Abstract
Dendritic cells (DCs) play a critical role in the generation of adaptive immunity via the efficient capture, processing, and presentation of antigen (Ag) to naïve T cells. Administration of Ag-pulsed DCs is also an effective strategy for enhancing immunity to tumors and infectious disease organisms. Studies have also demonstrated that targeting Ags to Fcγ receptors (FcγR) on Ag presenting cells can enhance humoral and cellular immunity in vitro and in vivo. Specifically, our studies using an F. tularensis (Ft) infectious disease vaccine model have demonstrated that targeting immunogens to FcγR via intranasal (i.n.) administration of monoclonal antibody (mAb)-inactivated Ft (iFt) immune complexes (ICs) enhances protection against Ft challenge. Ft is the causative agent of tularemia, a debilitating disease of humans and other mammals and a category A biothreat agent for which there is no approved vaccine. Therefore, using iFt Ag as a model immunogen, we sought to determine if ex vivo targeting of iFt to FcγR on DCs would enhance the potency of i.n. administered iFt-pulsed DCs. In this study, bone marrow-derived DCs (BMDCs) were pulsed ex vivo with iFt or mAb-iFt ICs. Intranasal administration of mAb-iFt-pulsed BMDCs enhanced humoral and cellular immune responses, as well as protection against Ft live vaccine strain (LVS) challenge. Increased protection correlated with increased iFt loading on the BMDC surface as a consequence of FcγR targeting. However, the inhibitory FcγRIIB had no impact on this enhancement. In conclusion, targeting Ag ex vivo to FcγR on DCs provides a method for enhanced Ag loading of DCs ex vivo, thereby reducing the amount of Ag required, while also avoiding the inhibitory impact of FcγRIIB. Thus, this represents a simple and less invasive strategy for increasing the potency of ex vivo-pulsed DC vaccines against chronic infectious diseases and cancer.
Keywords: Dendritic cell vaccine, Fcγ receptor-targeted vaccine, intranasal vaccine, vaccine platform, chronic infection, cancer
1. Introduction
Dendritic cells (DCs) play a central role in generating immunity to infection [1]. Specifically, DCs are highly efficient at taking up, processing, and presenting antigens (Ags) to naïve T cells [1]. This has lead to studies focused on DC-based vaccines against cancer and infectious diseases including HIV-1 and influenza [2–6]. Furthermore, numerous studies, including our own, have demonstrated that targeting Ag to Fcγ receptors (FcγRs) on Ag presenting cells (APCs), can enhance humoral and cellular immunity and protection against infectious diseases and cancer [6–12]. Consistent with these observations, engagement of FcγRs can induce DC maturation, a key event required for Ag processing and presentation to T cells [13, 14]. In this regard, studies from our laboratory have demonstrated that inactivated F. tularensis (iFt) Ag can induce enhanced protection against Ft challenge, when targeted to FcγRs as mAb-iFt complexes (ICs) administered intranasally (i.n.). The latter involves enhanced Ag binding to DCs, DC maturation, Ag processing/presentation by DCs, and iFt trafficking to lymphoid tissues [7, 12, 14]. Thus, we hypothesized that the ability of ex vivo Ag-pulsed DCs to induce immunity/protection could be significantly enhanced by pulsing DCs with FcγR-targeted Ag administered i.n. Importantly, while parenteral immunization generally utilizes needle injection and is not optimal for stimulating mucosal immunity, i.n. immunization is less invasive and stimulates strong parenteral and mucosal immune responses [15]. Furthermore, recent studies have demonstrated Ag-containing DCs administered i.n., like Ag-containing peripheral DCs, migrate to lymphoid tissues [16, 17].
In this report, we demonstrate that immunization of mice i.n. with ex vivo FcγR-targeted Ag (mAb-iFt)-pulsed bone marrow derived DCs (BMDCs) enhances humoral and cellular immune responses and protection against Ft challenge. Furthermore, despite the presence of the inhibitory FcγRIIB on DCs [18], protection is not impacted by FcγRIIB. Thus, these studies identify a vaccine strategy, which can be used to increase the potency of DC-based vaccines, as well as simultaneously induce mucosal and peripheral immunity, while bypassing the inhibitory impact of FcγRIIB.
2. Materials and Methods
2.1. Cells and reagents
RPMI 1640 medium (CellGro, Manassas, VA) supplemented with 10% FCS (HyClone, Logan, UT), 2mM L-glutamine, 1 mM sodium pyruvate, 1% MEM nonessential amino acids (CellGro), 100 U/ml penicillin, 100 µg/ml streptomycin (Gibco Life Technologies, Carlsbad, CA), and 50 µM 2-ME (Bio-Rad, Hercules, CA) was used to generate BMDCs. The Ft-specific T cell hybridoma (FT256D10) is specific for an Ft ribosomal protein-derived peptide, and was provided by Dr. Jeffrey Frelinger (University of North Carolina at Chapel Hill). The T cell hybridoma was maintained in RPMI 1640 medium containing 500 µg/ml of hygromycin B (CellGro). The mouse IgG2a anti-Ft LPS mAb used to make mAb-iFt ICs was purchased from Fitzgerald (cat # 10-F02, clone M0232621, Acton, MA). Mouse recombinant Flt3 ligand (Flt3L) was obtained from R&D systems (Minneapolis, MN). Abs for flow cytometry: Anti-mouse CD11c, CD11b, CD8a, B220, CD3, CD4, MHC class II (I-A/I-E), CD40, CD83, CD80, CD86, DEC205, or IFN-γ, as well as isotype control Abs, were purchased from eBioscience (San Diego, CA). Luminex Bio-Plex assay kits were purchased from Bio-Rad (Hercules, CA).
2.2. Mice
C57BL/6 and FcγRIIB knockout (KO) mice on a C57BL/6 background were purchased from Taconic Laboratories (Hudson, NY). Mice (6 to 8 weeks of age) were housed in the Animal Resources Facility at Albany Medical College and provided with ad lib water and food during the course of each experiment. Animal studies were reviewed and approved by the Institutional Animal Care and Use Committee utilizing NIH standards.
2.3. iFt (Ag) and mAb-iFt ICs
Inactivated Ft LVS (iFt) (immunogen) was prepared as previously described using endotoxin-free PBS [7, 14, 19]. The mAb-iFt ICs were generated by adding iFt to DCs in tissue culture medium, followed by mAb to a final concentration of 1 µg/ml. Unless otherwise indicated in the figure legend, iFt was used at a ratio of 10 iFt/cell. In all experiments comparing iFt versus mAb-iFt, iFt amounts were equalized between iFt and mAb-iFt preparations.
2.4. BMDCs
BMDCs were generated from mouse BM precursor as previously described [14, 20]. BMDC phenotype was verified by flow cytometry (> 93% CD11c+, high CD11b and B220, very low CD8a).
2.5. Immunization and challenge
BMDCs (3 × 106 cells/ml) in RPMI 1640 medium were pulsed with medium, iFt, or iFt plus 1 µg/ml anti-Ft LPS mAb at 37°C for 3 hours. BMDCs were then washed 3 times in PBS. Each mouse was then anesthetized and administered i.n. either 30 µl of PBS (vehicle) or 3 × 106 pulsed BMDCs. Before and after immunizations, serum was collected and analyzed for the presence of Ft-specific Ab. To measure Ft-specific Ab in BAL, immunized mice were sacrificed 2 and 4 weeks post-boost. In challenge experiments, immunized mice were infected i.n. 2 and 4 weeks post-boost with 4 × LD50 of live Ft LVS. Survival was monitored for 28 days.
2.6. BMDC maturation and cytokine secretion
BMDCs were cultured overnight in RPMI medium alone, or with iFt, or mAb-iFt ICs. Cells were then harvested, stained, and analyzed for maturation markers (CD40, CD80, CD86, CD205, and MHCII) via flow cytometry as previously described [14]. To monitor cytokine secretion, BMDCs were cultured with medium, iFt, or mAb-iFt for 3 days at 37°C. Culture supernatants were then collected and analyzed for cytokines/chemokines using the Luminex Bio-Plex assay system (Bio Rad).
2.7. Ag presentation
BMDCs were pulsed overnight at 37°C with medium, iFt, or mAb-iFt ICs. Cells were then washed twice with medium to remove unbound iFt and adjusted to 2 × 106 cells/ml. In a 96 well plate, 100 µl of 2 × 105 pulsed BMDCs were co-cultured with 100 µl of 1 × 105 Ft-specific T cells (FT256D10). The plate was then incubated at 37°C for 24 h and supernatants were collected. Supernatants were then assayed for IL-5 (secreted by these cells in response to Ag) using Luminex.
2.8. ELISA
The production of anti-Ft Ab in response to immunization was measured by ELISA as previously described [19].
2.9. Recall responses
Single cell suspensions from spleen and mediastinal lymph nodes (MLNs) were prepared from immunized mice at 2 and 4 weeks post-boost. In the case of MLN cells, due to limited numbers of cells, MLNs cells were pooled from mice in each immunization group. Cells were cultured in 96-well plates at 3 × 105 cells (200 µl)/well) for 3 days in the presence of medium or iFt. Supernatants were subsequently harvested and analyzed for cytokines by Luminex assay.
2.10. CD4 and CD8 T cell frequencies
Splenocytes and lung cells (3 × 106) were harvested from immunized mice and cultured overnight with iFt, ionomycin (50 ng/ml) (Sigma-Aldrich), and phorbol 12-myristate 13-acetate (PMA, Sigma-Aldrich) (750 ng/ml). Monensin solution (eBioscience) was then added to the cultures for 4 hours. Cells were then washed with FACS buffer (PBS containing 0.1% BSA and 0.02% Sodium azide) and stained for CD3, CD8, and CD4. Subsequently, cells were washed and fixed on ice with 2% paraformaldehyde in PBS for 30 minutes. Cells were then resuspended in permeablization buffer [PBS containing 0.1% saponin (Sigma) and 0.09% sodium azide (Sigma)] for another 30 minutes on ice and stained with anti-IFN-γ Ab. The number of CD4+ and CD8+ T cells positive for IFN-γ was then quantified using an LSRII flow cytometer.
2.11. Statistics
The log-rank (Mantel–Cox) test was used for survival curves. One-way analysis of variances or the unpaired, one-tailed Student’s t-test was used for the remaining figures. Data analyses were performed using GraphPad Prism 4 (San Diego, CA).
3. Results
3.1. Mice immunized with mAb-iFt-pulsed BMDCs are better protected against Ft LVS challenge
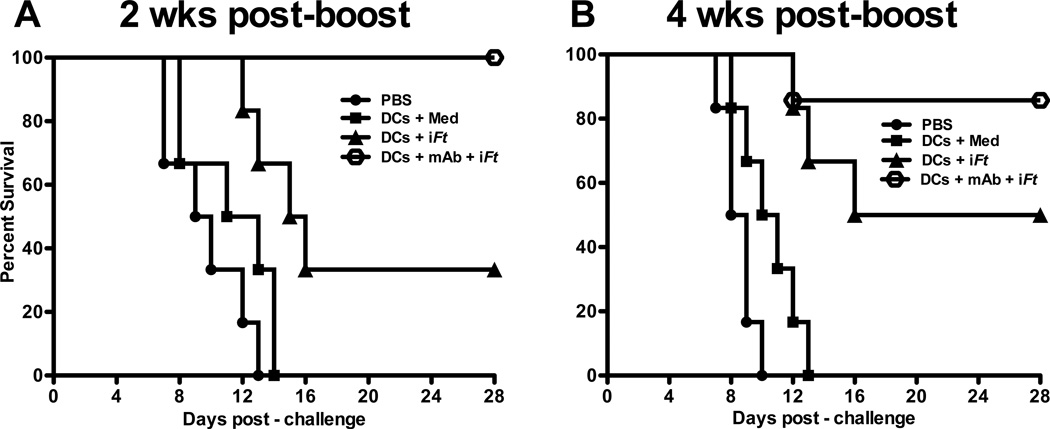
To evaluate the impact of administering BMDCs pulsed ex-vivo with FcγR-targeted mAb-iFt ICs versus non-FcγR-targeted iFt i.n. on protection, mice were immunized with PBS or BMDCs pulsed ex-vivo with medium, iFt, or mAb-iFt. Mice were subsequently challenged with a lethal dose of Ft LVS (4 × LD50) i.n. 2 and 4 weeks post-boost and monitored for 28 days for survival. As shown in Fig. 1A, mice immunized with mAb-iFt-pulsed BMDCs and challenged 2 weeks post-boost were 100% protected compared with mice immunized with iFt-pulsed BMDCs, which were 37% protected. No protection was observed when administering PBS or BMDCs pulsed with culture medium. Similarly, 4 weeks post-boost (Fig. 1B), mice immunized with mAb-iFt-pulsed BMDCs exhibited 87% survival compared to 50% survival for the iFt-pulsed BMDC group.
Figure 1. Mice immunized with mAb-iFt-pulsed BMDCs are better protected against a lethal Ft LVS challenge.
BMDCs pulsed with medium, iFt, or mAb-iFt (10 iFt/cell) were washed with PBS. Pulsed cells (3 × 106 cells/dose) were then suspended in 30 µl of PBS and administered to C57BL/6 mice (n=6) i.n. as droplets in alternating nares. Mice were primed and boosted with the Ag-pulsed BMDCs on days 0 and 14. Two weeks (A) and 4 weeks (B) post-boost, mice were challenged i.n. with 4 × LD50 (2 × 104 CFU) of Ft LVS in a volume of 20 µl of PBS. Survival was monitored for 28 days post-infection. The vehicle control groups received PBS only. Data presented are from a single experiment containing 6 mice per group. Each figure depicts results from a single experiment. The figure in panel A is representative of four independent experiments. The figure in panel B is representative of two independent experiments. In the case of panel A, the p value for the difference between iFt versus PBS is p=0.0045 and for iFt versus mAb-iFt 0.018. In the case of panel B, the p value for the difference between iFt versus PBS is p=0.0006 and for iFt versus mAb-iFt 0.2108.
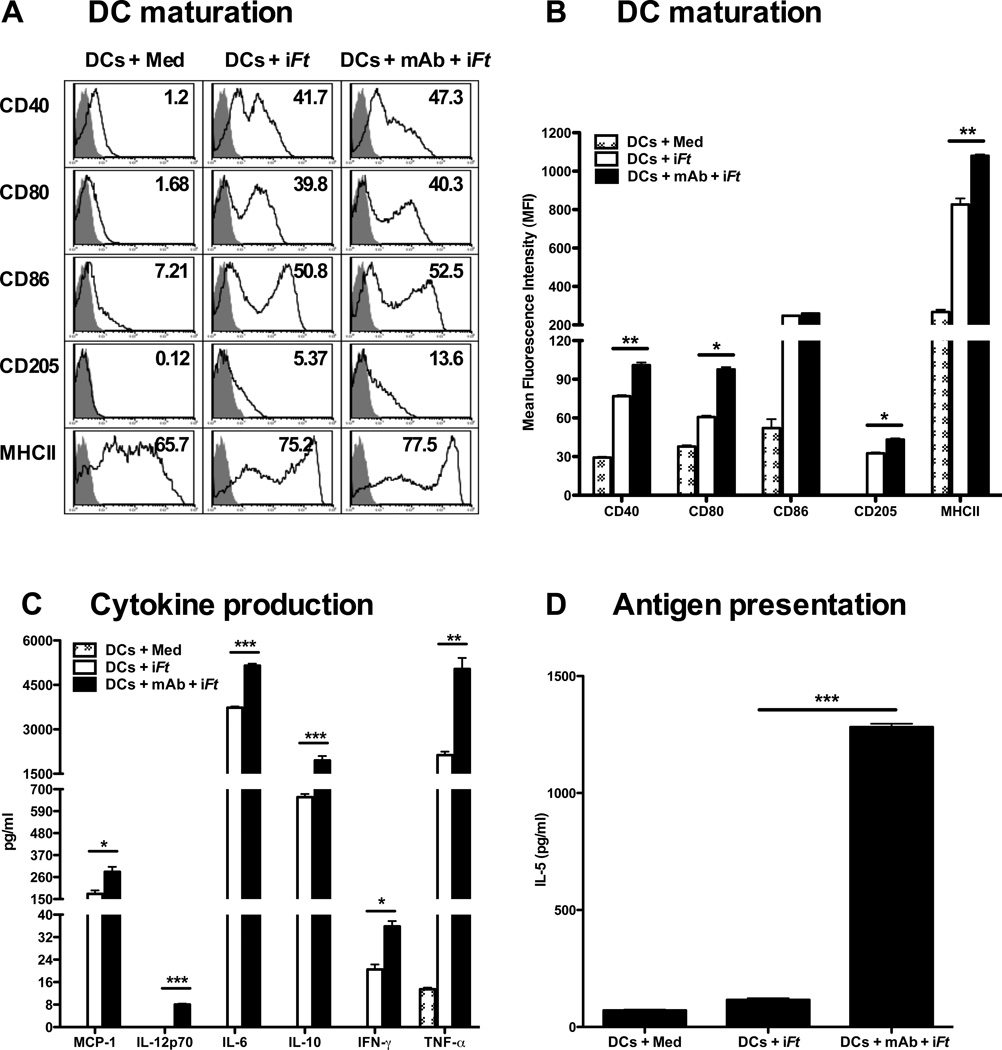
3.2. Pulsing BMDCs ex vivo with mAb-iFt ICs enhances DC maturation, cytokine secretion, and Ag presentation
In order to study the effect of FcγR-targeted mAb-iFt ICs versus non-FcγR-targeted iFt on BMDC function, we examined DC maturation following exposure to medium, iFt, or mAb-iFt for 24 hours in vitro. As demonstrated in Figs. 2A and 2B, the expression of the maturation markers CD40, CD80, CD86, CD205, and MHCII were substantially increased for iFt-pulsed BMDCs. However, a significant increase in CD40, CD80, CD205, and MHCII expression above that of iFt was also observed when pulsing BMDCs with mAb-iFt. Similar results were observed when measuring IL-12p70, IL-6, IL-10, IFN-γ, TNF-α and MCP-1 in response to iFt versus mAb-iFt-pulsed DCs (Fig. 2C). In contrast, as shown in Figure 2D, T cell activation in response to iFt alone was relatively low (approximately twice that of the medium control), while there was approximately an 80-fold increase in T cell activation compared to that of iFt, when BMDCs were pulsed with mAb-iFt.
Figure 2. Pulsing BMDCs ex-vivo with mAb-iFt enhances in vitro iFt-induced BMDC maturation and Ag presentation.
BMDCs (5 × 105) were pulsed overnight with medium or iFt (10 iFt/cell) in the presence or absence of mAb (1 µg/ml). Cells were then washed and stained for DC maturation markers. Acquisition was performed using an LSRII flow cytometer and data was analyzed using FlowJo software (Tree Star). (A) The grey peak represents isotype controls. Numbers in the right hand corner of each histogram indicate percent positive cells for the indicated DC maturation marker. (B) The maturation data are analyzed and expressed as Mean Fluorescence Intensity (MFI). Data in A and B are representative of four independent experiments. (C) BMDCs (2 × 105) were stimulated with medium or iFt (100 iFt/cell), in the presence or absence of mAb (1µg/ml) for 3 days. Culture supernatants were then collected on day 3 and screened for cytokines using the Luminex assay. Data presented are from a single experiment, but are representative of results from three independent experiments. (D) BMDCs were stimulated overnight with medium or iFt (2.5 iFt/cell) in the presence or absence of mAb (1 µg/ml). Pulsed BMDCs were then washed 3 times with medium and co-cultured with an Ft-specific T cell hybridoma (FT256D10) for 24 hours. Culture supernatants were subsequently harvested and assayed for the presence of IL-5, a cytokine secreted by this T cell hybridoma in response to Ag recognition. Figures presented are from single experiments and are representative of results from three independent experiments. Data are presented as the average of three replicate samples ± SD (*: P< 0.01, ** P< 0.005, *** P<0.0005).
3.3. Intranasal administration of BMDCs pulsed ex vivo with mAb-iFt ICs enhances Ag-specific B and T cell responses
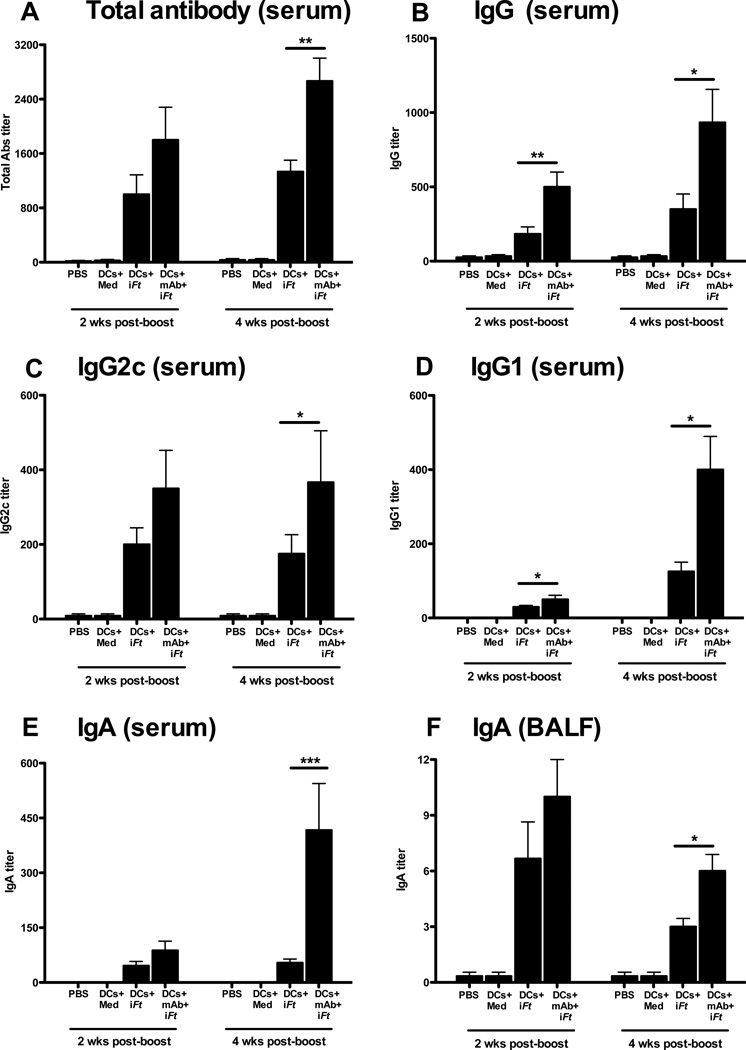
Humoral immunity can play a key role in protection against extracellular and intracellular bacterial infections, including Ft infection [7, 21–26]. In addition, IgA is the predominant Ab found at mucosal sites. Thus enhancement of pathogen-specific Abs provides one possible means of enhancing protection against mucosal infection [7]. Sera were thus collected from mice immunized i.n. with BMDCs pulsed with medium, iFt, or mAb-iFt 2 and 4 weeks post-boost, and Ft-specific Abs were measured by ELISA. In the case where mAb-iFt-pulsed BMDCs were administered, total Ft-specific Ab was enhanced above that of iFt-pulsed BMDCs (Figs. 3A). The case was similar when examining Ft-specific IgG (Fig. 3B), IgG2c (Fig. 3C), IgG1 (Fig. 3D), and IgA (Figs. 3E and 3F).
Figure 3. Mice immunized with mAb-iFt-pulsed BMDCs exhibit higher Ft-specific Ab titers.
Sera and BALF from immunized mice (n=4) were analyzed for total Ft-specific Ab (IgG, IgG1, IgG2c, and IgA) by ELISA. Ab titers were calculated using the reciprocal of the endpoint dilution of the test serum. Figures presented are from a single experiment, but are representative of results from two independent experiments. Data are presented as the average of four individual mice ± SD (*: P< 0.01, ** P< 0.005, *** P<0.0005).
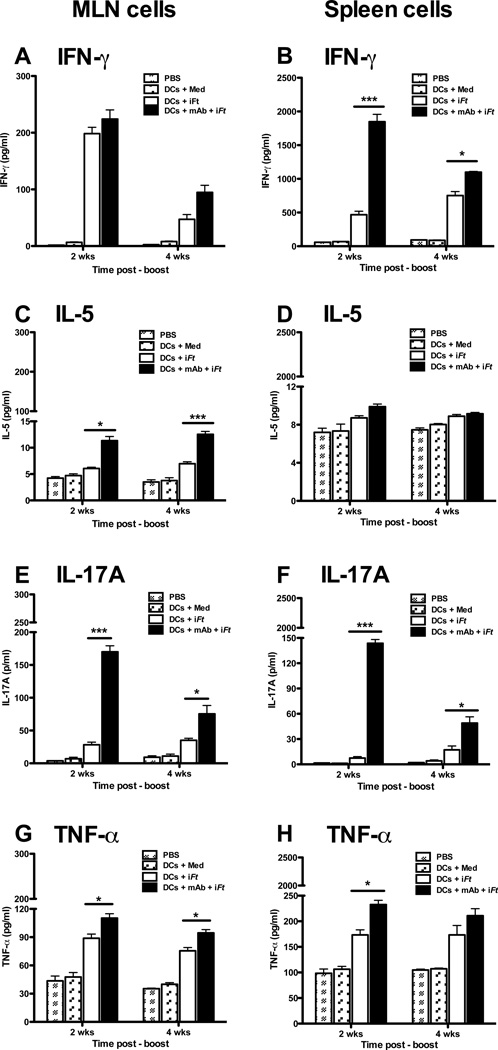
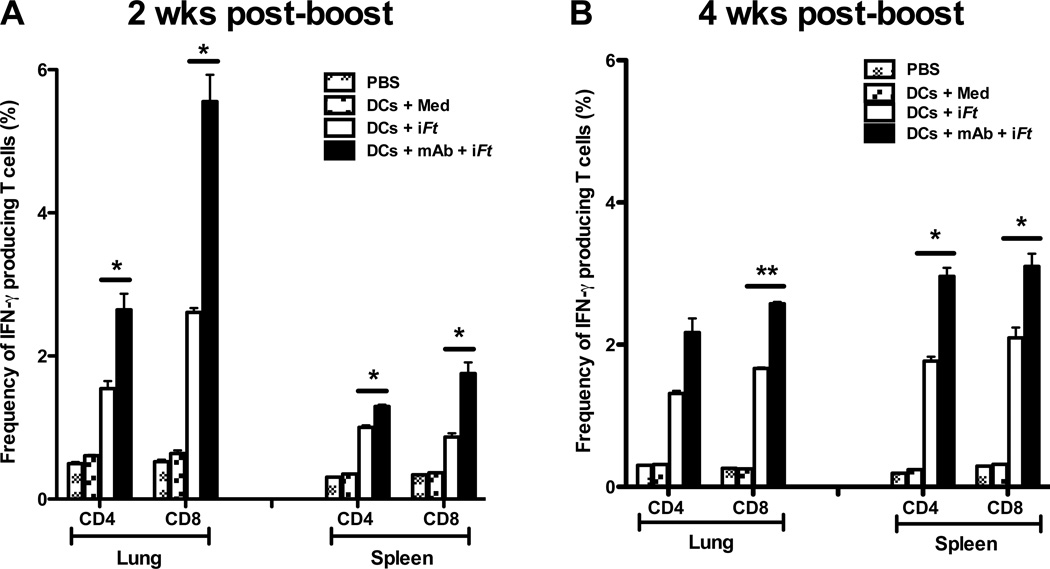
IFN-γ also plays a key role in controlling Ft infection [7, 19, 27, 28]. In addition, pro-inflammatory cytokines such as IL-17 and TNF-α can confer protection against a variety of extracellular and intracellular bacterial pathogens including Ft [29–32]. As shown in Figure 4, cells from MLN of mice immunized with mAb-iFt-pulsed BMDCs exhibited increased production of IFN-γ (Fig. 4A), IL-5 (Fig. 4C), IL-17A (Fig. 4E), and TNF-α (Fig. 4G), as compared to that of iFt-pulsed BMDCs. Similar enhancement of cytokine levels from splenocytes of mice immunized with mAb-iFt-pulsed BMDCs were also observed, with the exception of IL-5 (Figs. 4B, 4D, 4F, and 4H). To further define the IFN-γ-producing T cell subsets being enhanced, we analyzed IFN-γ+ CD4+ and IFN-γ+ CD8+ T cell populations from lung and spleen of immunized mice. In all cases, immunization with mAb-iFt-pulsed BMDCs increased the frequencies of IFN-γ+ CD4+ and IFN-γ+ CD8+ T cells over that of iFt-pulsed BMDCs (Figs. 5A and 5B).
Figure 4. MLN and spleen cells from mice immunized with mAb-iFt-pulsed BMDCs exhibit increased cytokine levels upon restimulation with iFt in vitro.
Single cell suspensions (3 × 105) of MLN or spleen cells from immunized mice (n=4) were added to wells of a 96 well plate and re-stimulated in vitro with medium or iFt (5 iFt/cell) for 3 days. Culture supernatants were harvested on day 3 and analyzed for cytokines using the Luminex assay. Figures presented are from a single experiment, but are representative of results from two independent experiments. Data are presented as the average of two (MLN cells) or four (Spleen cells) individual mice ± SD (*: P< 0.01, ** P< 0.005, *** P<0.0005).
Figure 5. The frequency of IFN-γ producing CD4 and CD8 T cells is enhanced in mice immunized i.n. with mAb-iFt-pulsed BMDCs.
Splenocytes and lung cells from immunized mice were stimulated in vitro with iFt (5 iFt/cell) combined with PMA and Ionomycine overnight, and then stained for CD4 and CD8 surface markers and intracellular IFN-γ. Samples were acquired using an LSRII flow cytometer and analyzed with FlowJo software. Percentages of IFN-γ+ CD4+ and IFN-γ+ CD8+ T lymphocytes at 2 weeks post-boost (A) and 4 weeks post-boost (B) are shown. Figures presented are from a single experiment, but are representative of results from two independent experiments. Data are presented as the average of four individual mice ± SD (*: P< 0.01, ** P< 0.005, *** P<0.0005).
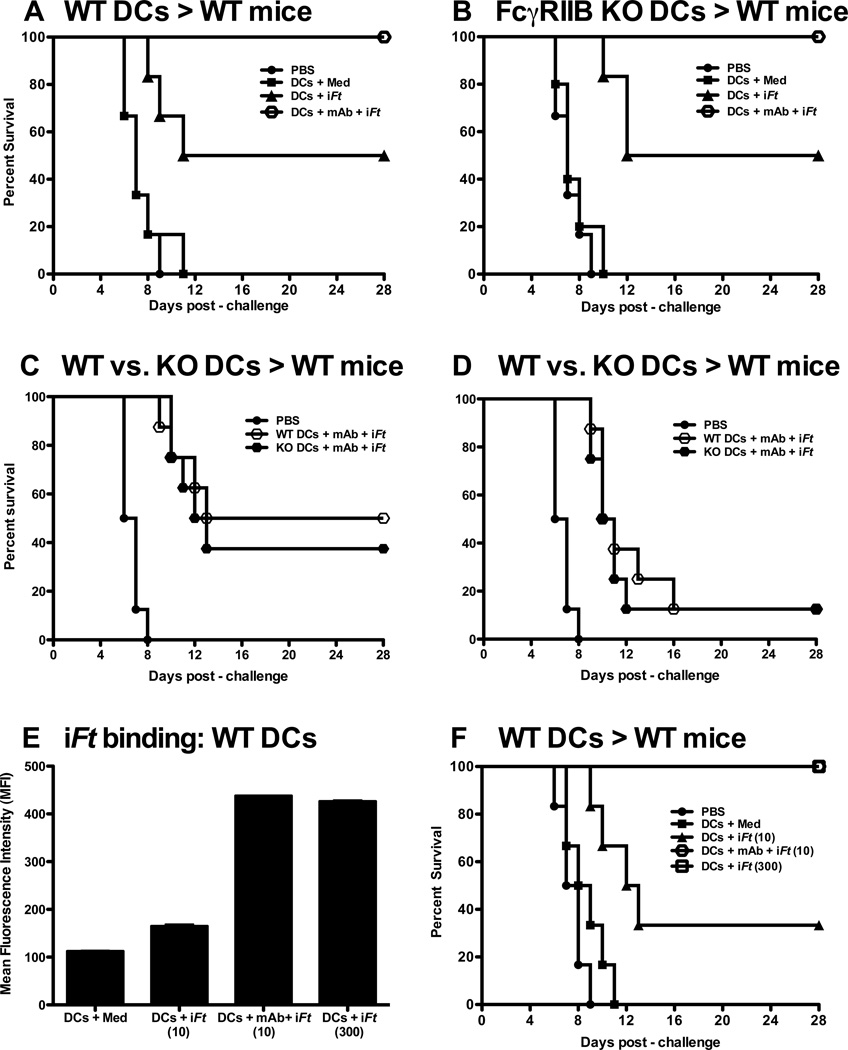
3.4. FcγRIIB has no impact on the enhanced protection induced by ex vivo mAb-iFt-pulsed BMDCs
FcγRIIB is the only inhibitory FcγR present in humans and mice [33, 34]. FcγRIIB mediates its inhibitory effect through an immunoreceptor tyrosine-based inhibitory motif (ITIM) in the cytoplasmic domain [35]. A number of studies have demonstrated that DCs derived from mice lacking FcγRIIB generate stronger immune responses in vitro and in vivo than that of WT mice [36, 37]. Since mAb-iFt ICs are capable of engaging FcγRIIB as well as activating FcγRs (I and III) on BMDCs, we wished to determine whether FcγRIIB limits the level protection we are able to achieve when administering mAb-iFt-pulsed BMDCs. As indicated in Figures 6A through 6D, the level of protection against Ft challenge is unchanged, regardless of whether FcγRIIB is present or absent on DCs pulsed with iFt (Figs 4A and 4B) or mAb-iFt ICs (Figs. 4C and 4D). Given the latter, we considered the possibility that FcγR signaling events initiated via the mAb-iFt IC pulse are not involved in the enhanced protection we observed, but rather that enhanced Ag (iFt) loading onto the DC surface is primarily responsible for the increased potency of the mAb-iFt-pulsed BMDC vaccine. Thus, we loaded BMDCs with equal amounts of iFt using either iFt or mAb-iFt ICs. As indicated in Figure 6E, by increasing the concentration of iFt 30 fold above that of mAb-iFt, BMDCs from both the iFt and mAb-iFt-pulsed DC groups bound equal amounts of iFt. Furthermore, in this case, DCs pulsed with either iFt or mAb-iFt were equally effective at inducing protection against Ft challenge (Fig. 6F).
Figure 6. The inhibitory receptor (FcγRIIB) has no impact on the enhanced protection induced by ex vivo mAb-iFt-pulsed BMDCs while enhanced iFt (Ag) loading plays the primary role in the enhanced protection observed with mAb-iFt-pulsed BMDCs.
C57BL/6 mice (n = 6) were i.n primed and boosted with Ag pulsed BMDCs differentiated from WT mice (A) or FcγRIIB KO mice (B). Two weeks post-boost, mice were challenged i.n. with 4 × LD50 of Ft LVS prepared in 20 µl of PBS. Survival was monitored for 28 days post-infection. Data are representative of two independent experiments. (C and D) C57BL/6 mice (n = 8) were immunized i.n. and boosted two weeks later with PBS or mAb-iFt pulsed BMDCs (10 iFt/cell) from WT or FcγRIIB KO mice. Two weeks post-boost, mice were challenged i.n. with 8 × LD50 of Ft LVS (C) or 16 × LD50 of Ft LVS (D) in 20 µl of PBS. DCs pulsed with PBS (the vehicle control group) were challenged with 4 × LD50 of Ft LVS. Survival was monitored for 28 days post-infection. (E) BMDCs were pulsed with medium, iFt (10 iFt/cell), mAb-iFt (10 iFt/cell) or an amount of iFt (300 iFt/cell), which produced iFt binding equivalent to that of mAb-iFt. In this case, GFP-expressing Ft was used to produce iFt in order to more easily monitor iFt binding by flow cytometry. Ft inactivation/fixation does not alter GFP fluorescence. Pulsed BMDCs were then washed 3 times with PBS and binding to BMDCs analyzed with an LSRII flow cytometer and FlowJo software. Data are presented from a single experiment as the average of three replicate samples ± SD. (F) C57BL/6 mice (n=6) were primed and boosted i.n. with BMDCs pulsed with iFt (10 iFt/cell or 300 iFt/cell) or mAb-iFt (10 iFt/cell). At days 0 and 14, 3 × 106 pulsed BMDCs in 30 µl of PBS were administered i.n. to mice. Two weeks post-boost, mice were challenged i.n. with 4 × LD50 of Ft LVS in 20 µl of PBS. Survival was monitored for 28 days post-infection. A vehicle control group received PBS alone. Figures presented in panels A, B, E, and F are from a single experiment, but are representative of results from two independent experiments.
4. Discussion
DCs play a critical role in generating immunity to infection [1]. Furthermore, the use of DCs pulsed ex vivo with Ag as a treatment strategy for cancer and chronic infections is currently a focus of intense investigation. Thus, the identification of approaches, which improve the effectiveness of DC-based vaccines could have a significant impact on DC-based vaccine development. Based on previous studies using soluble mAb-iFt ICs as i.n. immunogens [7], we postulated that targeting Ags to FcγR on DCs would enhance the efficacy of DC-based vaccines. Here, we describe for the first time the use of FcγR-targeted Ag-pulsed DCs administered i.n. to enhance the potency of ex-vivo Ag-pulsed DC vaccines. Specifically, targeting iFt to FcγR on BMDCs ex-vivo and administering them i.n., significantly enhances protection versus that of BMDCs pulsed with non-targeted iFt. However, somewhat surprisingly, FcγR signaling appears to play a minimal role in the enhanced protection observed. Specifically, when the amount of iFt loaded onto the surface of BMDCs is equalized to that of mAb-iFt-pulsed BMDCs, both iFt and mAb-iFt-pulsed BMDCs are equally effective at stimulating protection. The apparent lack of a requirement for FcγR signaling may be partially explained by the observation that non-targeted iFt stimulates substantial BMDC maturation and cytokine production. This is likely due to the presence of endotoxins, including Ft LPS and other bacterial products, which are iFt components. However, despite the stimulatory capacity of non-targeted iFt, DC maturation and cytokine secretion are further enhanced when iFt is targeted to FcγR on BMDCs. The latter is significant, since purified protein Ags are preferred for vaccine purposes but are poorly immunogenic and require adjuvant [38]. Importantly, this requirement for adjuvant can be overcome by targeting protein Ags to FcγR [7, 12], which, as observed, also stimulates DC maturation and cytokine secretion [13, 14, 39].
In regard to immune mechanisms involved, Ft-specific IgG and IgA Abs in sera and BALF of mice immunized with mAb-iFt-pulsed BMDCs, versus that of mice immunized with iFt-pulsed BMDCs, are significantly enhanced. Furthermore, production of IFN-γ, IL-17A, and TNF-α are also enhanced. While the role of Abs in protection against Ft infection is controversial, Abs do mediate protection against Ft infection under some circumstances [25, 26, 40]. In contrast, it is widely accepted that cellular immunity, in particular the production of IFN-γ and development of a Th1-type T cell response, is critical for generating protection against Ft [7, 19, 41–43]. A number of studies have also demonstrated a protective role for IL-17 following Ft infection [29–31, 44]. Thus, the fact Ab, IFN-γ, and IL-17 responses are enhanced via immunization with mAb-iFt-pulsed BMDCs, not only explains why enhanced protection against Ft is observed when utilizing mAb-iFt-pulsed BMDCs, but also suggests a broader potential for application of this strategy [2]. It is also important to note that another key advantage of utilizing DC-based vaccines in combination with FcγR targeting of Ag is the improved ability of DCs to process and present exogenous Ag through both the MHC Class II and MHC Class I pathways (cross-presentation), thereby stimulating CD4 and CD8 T cell responses, respectively [39]. CD8 T cell responses are not only important for controlling Ft infection [27], but also numerous intracellular infections and cancer. Thus, of substantial significance is the balanced induction of both CD4 and CD8 T cell responses induced by mAb-iFt-pulsed DCs.
Finally, the inhibitory receptor FcγRIIB present on DCs can negatively regulate Ag-specific immune responses [8, 45]. In our previous protection studies utilizing soluble mAb-iFt ICs administered i.n., Ft-specific IgA production was enhanced while production of Ft-specific IgG was inhibited [7]. This observation is consistent with mAb-iFt ICs engaging FcγRIIB and thereby negatively regulating IgG production. However, when utilizing mAb-iFt-pulsed DCs administered i.n., FcγRIIB had no significant impact on the level of protection, which is also reflected by the enhanced production of Ft-specific IgG, in direct contrast to what we observed using soluble mAb-iFt ICs. The latter is a critical distinction between these two approaches. Specifically, a significant additional advantage of using FcγR-targeted Ags in combination with this DC-based vaccine strategy is the ability to bypass FcγRIIB-mediated inhibition and enhance both Ag-specific IgG and IgA production. The ability to do the latter may be explained in part by observations that FcγRIIB expression is reduced as a consequence of DC maturation, which is enhanced by mAb-iFt. In addition, under some circumstances, FcγRIIB can act to enhance Ag presentation by DCs [18, 46, 47]. Alternatively, studies have suggested that in the case of DC-based vaccines, transfer of Ag to endogenous APCs is required in vivo [48], thus eliminating a direct role for FcγRIIB signaling in APC-mediated T cell activation. In addition, the latter may be further enhanced by DC lysis via DC-CD8 T cell interaction, thereby also contributing to reduced CD8 T cell responses over time [49]
5. Conclusions
These studies demonstrate the efficacy of a strategy for increasing the potency of DC-based vaccines by targeting immunogens to FcγR ex-vivo, prior to i.n. administration. Advantages of this approach include: Enhanced protection against infection; Enhanced mucosal, peripheral, humoral, and cellular immune responses; The ability to overcome FcγRIIB-mediated inhibition; The ability of DCs to act as “natural adjuvants” [50]; Less invasive administration [(i.n.) versus needle-based injection. In conclusion, this approach provides a unique ability to utilize FcγR-targeting to enhance Ag loading, immune responses, and protection generated by DC-based vaccines, while avoiding the inhibitory impact of FcγRIIB.
Highlights.
Efficacy: Effective i.n. vaccination via DCs pulsed ex-vivo with FcγR-targeted Ag is demonstrated.
Immune Responses: Mucosal, peripheral, humoral, and cellular immune responses are enhanced.
Immune Advantages: Traditional adjuvant is not required and inhibition via FcγRIIB is absent.
Clinical Advantages: Ability to use a less invasive i.n. route and stimulate CD4 and CD8 responses.
Applications: Vaccines and therapeutics against chronic infectious diseases and cancer.
Acknowledgments
This work was supported by grants from National Institute of Health 2P01 AI056320 and R01 AI076408. We thank the Animal Research Facility and CIMD Immunology Core for expert technical assistance during these studies.
Abbreviations
- DCs
Dendritic cells
- BMDCs
Bone Marrow-Derived Dendritic cells
- mAb
Monoclonal Antibody
- Ft
F. tularensis
- iFt
Inactivated Ft
- IC
immune complex
- Ag
Antigen
- Ab
Antibody
- FcγR
Fcγ receptors
- APCs
Ag presenting cells
- i.n.
intranasal
- BALF
bronchial alveolar lavage fluid
- MLN
mediastinal lymph node
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
None of the other authors have conflicts of interest to disclose.
References
- 1.Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392:245–252. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- 2.Apostolopoulos V, Thalhammer T, Tzakos AG, Stojanovska L. Targeting Antigens to Dendritic Cell Receptors for Vaccine Development. J Drug Delivery. 2013;2013:869718. doi: 10.1155/2013/869718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Garcia F, Plana M, Climent N, Leon A, Gatell JM, Gallart T. Dendritic cell based vaccines for HIV infection: The way ahead. Hum Vac & Immunother. 2013;9 doi: 10.4161/hv.25876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boonnak K, Vogel L, Orandle M, Zimmerman D, Talor E, Subbarao K. Antigen-activated dendritic cells ameliorate influenza A infections. Journal Clin Inv. 2013;123:2850–2861. doi: 10.1172/JCI67550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tacken PJ, de Vries IJ, Torensma R, Figdor CG. Dendritic-cell immunotherapy: from ex vivo loading to in vivo targeting. Nat Rev Immunol. 2007;7:790–802. doi: 10.1038/nri2173. [DOI] [PubMed] [Google Scholar]
- 6.Gil M, Bieniasz M, Wierzbicki A, Bambach BJ, Rokita H, Kozbor D. Targeting a mimotope vaccine to activating Fcgamma receptors empowers dendritic cells to prime specific CD8+ T cell responses in tumor-bearing mice. J Immunol. 2009;183:6808–6818. doi: 10.4049/jimmunol.0900364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rawool DB, Bitsaktsis C, Li Y, Gosselin DR, Lin Y, Kurkure NV, et al. Utilization of Fc receptors as a mucosal vaccine strategy against an intracellular bacterium, Francisella tularensis. J Immunol. 2008;180:5548–5557. doi: 10.4049/jimmunol.180.8.5548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Heyman B. Regulation of antibody responses via antibodies, complement, and Fc receptors. Ann. Rev Immunol. 2000;18:709–737. doi: 10.1146/annurev.immunol.18.1.709. [DOI] [PubMed] [Google Scholar]
- 9.Keler T, Guyre PM, Vitale LA, Sundarapandiyan K, van De Winkel JG, Deo YM, et al. Targeting weak antigens to CD64 elicits potent humoral responses in human CD64 transgenic mice. J Immunol. 2000;165:6738–6742. doi: 10.4049/jimmunol.165.12.6738. [DOI] [PubMed] [Google Scholar]
- 10.Heijnen IA, van Vugt MJ, Fanger NA, Graziano RF, de Wit TP, Hofhuis FM, et al. Antigen targeting to myeloid-specific human Fc gamma RI/CD64 triggers enhanced antibody responses in transgenic mice. J Clin Invest. 1996;97:331–338. doi: 10.1172/JCI118420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gosselin EJ, Wardwell K, Gosselin DR, Alter N, Fisher JL, Guyre PM. Enhanced antigen presentation using human Fc gamma receptor (monocyte/macrophage)-specific immunogens. J Immunol. 1992;149:3477–3481. [PubMed] [Google Scholar]
- 12.Bitsaktsis C, Iglesias BV, Li Y, Colino J, Snapper CM, Hollingshead SK, et al. Mucosal immunization with an unadjuvanted vaccine that targets Streptococcus pneumoniae PspA to human Fcgamma receptor type I protects against pneumococcal infection through complement- and lactoferrin-mediated bactericidal activity. Infect Immun. 2012;80:1166–1180. doi: 10.1128/IAI.05511-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sedlik C, Orbach D, Veron P, Schweighoffer E, Colucci F, Gamberale R, et al. A critical role for Syk protein tyrosine kinase in Fc receptor-mediated antigen presentation and induction of dendritic cell maturation. J Immunol. 2003;170:846–852. doi: 10.4049/jimmunol.170.2.846. [DOI] [PubMed] [Google Scholar]
- 14.Iglesias BV, Bitsaktsis C, Pham G, Drake JR, Hazlett KR, Porter K, et al. Multiple mechanisms mediate enhanced immunity generated by mAb-inactivated F. tularensis immunogen. Immunol Cell Biol. 2013;91:139–148. doi: 10.1038/icb.2012.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Davis SS. Nasal vaccines. Adv Drug Delivery Rev. 2001;51:21–42. doi: 10.1016/s0169-409x(01)00162-4. [DOI] [PubMed] [Google Scholar]
- 16.Vilekar P, Awasthi V, Lagisetty P, King C, Shankar N, Awasthi S. In vivo trafficking and immunostimulatory potential of an intranasally-administered primary dendritic cell-based vaccine. BMC Immunol. 2010;11:60. doi: 10.1186/1471-2172-11-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Platt AM, Randolph GJ. Dendritic cell migration through the lymphatic vasculature to lymph nodes. Adv Immunol. 2013;120:51–68. doi: 10.1016/B978-0-12-417028-5.00002-8. [DOI] [PubMed] [Google Scholar]
- 18.Liu Y, Gao X, Masuda E, Redecha PB, Blank MC, Pricop L. Regulated expression of FcgammaR in human dendritic cells controls cross-presentation of antigen-antibody complexes. J Immunol. 2006;177:8440–8447. doi: 10.4049/jimmunol.177.12.8440. [DOI] [PubMed] [Google Scholar]
- 19.Bitsaktsis C, Rawool DB, Li Y, Kurkure NV, Iglesias B, Gosselin EJ. Differential requirements for protection against mucosal challenge with Francisella tularensis in the presence versus absence of cholera toxin B and inactivated F. tularensis. J Immunol. 2009;182:4899–4909. doi: 10.4049/jimmunol.0803242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Periasamy S, Singh A, Sahay B, Rahman T, Feustel PJ, Pham GH, et al. Development of tolerogenic dendritic cells and regulatory T cells favors exponential bacterial growth and survival during early respiratory tularemia. J Leuk Biol. 2011;90:493–507. doi: 10.1189/jlb.0411197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yager E, Bitsaktsis C, Nandi B, McBride JW, Winslow G. Essential role for humoral immunity during Ehrlichia infection in immunocompetent mice. Infect Imm. 2005;73:8009–8016. doi: 10.1128/IAI.73.12.8009-8016.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Feng HM, Whitworth T, Olano JP, Popov VL, Walker DH. Fc-dependent polyclonal antibodies and antibodies to outer membrane proteins A and B, but not to lipopolysaccharide, protect SCID mice against fatal Rickettsia conorii infection. Infect Imm. 2004;72:2222–2228. doi: 10.1128/IAI.72.4.2222-2228.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Edelson BT, Unanue ER. Intracellular antibody neutralizes Listeria growth. Immunity. 2001;14:503–512. doi: 10.1016/s1074-7613(01)00139-x. [DOI] [PubMed] [Google Scholar]
- 24.Casadevall A. Antibody-mediated protection against intracellular pathogens. Trends Microbiol. 1998;6:102–107. doi: 10.1016/s0966-842x(98)01208-6. [DOI] [PubMed] [Google Scholar]
- 25.Klimpel GR, Eaves-Pyles T, Moen ST, Taormina J, Peterson JW, Chopra AK, et al. Levofloxacin rescues mice from lethal intra-nasal infections with virulent Francisella tularensis and induces immunity and production of protective antibody. Vaccine. 2008;26:6874–6882. doi: 10.1016/j.vaccine.2008.09.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mara-Koosham G, Hutt JA, Lyons CR, Wu TH. Antibodies contribute to effective vaccination against respiratory infection by type A Francisella tularensis strains. Infect Imm. 2011;79:1770–1778. doi: 10.1128/IAI.00605-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Elkins KL, Cowley SC, Bosio CM. Innate and adaptive immunity to Francisella. Ann NY Acad Sci. 2007;1105:284–324. doi: 10.1196/annals.1409.014. [DOI] [PubMed] [Google Scholar]
- 28.Ryden P, Twine S, Shen H, Harris G, Chen W, Sjostedt A, et al. Correlates of protection following vaccination of mice with gene deletion mutants of Francisella tularensis subspecies tularensis strain, SCHU S4 that elicit varying degrees of immunity to systemic and respiratory challenge with wild-type bacteria. Mol Immunol. 2013;54:58–67. doi: 10.1016/j.molimm.2012.10.043. [DOI] [PubMed] [Google Scholar]
- 29.Lin Y, Ritchea S, Logar A, Slight S, Messmer M, Rangel-Moreno J, et al. Interleukin-17 is required for T helper 1 cell immunity and host resistance to the intracellular pathogen Francisella tularensis. Immunity. 2009;31:799–810. doi: 10.1016/j.immuni.2009.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Markel G, Bar-Haim E, Zahavy E, Cohen H, Cohen O, Shafferman A, et al. The involvement of IL-17A in the murine response to sub-lethal inhalational infection with Francisella tularensis. PloS one. 2010;5:e11176. doi: 10.1371/journal.pone.0011176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Skyberg JA, Rollins MF, Samuel JW, Sutherland MD, Belisle JT, Pascual DW. Interleukin-17 Protects against the Francisella tularensis Live Vaccine Strain but Not against a Virulent F. tularensis Type A Strain. Infect Imm. 2013;81:3099–3105. doi: 10.1128/IAI.00203-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Green SJ, Nacy CA, Schreiber RD, Granger DL, Crawford RM, Meltzer MS, et al. Neutralization of gamma interferon and tumor necrosis factor alpha blocks in vivo synthesis of nitrogen oxides from L-arginine and protection against Francisella tularensis infection in Mycobacterium bovis BCG-treated mice. Infect Imm. 1993;61:689–698. doi: 10.1128/iai.61.2.689-698.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Takai T, Ono M, Hikida M, Ohmori H, Ravetch JV. Augmented humoral and anaphylactic responses in Fc gamma RII-deficient mice. Nature. 1996;379:346–349. doi: 10.1038/379346a0. [DOI] [PubMed] [Google Scholar]
- 34.Sharp PE, Martin-Ramirez J, Boross P, Mangsbo SM, Reynolds J, Moss J, et al. Increased incidence of anti-GBM disease in Fcgamma receptor 2b deficient mice, but not mice with conditional deletion of Fcgr2b on either B cells or myeloid cells alone. Mol Immunol. 2012;50:49–56. doi: 10.1016/j.molimm.2011.12.007. [DOI] [PubMed] [Google Scholar]
- 35.Williams EL, Tutt AL, Beers SA, French RR, Chan CH, Cox KL, et al. Immunotherapy targeting inhibitory Fcgamma receptor IIB (CD32b) in the mouse is limited by monoclonal antibody consumption and receptor internalization. J Immunol. 2013;191:4130–4140. doi: 10.4049/jimmunol.1301430. [DOI] [PubMed] [Google Scholar]
- 36.Smyth MJ, Kershaw MH. Immunology. The adjuvant effects of antibodies. Science. 2011;333:944–945. doi: 10.1126/science.1210801. [DOI] [PubMed] [Google Scholar]
- 37.Kalergis AM, Ravetch JV. Inducing tumor immunity through the selective engagement of activating Fcgamma receptors on dendritic cells. Journal Exp Med. 2002;195:1653–1659. doi: 10.1084/jem.20020338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kensil CR, Mo AX, Truneh A. Current vaccine adjuvants: an overview of a diverse class. Front Biosci. 2004;9:2972–2988. doi: 10.2741/1452. [DOI] [PubMed] [Google Scholar]
- 39.Schuurhuis DH, Ioan-Facsinay A, Nagelkerken B, van Schip JJ, Sedlik C, Melief CJ, et al. Antigen-antibody immune complexes empower dendritic cells to efficiently prime specific CD8+ CTL responses in vivo. J Immunol. 2002;168:2240–2246. doi: 10.4049/jimmunol.168.5.2240. [DOI] [PubMed] [Google Scholar]
- 40.Lavine CL, Clinton SR, Angelova-Fischer I, Marion TN, Bina XR, Bina JE, et al. Immunization with heat-killed Francisella tularensis LVS elicits protective antibody-mediated immunity. Eur J Immunol. 2007;37:3007–3020. doi: 10.1002/eji.200737620. [DOI] [PubMed] [Google Scholar]
- 41.Dreisbach VC, Cowley S, Elkins KL. Purified lipopolysaccharide from Francisella tularensis live vaccine strain (LVS) induces protective immunity against LVS infection that requires B cells and gamma interferon. Infect Imm. 2000;68:1988–1996. doi: 10.1128/iai.68.4.1988-1996.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rhinehart-Jones TR, Fortier AH, Elkins KL. Transfer of immunity against lethal murine Francisella infection by specific antibody depends on host gamma interferon and T cells. Infect Imm. 1994;62:3129–3137. doi: 10.1128/iai.62.8.3129-3137.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Conlan W, Shen H, Kuolee R, Zhao X, Chen W. Aerosol-, but not intradermal-immunization with the live vaccine strain of Francisella tularensis protects mice against subsequent aerosol challenge with a highly virulent type A strain of the pathogen by an alphabeta T cell- and interferon gamma- dependent mechanism. Vaccine. 2005;23:2477–2485. doi: 10.1016/j.vaccine.2004.10.034. [DOI] [PubMed] [Google Scholar]
- 44.Cowley SC, Meierovics AI, Frelinger JA, Iwakura Y, Elkins KL. Lung CD4-CD8- double-negative T cells are prominent producers of IL–17A and IFN-gamma during primary respiratory murine infection with Francisella tularensis live vaccine strain. J Immunol. 2010;184:5791–5801. doi: 10.4049/jimmunol.1000362. [DOI] [PubMed] [Google Scholar]
- 45.Boruchov AM, Heller G, Veri MC, Bonvini E, Ravetch JV, Young JW. Activating and inhibitory IgG Fc receptors on human DCs mediate opposing functions. Journal Clin Invest. 2005;115:2914–2923. doi: 10.1172/JCI24772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bergtold A, Desai DD, Gavhane A, Clynes R. Cell surface recycling of internalized antigen permits dendritic cell priming of B cells. Immunity. 2005;23:503–514. doi: 10.1016/j.immuni.2005.09.013. [DOI] [PubMed] [Google Scholar]
- 47.Yada A, Ebihara S, Matsumura K, Endo S, Maeda T, Nakamura A, et al. Accelerated antigen presentation and elicitation of humoral response in vivo by FcgammaRIIB- and FcgammaRI/III-mediated immune complex uptake. Cell Immunol. 2003;225:21–32. doi: 10.1016/j.cellimm.2003.09.008. [DOI] [PubMed] [Google Scholar]
- 48.Yewdall AW, Drutman SB, Jinwala F, Bahjat KS, Bhardwaj N. CD8+ T cell priming by dendritic cell vaccines requires antigen transfer to endogenous antigen presenting cells. PloS one. 2010;5:e11144. doi: 10.1371/journal.pone.0011144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ronchese F, Hermans IF. Killing of dendritic cells: a life cut short or a purposeful death? J Ex Med. 2001;194:F23–F26. doi: 10.1084/jem.194.5.f23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fajardo-Moser M, Berzel S, Moll H. Mechanisms of dendritic cell-based vaccination against infection. Int J Med Microbiol. 2008;298:11–20. doi: 10.1016/j.ijmm.2007.07.003. [DOI] [PubMed] [Google Scholar]