia, including asplenia or sickle cell anemia; chronic illness, including chronic lung, renal, liver, cardiac disease, and diabetes; other immunocompromising conditions (excluding HIV), including organ transplant, primary immunodeficiency, immunotherapy, and malignancy; and other risk factors, including head injury with possible cerebrospinal fluid leak, neurological disorders, burns, and chromosomal abnormalities.
f Reference period is the 1 month preceding the date of pneumococcal specimen collection.
Among HIV-uninfected children aged ≥16 weeks (ie, post–primary series), the adjusted effectiveness of ≥2 doses of PCV7 was 74% (95% CI, 25%–91%) against VT disease, 70% (28%–88%) against VTs plus serotype 6A, and 29% (95% CI −27% to 60%) against all-serotype IPD (Table 2). Among HIV-uninfected children aged ≥41 weeks, the adjusted effectiveness of ≥3 doses of PCV7 was 90% (95% CI, 14%–99%) against VT IPD and 63% (95% CI, −1% to 87%) against all-serotype IPD. There was no significant VE against non-VT disease. Among HIV-infected children aged ≥16 weeks, the adjusted effectiveness of ≥2 doses of PCV7 was −12% (95% CI, −449% to 77%) against VT disease and 6% (95% CI, −194% to 70%) for all-serotype IPD, and confidence intervals were wide. VE confidence intervals for VT and all-serotype IPD following ≥3 doses at ≥41 weeks were also wide (Table 2).
Table 2.
Effectiveness of 7-Valent Pneumococcal Conjugate Vaccine Against Invasive Pneumococcal Disease in HIV-Infected and -Uninfected Children by Pneumococcal Serotype
| Outcome (No. of Cases/No. of Controls) | Unadjusted VE% (95% CI) | Adjusted VE% (95% CI)a |
|---|---|---|
| HIV-uninfected, ≥16 wk, ≥2 doses vs 0 doses | ||
| PCV7 serotypes (48/194) | 77 (40–91) | 74 (25–91) |
| PCV7 serotypes plus 6A (71/289) | 71 (35–87) | 70 (28–88) |
| All serotypes (187/752) | 35 (−13 to 63) | 29 (−27 to 60) |
| Nonvaccine serotypes (101/403) | −56 (−315 to 41) | −76 (−384 to 36) |
| HIV-uninfected, ≥41 wk, ≥3 doses vs 0 doses | ||
| PCV7 serotypes (23/86) | 57 (−100 to 91) | 90 (14 to 99) |
| PCV7 serotypes plus 6A (31/122) | 47 (−109 to 87) | 78 (−15 to 96) |
| All serotypes (89/353) | 47 (−37 to 79) | 63 (−1 to 87) |
| Nonvaccine serotypes (48/195) | 2 (−433 to 82) | 21 (−390 to 87) |
| HIV-infected, ≥16 wk, ≥2 doses vs 0 doses | ||
| PCV7 serotypes (43/137) | 15 (−145 to 71) | −12 (−449 to 77) |
| PCV7 serotypes plus 6A (60/188) | 34 (−94 to 78) | 29 (−174 to 81) |
| All serotypes (109/347) | 31 (−42 to 67) | 6 (−194 to 70) |
| Nonvaccine serotypes (44/136) | 20 (−197 to 79) | −190 (−2997 to 73) |
| HIV-infected, ≥41 wk, ≥3 doses vs 0 doses | ||
| PCV7 serotypes (28/86) | 43 (−108 to 85) | 57 (−371 to 96) |
| PCV7 serotypes + 6A (37/116) | 53 (−49 to 85) | 76 (−87 to 97) |
| All serotypes (68/223) | 26 (−84 to 70) | 46 (−122 to 87) |
| Nonvaccine serotypes (26/87) | −72 (−966 to 72) | 76 (−166 to 318) |
Abbreviations: CI, confidence interval; HIV, human immunodeficiency virus; PCV7, 7-valent pneumococcal conjugate vaccine; VE, vaccine effectiveness.
a Adjusted for use of a wood fire in the home, number of children in the home aged <5 years, and maternal education level for HIV-uninfected children. Adjusted for receipt of trimethoprim-sulfamethoxazole prophylaxis, malnutrition, presence of severe immunosuppression on CD4+ T-cell count, and whether the patient had received 3 doses of hepatitis B vaccine at 16 weeks of age for HIV-infected children.
The adjusted VE for ≥2 doses among HIV-exposed but -uninfected children aged ≥16 weeks was 92% (95% CI, 47%–99%) against VT IPD (Table 3). The adjusted VE of ≥2 doses for HIV-uninfected children aged ≥16 weeks against all IPD due to penicillin-nonsusceptible disease was 50% (95% CI, −15% to 79%) and against MDR IPD was 96% (95% CI, 62%–100%). Point estimates of VE were lower for malnourished children than for nonmalnourished children and for HIV-infected children with severe immunosuppression compared to others, but numbers in each subgroup for these analyses were small and differences were not statistically significant. Among HIV-uninfected children, receipt of 2 primary doses alone or 2 primary doses plus a booster dose had similar effectiveness against VT disease (Table 4). A single dose of PCV7 given at about 6 weeks provided no protection against VT IPD.
Table 3.
Effectiveness of ≥2 Doses of 7-Valent Pneumococcal Conjugate Vaccine Versus 0 Doses Against Invasive Pneumococcal Disease in HIV-Uninfected and -Infected Children Aged ≥16 Weeks by HIV Exposure, Malnutrition Status, and Type of Disease
| Risk Groupa | No. of Cases/No. of Controls | Outcome | Unadjusted VE% (95% CI) | Adjusted VE% (95% CI)b |
|---|---|---|---|---|
| HIV uninfected | ||||
| HIV exposed | 21/57 | PCV7 serotypes | 91 (54–98) | 92 (47–99) |
| HIV unexposed | 27/133 | PCV7 serotypes | 72 (1–92) | 58 (−73 to 90) |
| HIV exposed | 79/217 | All serotypes | 12 (−87 to 58) | 8 (−102 to 16) |
| HIV unexposed | 102/508 | All serotypes | 57 (−3 to 82) | 51 (−25 to 86) |
| Meningitis | 13/55 | PCV7 serotypes | 85 (−12 to 98) | 93 (−6 to 100) |
| Bacteremic pneumonia | 20/85 | PCV7 serotypes | 39 (−194 to 87) | 78 (−60 to 97) |
| Malnourishedc | 19/49 | PCV7 serotypes | 57 (−79 to 90) | 66 (−79 to 80) |
| Not malnourished | 28/121 | PCV7 serotypes | 84 (41–96) | 81 (19–96) |
| Multidrug-resistant IPD | 161/637 | All serotypes | 94 (55–99) | 96 (62–100) |
| Penicillin-nonsusceptible IPD | 161/637 | All serotypes | 54 (−2 to 79) | 50 (−15 to 79) |
| HIV infected | ||||
| Severe immunosuppressiond | 26/73 | PCV7 serotypes | −146 (−2119 to 73) | −202 (−3199 to 72) |
| No severe immunosuppression | 7/48 | PCV7 serotypes | 81 (−32 to 97) | 67 (−222 to 97) |
| Malnourished | 31/53 | PCV7 serotypes | −53 (−547 to 64) | −35 (−814 to 80) |
| Not malnourished | 10/68 | PCV7 serotypes | 36 (−790 to 95) | 24 (−1358 to 96) |
Abbreviations: CI, confidence interval; HIV, human immunodeficiency virus; IPD, invasive pneumococcal disease; VE, vaccine effectiveness.
a VE in subgroups for which cases and controls were not matched (HIV exposure, malnutrition, severe immunosuppression) was evaluated by inclusion of an interaction term for the subgroup of interest in the multivariable model. P > .1 for all interactions evaluated except for HIV exposure where P = .081.
b Adjusted for use of a wood fire in the home, number of children in the home aged <5 years, and maternal education level for HIV-uninfected children. Adjusted for receipt of trimethoprim-sulfamethoxazole prophylaxis, malnutrition, presence of severe immunosuppression on CD4+ T- cell count, and whether the patient had received 3 doses of hepatitis B vaccine at 16 weeks of age for HIV-infected children.
c Only children with available data on malnutrition status in the reference period were included in this analysis.
d Based on CD4+ percentage of total lymphocyte cell count according to World Health Organization categories [20].
Table 4.
Effectiveness of 7-Valent Pneumococcal Conjugate Vaccine Against Invasive Pneumococcal Disease Caused by Vaccine Serotypes in HIV-Uninfected Children by Number and Timing of Doses
| Schedule (No. of Cases/No. of Controls) | Age Group | Unadjusted VE% (95% CI) | Adjusted VE% (95% CI)a |
|---|---|---|---|
| 1 + 0 vs 0 (64/255) | ≥8 wk | 13 (−90 to 60) | −11 (−167 to 54) |
| 2 + 0 vs 0 (48/194) | ≥16 wk | 82 (48–97) | 76 (27–92) |
| 2 + 0 vs 0 (25/108) | 16–40 wk | 83 (36–96) | 73 (−18 to 94) |
| 2 + 1 vs 0 (23/86) | ≥41 wk | 55 (−117 to 91) | 88 (−3 to 99) |
Abbreviations: CI, confidence interval; VE, vaccine effectiveness.
a Adjusted for use of a wood fire in the home, number of children in the home <5 years, and maternal education level.
DISCUSSION
We have demonstrated effectiveness of 2 doses of PCV7 administered at 6 and 14 weeks of age with a booster dose at 9 months in a low- to middle-income country. A 2 + 1 schedule has been demonstrated to be effective in Europe and North America administered at 2 and 4 or 3 and 5 months of age with a booster dose in the second year of life [7, 10, 24, 25]. Although we were unable to demonstrate effectiveness of this schedule in HIV-infected children, VE in HIV-exposed but -uninfected children was high [16, 26]. The effectiveness against penicillin-nonsusceptible and MDR IPD caused by any serotype was high, indicating that PCV may have a substantial impact in reducing the prevalence of MDR pneumococcal disease, as has been demonstrated in other settings [27].
Effectiveness of ≥2 doses in HIV-uninfected children was 74% (95% CI, 25%–91%) against VT disease, similar to estimates of PCV9 efficacy in HIV-uninfected children administered a 3-dose primary schedule at 6, 10, and 14 weeks of age in South Africa (83%; 95% CI, 39%–97%) and The Gambia (77%; 95% CI, 51%–90%) [2, 28]. This is also similar to the approximately 85% reduction in VT IPD observed in HIV-uninfected children aged <2 years from surveillance data in South Africa (A. von Gottberg, unpublished data). Two primary doses are not as immunogenic as 3 primary doses during infancy, but the differences overall are small [29]. A 2 + 1 schedule is feasible for implementation in low- to middle-income countries with high measles vaccine coverage at 9 months and provides cost savings and reduced number of injections compared with a 4-dose schedule, but still includes a booster dose [11].
The magnitude of the all-serotype IPD VE estimate (29%; 95% CI, −27% to 60%) should not be misinterpreted to mean that PCV confers limited overall impact. The vaccine is effective against VT, but not against non-VT, and the measured all-serotype IPD vaccine effectiveness is a combination of effectiveness against VT and non-VT together. When PCV is highly effective, the majority of remaining cases available to be included in a case-control study are non-VT, therefore resulting in a lower measured VE estimate for all-IPD than efficacy against all-IPD as measured in a randomized clinical trial.
We were unable to demonstrate statistically significant effectiveness of ≥2 PCV7 doses in HIV-infected children. This could reflect a lack of statistical power to detect a lower VE than anticipated. Surveillance data from South Africa have shown a 55% relative reduction in VT compared with non-VT among HIV-infected children aged <2 years following PCV7 introduction (A. von Gottberg, unpublished data). At least some of this reduction likely results from indirect protection [30, 31]. HIV-infected children with CD4+ T-cell percentage ≥25% and delayed highly active antiretroviral therapy (HAART) initiation had similar immunoglobulin G (IgG) antibody responses to HIV-uninfected children for PCV administered at 6 and 10 weeks of age; however, this subgroup had functionally impaired antibody responses as measured by opsonophagocytic activity (OPA) compared to children with early HAART initiation [15, 32]. In the latter study, IgG and OPA (serotype 23F) responses were substantially improved in HIV-infected and -uninfected children following a third PCV7 dose at 14 weeks of age, particularly for serotypes 6B and 23F, for which responses were generally lowest. HIV-infected children may benefit from a full 3-dose infant primary series, as was demonstrated to be effective in the South African clinical trial [2]. Practical implementation of a different vaccination schedule by HIV status may, however, not be feasible in settings where HIV status is not known at 10 weeks of age.
Numbers of HIV-exposed but -uninfected children in South Africa remain high (30% of pregnant women in 2011 were HIV infected) following widespread PMTCT implementation, and this group has an increased risk of severe infections [14, 16, 33, 34]. Importantly, the VE in HIV-exposed but -uninfected children was similar to HIV-unexposed children. Antibody responses have been found to be slightly higher in HIV-exposed but -uninfected children compared with HIV-unexposed children after 2 and 3 doses of PCV, possibly related to less interference from maternal antibodies [15, 32].
Our study has limitations. Controls were enrolled from hospitals and clinics rather than the community and thus may differ in their vaccination and disease risk factor status in unmeasured ways from the general population. In our setting, where barriers may exist to access hospital care, hospital controls may, however, be more similar to cases than community controls with respect to unmeasured factors associated with access to care. Low numbers of HIV-infected hospitalized children led to delays in identification of suitable controls and the potential for poor information recall; vaccination histories were gathered from written records and thus would not have been affected, but this might have been a concern for potential confounder variables. HIV-infected controls enrolled from HIV clinics may have had better access to care, which would have biased toward an overestimate of VE. In addition, this group of controls were less immunosuppressed and more likely to receive HAART than cases. Because controls are more likely to be vaccinated than cases, proportionately more vaccinated controls than cases who had received PCV13 were excluded. This should not have substantially affected our estimate of VE but may have reduced our power to detect an effect. Boys were more common among controls, likely because of high numbers of surgical controls [35]. Although we evaluated a large number of potential confounders in the analysis, residual confounding is possible. Unadjusted and adjusted VE estimates were similar in children aged <41 weeks but differed in older children. This is likely because hospitalization is relatively common in younger children; thus, hospitalized children in this group are probably representative of the general population. Older hospitalized children, however, may have specific risk factors for hospitalization, leading to them being less representative of the source population and therefore more confounding in this age group. For some subanalyses, few cases were observed, limiting our ability to evaluate VE and precluding estimation of effectiveness against individual serotypes.
We were not able to definitively assess the effectiveness of a 2 + 1 schedule in HIV-infected children, but based on existing clinical trial data [2], 3 primary doses should be considered. As coverage with PCV increases among South African children, indirect effects may enhance protection of HIV-infected children [31]. Our study demonstrates that a 2 + 1 schedule of PCV7 aligned with the EPI schedule is effective against VT IPD and MDR IPD in HIV-uninfected and HIV-exposed, -uninfected children, supporting the recent WHO statement indicating use of this alternative schedule in some settings [1].
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online (http://cid.oxfordjournals.org). Supplementary materials consist of data provided by the author that are published to benefit the reader. The posted materials are not copyedited. The contents of all supplementary data are the sole responsibility of the authors. Questions or messages regarding errors should be addressed to the author.
Notes
Acknowledgments. We thank all the participants and their caregivers who kindly agreed to be included in this study; Group for Enteric, Respiratory and Meningeal pathogens Surveillance - South Africa surveillance officers for their tireless efforts to enroll participants and to obtain vaccination histories; laboratory staff throughout the country for submitting isolates to the National Institute for Communicable Diseases (NICD); and the staff at the NICD, Centre for Respiratory Diseases and Meningitis laboratory for their efforts in processing and characterizing these isolates.
Author contributions. Conception and design of study: C. C., S. A. M., K. O. B., E. R. Z., K. K., C. G. W., A. v. G. Data collection and laboratory processing: C. C., C. v. M., L. d. G., N. N., S.M., V. Q., V. N., M. F. d. S., B. M., D. M., G. R., M. M., B. E., U. H., R. K., L. C., A. v. G. Analysis and interpretation: C. C., C. v. M., L. d. G., N. N., S. M., V. Q., V. N., M. F. d. S., B. M., D. M., G. R., M. M., S. A. M., B. E., U. H., R. K., L. C., K. O. B., E. R. Z., K. K., C. G. W., A. v. G. Drafting or critical review of the article: C. C., C. v. M., L. d. G., N. N., S. M., V. Q., V. N., M. F. d. S., B. M., D. M., G. R., M. M., S. A. M., B. E., U. H., R. K., L. C., K. O. B., E. R. Z., K. K., C. G. W., A. v. G.
IPD Case-Control Study Group. Chris Hani Baragwanath Hospital, Paediatrics Department, University of the Witwatersrand, Johannesburg, Gauteng, South Africa: David Moore, Charl Verwey; Charlotte Maxeke Johannesburg Academic Hospital, Paediatrics Department, University of the Witwatersrand, Johannesburg, Gauteng, South Africa: Sheeba Varughese; Nelson R. Mandela School of Medicine, Department of Paediatrics and Child Health, University of KwaZulu-Natal, Durban; and Pietermaritzburg Metropolitan Hospitals Complex, Department of Paediatrics, Pietermaritzburg, KwaZulu-Natal, South Africa: Moherndran Archary, Fathima Naby, Khathija Dawood, Ramola Naidoo; Steve Biko (Pretoria Academic Hospital) and Kalafong Hospital, Paediatric Infectious Diseases Unit, University of Pretoria, Pretoria, Gauteng, South Africa: Theunis Avenant, Nicolette du Plessis; Universitas and Pelonomi Hospitals, Department of Paediatrics and Child Health, and Department of Microbiology, University of the Free State, Bloemfontein, Free State, South Africa: Gene Elliott, Ute Hallbauer; Red Cross War Memorial Children's Hospital, and the Department of Paediatrics and Child Health, University of Cape Town, Cape Town, Western Cape, South Africa: Brian Eley, James Nuttall; Tygerberg Hospital, Department of Paediatric Infectious Diseases, University of Stellenbosch, Cape Town, Western Cape, South Africa: Louise Cooke, Heather Finlayson, Helena Rabie; NHLS/Division of Medical Microbiology, University of Cape Town, Cape Town, Western Cape, South Africa: Andrew Whitelaw; Nelson Mandela Academic Hospital, Paediatric Department, Walter Sisulu University, Mthatha, Eastern Cape, South Africa: Dania Perez; Kimberley Hospital, Paediatrics Department, Kimberley, Northern Cape, South Africa: Pieter Jooste, Dhamiran Naidoo; Rahima Moosa Mother and Child Hospital, Departments of Clinical Microbiology and Infectious Diseases and Paediatrics, Faculty of Health Sciences, University of the Witwatersrand and National Health Laboratory Service, Johannesburg, Gauteng, South Africa: Ranmini Kularatne, Gary Reubenson; National Institute for Communicable Diseases, Sandringham, Johannesburg, Gauteng, South Africa: Cheryl Cohen, Linda de Gouveia, Mignon du Plessis, Nevashan Govender, Susan Meiring, Vanessa Quan, Claire von Mollendorf, Melony Fortuin-de Smit, Nireshni Naidoo, Babatyi Malope-Kgokong, Vusi Nokeri, Relebohile Ncha, Sonwabo Lindani, Anne von Gottberg; Rob Ferreira Hospital, Paediatrics Department, Nelspruit, Mpumalanga, South Africa: Barry Spies; Rustenberg Hospital, Paediatrics Department, Rustenberg, North-West Province, South Africa: Lino Sono; Polokwane & Mankweng Hospitals, Paediatrics Department, Polokwane. Limpopo Province, South Africa: Phasweni Maredi, Ken Hamese; Dr George Mukhari Hospital, Paediatrics Department and Department of Pathology, Medunsa University, Gauteng Province, South Africa: Mamokgethi Moshe, Maphosane Nchabeleng; National Department of Health, Expanded Programme on Immunisation, Pretoria, Gauteng, South Africa: Ntombenhle Ngcobo, Johann van den Heever; Department of Science and Technology/ National Research Foundation: Vaccine Preventable Diseases, Gauteng, South Africa: Shabir Madhi; National Center for Immunization and Respiratory Diseases, Centers for Disease Control and Prevention, Atlanta, Georgia: Laura Conklin, Jennifer Verani, Cynthia Whitney, Elizabeth Zell, Jennifer Loo, George Nelson; Emory University, Atlanta, Georgia: Keith Klugman; Johns Hopkins Bloomberg School of Public Health, Johns Hopkins University, Baltimore: Katherine O'Brien.
Disclaimer. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. The views expressed by the authors do not necessarily reflect the views of the GAVI Alliance (GAVI), the Centers for Disease Control and Prevention, or the Program for Appropriate Technology in Health (PATH).
Financial support. This work was supported by GAVI through PATH.
Potential conflicts of interest. C. v. M. has received honoraria from Pfizer. V. N. was employed by GlaxoSmithKline following his involvement in the study. G. R. has received speakers' fees and local and international conference support from Pfizer and local conference support from Sanofi Aventis. S. A. M. received grant support and also received honoraria for participation on speaker's bureaus and as a scientific advisor to GlaxoSmithKline and Pfizer. K. O. B. has had grant support from Pfizer and GlaxoSmithKline. K. K. has received research funding and honoraria from Pfizer and GlaxoSmithKline. A. v. G. has had grant support from Pfizer. All other authors report no potential conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.World Health Organization Pneumococcal vaccines WHO position paper—2012—recommendations. Vaccine. 2012;30:4717–8. doi: 10.1016/j.vaccine.2012.04.093. [DOI] [PubMed] [Google Scholar]
- 2.Klugman KP, Madhi SA, Huebner RE, Kohberger R, Mbelle N, Pierce N. A trial of a 9-valent pneumococcal conjugate vaccine in children with and those without HIV infection. N Engl J Med. 2003;349:1341–8. doi: 10.1056/NEJMoa035060. [DOI] [PubMed] [Google Scholar]
- 3.Ingels H, Rasmussen J, Andersen PH, et al. Impact of pneumococcal vaccination in Denmark during the first 3 years after PCV introduction in the childhood immunization programme. Vaccine. 2012;30:3944–50. doi: 10.1016/j.vaccine.2012.03.060. [DOI] [PubMed] [Google Scholar]
- 4.Pilishvili T, Lexau C, Farley MM, et al. Sustained reductions in invasive pneumococcal disease in the era of conjugate vaccine. J Infect Dis. 2010;201:32–41. doi: 10.1086/648593. [DOI] [PubMed] [Google Scholar]
- 5.Leal J, Vanderkooi OG, Church DL, MacDonald J, Tyrrell GJ, Kellner JD. Eradication of invasive pneumococcal disease due to the seven-valent pneumococcal conjugate vaccine serotypes in Calgary, Alberta. Pediatr Infect Dis J. 2012;31:e169–75. doi: 10.1097/INF.0b013e3182624a40. [DOI] [PubMed] [Google Scholar]
- 6.Johnson DR, D'Onise K, Holland RA, Raupach JC, Koehler AP. Pneumococcal disease in South Australia: vaccine success but no time for complacency. Vaccine. 2012;30:2206–11. doi: 10.1016/j.vaccine.2011.12.119. [DOI] [PubMed] [Google Scholar]
- 7.Vestrheim DF, Lovoll O, Aaberge IS, et al. Effectiveness of a 2+1 dose schedule pneumococcal conjugate vaccination programme on invasive pneumococcal disease among children in Norway. Vaccine. 2008;19:3277–81. doi: 10.1016/j.vaccine.2008.03.087. [DOI] [PubMed] [Google Scholar]
- 8.Whitney CG, Pilishvili T, Farley MM, et al. Effectiveness of seven-valent pneumococcal conjugate vaccine against invasive pneumococcal disease: a matched case-control study. Lancet. 2006;368:1495–502. doi: 10.1016/S0140-6736(06)69637-2. [DOI] [PubMed] [Google Scholar]
- 9.Palmu AA, Jokinen J, Borys D, et al. Effectiveness of the ten-valent pneumococcal Haemophilus influenzae protein D conjugate vaccine (PHiD-CV10) against invasive pneumococcal disease: a cluster randomised trial. Lancet. 2013;381:214–22. doi: 10.1016/S0140-6736(12)61854-6. [DOI] [PubMed] [Google Scholar]
- 10.Miller E, Andrews NJ, Waight PA, Slack MP, George RC. Herd immunity and serotype replacement 4 years after seven-valent pneumococcal conjugate vaccination in England and Wales: an observational cohort study. Lancet Infect Dis. 2011;11:760–8. doi: 10.1016/S1473-3099(11)70090-1. [DOI] [PubMed] [Google Scholar]
- 11.Madhi SA, Cohen C, von Gottberg A. Introduction of pneumococcal conjugate vaccine into the public immunization program in South Africa: translating research into policy. Vaccine. 2012;30(suppl 3) doi: 10.1016/j.vaccine.2012.05.055. C21–7. [DOI] [PubMed] [Google Scholar]
- 12.Goldblatt D, Southern J, Ashton L, et al. Immunogenicity and boosting after a reduced number of doses of a pneumococcal conjugate vaccine in infants and toddlers. Pediatr Infect Dis J. 2006;25:312–9. doi: 10.1097/01.inf.0000207483.60267.e7. [DOI] [PubMed] [Google Scholar]
- 13.Madhi SA, Adrian P, Kuwanda L, et al. Long-term immunogenicity and efficacy of a 9-valent conjugate pneumococcal vaccine in human immunodeficient virus infected and non-infected children in the absence of a booster dose of vaccine. Vaccine. 2007;25:2451–7. doi: 10.1016/j.vaccine.2006.09.019. [DOI] [PubMed] [Google Scholar]
- 14.Actuarial Society of South Africa (ASSA) AIDS and demographic model. Available at: http://aids.actuarialsociety.org.za/ASSA2008-Model-3480.htm. Accessed 3 July 2014.
- 15.Madhi SA, Adrian P, Cotton MF, et al. Effect of HIV infection status and anti-retroviral treatment on quantitative and qualitative antibody responses to pneumococcal conjugate vaccine in infants. J Infect Dis. 2010;202:355–61. doi: 10.1086/653704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Slogrove A, Reikie B, Naidoo S, et al. HIV-exposed uninfected infants are at increased risk for severe infections in the first year of life. J Trop Pediatr. 2012;58:505–8. doi: 10.1093/tropej/fms019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ruoff KL, Whiley RA, Beighton D. Streptococcus. In: Murray PR, Baron EJ, Jorgensen JH, Yolken RH, editors. Manual of clinical microbiology. Washington, DC: ASM Press; 2006. pp. 405–21. [Google Scholar]
- 18.Crowther-Gibson P, Cohen C, Klugman KP, de Gouveia L, von Gottberg A. Risk factors for multidrug-resistant invasive pneumococcal disease in South Africa, a setting with high HIV prevalence, in the prevaccine era from 2003 to 2008. Antimicrob Agents Chemother. 2012;56:5088–95. doi: 10.1128/AAC.06463-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Glencross D, Scott LE, Jani IV, Barnett D, Janossy G. CD45-assisted PanLeucogating for accurate, cost-effective dual-platform CD4+ T-cell enumeration. Cytometry. 2002;50:69–77. doi: 10.1002/cyto.10068. [DOI] [PubMed] [Google Scholar]
- 20.World Health Organisation HIV/AIDS programme. Geneva, Switzerland: WHO; 2007. WHO case definitions of HIV for surveillance and revised clinical staging and immunologic classification of HIV-related disease in adults and children. [Google Scholar]
- 21.World Health Organization, United Nations Children's Fund. Geneva, Switzerland: WHO; 2009. WHO child growth standards and the identification of severe malnutrition in infants and children. [PubMed] [Google Scholar]
- 22.Dupont WD. Power calculations for matched case-control studies. Biometrics. 1988;44:1157–68. [PubMed] [Google Scholar]
- 23.Rothman KJ, Greenland S. Modern epidemiology. 2nd ed. Philadelphia: Lippincott-Raven; 1998. [Google Scholar]
- 24.Deceuninck G, De Wals P, Boulianne N, De Serres G. Effectiveness of pneumococcal conjugate vaccine using a 2+1 infant schedule in Quebec, Canada. Pediatr Infect Dis J. 2010;29:546–9. doi: 10.1097/INF.0b013e3181cffa2a. [DOI] [PubMed] [Google Scholar]
- 25.Schonberger K, Kirchgassner K, Riedel C, von KR. Effectiveness of 2+1 PCV7 vaccination schedules in children under 2 years: a meta-analysis of impact studies. Vaccine. 2013;31:5948–52. doi: 10.1016/j.vaccine.2013.10.042. [DOI] [PubMed] [Google Scholar]
- 26.Koyanagi A, Humphrey JH, Ntozini R, et al. Morbidity among human immunodeficiency virus-exposed but uninfected, human immunodeficiency virus-infected, and human immunodeficiency virus-unexposed infants in Zimbabwe before availability of highly active antiretroviral therapy. Pediatr Infect Dis J. 2011;30:45–51. doi: 10.1097/INF.0b013e3181ecbf7e. [DOI] [PubMed] [Google Scholar]
- 27.Kyaw MH, Lynfield R, Schaffner W, et al. Effect of introduction of the pneumococcal conjugate vaccine on drug-resistant Streptococcus pneumoniae. N Engl J Med. 2006;354:1455–63. doi: 10.1056/NEJMoa051642. [DOI] [PubMed] [Google Scholar]
- 28.Cutts FT, Zaman SM, Enwere G, et al. Efficacy of nine-valent pneumococcal conjugate vaccine against pneumonia and invasive pneumococcal disease in The Gambia: randomised, double-blind, placebo-controlled trial. Lancet. 2005;365:1139–46. doi: 10.1016/S0140-6736(05)71876-6. [DOI] [PubMed] [Google Scholar]
- 29.Scott P, Rutjes AW, Bermetz L, et al. Comparing pneumococcal conjugate vaccine schedules based on 3 and 2 primary doses: systematic review and meta-analysis. Vaccine. 2011;29:9711–21. doi: 10.1016/j.vaccine.2011.07.042. [DOI] [PubMed] [Google Scholar]
- 30.Flannery B, Heffernan RT, Harrison LH, et al. Changes in invasive pneumococcal disease among HIV-infected adults living in the era of childhood pneumococcal immunization. Ann Intern Med. 2006;144:1–9. doi: 10.7326/0003-4819-144-1-200601030-00004. [DOI] [PubMed] [Google Scholar]
- 31.Whitney CG, Farley MM, Hadler J, et al. Decline in invasive pneumococcal disease after the introduction of protein-polysaccharide conjugate vaccine. N Engl J Med. 2003;348:1737–46. doi: 10.1056/NEJMoa022823. [DOI] [PubMed] [Google Scholar]
- 32.Madhi SA, Izu A, Violari A, et al. Immunogenicity following the first and second doses of 7-valent pneumococcal conjugate vaccine in HIV-infected and -uninfected infants. Vaccine. 2013;31:777–83. doi: 10.1016/j.vaccine.2012.11.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.National Department of Health. Policy and guidelines for the implementation of the PMTCT programme. 2008. Available at: http://southafrica.usembassy.gov/root/pdfs/2008-pmtct.pdf . Accessed 3 July 2014.
- 34.National Department of Health. The 2011 National Antenatal Sentinel HIV & Syphilis Prevalence Survey in South Africa. 2012. Available at: http://www.hst.org.za/publications/2011-national-antenatal-sentinel-hiv-syphilis-prevalence-survey-south-africa . Accessed 20 June 2014.
- 35.Herbert HK, van As AB, Bachani AM, et al. Patterns of pediatric injury in South Africa: an analysis of hospital data between 1997 and 2006. J Trauma Acute Care Surg. 2012;73:168–74. doi: 10.1097/TA.0b013e31824d67c3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.