Abstract
Background and Purpose
Parenchymal arterioles (PAs) are high resistance vessels in the brain that connect pial vessels to the microcirculation. We previously showed that PAs have increased vasoconstriction after ischemia and reperfusion that could increase perfusion deficit. Here, we investigated underlying mechanisms by which early post-ischemic reperfusion causes increased vasoconstriction of PAs.
Methods
Isolated and pressurized PAs from within the MCA territory were studied in male Wistar rats that were either nonischemic control (CTL; n=34) or after exposure to transient MCAO by filament occlusion for 2 hours with 30 minutes of reperfusion (MCAO; n=38). The relationships between pressure-induced tone, smooth muscle calcium (using Fura 2) and membrane potential were determined. Sensitivity of the contractile apparatus to calcium was measured in permeabilized arterioles using Staphaloccus aureus α-toxin. Reactivity to inhibition of TRPM4 (9-phenanthrol), Rho kinase (Y27632) and protein kinase C (Gö6976) was also measured.
Results
After MCAO, PAs had increased myogenic tone compared to controls (47±2% vs. 35±2% at 40 mmHg; p<0.01), without an increase in smooth muscle calcium (177±21 vs. 201±16 nmol/L; p>0.05) or membrane depolarization (−38±4 vs. −36±1 mV;p>0.05). In α-toxin permeabilized vessels, MCAO caused increased sensitivity of the contractile apparatus to calcium. MCAO did not affect dilation to TRPM4- or PKC-inhibition, but diminished dilation to Rho kinase inhibition.
Conclusions
The increased vasoconstriction of PAs during early post-ischemic reperfusion appears to be due to calcium sensitization of smooth muscle and could contribute to infarct expansion and limit neuroprotective agents from reaching their target tissue.
Keywords: post-ischemic reperfusion, no reflow, myogenic tone, brain arterioles, calcium
Introduction
Restoration of blood flow to the ischemic brain is the most potent and efficacious means to improve outcome from ischemic stroke. Recanalization therapies, such as thrombolysis and mechanical removal or disruption of the clot, have shown clear benefit if reperfusion occurs within a narrow time window of ~4.5 hours.1,2 However, recanalization after this time period can cause deleterious effects including hemorrhagic transformation and edema that worsens outcome.1-3 Thus, early postischemic reperfusion to the brain parenchyma is one of the most important treatments for acute ischemic stroke.
Studies in animals and humans have shown that recanalization of an occluded artery does not necessarily lead to complete reperfusion or improvement of outcome.4-7 Incomplete microcirculatory reperfusion occurs in response to both global and focal ischemia in the brain and may be a primary factor that increases perfusion deficit, decreases efficacy of early thrombolysis, and limits neuroprotective agents from reaching their target.5,8-12 This “no-reflow” phenomenon occurs in other circulations in response to postischemic reperfusion including the coronary circulation, and is thought to be a major contributor to ischemic tissue injury.13 Importantly, a recent study in stroke patients found that reperfusion was a more accurate predictor of final infarct volume than recanalization.4 The dissociation between recanalization and reperfusion is not entirely clear and may be due to distal embolization of thrombus or to incomplete reperfusion of the brain parenchyma.14,15 Incomplete postischemic reperfusion has been mostly attributed to microcirculatory disturbances (for review see 14), including perivascular glial cells swelling that occludes capillaries6,16 and clogging of capillaries by microthrombi and immune cells.5,7,17,18 However, evidence from earlier studies also suggests that upstream vasoconstriction and increased cerebrovascular resistance occur in no-reflow zones.8-10,19 More recently, Shih et al. used two-photon microscopy to measure red blood cell flux and diameters of penetrating brain arterioles during early post-ischemic reperfusion and found both flux and diameters to be decreased below baseline, suggesting vasoconstriction occurs during reperfusion that may contribute to incomplete restoration of blood flow.20
Our own studies using isolated and pressurized parenchymal arterioles have shown that these vessels have increased vasoconstriction in response to early post-ischemic reperfusion.21 While this previous study mainly focused on endothelial cell changes, we found that vascular smooth muscle was also more contractile after ischemia and reperfusion.21 In the present study, we investigated mechanisms by which vascular smooth muscle of parenchymal arterioles has increased vasoconstriction compared to non-ischemic vessels, focusing on the relationship between myogenic tone, smooth muscle calcium and membrane potential. Understanding this relationship may provide a therapeutic target to improve blood flow to the ischemic brain during reperfusion.
Materials and Methods
Animal Model of Transient Focal Ischemia
Experiments were performed using male Wistar rats (Harlan) that were ~350-380 g. All procedures involving animals were approved by the Institutional Animal Care and Use Committee at the University of Vermont and complied with the National Institutes of Health guidelines for the care and use of laboratory animals. Rats were housed in the Animal Care Facility at the University of Vermont, an Association for Assessment and Accreditation of Laboratory Animal Care accredited facility, and were allowed food and water ad libitum. Proximal middle cerebral artery occlusion (MCAO) was performed using the filament technique, as previously described.21 Animals were anesthetized with isoflurane (1.5% in oxygen) and intubated and mechanically ventilated to maintain blood gases within a normal physiological range (Supplemental Table S1). The MCA was occluded for 2 hours followed by suture removal for 30 minutes. Animals were randomized to either sham, naïve or MCAO groups and individuals performing the experiments were blinded to group and/or outcome. Animals were excluded if the drop in cerebral blood flow was <60% from baseline. Sham control animals underwent anesthesia for 2.5 hours and received a midline neck incision, but no filament was inserted.
Preparation of Isolated Parenchymal Arterioles and the Relationship between Myogenic Vasoconstriction and Smooth Muscle Calcium
Animals were decapitated under isoflurane anesthesia and the brain removed and placed in cold physiologic saline solution (PSS). PAs, branching off the MCA at right angles and penetrating into the brain tissue, were dissected and mounted in an arteriograph chamber, as previously described.21 Myogenic activity was compared between control (n=12) and MCAO (n=12) arterioles by measuring diameter and tone at pressures from 40 to 80 mmHg. To investigate the relationship between myogenic tone and calcium in PAs from control animals (n=6) and after MCAO (n=6), the calcium-sensitive dye, Fura 2-AM was used, as previously described22 and in Supplemental Materials. Arteriolar diameter and calcium were simultaneously recorded using IonWizard software (IonOptix). Briefly, PAs were pressurized to 40 mmHg and equilibrated for 30 minutes to allow spontaneous development of myogenic tone. Changes in smooth muscle calcium and diameter were evaluated by stepwise increases in pressure from 40 to 80 mmHg. At the conclusion of the experiment, diltiazem (10 µmol/L) in calcium-free PSS was added to obtain fully relaxed diameters.
Smooth Muscle Membrane Potential Measurement
Myogenic tone and smooth muscle membrane potential were simultaneously measured in isolated and pressurized PAs from sham control animals (n=5) and after MCAO (n=5), as previously described.23,24 Smooth muscle membrane potential was measured by insertion of a sharp glass microelectrode (~100 MΩ resistance) filled with 0.5 M KCl into the vessel wall. Impalement was considered successful if there was an abrupt deflection to negative membrane potential upon electrode entry, membrane potential was stable for ≥30 s, and the voltage returned abruptly to 0 mV upon removal of the electrode23,24. Membrane potential measurements were made with an electrometer (World Precision Instruments) and recorded via computer with Axotape and Dataq software.
Measurement of Calcium Sensitivity in Permeabilized Arterioles using Staphalococcus aureus α-toxin
The sensitivity of the contractile apparatus to calcium in arterioles from control animals (n=6) and after MCAO (n=7) was determined by permeabilizing the myocyte membrane with Staphylococcus (S.) aureus α-toxin and measuring the contractile response to addition of calcium, as previously described22 and in Supplemental Materials. S. aureus α-toxin forms small (1-2 nm diameter) pores in the plasma membrane that allows ions but not proteins to pass. This technique is commonly used to study calcium sensitization since under these conditions intracellular calcium in smooth muscle can be tightly controlled. Briefly, PAs were carefully dissected and mounted in an arteriograph filled with HEPES-buffered PSS, pressurized to 40 mmHg and equilibrated for 30 minutes. Vessels were permeabilized with S. aureus α-toxin (800 U/ml) in relaxing solution at room temperature for 20 minutes. The S. aureus α-toxin was then washed from the bath and vessels were equilibrated in relaxing solution at 37 °C for 30 minutes. The vasoactive response to calcium was determined by replacing relaxing solution with activating solution containing known concentrations of free ionic calcium (pCa or -log [Ca]: 7.0-6.0). For each concentration of calcium, the inner diameters were recorded once stable (5-7 min).
Reactivity of PAs to 9-phenanthrol, Y27632 and Gö6976
In a separate set of PAs from sham control animals (n=6) or after MCAO (n=7), dilator responses to the transient receptor potential melastanin receptor type 4 (TRPM4) inhibitor, 9-phenanthrol, were determined. Arterioles were dissected and mounted in an arteriograph chamber, equilibrated at 40 mmHg and an air bubble passed through the lumen to remove the endothelium. Endothelial denudation was confirmed by lack of dilation to NS309. 9-phenanthrol was cumulatively added to the bath and lumen diameters measured at each concentration, once stable. In a separate set of arterioles that were intact (not denuded of endothelium) from control animals (n=6) or after MCAO (n=6), reactivity to inhibitors of Rho kinase (Y27632) and protein kinase C (PKC, Gö6976) was determined by cumulative addition to the bath and measuring diameters at each concentration. Y27632 is a selective inhibitor of ROCK1 (IC50 = 140 nmol/L) that exhibits >200-fold selectivity over other kinases including PKC and myosin light chain kinase (MLCK). Gö6976 is a selective inhibitor of conventional PKCα (IC50 = 2.3 nmol/L) and PKCβ (IC50 = 6.2 nmol/L) and does not inhibit unconventional PKC isoforms (PKCδ, γ or ε).
Drugs and Solutions
All isolated vessel experiments, except calcium sensitivity measurements in S. aureus α-toxin, were performed using a bicarbonate-based Ringer's PSS, the ionic composition of which was (in mmol/L): NaCl 119.0, NaHCO3 24.0, KCl 4.7, KH2PO4 1.18, MgSO4x7H2O 1.17, CaCl2 1.6, EDTA 0.026, and glucose 5.5. PSS was made each week and stored without glucose at 4 °C. Glucose was added to the PSS prior to each experiment. PSS was aerated with 5% CO2, 10% O2 and 85% N2 to maintain pH. S. aureus α-toxin was purchased from Calbiochem (La Jolla, CA), and aliquoted in relaxing buffer and stored at −20 °C until use. All other chemicals were purchased from Sigma (St. Louis, MO). Please see Supplemental Materials for details on Fura 2 measurements.
Data calculations
Please see Supplemental Materials for all data calculations.
Statistical analysis
All data are presented as mean ± SEM. Unpaired t-test with Welch's correction was used to compare differences between control and MCAO arterioles.
Results
Effect of Early Post-ischemic Reperfusion on Myogenic Tone and Smooth Muscle Calcium
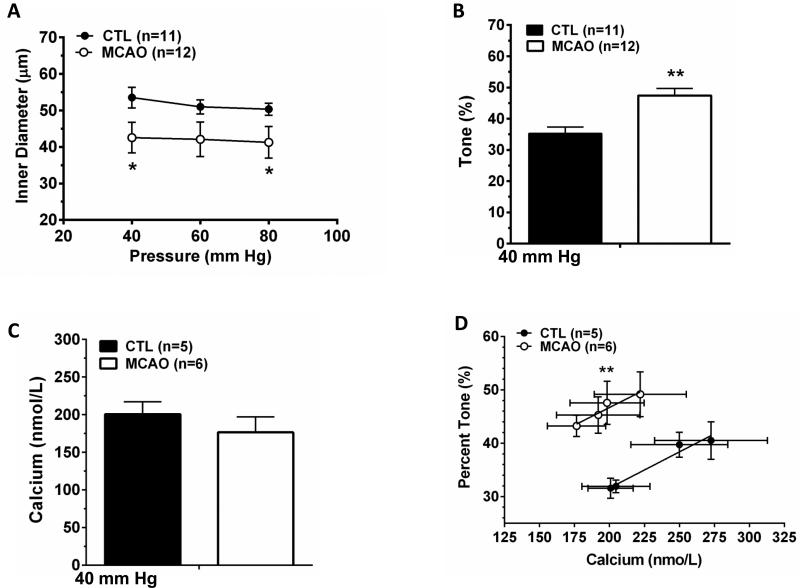
Figure 1 shows the relationship between myogenic tone and smooth muscle calcium in PAs from control animals and after transient MCAO. One vessel from control animals was excluded for technical reasons. Unlike pial arteries that have been shown to have diminished myogenic tone after post-ischemic reperfusion,25 PAs were smaller in diameter (Figure 1A) due to increased myogenic tone (Figure 1B). Passive diameters were not different between groups (data not shown). Interestingly, there was no difference in smooth muscle calcium in arterioles after MCAO despite greater vasoconstriction (Figure 1C). When the level of tone was plotted as a function of calcium concentration for both groups of vessels, arterioles after MCAO had greater tone for the same level of calcium (Figure 1D). For example, at ~200 nmol/L calcium arterioles from control animals had 32±2% tone whereas arterioles that were exposed to transient MCAO had 48±4% tone (p<0.01).
Figure 1. Effect of early post-ischemic reperfusion on myogenic tone and smooth muscle calcium in PAs.
(A) Arteriolar diameter in response to pressure. Arterioles displayed myogenic vasoconstriction and were smaller actively after MCAO. (B) Percent tone at 40 mm Hg. Arterioles had increased tone that contributed to their smaller diameter. Passive diameters were not different between groups (not shown). (C) Smooth muscle calcium, measured using Fura 2 at 40 mm Hg, was not different in arterioles after MCAO. (D) Relationship between percent tone and smooth muscle calcium. Arterioles were more sensitive to calcium after MCAO. *p<0.05 vs Control; **p<0.01 vs. Control.
Effect of Early Post-ischemic Reperfusion on Smooth Muscle Membrane Potential and TRPM4 Activity
The increase in tone in parenchymal arterioles after MCAO could be due to greater pressure-induced depolarization and activation of voltage-dependent calcium channel (VDCC) activity. To test this possibility, we simultaneously measured smooth muscle membrane potential and tone of isolated arterioles pressurized to 40 mm Hg. Figure 2A shows the relationship between myogenic tone and smooth muscle membrane potential. PAs exposed to post-ischemic reperfusion had increased tone, but this was not due to greater smooth muscle depolarization as membrane potential of arterioles from the two groups was similar, and very close to the values observed in these vessels previously under control conditions.23 We also tested the possibility that greater activation of TRPM4, a mechanosensitive ion channel that promotes depolarization in response to pressure,26 could account for the increased tone of PAs after MCAO. Figure 2B shows that both types of arterioles dilated to TRPM4 inhibition, but there was no difference in sensitivity to 9-phenanthrol, suggesting MCAO did not affect these channels.
Figure 2. PA smooth muscle membrane potential and reactivity to TRPM4 inhibition after early post-ischemic reperfusion.
(A) Percent tone and smooth muscle membrane potential (Vm) of pressurized PAs at 40 mm Hg. There was no difference in membrane potential of arterioles after MCAO despite increased myogenic tone. (B) Dilation in response to TRPM4 inhibition with 9-phenanthrol. TRPM4 inhibition caused equivalent dilations in Control and MCAO arterioles. *p<0.05 vs. Control.
Calcium Sensitization of Permeabilized Arterioles after Post-ischemic Reperfusion
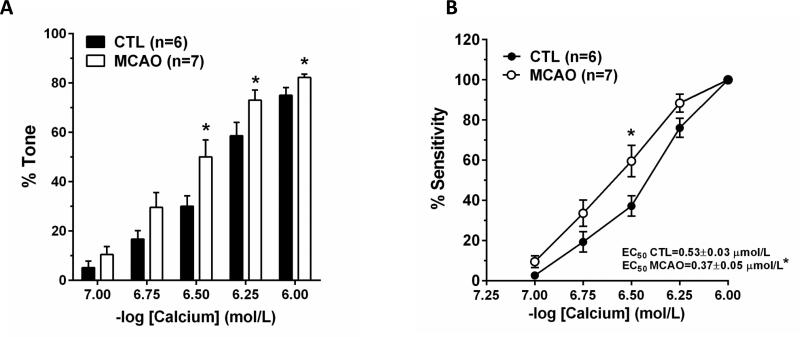
To determine if the contractile apparatus of smooth muscle was more sensitive to calcium after MCAO, which could explain the increased tone, a permeabilized vessel preparation was used. The use of S. aureus α-toxin effectively eliminates the contribution of plasma membrane ion channels and measures the contractile response to addition of calcium.22 Figure 3A shows that arterioles after MCAO had increased tone at calcium concentrations ≥ 0.3 μmol/L. Figure 3A also shows that after MCAO, PAs were physically more constricted compared to controls at the highest level of calcium. When calcium sensitivity was compared between the two groups of arterioles (Figure 3B), there was an increase in calcium sensitivity after MCAO. The effective concentration that produced half maximal constriction (EC50) was significantly less for arterioles after MCAO than controls.
Figure 3. Effect of early post-ischemic reperfusion on calcium sensitivity of smooth muscle in PAs.
Arterioles permeabilized with S. aureus α-toxin were more sensitive to calcium after MCAO and had increased tone (A) and sensitivity to calcium (B). *p<0.05 vs. Control.
Effect of Early Post-ischemic Reperfusion on Reactivity to Rho Kinase and PKC Inhibition
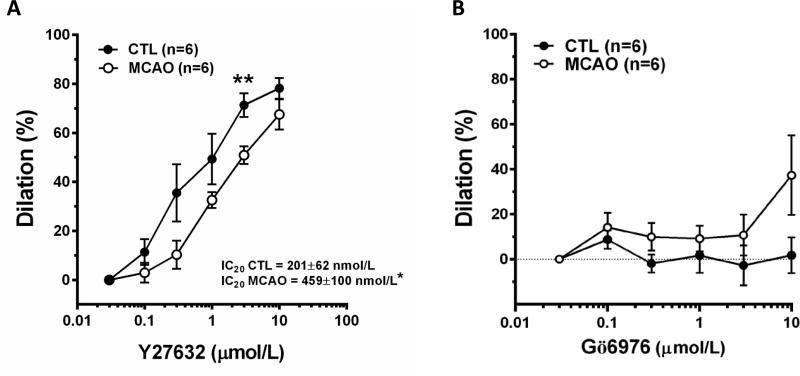
To begin to investigate the mechanism by which calcium sensitization occurs in arterioles after MCAO, the sensitivity to inhibition of ROCK and PKC was compared between groups. Figure 4A shows the sensitivity of PAs in response to ROCK inhibition with Y27632. Both types of arterioles dilated in response to Y27632, however, after MCAO arterioles were less sensitive to its effects. Figure 4A also shows that the IC20 value for Y27632 was significantly larger in arterioles after MCAO. We compared the IC20 values since at these concentrations Y27632 is not likely to be inhibiting other kinases that also promote vasodilation such as PKC or myosin light chain kinase (MLCK) as the IC50 for this compound is 140 nmol/L. In contrast, inhibition of PKCα and β with Gö6976 had little effect on either vessel type. Figure 4B shows there was almost no change in diameter in response to Gö6976 regardless of exposure to ischemia and reperfusion.
Figure 4. Reactivity of PAs to ROCK and PKC inhibition after post-ischemic reperfusion.
Inhibition of ROCK with Y27632 caused dilation of both types of arterioles (A) that were less sensitive after MCAO. Inhibition of PKC with Gö6976 had little effect on either group of arterioles (B). *p<0.05 vs Control; **p<0.01 vs. Control.
Discussion
In the present study, we show that after early post-ischemic reperfusion, PAs had increased pressure-induced tone that was not due to smooth muscle membrane depolarization or increased cell calcium, but was related to an increase in calcium sensitivity. In fact, the capacity of PAs to constrict in response to calcium was also increased after MCAO, suggesting that other vasoconstrictor mechanisms (e.g., ET-1) may also be enhanced. Increased vasoconstriction after ischemia and reperfusion has been shown previously in these arterioles,9,19-21 a response that is distinct from that of pial arteries that undergo vasodilation and decreased tone.25 However, this is the first study we are aware of to investigate the underlying mechanism by which increased vasoconstriction of PAs occurs with ischemia and reperfusion. Although capillary disturbances are well-known to occur after ischemia and reperfusion that can limit reperfusion,5-7,17,18 upstream vasoconstriction of PAs may be another factor that contributes to heterogeneity of tissue recovery and incomplete perfusion.
This study focused on smooth muscle cell calcium and changes in membrane potential as underlying mechanism by which myogenic tone could be increased in PAs after ischemia and reperfusion because it is well-established that pressure induces membrane depolarization that opens VDCC to cause vasoconstriction.24 Thus, one means by which tone could be increased is through greater smooth muscle depolarization and increased cell calcium. However, we found that despite increased tone, smooth muscle calcium was not elevated compared to control vessels (177±21 nmol/L in MCAO vs. 201±16 nmol/L in control; p>0.05). In addition, membrane potential of PAs pressurized to 40 mmHg was not different (−38±4 mV in MCAO vs. −36±1 mV in control;p>0.05). These values are in agreement with what has been published by our group previously in control arterioles pressurized to 40 mmHg (-35±1 mV).23 In addition, sensitivity to 9-phenanthrol, a selective TRPM4 inhibitor, dilated both types of PAs to a similar extent, suggesting that a difference in TRPM4 activity in response to pressure was not the underlying cause by which ischemia and reperfusion increased tone in PAs.
The findings described above suggest that the contractile apparatus of smooth muscle from PAs after ischemia and reperfusion may be more sensitive to calcium such that less calcium was needed to cause vasoconstriction at the same level of pressure. To directly measure calcium sensitivity, we used a permeabilized vessel preparation that eliminated the role of the membrane potential and ionic fluxes and allowed direct and controlled access of calcium to the contractile apparatus. Calcium sensitization of smooth muscle occurs via several mechanisms, including phosphorylation of myosin light chain kinase by PKC, decreased myosin phosphatase activity and increased Rho A-induced actin polymerization.27,28 We therefore compared the vasoactive response of arterioles to both ROCK and PKC inhibition. PKC inhibition with Gö6976, a conventional PKC inhibitor, had little effect on basal tone of PAs, regardless of ischemia and reperfusion, suggesting PKC activation was not involved in calcium sensitization with MCAO. Conversely, ROCK inhibition with Y27632 caused significant vasodilation in both vessel types, confirming our previous study and others that the Rho A-ROCK pathway is involved in myogenic tone in these arterioles.22,29,30 However, sensitivity to Y27632 was decreased in arterioles after MCAO, suggesting ROCK was less activated and contributes less to tone in those arterioles and may not be the mechanism by which calcium sensitization occurs after ischemia and reperfusion either. Calcium sensitization by ROCK depends on its phosphorylation of MYPT1 at Thr696 and Thr853 to suppress myosin light chain phosphatase activity.31 However, other kinases such as zipper-interacting protein kinase and integrin-linked kinase also have the ability to phosphorylate MYPT1 at Thr696.32,33 The role of these kinases in calcium sensitization of PAs after MCAO clearly needs to be addressed with additional biochemical studies.
Conclusions
We show that PAs supplying the subcortical brain regions and striatum have increased vasoconstriction in response to early post-ischemic reperfusion, which could limit perfusion to these vulnerable brain regions including white matter. This appears to be due to ischemia and reperfusion-induced smooth muscle calcium sensitization. The main target of acute stroke treatment is the ischemic penumbra and peri-infarcted tissue – regions where the brain tissue is not dead, and where reperfusion and neuroprotective agents can still provide benefit. Without restoration of blood flow, tissue within the penumbra dies and the core infarct expands to include the penumbra.34 Understanding the mechanisms by which post-ischemic reperfusion preferentially promotes vasoconstriction of PAs is of interest to acute stroke treatment, as vasoconstriction in these vessels under these conditions would not seem to be beneficial. Therapeutic interventions opposing parenchymal arteriolar constriction during reperfusion should enhance local blood flow and aid recovery and delivery of neuroprotective agents to the penumbra.
Supplementary Material
Acknowledgments
Sources of Funding: National Institute of Neurologic Disorders and Stroke grant NS045940, National Heart Lung and Blood Institute grants PO1 HL095488 and R01 HL088245, and the Totman Medical Research Trust.
Footnotes
Conflict of Interest/Disclosures: None.
References
- 1.Ahmed N, Wahlgren N, Grond M, Hennerici M, Lees KR, Mikulik R, et al. SITS investigators. Implementation and outcome of thrombolysis with alteplase 3-4.5 h after an acute stroke: an updated analysis from SITS-ISTR. Lancet Neurol. 2010;9:866–874. doi: 10.1016/S1474-4422(10)70165-4. [DOI] [PubMed] [Google Scholar]
- 2.The National Institute of Neurological Disorders and Stroke rt-PA Stroke Study Group Tissue plasminogen activator for acute ischemic stroke. N Engl J Med. 1995;333:1581–1587. doi: 10.1056/NEJM199512143332401. [DOI] [PubMed] [Google Scholar]
- 3.Donnan GA, Davis SM, Parsons MW, Ma H, Dewey HM, Howells DW. How to make better use of thrombolytic therapy in acute ischemic stroke. Nat Rev Neurol. 2011;7:400–409. doi: 10.1038/nrneurol.2011.89. [DOI] [PubMed] [Google Scholar]
- 4.Soares BP, Tong E, Hom J, Cheng SC, Bredno J, Boussel L, et al. Reperfusion is a more accurate predictor of follow-up infarct volume than recanalization: a proof of concept using CT in acute ischemic stroke patients. Stroke. 2010;41:e34–e40. doi: 10.1161/STROKEAHA.109.568766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Busch E, Kruger K, Allegrini PR, Kerskens CM, Gyngell ML, Hoehn-Berlage M, et al. Reperfusion after thrombolytic therapy of embolic stroke in the rat: magnetic resonance and biochemical imaging. J Cereb Blood Flow Metab. 1998;18:407–418. doi: 10.1097/00004647-199804000-00009. [DOI] [PubMed] [Google Scholar]
- 6.Garcia JH, Liu KF, Yoshida Y, Chen S, Lian J. Brain microvessels: factors altering their patency after the occlusion of a middle cerebral artery (Wistar rat). Am J Pathol. 1994;145:728–740. [PMC free article] [PubMed] [Google Scholar]
- 7.del Zoppo GJ, Mabuchi T. Cerebral microvessel responses to focal ischemia. J Cereb Blood Flow Metab. 2003;23:879–894. doi: 10.1097/01.WCB.0000078322.96027.78. [DOI] [PubMed] [Google Scholar]
- 8.Alexandrov AV, Hall CE, Labiche LA, Wojner AW, Grotta JC. Ischemic stunning of the brain: early recanalization without immediate clinical improvement in acute ischemic stroke. Stroke. 2004;35:449–452. doi: 10.1161/01.STR.0000113737.58014.B4. [DOI] [PubMed] [Google Scholar]
- 9.Fischer EG, Ames A, 3rd, Hedley-Whyte ET, O'Gorman S. Reassessment of cerebral capillary changes in acute global ischemia and their relationship to the “no-reflow phenomenon”. Stroke. 1977;8:36–39. doi: 10.1161/01.str.8.1.36. [DOI] [PubMed] [Google Scholar]
- 10.Ames A, 3rd, Wright RL, Kowada M, Thurston JM, Majno G. Cerebral ischemia. II. The no-reflow phenomenon. Am J Pathol. 1968;52:437–453. [PMC free article] [PubMed] [Google Scholar]
- 11.Franke C, Brinker G, Pillekamp F, Hoehn M. Probability of metabolic tissue recovery after thrombolytic treatment of experimental stroke: a magnetic resonance spectroscopic imaging study in rat brain. J Cereb Blood Flow Metab. 2000;20:583–591. doi: 10.1097/00004647-200003000-00016. [DOI] [PubMed] [Google Scholar]
- 12.Niessen F, Hilger T, Hoehn M, Hossmann KA. Thrombolytic treatment of clot embolism in rat: comparison of intra-arterial and intravenous application of recombinant tissue plasminogen activator. Stroke. 2002;33:2999–3005. doi: 10.1161/01.str.0000038096.60932.f4. [DOI] [PubMed] [Google Scholar]
- 13.Rezkalla SH, Kloner RA. No-reflow phenomenon. Circulation. 2002;105:656–662. doi: 10.1161/hc0502.102867. [DOI] [PubMed] [Google Scholar]
- 14.Janjua N, Alkawi A, Suri MF, Qureshi AI. Impact of arterial reocclusion and distal fragmentation during thrombolysis among patients with acute ischemic stroke. Am J Neuroradiol. 2008;29:253–258. doi: 10.3174/ajnr.A0825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dalkara T, Arsava EM. Can restoring incomplete microcirculatory reperfusion improve stroke outcome after thrombolysis? J Cereb Blood Flow Metab. 2012;32:2091–2099. doi: 10.1038/jcbfm.2012.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.del Zoppo GJ, Schmid-Schonbein GW, Mori E, Copeland BR, Chang CM. Polymorphonuclear leukocytes occlude capillaries following middle cerebral artery occlusion and reperfusion in baboons. Stroke. 1991;22:1276–1283. doi: 10.1161/01.str.22.10.1276. [DOI] [PubMed] [Google Scholar]
- 17.Hallenbeck JM, Dutka AJ, Tanishima T, Kochanek PM, Kumaroo KK, Thompson CB, et al. Polymorphonuclear leukocyte accumulation in brain regions with low blood flow during the early postischemic period. Stroke. 1986;17:246–253. doi: 10.1161/01.str.17.2.246. [DOI] [PubMed] [Google Scholar]
- 18.Mori E, del Zoppo GJ, Chambers JD, Copeland BR, Arfors KE. Inhibition of polymorphonuclear leukocyte adherence suppresses no-reflow after focal cerebral ischemia in baboons. Stroke. 1992;23:712–718. doi: 10.1161/01.str.23.5.712. [DOI] [PubMed] [Google Scholar]
- 19.Hart MN, Sokoll MD, Davies LR, Henriquez E. Vascular spasm in cat cerebral cortex following ischemia. Stroke. 1978;9:52–57. doi: 10.1161/01.str.9.1.52. [DOI] [PubMed] [Google Scholar]
- 20.Shih AY, Friedman B, Drew PJ, Tsai PS, Lyden PD, Kleinfeld D. Active dilation of penetrating arterioles restores red blood cell flux to penumbral neocortex after focal stroke. J Cereb Blood Flow Metab. 2009;29:738–751. doi: 10.1038/jcbfm.2008.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cipolla MJ, Sweet JG, Gokina NI, White SL, Nelson MT. Mechanisms of enhanced basal tone of brain parenchymal arterioles during early postischemic reperfusion: role of ET-1-induced peroxynitrite generation. J Cereb Blood Flow Metab. 2013;33:1486–1492. doi: 10.1038/jcbfm.2013.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gokina NI, Park KM, McElroy-Yaggy K, Osol G. Effects of Rho kinase inhibition on cerebral artery myogenic tone and reactivity. J Appl Physiol. 2005;98:1940–1948. doi: 10.1152/japplphysiol.01104.2004. [DOI] [PubMed] [Google Scholar]
- 23.Nystoriak MA, O'Connor KP, Sonkusare SK, Brayden JE, Nelson MT, Wellman GC. Fundamental increase in pressure-dependent constriction of brain parenchymal arterioles from subarachnoid hemorrhage model rats due to membrane depolarization. Am J Physiol Heart Circ Physiol. 2011;300:H803–H812. doi: 10.1152/ajpheart.00760.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Knot HJ, Nelson MT. Regulation of arterial diameter and wall [Ca2+] in cerebral arteries of rat by membrane potential and intravascular pressure. J Physiol. 1998;508:199–209. doi: 10.1111/j.1469-7793.1998.199br.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cipolla MJ, Curry AB. Middle cerebral artery function after stroke: the threshold duration of reperfusion for myogenic activity. Stroke. 2002;33:2094–2099. doi: 10.1161/01.str.0000020712.84444.8d. [DOI] [PubMed] [Google Scholar]
- 26.Earley S, Waldron BJ, Brayden JE. Critical role for transient receptor potential channel TRPM4 in myogenic constriction of cerebral arteries. Circ Res. 2004;95:922–929. doi: 10.1161/01.RES.0000147311.54833.03. [DOI] [PubMed] [Google Scholar]
- 27.Kimura K, Ito M, Amano M, Chihara K, Fukata Y, Nakafuku M, et al. Regulation of myosin phosphatase by Rho and Rho-associated kinase (Rho-kinase). Science. 1996;273:245–248. doi: 10.1126/science.273.5272.245. [DOI] [PubMed] [Google Scholar]
- 28.Kureishi Y, Kobayashi S, Amano M, Kimura K, Kanaide H, Nakano T, et al. Rho-associated kinase directly induces smooth muscle contraction through myosin light chain phosphorylation. J Biol Chem. 1997;272:12257–12260. doi: 10.1074/jbc.272.19.12257. [DOI] [PubMed] [Google Scholar]
- 29.Chrissobolis S, Sobey CG. Recent evidence for an involvement of rho-kinase in cerebral vascular disease. Stroke. 2006;37:2174–2180. doi: 10.1161/01.STR.0000231647.41578.df. [DOI] [PubMed] [Google Scholar]
- 30.Chrissobolis S, Sobey CG. Evidence that Rho-kinase activity contributes to vascular tone in vivo and is enhanced during chronic hypertension: Comparison with protein kinase C. Circ Res. 2001;88:774–779. doi: 10.1161/hh0801.090441. [DOI] [PubMed] [Google Scholar]
- 31.Muranyi A, Derkach D, Erdodi F, Kiss A, Ito M, Hartshorne DJ. Phosphorylation of Thr695 and Thr850 on the myosin phosphatase target subunit: inhibitory effects and occurrence in A7r5 cells. FEBS Lett. 2005;579:6611–6615. doi: 10.1016/j.febslet.2005.10.055. [DOI] [PubMed] [Google Scholar]
- 32.MacDonald JA, Borman MA, Muranyi A, Somlyo AV, Hartshorne DJ, Haystead TA. Identification of the endogenous smooth muscle myosin phosphatase-associated kinase. Proc Natl Acad Sci USA. 2001;98:2419–2424. doi: 10.1073/pnas.041331498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Muranyi A, MacDonald JA, Deng JT, Wilson DP, Haystead TA, Walsh MP, et al. Phosphorylation of the myosin phosphatase target subunit by integrin- linked kinase. Biochem J. 2002;366:211–216. doi: 10.1042/BJ20020401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hossmann KA. Pathophysiology and therapy of experimental stroke. Cell Mol Neurobiol. 2006;26:1055–1081. doi: 10.1007/s10571-006-9008-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.