Abstract
It is becoming increasingly clear that a dysfunction of the GABAergic/glutamatergic network in telencephalic brain structures may be the pathogenetic mechanism underlying psychotic symptoms in schizophrenia (SZ) and bipolar (BP) disorder patients. Data obtained in Costa’s laboratory (1996–2009) suggest that this dysfunction may be mediated primarily by a downregulation in the expression of GABAergic genes (e.g., glutamic acid decarboxylase67 [GAD67] and reelin) associated with DNA-methyltransferase (DNMT)-dependent hypermethylation of their promoters.
A pharmacological strategy to reduce the hypermethylation of GABAergic promoters is to administer drugs, such as the histone deacetylase (HDAC) inhibitor valproate (VPA), that induce DNA-demethylation when administered at doses that facilitate chromatin remodeling. The benefits elicited by combining VPA with antipsychotics in the treatment of BP disorder suggest that an investigation of the epigenetic interaction of these drugs is warranted.
Our studies in mice suggest that when associated with VPA, clinically relevant doses of clozapine elicit a synergistic potentiation of VPA-induced GABAergic promoter demethylation. Olanzapine and quetiapine (two clozapine congeners) also facilitate chromatin remodeling but at doses higher than used clinically, whereas haloperidol and risperidone are inactive. Hence, the synergistic potentiation of VPA’s action on chromatin remodeling by clozapine appears to be a unique property of the dibenzepines and is independent of their action on catecholamine or serotonin receptors.
By activating DNA-demethylation, the association of clozapine or its derivatives with VPA or other more potent and selective HDAC inhibitors may be considered a promising treatment strategy for normalizing GABAergic promoter hypermethylation and the GABAergic gene expression downregulation detected in the postmortem brain of SZ and BP disorder patients.
Introduction
This review summarizes our present understanding of the topic of neuroepigenetics in major psychotic disorders. To elucidate the molecular mechanisms whereby nurture (biological or environmental epigenetic factors) and nature (genetic factors) interact to cause major psychiatric disorders such as schizophrenia (SZ) and bipolar (BP) disorder was at the center of Dr. Costa’s mission for the last 15 years of his research at the Psychiatric Institute at the University of Illinois at Chicago (Costa et al., 2002).
Existing drugs used to treat major psychiatric disorders have limited efficacy and substantial side effects. Hence, the challenge for Dr. Costa and his colleagues has been to find new ways to prevent and treat psychiatric disorders with pharmacological agents that fail to have major unwanted side effects.
The challenge to identify the core pathophysiological mechanisms underlying schizophrenia (SZ) and bipolar (BP) disorder that are targeted by antipsychotics
Unfortunately, there are few new leads for future drug development for the treatment of major psychiatric disorders (Miller, 2010a). A fundamental barrier to the identification of more efficacious, less toxic, and faster acting drugs than those presently available to treat BP disorder and SZ is the incomplete understanding of the etiopathogenetic mechanisms underlying the symptomatology of these diseases.
Population, family, and twin studies indicate that SZ and BP disorders are highly heritable but a single allele conferring increased risk has been identified in only a small proportion of the observed phenotypic variants (Li, 2010, Sullivan et al., 2008). Hence, the hypothesis that complex psychiatric disorders are attributable to a relatively few common genetic variants has been questioned. Rather, it appears that these disorders are the consequence of synergistic interactions of multiple susceptibility genes with environmental neuroepigenetic factors (Ptak and Petronis, 2008).
“Neuroepigenetics” refers to the reversible regulation of various genomic functions mediated principally through changes in DNA methylation and chromatin structure in neurons.
The findings reviewed in this article marshaled by the pioneering work of Costa and his collaborators (1996–2010) suggest that an epigenetic downregulation of telencephalic GABAergic genes may represent a contributing factor to the behavioral and cognitive impairments experienced by SZ and BP disorder patients.
Despite the large number of pharmacological studies performed in recent years to delineate the receptor affinity profile of different antipsychotics (Table 1) (Gary and Roth 2007, Jarskog et al. 2007, Roth et al. 2004), little is known about the action of antipsychotics on specific epigenetic mechanisms in GABAergic or glutamatergic neurons. In fact, the antipsychotic medications presently used clinically were not designed to target altered GABAergic or glutamatergic neurotransmission.
Table 1.
Relative Neurotransmitter Receptor Affinity and GABAergic Promoters Demethylation for Antipsychotics and Valproate (VPA)
| Drug | Receptors | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| D1 | D2 | D4 | 5HT 2A |
5HT 1A |
5HT2 C |
Α1 | H1 | M1 | mGlur 2/3 | GABAergic* promoter demethylation |
|
| Haloperidol | + | ++++ | +++ | + | − | − | +++ | − | − | − | − |
| Perphenazine | ? | ++++ | + | +++ | − | ? | ++ | − | − | − | ? |
| Olanzapine | ++ | ++ | ++ | +++ | − | ++ | ++ | +++ | ++ | − | + |
| Clozapine | + | ++ | ++ | +++ | + | ++ | +++ | ++ | ++++ | − | ++ |
| Quetiapine | − | + | − | + | − | _ | +++ | ++ | + | − | + |
| Risperidone | + | ++++ | +++ | ++++ | − | ++ | +++ | + | − | − | − |
| Aripiprazol | + | ++++ | ++ | ++ | +++ | ++ | − | + | − | − | ? |
| Sulpiride | ? | ++ | − | − | − | ? | − | − | − | − | ++ |
| VPA | − | − | − | − | − | − | − | − | − | − | +++ |
| LY2140023 | − | − | − | − | − | − | − | − | − | +++ | ? |
Data from Fig. 4, Table 4, and Dong et al., 2008
A primary objective of our studies has been to understand more about the specific intracellular signal transduction processes and nuclear events involved in the pharmacological action of antipsychotics by testing whether antipsychotic drugs elicit functionally relevant neuroepigenetic changes at GABAergic and glutamatergic gene promoters.
Understanding more about the action of antipsychotic drugs on neuroepigenetic mechanisms will not only accelerate the development of novel pharmacological agents to treat SZ and BP disorder but should also provide insights into the underlying neurobiological causes of these disorders.
The reciprocal interaction between GABAergic interneurons and glutamatergic pyramidal principal neurons is altered in the cortex or hippocampus of SZ or BP disorder patients
When the postmortem brain of SZ and BP disorder patients is compared to that of non-psychiatric subjects, GABAergic neuropathology is detected in the hippocampus and cortex (Akbarian et al., 1995; Benes et al., 1992; Benes and Berretta, 2001, Fatemi et al., 2000, Guidotti et al., 2000; 2005, Impagnatiello et al., 1998; Lewis et al., 2005; Veldic et al., 2007). The GABAergic neuropathology found in the brain of SZ and BP disorder patients is characterized by a decrease in the expression of glutamic acid decarboxylase67 (GAD67). GABAergic pathology is also characterized by decreased expression of nicotine acetylcholine receptor (nAChR) α4 and α7 subunits (Breese et al., 2000) and by a decrease in other proteins abundantly expressed in GABAergic neurons, such as the NMDA receptor subunit NR2A and the kainate receptor subunit GluR5 (Bitanihirwe et al., 2009; Woo et al., 2004, 2008). Further, there are decreases in somatostatin, tyrosine kinase B (TrkB) receptors, cholecystokinin, GABA transporter-1, parvalbumin (Lewis et al., 2005), and reelin (for a review see Guidotti et al., 2005).
Reelin is a large (400 kDa) extracellular matrix protein that is most abundantly synthesized in GABAergic neurons of cortical layers I and II, and in the hippocampus, caudate, and putamen (Guidotti et al. 2000,Costa et al., 2001, Veldic et al. 2007, Guidotti et al., 2009, Levenson et al., 2008). In the cortex, upon secretion reelin adheres to postsynaptic densities located on dendritic spines and shafts of pyramidal neurons (Costa et al., 2001). This protein binds to specific receptors [apolipoprotein E 2 (ApoE2), very low density lipoprotein (VLDL), and integrin], harmonizing local dendritic protein synthesis rates necessary for: 1) dendritic spine formation, 2) spine maturation, and 3) the regulation of glutamate receptor structure and function (Costa et al., 2001; Guidotti et al., 2009, Levenson et al., 2008, Pujadas et al., 2010; Tueting et al., 1999, 2010). It is important to note that a decrease of GAD67 and reelin expression in brain of SZ or BP disorder patients has not been always confirmed. For example in one study an increase instead of a decrease of GAD67 was reported (Dracheva et al. 2004) and in another study lack of a significant decrease of reelin mRNA has been reported (Lipska et al. 2006). Presumably differences in the demographic characteristics, drug treatment, methodology, and composition of brain tissue samples including the ratio of white vs gray matter may actually account for these apparent discrepancies. The later point is highlighted by our more recent studies that identified GAD67 and reelin downregulation occurring specifically in GABAergic interneurons of layer I and II (Ruzika et al. 2007, Veldic et al. 2007) suggesting that different cell populations within the same brain areas may have a quite distinct GABAergic deficit profile.
At the molecular level, in addition to reelin, brain-derived neurotrophic factor (BDNF) could be a potential mediator of the reduction of the neuropil expression detected in the prefrontal cortex (PFC) of SZ and BP disorder patients (Lewis et al., 2005). BDNF stimulates the growth of dendrites and increases spine density on dendrites of cortical pyramidal neurons (McAllister et al., 1996). The expression of BDNF mRNA and protein is lower in the PFC of subjects with SZ (for a review see Roth et al. 2009) and BDNF levels and spine density on the basilar dendrites of deep layer 3 pyramidal neurons are positively correlated in SZ patients (Hill et al., 2005). It is important to mention that in SZ patients, reduced BDNF expression in pyramidal neurons is accompanied by a significant decrease in TrkB mRNA levels in GABAergic interneurons (Lewis et al., 2005). Therefore, reduced levels of BDNF and TrkB as well as reelin may be required to produce the decrease in spine density observed in the brain of SZ patients.
An important new finding is that the selective partial elimination of the essential NR1 subunit of the NMDA receptor in GABAergic cortical and hippocampal neurons of mice during early adolescence generates a phenotype characteristic of the GABAergic pathology in SZ (decreased GAD67, decreased parvalbumin, disinhibition of cortical excitatory neurons, reduced neuronal synchrony, and SZ-like behaviors) (Belforte et al., 2010).
GABAergic neuropathologies could explain the disturbance of the reciprocal interaction between GABAergic interneurons and principal glutamatergic pyramidal neurons that likely underlies the appearance of the positive and negative symptoms and cognitive dysfunctions seen in psychotic patients (Lewis and Gonzales-Burgos, 2008, Lisman et al., 2008). We and others have suggested that SZ and BP disorder are diseases characterized by a deficit of GABAergic transmission with consequent glutamatergic and monoaminergic network dysfunction (Benes et al., 2007, Guidotti et al., 2005, 2009, Lewis and Gonzalez-Burgos, 2008, Lisman et al., 2008, Moghaddam, 2003, Woo et al., 2004).
Is an altered epigenetic regulation of gene expression the molecular mechanism mediating the GABAergic and glutamatergic dysfunction in SZ and BP disorder?
In SZ and BP disorder patients, the downregulation of GAD67, reelin, and other genes expressed in GABAergic neurons could be a sign of a genetic abnormality. Although a highly conserved single nucleotide polymorphism (SNP) has been identified in the vicinity of the regulatory region of GAD67 (Straub et al., 2007) and of the reelin gene (Shifman et al., 2008, Wedenoja et al., 2008), it is possible that these polymorphisms are associated with an increased risk of psychotic symptoms in a small number of cases.
However, there are several epidemiological, clinical, and molecular peculiarities associated with SZ or BP disorder that are difficult to reconcile with Mendelian genetic disorders and in contrast, correspond to features of an altered epigenetic homeostasis (Ptak and Petronis, 2008). Such features include: 1) incomplete phenotypic concordance between monozygotic twins, 2) fluctuating disease course with periods of remission and relapse, 3) peaks of susceptibility to disease coinciding with major hormonal changes, and 4) parent-of-origin effects. These observations have led to speculation about the importance of epigenetic factors in mediating psychosis susceptibility.
In support of a role for aberrant epigenetic mechanisms in the pathogenesis of SZ and BP disorder, we have recently reported that the downregulation of GAD67 or reelin expression in GABAergic neurons of SZ and BP patients is associated with an overexpression of DNA methyltransferase 1 (DNMT1) and DNA methyltransferase 3a (DNMT3a) in layers I and II of BA9, BA10, BA17, but not in layers V and VI (Costa et al., 2007, Ruzicka et al., 2007, Veldic et al., 2004, 2005, 2007, Zhubi et al., 2009) or in the striatum of BP disorder patients (Veldic et al., 2007). DNMTs belong to a family of enzymes that catalyze the transfer of a methyl group from the methyl donor S-adenosylmethionine (SAM) to the carbon 5’ of cytosines embedded in cytosine phosphodiester guanine (CpG) islands of many gene promoters (Van Emburg and Robertson, 2008). Although increased promoter methylation induced by the overexpression of DNMTs in SZ and BP disorder patients may be the cause of the downregulation of GABAergic genes, the inhibitory action of DNMTs on gene expression may also occur through the formation of repressor complexes. These chromatin complexes may contain other specific proteins (e.g., methyl CpG binding domain proteins, SIN3A, and histone deacetylases) that may repress transcription via a modification of chromatin structure, shifting chromatin from a permissive open conformation to a repressive closed conformation (Chen et al., 2007, Costa et al., 2007, Dong et al., 2005, Grayson et al., 2006, Kundakovic et al., 2009, Van Emburg and Robertson, 2008).
In addition to the increased expression of DNMT1 and DNMT3a, which is associated with a decrease of reelin and GAD67 expression in a selected population of GABAergic interneurons of the prefrontal cortex (PFC), the hypothesis that an epigenetic pathology of GABAergic promoters is operative in the transcriptional downregulation of several genes in SZ or BP disorder patients is supported by the following evidence: 1) increased S-adenosyl-methionine (SAM) in the PFC (Guidotti et al., 2007), 2) hypermethylation of cytosines in CpG islands of reelin (Abdolmaleky et al., 2005, Grayson et al., 2005, 2006) and other selected promoters (Mill et al., 2008) and the associated downregulation of cognate protein expression in the PFC of psychotic patients, although negative findings for reelin have also been reported (Mill et al., 2008)., 3) decreased histone methylation at GABAergic gene promoters (Huang et al., 2007), 4) increased histone deacetylase expression in the PFC (Benes et al., 2007; Sharma et al., 2008), 5) an inverse correlation between DNA methylation of the BDNF gene and the level of its expression in the PFC (Mill et al., 2008), and 6) evidence of epigenetic dysregulation of several other GABAergic and glutamatergic genes in major psychosis (Mill et al., 2008). For example, Vglut-1 is hypermethylated and downregulated in SZ postmortem brain samples from females (Eastwood and Harrison, 2005, Mill et al., 2008).
These data are consistent with the epigenetic theory of major psychosis (Costa et al., 2002) and suggest that DNA-methylation or histone tail covalent modifications associated with GABAergic and glutamatergic gene promoters are important casual events in the pathogenesis of SZ and BP disorder. Furthermore, support for the hypothesis that an epigenetic chromatin remodeling pathology contributes to the downregulation of GABAergic or glutamatergic genes in psychotic patients is sustained by clinical studies conducted in the early 1970s (for review see Wyatt et al., 1971).
Methionine, the precursor of SAM (the universal methyl donor utilized by DNMTs to methylate cytosine in CpG islands), administered in large doses (10–20 g/day) for 3–4 weeks to SZ patients was reported to exacerbate psychotic symptomatology. In both mouse FC and neuronal cultures, the administration of large doses of methionine induces an increase in SAM and the hypermethylation of selective CpG-rich GABAergic promoters, including GAD67 and reelin, and facilitates the downregulation of their expression (Chen et al., 2007, Noh et al., 2005, Mitchell et al., 2005, Tremolizzo et al., 2002, 2005). Importantly, the brain levels of GAD65 and that of other housekeeping genes were not affected (Tremolizzo et al., 2002).
DNA promoter methylation patterns in neurons constitute a dynamic process
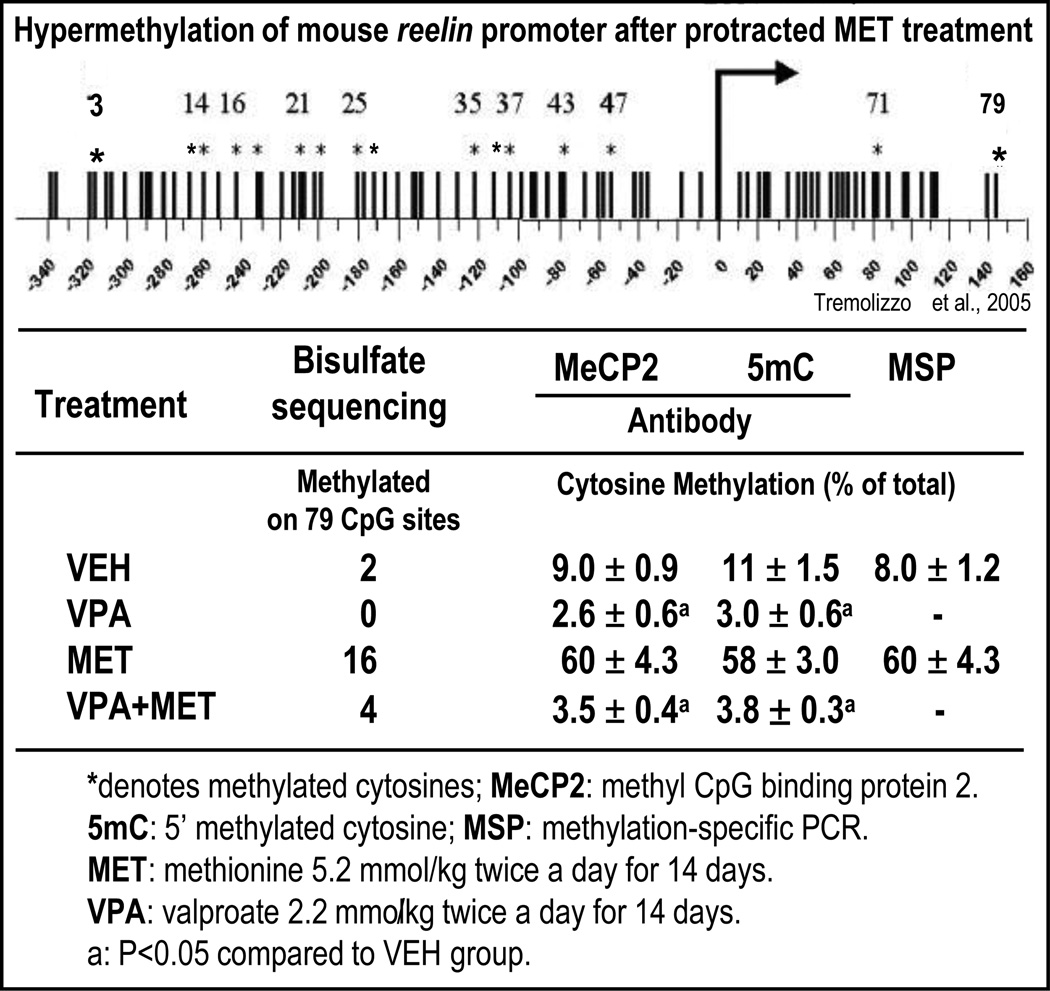
It was thought that in neurons, DNA methylation patterns were established during development and remained stable thereafter (Razin and Shemer, 1995). However, there is increasing evidence supporting the concept that in adult neurons, methylation patterns of specific cytosine/guanine (CpG) dinucleotide-rich promoters change rapidly. Thus, throughout life, DNA methylation provides a platform on which the environment can sculpt the genome and affect neuronal phenotype profiles without altering genotypes (Szyf, 2005). We tested this hypothesis in a behavioral, neuroanatomical, and biochemical epigenetic mouse model of SZ (Tremolizzo et al., 2002, 2005, Tueting, 2010), treating mice protractedly with methionine to induce reelin and GAD67 promoter hypermethylation (Fig. 1, left side). We quantified the ratio of 5 methyl cytosine (5mC) to the unmethylated cytosine (C) of the murine reelin CpG-enriched promoter region from −340 to + 160 bp (Fig. 2) or the murine GAD67 CpG-enriched promoter region from −760 to – 311 (Satta et al., 2008) by measuring the fraction of reelin or GAD67 promoter immunoprecipitated by specific anti-5mC or anti-methylcytosine binding protein-2 (MeCP2) antibodies with competitive RT-PCR and mutated internal standards (Dong et al., 2005). The results reported in Fig. 2 show that in the FC of mice treated for 14 days with methionine, the ratio of 5mC/C promoters measured with these two antibodies is virtually identical. These results were duplicated using methylation-specific PCR primers (MSP) (Dong et al., 2007). We found that the ratio of 5mC/C in the reelin promoter region −340 to + 160 bp was approximately proportional to the number of methylated CpG dinucleotides measured with sodium bisulfate mapping (Fig. 2), (Dong et al., 2007, 2008, Tremolizzo et al., 2005).
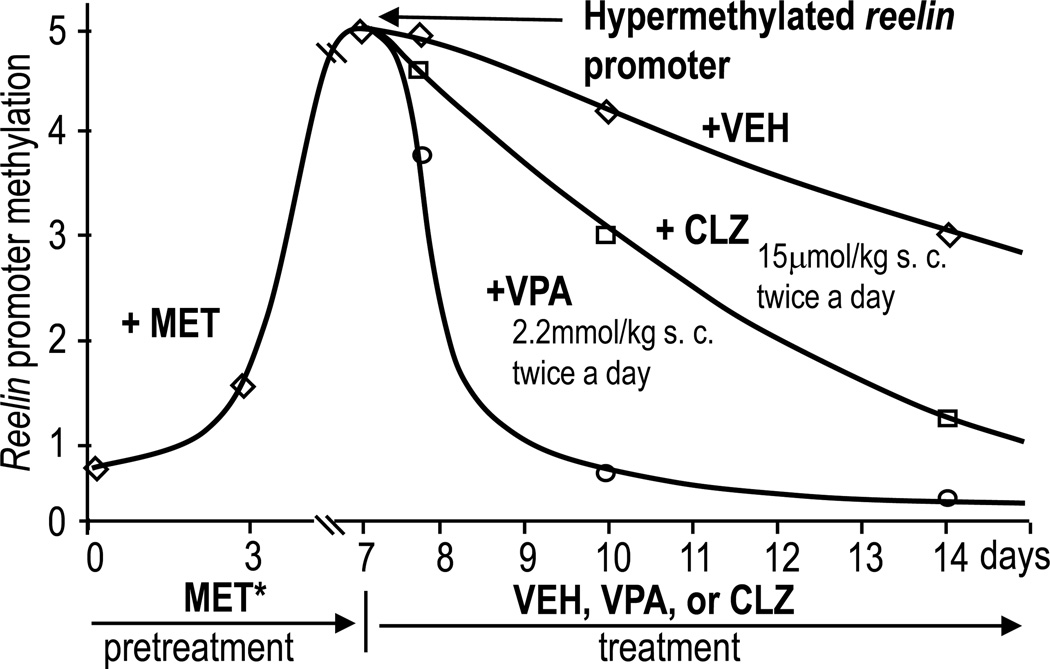
FIG 1. Valproate (VPA) and clozapine (CLZ) accelerate the demethylation of methionine (MET*)-induced hypermethylation of reelin promoter.
Met*: mice were pretreated with L-methionine (5.2 mmol/kg s.c. twice a day) for 7 days to hypermethylate the reelin promoter. At 7 days MET was withdrawn and mice received vehicle (VEH) or VPA or CLZ as indicated. Reelin promoter methylation was measured using MeDIP and quantitative PCR assays. Similar results were obtained for the GAD67 promoter.
FIG 2. Cytosine methylation in a CpG island-enriched promoter region of reelin comparing various methods.
Methionine induces an increase of brain levels of 1) SAM (Table 2); 2) reelin and GAD67 promoter methylation (Fig. 2 and Dong et al., 2007, 2008), and 3) downregulation of reelin and GAD67 mRNA (Fig. 3) and cognate protein expression (Tremolizzo et al., 2002, 2005).
Table 2.
Time course of the increase of S-adenosyl methionine (SAM) and of its metabolite S-adenosyl homocysteine (SAH) in the cortex of mice receiving methionine (6.6 mmol/kg s.c.)
| Treatment | Hours after treatment |
SAM | SAH |
|---|---|---|---|
| pmol/mg protein | |||
| Vehicle | 1–4 | 200 ± 7.1 | 70 ± 10.0 |
| Methionine | 1 | 305 ± 4.0* | 142 ± 8.0* |
| 2 | 280 ± 3.8* | 120 ± 4.0 | |
| 4 | 295 ± 2.1* | 100 ± 2.0* | |
Each value is the mean ± SE of 4–5 mice
P <0.01 vs vehicle
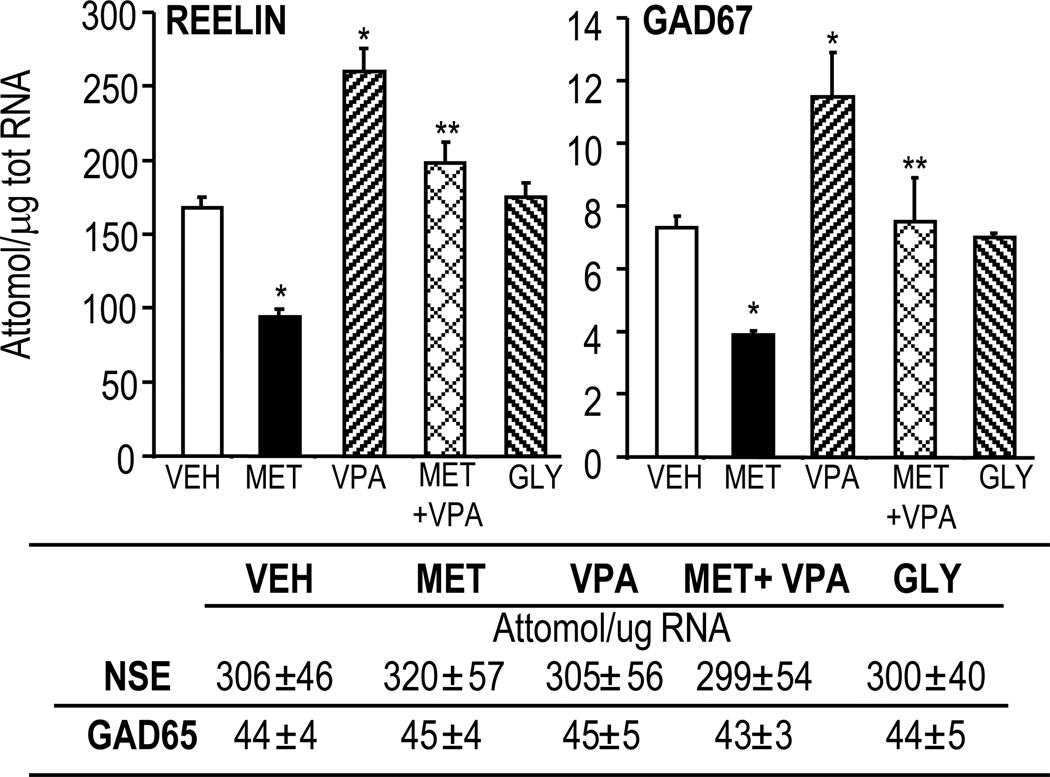
FIG 3. Valproate (VPA) increases reelin and GAD67 mRNAs and reverses L-methionine (MET)-induced downregulation of these mRNAs.
MET 5.2 mmol/kg; GLY (glycine) 5.2 mmol/kg; VPA 2.2 mmol/kg s.c. twice daily for 15 days.
*p< 0.05 vs VEH; **p< 0.05 vs MET.
GAD67 and reelin are not the only gene promoters hypermethylated by methionine treatment. ChIP-on-chip assays show that in mice receiving methionine, about 5% of the genome promoters are hypermethylated in the FC (Dong et al., 2008). Interestingly, the GAD65, NSE (Fig. 3), and glyceraldehyde-3-phosphate dehydrogenase (G3PDH) promoters are not hypermethylated by the administration of methionine, suggesting that promoter hypermethylation is cell- and gene-specific.
As shown in Fig. 1, if methionine is withdrawn after seven days of treatment, reelin promoter hypermethylation decreases by ~50% after the seventh day and returns to control levels after 12–14 days of methionine withdrawal.
Methyl donors have been identified as important epigenetic factors contributing to the aberrant regulation of reelin and other gene promoters. For example, maternal methionine supplementation in rats induces epigenetic variations including DNA methylation alterations in offspring (Weaver et al., 2006). Further, there is an epigenomic reprogramming of reelin and glucocorticoid receptors in hippocampal pyramidal neurons after methionine administration (Weaver et al., 2006). Our studies in cultured cortical neurons (Kundakovic et al., 2009, Noh et al., 2005) not only show that the hypermethylation of promoters induced by methionine is blocked by siRNA-mediated DNMT-KO or by DNMT antagonists but also that this blockade induces the overexpression of reelin, GAD67, or BDNF (Kundakovic et al., 2009).
Collectively, these data challenge the classic concept that DNA 5-mC patterns remain stable in postmitotic cells and strongly suggest that by increasing SAM brain content, methionine facilitates the promoter methylation mediated by DNMT1 or DNMT3a in the CNS. Unlike the DNA sequence of a cell, which is stable and strongly conserved, epigenetic processes are highly dynamic: they can be tissue-specific, developmentally-regulated, and modified by exposure to a range of drugs or environmental factors (Ptak and Petronis, 2008, Szyf, 2005).
Valproate (VPA) and other histone deacetylase (HDAC) inhibitors promote chromatin remodeling, induce DNA-demethylation, and regulate cognitive function
The dynamic nature of the epigenome means that, unlike pathogenic DNA sequence mutations, epigenetic disruptions are potentially reversible and thus a realistic target for pharmacological intervention.
In psychiatry, the use of VPA as a drug that enhances GABAergic transmission is based on the observation that protracted VPA treatment in rodents induces an increase of GAD67 expression (Fig. 3) (see also Loscher et al., 1999, Tremolizzo et al. 2002). Recent studies from our group and others suggest that GAD67 and reelin are increased by VPA because this drug not only inhibits HDACs and modifies the “histone code” but also decreases methylated sites at reelin and GAD67 promoters, [(Fig. 1) and Dong et al., 2007], thus preventing the recruitment of a corepressor protein complex. This complex includes methyl-CpG-binding domain proteins (MeCP2, MDB2, MBD4), DNMTs, and other corepressor proteins (Dong et al., 2007). We have shown that if HDAC inhibitors such as VPA and the benzamide derivative MS-275 are given to mice after methionine withdrawal, they dramatically accelerate reelin and GAD67 promoter demethylation during the subsequent 24–48 hours (Fig. 1 and Dong et al., 2007).
The ability of VPA to facilitate the demethylation of reelin and GAD67 promoters was not caused by a direct inhibitory action of this drug on DNMT1 or DNMT3a expression (Table 3) or DNMT activity (DNMT activity in nuclear extract of NT2 cells is 5.0 ± 0.1 × 103 cpm/µg protein in control and 5.5 ±0.6 × 103 cpm/µg protein in cells treated with 1 mM VPA for 12 hrs n=3) or on an inhibitory action of VPA on SAM biosynthesis (Dong et al., 2008). Moreover, these data are in line with a previous report by Detich et al. (2003) demonstrating that in human embryonic kidney cells (HEK-293), VPA triggers a replication-independent DNA demethylation that may be associated with an increase of histone acetylation. Since the biochemical identity of the catalytic process mediating DNA demethylation in mammalian cells remains unclear (Ma et al., 2009, Zhu, 2009), one cannot conclude that the accelerated reelin and GAD67 promoter demethylation elicited by HDAC inhibitors in the mouse FC (Fig. 1) is 1) the result of a direct induction of a DNA demethylase activity, or 2) the result of an indirect recruitment or activation of a preexisting demethylation mechanism related to “histone code” remodeling (Jenuwein and Allis, 2001).
Table 3.
VPA at a dose that blocks HDAC fails to reduce DNMT1 and DNMT3a mRNA expression in the mouse frontal cortex.
| mRNA (fmol/0.1 pmol NSE) | ||
|---|---|---|
| VEH | VPA | |
| DNMT1 | 3.2 ± 0.3 | 3.1 ± 0.2 |
| DNMT3a | 4.8 ± 0.4 | 4.6 ± 0.8 |
Mice were treated with vehicle (VEH) or with VPA (2.2 mmol/kg s.c. twice a day for 3 days) and were killed 2 hr after the last injection. Total mRNA isolated from the frontal cortex was analyzed using quantitative competitive PCR. The value was corrected by NSE mRNA, which was co-amplified with DNMT mRNA. Each value use the mean ± SE of five mice.
Evidence has been obtained in rodents that methionine-induced reelin, GAD67, and exon17 glucocorticoid receptor promoter hypermethylation can be prevented or effectively reversed by VPA and other HDAC inhibitors (Dong et al., 2007, Weaver et al., 2006) in the absence of an action on DNMT. This supports the concept that a putative DNA-demethylase activity may also play a pivotal role in regulating the appropriate dynamic balance of DNA cytosine-5 methylation patterns in the mammalian brain.
Recently, cortical DNA demethylation induced by chromatin modifications, especially histone tail acetylation, has been implicated in memory formation (Miller and Sweatt, 2007; Miller et al. 2010b). Increased histone tail acetylation induced by repeated administration of histone deacetylase inhibitors facilitates cognitive function in normal (Levenson et al., 2004) and aging mice (Peleg et al., 2010) and in a mouse model of neurodegeneration (Alarcon et al., 2004, Chuang et al., 2009). Taken together, these data suggest that pan-HDAC inhibitors such as VPA and other more selective chromatin remodeling agents may provide a potential adjuvant treatment for the cognitive deficits present in SZ and BP disorder patients or other neuropsychiatric disorders.
Epigenetic processes can be a target of antipsychotic drug action
Recent work has demonstrated that methylation of a promoter CpG island located ~30kb upstream of the gene encoding mitogen-activated protein kinase I (MEK1) is significantly correlated with lifetime antipsychotic use in postmortem PFC samples, with greater lifetime antipsychotic use associated with lower levels of DNA methylation (Mill et al., 2008). This finding is interesting given the involvement of (MAPK1) signaling pathways in mediating intraneuronal signaling and the observation that clozapine, a widely used medication in the treatment of schizophrenia, selectively activates this pathway via an interaction with MEK1 (Browning et al., 2005).
Recently, Huang et al. (2007) reported that clozapine but not haloperidol increases histone3-lysine4 (H3K4) (tri) methylation at the GAD67 promoter. These effects were not mimicked by genetic ablation of D2 and D3 receptors, suggesting that dopamine receptor signaling is not required for clozapine-induced histone methylation.
To address the issue of whether antipsychotic drugs acting on epigenetic mechanisms alter DNA methylation of GABAergic gene promoters, experiments were carried out in which Swiss albino mice were treated with typical and atypical antipsychotics, including haloperidol, clozapine, olanzapine, quetiapine, and risperidone. We found that the reelin and GAD67 promoters were significantly demethylated in the FC of mice receiving three days of treatment with clinically relevant doses of clozapine and relatively high doses of quetiapine and olanzapine but not in mice receiving haloperidol or risperidone (Fig. 4, Table 4, and Dong et al., 2008).
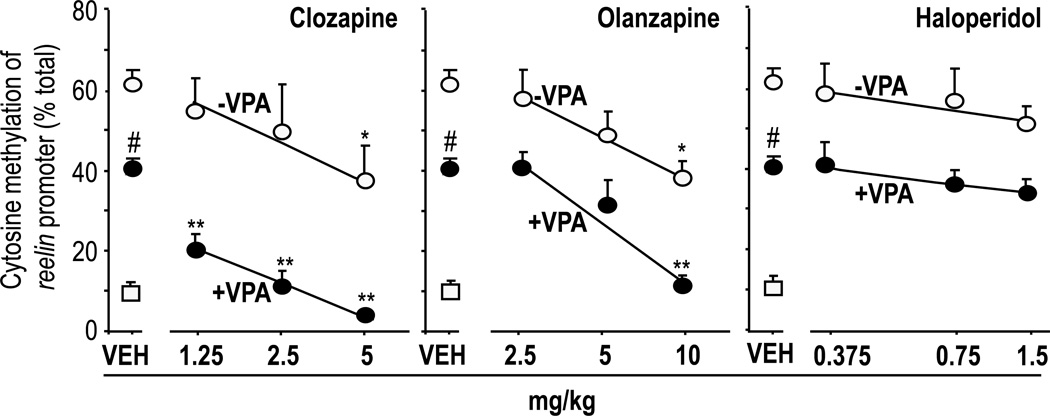
FIG 4. Clozapine or olanzapine alone or in combination with valproate (VPA) but not haloperidol induce reelin promoter demethylation in the mouse frontal cortex.
VPA (70 mg/kg) and antipsychotics were given s.c. twice a day for three days after MET withdrawal. Open circles denote MET-pretreated mice that did not receive VPA. Filled circles denote MET-pretreated mice that received VPA. Open squares denote mice never treated with MET.
*p< 0.05 when clozapine or olanzapine in absence of VPA were compared with the respective VEH-treated mice.
**p<0.01 when clozapine + VPA or olanzapine + VPA-treated mice were compared with VEH + VPA-treated mice.
# p< 0.05 when VEH + VPA-treated mice were compared with the respective VEH-treated mice.
Table 4.
Effects of VPA and various antipsychotics on GAD67 or reelin promoter demethylation in the mouse brain (frontal cortex).
| Drugs | mg/kg | GAD67 or reelin promoter cytosine demethylation | |
|---|---|---|---|
| −VPA | +VPA | ||
| Vehicle | − | +/− | + |
| Clozapine | 1.25 | + | +++ |
| Risperidone | 10 | inactive | inactive |
| Haloperidol | 1.5 | inactive | inactive |
| Olanzapine | 10 | + | ++ |
| Quetiapine | 10 | + | +++ |
VPA: 70 mg/kg
Drugs were given s.c. twice a day for three days
Results similar to those in the FC were obtained in the striatum, which expresses an almost homogeneous population (~90%) of GABAergic medium spiny neurons that also synthesize reelin (Dong et al., 2008). Because in the same mice reelin promoter methylation in the liver fails to change, we infer that clozapine and congeners modify methylation in the CNS and specifically in GABAergic neurons. Importantly, the action of clozapine on GABAergic promoter demethylation appears to be independent of an inhibitory effect on DNMT (Satta, personal communication).
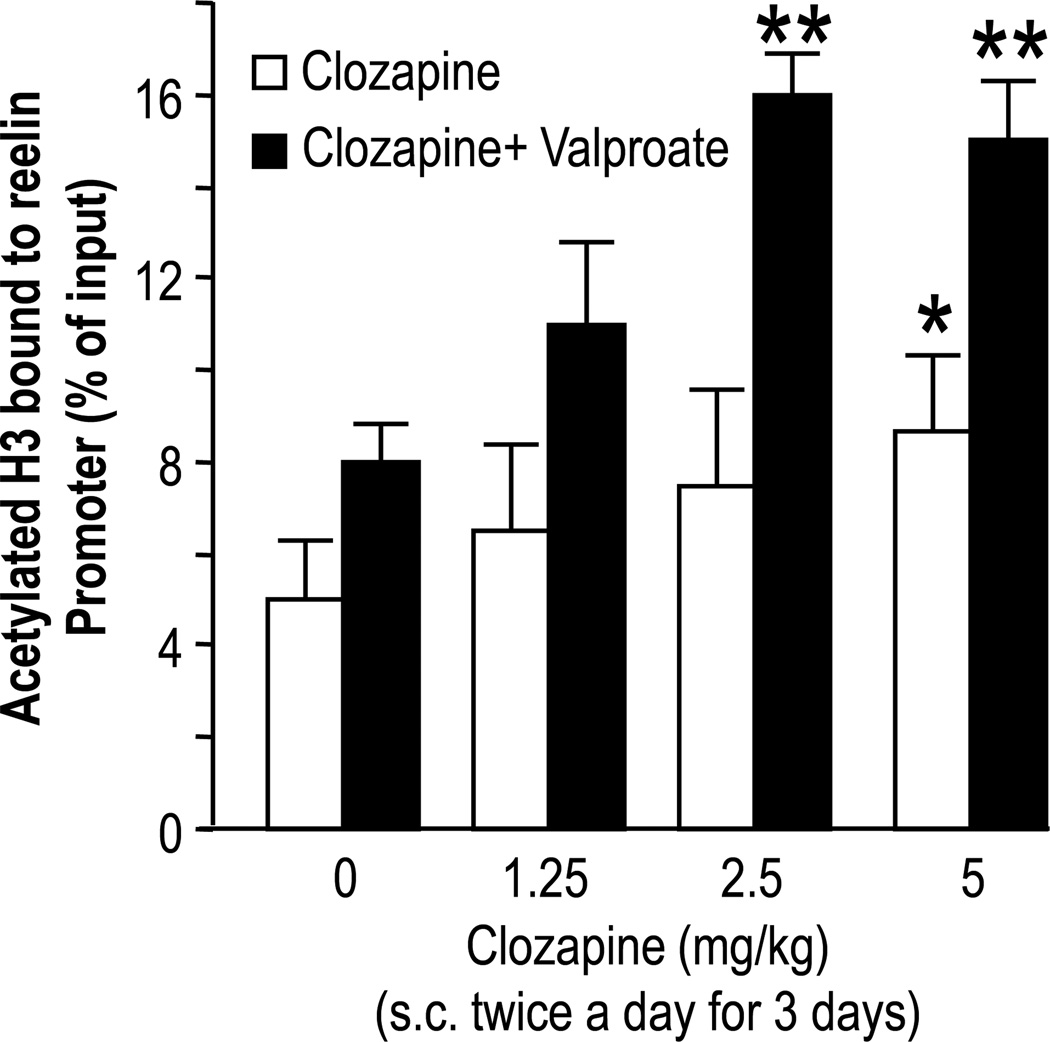
The administration of clozapine but not that of haloperidol, in parallel with the increase of DNA demethylation, induces increased nuclear H3 hyperacetylation at the reelin (Fig. 5) or GAD67 promoters in the FC (Dong et al., 2008). It is conceivable that antipsychotics that induce chromatin remodeling act by increasing histone acetylation, thereby recruiting putative DNA demethylating enzymes. This would convert chromatin at specific loci from an inactive/silenced state to an active/state positively modulating synaptic plasticity and regulating cognitive function (for a review see Guidotti et al., 2009). The precise mechanism whereby clozapine modulates the “histone code” and induces DNA-demethylation in GABAergic neurons remains to be elucidated. However, clozapine is marginally active by itself and we found it difficult to reconcile the structure of clozapine with that of any other currently known HDAC inhibitor.
FIG 5. Clozapine increases acetylated H3-lysine9 frontal cortex levels at the reelin promoter.
Open bars denote mice that did not receive valproate. Closed bars denote mice that received valproate (70 mg/kg s.c. twice a day for three days)
*p< 0.05; **p< 0.01 when compared with the respective controls.
Controls are mice that did not receive valproate or clozapine.
For details see Dong et al., 2008.
Effect of VPA and clozapine on DNA-demethylation
Important for the translational implications, the DNA-demethylating actions of clozapine, olanzapine and quetiapine were synergistically potentiated by the co-administration of a threshold inhibitory dose of VPA (Fig 4, Table 4, Dong et al. 2008). Furthermore as shown in Table 5, the administration of clozapine (5 mg/kg s.c./three days/twice a day) in conjunction with VPA (70 mg/kg s.c./three days/twice a day) reverses the downregulation of GAD67 expression induced in mice by seven days of methionine administration, suggesting an epigenetic action of this drug combination through histone acetylation and promoter demethylation.
Table 5.
Clozapine and valproate (VPA) co-administration reverses the methionine-induced downregulation of frontal cortex GAD67 expression in a methionine-induced epigenetic mouse model of schizophrenia
| Treatments | GAD67 protein (vs β actin) |
|---|---|
| Methionine | 0.15 ± 0.020* |
| Methionine + clozapine | 0.19 ± 0.015 |
| Methionine + clozapine + VPA | 0.35 ± 0.032** |
| Methionine + VPA | 0.29 ± 0.070 |
| VEH | 0.38 ± 0.030 |
GAD67 protein levels (OD ratio of GAD67 to β actin) were determined by Western blot analyses in the frontal cortex of mice treated with methionine (5.2 mmol/kg s.c./twice a day/for 7 days)
VEH, VPA (70 mg/kg), clozapine (5 mg/kg) were administered to mice s.c./twice a day/for 3 days after methionine treatment termination.
Data represent the mean ± SE of 4 mice
P<0.01 for methionine vs VEH
P<0.01 for methionine vs methionine + clozapine + VPA
In mammalian cells, active DNA-demethylation is achieved, at least in part, by a base-excision repair pathway that first requires the conversion of 5mC to thymine (T) through deamination (Ooi and Bestor 2008, Zhu, 2009). Recently, human breast cancer cell research suggested that DNMT3a and DNMT3b can convert 5mC to T through deamination; the resulting T is then removed by a G/T mismatch base-excision repair pathway (for a review see Zhu, 2009). Indeed, recent studies suggest that in mammalian cells including neurons, DNA-demethylation at promoter genes involved in memory and cognition or neurogenesis can be achieved by the coupled action of 5mC deamination [i.e., elicited by DNMT3a, 3b, AID (activation induced deaminase), or Apobec (apolipoprotein B RNA editing catalytic component)] and G/T mismatch DNA glycosylation presumably catalyzed by the methyl binding protein-4 (MBD4) (Ma et al. 2009, Kim et al., 2009).
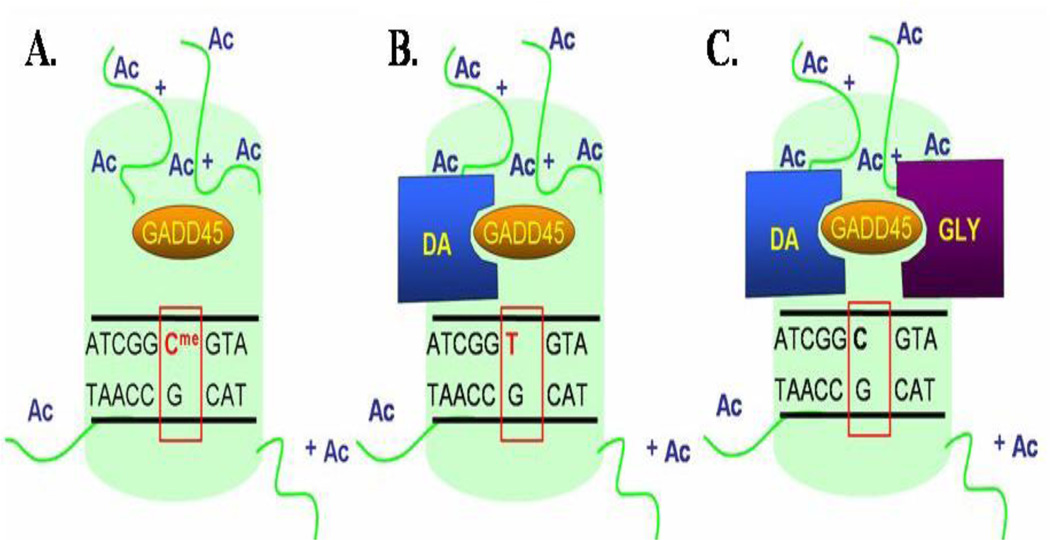
It has been proposed that coupling between 5mC deaminase and G/T mismatch DNA glycosylase is favored by the presence of “growth arrest and DNA-damage-inducible protein 45 (Gadd45) α and β (Fig. 6). These are small active nuclear acidic proteins that are induced in neurons by stress or by drugs that increase neuronal activity (Gavin et al., 2010).
FIG 6. Proposed mechanism of activity-dependent CpG-rich promoter demethylation.
A. Following depolarization GADD45 α, β protein levels increase and GADD45 α, β bind to a methylated promoter region proximal to an acetylated histone (green).
B. GADD45 recruits a deaminase (DA), which converts 5-methylcytosine to thymine leading to a T:G mismatch.
C. GADD45 recruits a DNA glycosylase (GLY), which removes thymine from the T:G mismatch. Thymine is later replaced with an unmethylated cytosine.
Recently, it has been reported that electroconvulsive treatment a) induces Gadd45 β expression, b) increases Gadd 45 β binding to cytosine deaminase or G/T mismatch glycosylase, and c) induces DNA-demethylation at specific gene promoters (i.e., BDNF, Fgf-1) that is abolished in Gadd45 KO mice (Ma et al., 2009). Hence, it is thought that Gadd45 proteins exert a putative regulatory role on DNA-methylation.
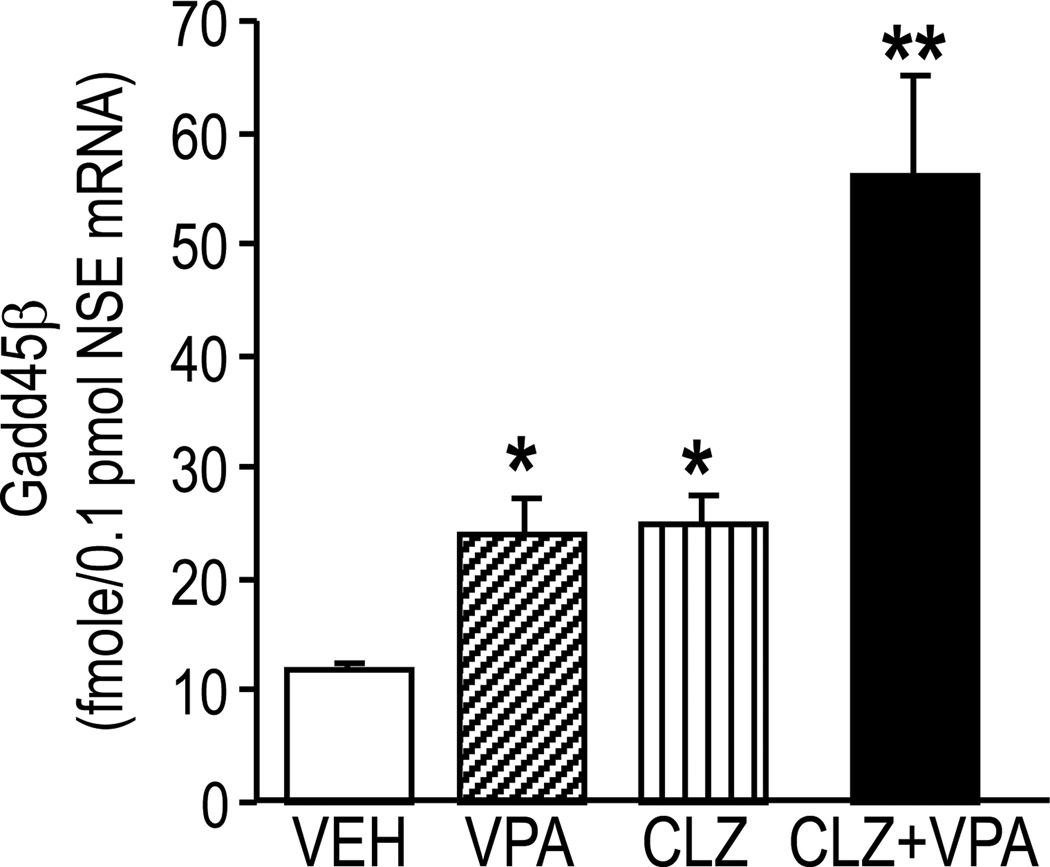
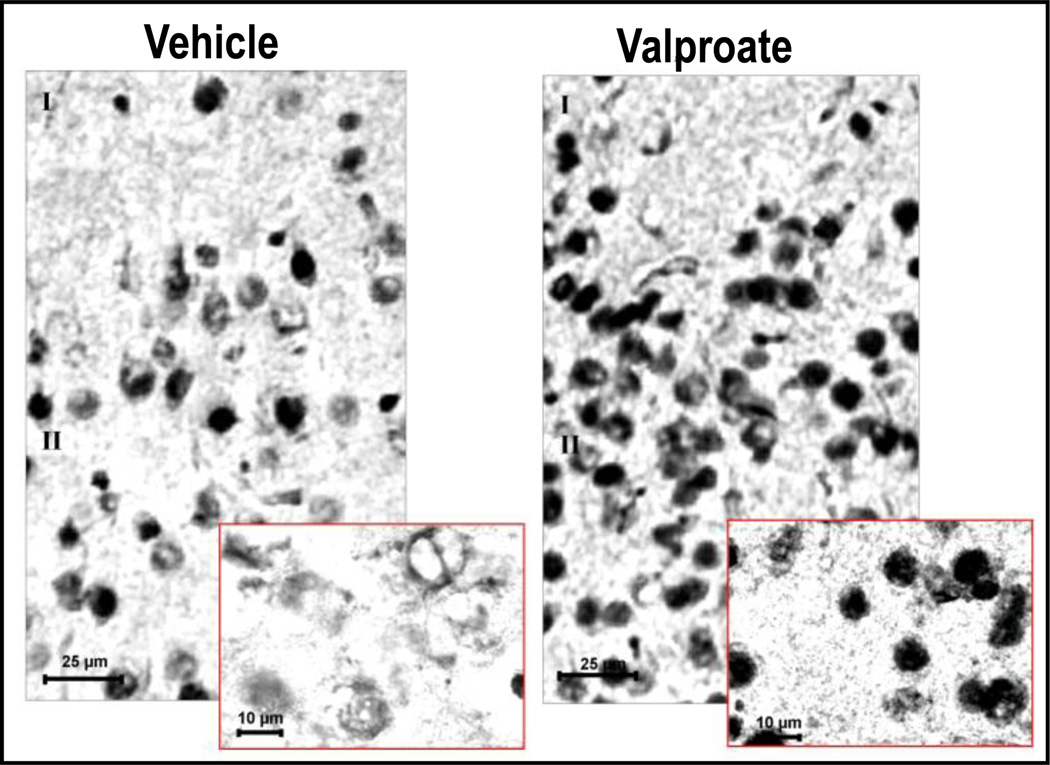
Given our data suggesting that subtypes of antipsychotic medications and VPA can synergistically interact to activate DNA-demethylation (Fig 3 and table 4), we examined the possibility that VPA and antipsychotic drugs elicit functionally-relevant DNA-demethylation changes altering the expression or activity of Gadd45 α or β. We found that in the FC of mice that had been given 70 mg/kg of VPA/three days/twice a day, Gadd45 β mRNA expression is increased compared to vehicle-treated controls (Fig. 7). Histochemical studies with specific Gadd45 β antibodies also show that cortical pyramidal neurons of mice treated with VPA (70 mg/kg/three days) exhibit increased nuclear Gadd45 β expression compared with vehicle-treated mice (Fig. 8). Moreover, clozapine in a dose that per se increases Gadd45 β potentiates the action of VPA (Fig. 7). Since these doses of clozapine and VPA induce promoter-demethylation (Fig. 4), taken together, the data suggest that in addition to DNMTs, neuronal promoter methylation can be regulated by the activity of a putative DNA demethylase, which can remove a methyl group from the carbon 5 of C. Hence, evidence suggests that in neurons, promoter methylation is a dynamic process that can be altered in response to environmental factors, such as stress, drugs, and various psychopathologies.
FIG 7. Clozapine (CLZ) alone or in combination with valproate (VPA) increases GADD45 β mRNA expression in FC of mice.
CLZ (5 mg/kg s.c./3 days/twice a day); VPA (70 mg/kg s.c./3 days/twice a day); VEH, vehicle. GADD45 β mRNA expression was measured two hours after the last drug injection.
*p< 0.05, **p< 0.01 vs VEH group. ANOVA followed by Bonferroni comparison; n=4–5 mice per group.
FIG 8. GADD45 β protein levels increase in FC neurons and coalesce in nuclei of mice treated with valproate.
Mice were treated with either vehicle or 70 mg/kg s.c. valproate twice a day for three days. Samples were analyzed two hours after the last drug injection.
GADD45 β antibody (Santa Cruz) recognized a major band (~ 18 kDa) of immune-reactive material in western blot of FC extracts.I and II denote layers I and II of cortex.
Concluding remarks
Recent breakthroughs in the study of aberrant molecular mechanisms operative in SZ and BP disorder point to a downregulation in the expression of several genes in GABAergic interneurons, most likely caused by gene promoter hypermethylation mediated by overexpression of DNMT in these cells (Costa et al., 2007).
The epigenetic downregulation of telencephalic GABAergic function may be responsible for disinhibiting pyramidal neurons that in turn could provide an excitatory input to dopamine cells in the ventral tegmental area (VTA) or serotonin cells in the raphe nucleus and drive a hyper-dopaminergic or -serotoninergic state that further increases pyramidal neuron excitability and presumably induces psychotic symptoms in SZ patients (Lewis and Gonzales Burgos, 2008, Lisman et al., 2008).
Taken together, these data suggest that to produce a significant symptomatic improvement of SZ or BP disorder morbidity, it may be desirable to pharmacologically reverse the promoter hypermethylation in GABAergic neurons.
We have attempted to establish a preclinical strategy for evaluating drugs that facilitate DNA-demethylation either 1) by reducing DNMT activity (i.e., administering DNMT inhibitors), or 2) by promoting the nuclear recruitment of DNA demethylation machinery associated with changes in chromatin remodeling.
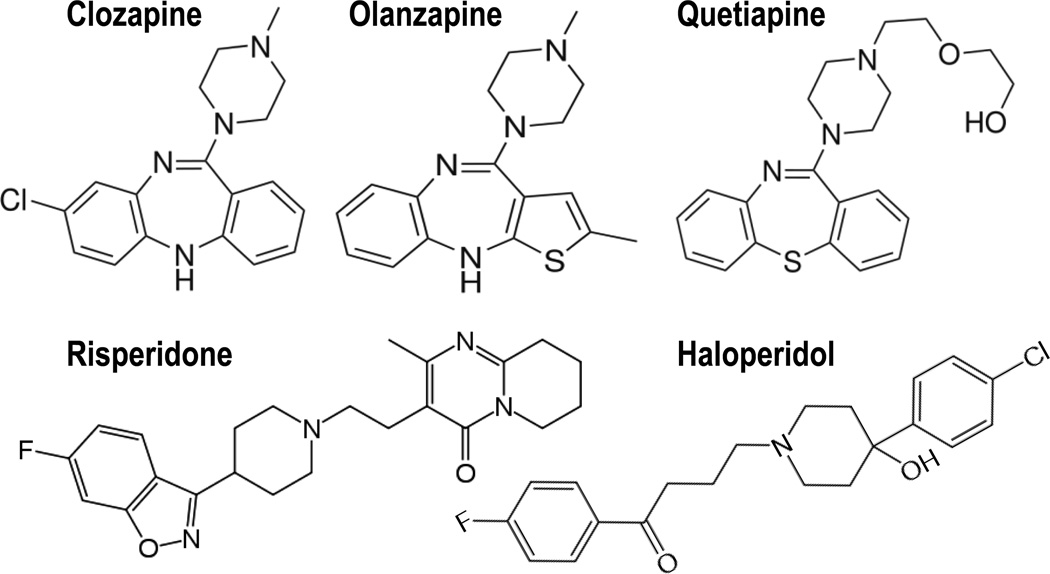
DNMT inhibitors that easily cross the blood-brain-barrier and are devoid of toxicity are presently not available. However, we have shown that the dibenzepine derivatives clozapine, quetiapine, and olanzapine but not the butyrophenone derivative haloperidol and the piperidyl-benzisoxazole derivative risperidone (Fig. 9) induce chromatin remodeling changes and activate DNA-demethylation of GABAergic gene promoters (Table 1), perhaps via this mechanism contributing to correction of the downregulation of GABAergic transmission present in the brain of BP and SZ patients.
FIG 9. Structures of the antipsychotics used in the study.
An analysis of the data of Table 1 suggests that the action of clozapine and its derivatives on chromatin remodeling is independent of its action on catecholamine or serotonin receptors. To validate this concept, the effect of clozapine and congeners on chromatin remodeling should be studied in mice with a genetic ablation of dopamine or 5HT receptor subtypes.
Although double-blind studies with VPA in SZ patients offer contrasting results (Casey et al. 2008), this drug has been co-administered for over a decade with typical and atypical antipsychotics to medicate BP and SZ disorder patients (Kelly et al., 2006, Wassef et al., 2000). The data presented in this review strongly support the provocative concept that the co-administration of VPA with clozapine, by activating DNA demethylation, can reverse a repressed nuclear epigenetic function expressed in the postmitotic cortical GABAergic neurons of SZ or BP disorder patients.
To test the concept that chromatin remodeling modifications may be a possible mechanism operative in the VPA augmentation of antipsychotic efficacy, it seemed appropriate to associate antipsychotics with other more potent and chemically unrelated HDAC inhibitors, such as the benzamide MS-275, which is now in phase II clinical trials and elicits brain histone hyperacetylation and activates DNA-demethylation in a manner similar to that of VPA (Dong et al., 2007, Simonini et al., 2006; Chen et al., 2010).
Presently, the overarching goal of the studies inspired by the pioneer work of Dr. Costa is to develop a classification of antipsychotic drugs based on their action on chromatin remodeling and DNA demethylation in GABAergic neurons. However, the biochemical identity and function of the process mediating DNA demethylation in mammalian cells requires further clarification (Szyf, 2005, Zhu, 2009).
Recently, the search for active DNA demethylation activity in mammals has been characterized by the identification of several different mechanisms. Interestingly, various reports suggest that DNA demethylation is initiated by molecules that either stabilize (methyl binding domain 2 [MBD2]) or induce (DNMT3a) DNA-methylation marks (Ooi and Bestor 2008, Szyf, 2005). Although the complete characterization of this activity is in progress (Dong et al., 2010), the identification of the biochemical nature of brain DNA-demethylation and an understanding of how drugs induce DNA demethylase activity are crucial to the progress of a new line of pharmacological interventions to treat major psychiatric disorders.
Acknowledgements
This research was partially supported by NIH grants MH071667 to E. Costa and by MH070855 to A. Guidotti
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abdolmaleky HM, Cheng KH, Russo A, Smith CL, Faraone SV, Wilcox M, Shafa R, Glatt SJ, Nguyen G, Ponte JF, Thiagalingam S, Tsuang MT. Hypermethylation of the reelin (RELN) promoter in the brain of schizophrenic patients: a preliminary report. Am. J. Med. Genet. B Neuropsychiatr. Genet. 2005;134B:60–66. doi: 10.1002/ajmg.b.30140. [DOI] [PubMed] [Google Scholar]
- Akbarian S, Kim JJ, Potkin SG, Hagman JO, Tafazzoli A, Bunney WE, Jr, Jones EG. Gene expression for glutamic acid decarboxylase is reduced without loss of neurons in prefrontal cortex of schizophrenics. Arch. Gen. Psychiatry. 1995;52:258–266. doi: 10.1001/archpsyc.1995.03950160008002. [DOI] [PubMed] [Google Scholar]
- Alarcón JM, Malleret G, Touzani K, Vronskaya S, Ishii S, Kandel ER, Barco A. Chromatin acetylation, memory, and LTP are impaired in CBP+/− mice: a model for the cognitive deficit in Rubinstein-Taybi syndrome and its amelioration. Neuron. 2004;42:947–959. doi: 10.1016/j.neuron.2004.05.021. [DOI] [PubMed] [Google Scholar]
- Belforte JE, Zsiros V, Sklar ER, Jiang Z, Yu G, Li Y, Quinlan EM, Nakazawa K. Postnatal NMDA receptor ablation in corticolimbic interneurons confers schizophrenia-like phenotypes. Nature Neurosci. 2010;12:76–83. doi: 10.1038/nn.2447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benes FM, Beretta S. GABAergic interneurons: implications for understanding schizophrenia and bipolar disorder. Neuropsychopharmacology. 2001;25:1–27. doi: 10.1016/S0893-133X(01)00225-1. [DOI] [PubMed] [Google Scholar]
- Benes FM, Lim B, Matzilevich D, Walsh JP, Subburaju S, Minns M. Regulation of the GABA cell phenotype in hippocampus of schizophrenics and bipolars. Proc. Natl. Acad. Sci. U.S.A. 2007;104:10164–10169. doi: 10.1073/pnas.0703806104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benes FM, Vincent SL, Alsterberg G, Bird ED, SanGiovanni JP. Increased GABAA receptor binding in superficial layers of cingulate cortex in schizophrenics. J. Neurosci. 1992;12:924–929. doi: 10.1523/JNEUROSCI.12-03-00924.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bitanihirwe BKY, Lim MP, Kelley JF, Kaneko T, Woo TUW. Glutamatergic deficits and parvalbumin-containing inhibitory neurons in the prefrontal cortex in schizophrenia. B.M.C. Psychiatry. 2009;9:71. doi: 10.1186/1471-244X-9-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breese CR, Lee MJ, Adams CE, Sullivan B, Logel J, Gillen KM, Marks MJ, Collins AC, Leonard S. Abnormal regulation of high affinity nicotinic receptors in subjects with schizophrenia. Neuropsychopharmacology. 2000;23:351–364. doi: 10.1016/S0893-133X(00)00121-4. [DOI] [PubMed] [Google Scholar]
- Browning JL, Patel T, Brandt PC, Young KA, Holcomb LA, Hicks PB. Clozapine and the mitogen-activated protein kinase signal transduction pathway: implications for antipsychotic actions. Biol. Psychiatry. 2005;57:617–623. doi: 10.1016/j.biopsych.2004.12.002. [DOI] [PubMed] [Google Scholar]
- Casey DE, Daniel DG, Tamminga C, Kane JM, Tran-Johnson T, Wozniak P, Abi-Saab W, Baker J, Redden L, Greco N, Saltarelli M. Divalproex ER combined with olanzapine or risperidone for treatment of acute exacerbations of schizophrenia. Neuropsychopharmacology. 2008 Dec;3 doi: 10.1038/npp.2008.209. [DOI] [PubMed] [Google Scholar]
- Chen Y, Dong E, Grayson DR. Analysis of the GAD1 promoter: Trans-acting factors and DNA methylation converge on the 5’ untranslated region. Neuropharmacology. 2010 Sept. doi: 10.1016/j.neuropharm.2010.09.017. (Epub ahead of print, PMID20869372). [DOI] [PubMed] [Google Scholar]
- Chen Y, Kundakovic M, Agis-Balboa RC, Pinna G, Grayson DR. Induction of the reelin promoter by retinoic acid is mediated by Sp1. J. Neurochem. 2007;183:650–665. doi: 10.1111/j.1471-4159.2007.04797.x. [DOI] [PubMed] [Google Scholar]
- Chuang DM, Leng Y, Marinova Z, Kim HJ, Chiu CT. Multiple roles of HDAC inhibition in neurodegenerative conditions. Trends Neurosci. 2009;32:591–601. doi: 10.1016/j.tins.2009.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa E, Chen Y, Davis J, Dong E, Noh JS, Tremolizzo L, Veldic M, Grayson DR, Guidotti A. Reelin and schizophrenia: a disease at the interface of the genome and the epigenome. Mol Interv. 2002;2002:47–57. doi: 10.1124/mi.2.1.47. [DOI] [PubMed] [Google Scholar]
- Costa E, Davis J, Grayson DR, Guidotti A, Pappas GD, Pesold C. Dendritic spine hypoplasticity and downregulation of reelin and GABAergic tone in schizophrenia vulnerability. Neurobiol. Dis. 2001;8:723–742. doi: 10.1006/nbdi.2001.0436. [DOI] [PubMed] [Google Scholar]
- Costa E, Dong E, Grayson DR, Guidotti A, Ruzicka W, Veldic M. Reviewing the role of DNA (cytosine-5) methyltransferase overexpression in the cortical GABAergic dysfunction associated with psychosis vulnerability. Epigenetics. 2007;2:29–36. doi: 10.4161/epi.2.1.4063. [DOI] [PubMed] [Google Scholar]
- Detich N, Bovenzi V, Szyf M. Valproate induces replication-independent active DNA demethylation. J. Biol. Chem. 2003;278:27586–27592. doi: 10.1074/jbc.M303740200. [DOI] [PubMed] [Google Scholar]
- Dong E, Agis-Balboa RC, Simonini MV, Grayson DR, Costa E, Guidotti A. Reelin and glutamic acid decarboxylase67 promoter remodeling in an epigenetic methionine-induced mouse model of schizophrenia. Proc. Natl. Acad. Sci. U.S.A. 2005;102:12578–125823. doi: 10.1073/pnas.0505394102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong E, Guidotti A, Grayson DR, Costa E. Histone hyperacetylation induces demethylation of reelin and 67-kDa glutamic acid decarboxylase promoters. Proc. Natl. Acad. Sci. U.S.A. 2007;104:4676–4681. doi: 10.1073/pnas.0700529104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong E, Nelson M, Grayson DR, Costa E, Guidotti A. Clozapine and sulpiride but not haloperidol or olanzapine activate nuclear DNA-demethylation in the brain. Proc. Natl. Acad. Sci. U.S.A. 2008;105:13614–13619. doi: 10.1073/pnas.0805493105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong E, Chen Y, Gavin DP, Grayson Dr, Guidotti A. Valproate induces DNA demethylation in nuclear extracts from adult mouse brain. Epigenetics. 2010 Nov.5(8) doi: 10.4161/epi.5.8.13053. [DOI] [PubMed] [Google Scholar]
- Dracheva S, Elhakem SL, McGurk SR, Davis KL, Haroutunian V. GAD67 and GAD65 mRNA and protein expression in cerebrocortical regions of elderly patients with schizophrenia. J. Neu. Res. 2004;76:581–592. doi: 10.1002/jnr.20122. [DOI] [PubMed] [Google Scholar]
- Eastwood SL, Harrison P. Decreased expression of vesicular glutamate transporter 1 and complexin II mRNAs in schizophrenia: further evidence for a synaptic pathology affecting glutamate neurons. Schizophr. Res. 2005;73:159–172. doi: 10.1016/j.schres.2004.05.010. [DOI] [PubMed] [Google Scholar]
- Fatemi SH, Earle JA, McMenomy T. Reduction in reelin immunoreactivity in hippocampus of subjects with schizophrenia, bipolar disorder and major depression. Mol. Psychiatry. 2000;5:654–665. doi: 10.1038/sj.mp.4000783. [DOI] [PubMed] [Google Scholar]
- Gavin D. Gadd45b, a putative component of DNA-demethylation pathway, is induced by HDAC inhibitors. ACNP Abstract. 2009 [Google Scholar]
- Gavin DP, Chase K, Matrisciano F, Grayson DR, Guidotti A, Sharma RP. Growth arrest and DNA-damage-inducible, beta (GADD45b) expression and function in the brain: an examination of a putative member of a DNA demethylation pathway abnormally expressed in psychosis. Soc. for Neurosci. Abstract. 2010 [Google Scholar]
- Gray JA, Roth BL. The pipeline and future of drug development in schizophrenia. Mol. Psychiatry. 2007;12:904–922. doi: 10.1038/sj.mp.4002062. [DOI] [PubMed] [Google Scholar]
- Grayson DR, Chen Y, Costa E, Dong E, Guidotti A, Kundakovic M, Sharma RP. The human reelin gene: transcription factors (+), repressors (−) and the methylation switch (+/−) in schizophrenia. Pharmacol. Ther. 2006;111:272–286. doi: 10.1016/j.pharmthera.2005.01.007. [DOI] [PubMed] [Google Scholar]
- Grayson DR, Jia X, Chen Y, Sharma RP, Mitchell CO, Guidotti A, Costa E. Reelin promoter hypermethylation in schizophrenia. Proc. Natl. Acad. Sci. U.S.A. 2005;102:9341–9346. doi: 10.1073/pnas.0503736102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guidotti A, Auta J, Davis JM, Di-Giorgi-Gerevini V, Dwivedi Y, Grayson DR, Impagnatiello F, Pandey G, Pesold C, Sharma R, Uzunov D, Costa E. Decrease in reelin and glutamic acid decarboxylase67(GAD67) expression in schizophrenia and bipolar disorder: a postmortem brain study. Arch. Gen. Psychiatry. 2000;57:1061–1069. doi: 10.1001/archpsyc.57.11.1061. [DOI] [PubMed] [Google Scholar]
- Guidotti A, Auta J, Davis JM, Dong E, Grayson DR, Veldic M, Zhang X, Costa E. GABAergic dysfunction in schizophrenia: new treatment strategies on the horizon. Psychopharmacology. 2005;180:191–205. doi: 10.1007/s00213-005-2212-8. [DOI] [PubMed] [Google Scholar]
- Guidotti A, Dong E, Kundakovic M, Satta R, Grayson DR, Costa E. Characterization of the action of antipsychotic subtypes on valproate-induced chromatin remodeling. Trends Pharmacol. Sci. 2009;30:55–60. doi: 10.1016/j.tips.2008.10.010. [DOI] [PubMed] [Google Scholar]
- Guidotti A, Ruzicka W, Grayson DR, Veldic M, Pinna G, Davis JM, Costa E. S-adenosyl methionine and DNA methyltransferase-1 mRNA overexpression in psychosis. Neuroreport. 2007;18:57–60. doi: 10.1097/WNR.0b013e32800fefd7. [DOI] [PubMed] [Google Scholar]
- Hill JJ, Kolluri N, Hashimoto T, Wu Q, Sampson AR, Monteggia LM. Analysis of pyramidal neuron morphology in an inducible knockout of brain derived neurotrophic factors. Biol. Psychiatry. 2005;57:923–934. doi: 10.1016/j.biopsych.2005.01.010. [DOI] [PubMed] [Google Scholar]
- Huang HS, Matevossian A, Whittle C, Kim SY, Shumacker A, Baker SP, Akbarian S. Prefrontal dysfunction in schizophrenia involves mixed-lineage leukemia 1-regulated histone methylation at GABAergic gene promoters. J. Neurosci. 2007;27:11254–11262. doi: 10.1523/JNEUROSCI.3272-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Impagnatiello F, Guidotti A, Pesold C, Dwivedi Y, Caruncho H, Pisu M, Uzunov D, Smalheiser N, Davis J, Pandey G, Pappas G, Tueting P, Sharma R, Costa E. A decrease of reelin expression as a putative vulnerability factor in schizophrenia. Proc. Natl. Acad. Sci. U.S.A. 1998;95:15718–15723. doi: 10.1073/pnas.95.26.15718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarskog LF, Miyamoto S, Lieberman JA. Schizophrenia: new pathological insights and therapies. Ann. Rev. Med. 2007;58:49–61. doi: 10.1146/annurev.med.58.060904.084114. [DOI] [PubMed] [Google Scholar]
- Jenuwein T, Allis CD. Translating the histone code. Science. 2001;293:1074–1080. doi: 10.1126/science.1063127. [DOI] [PubMed] [Google Scholar]
- Kelly DL, Conley RR, Feldman S, Yu Y, McMahon RP, Richardson CM. Adjunct divalproex or lithium to clozapine in treatment-resistant schizophrenia. Psychiatr. Q. 2006;77:81–95. doi: 10.1007/s11126-006-7963-9. [DOI] [PubMed] [Google Scholar]
- Kim MS, Kondo T, Takada I, Youn MY, Yamamoto Y, Takahashi S, Matsumoto T, Fujiyama S, Shirode Y, Yamaoka I, Kitagawa H, Takeyama K, Shibuya H, Ohtake F, Kato S. DNA demethylation in hormone-induced transcriptional derepression. Nature. 2009;461:1007–1012. doi: 10.1038/nature08456. [DOI] [PubMed] [Google Scholar]
- Kundakovic M, Chen Y, Guidotti A, Grayson DR. The reelin and GAD67promoters are activated by epigenetic drugs that facilitate the disruption of local repressor complexes. Mol. Pharmacol. 2009;75:342–354. doi: 10.1124/mol.108.051763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levenson JM, O'Riordan KJ, Brown KD, Trinh MA, Molfese DL, Sweatt JD. Regulation of histone acetylation during memory formation in the hippocampus. J. Biol. Chem. 2004;279:40545–40559. doi: 10.1074/jbc.M402229200. [DOI] [PubMed] [Google Scholar]
- Levenson JM, Qiu S, Weeber EF. The role of reelin in adult synaptic function and the genetic and epigenetic regulation of the reelin gene. Biochim. Biophys. Acta. 2008;1779:422–431. doi: 10.1016/j.bbagrm.2008.01.001. [DOI] [PubMed] [Google Scholar]
- Lewis DA, González-Burgos G. Neuroplasticity of neocortical circuits in schizophrenia. Neuropsychopharmacology. 2008;33:141–165. doi: 10.1038/sj.npp.1301563. [DOI] [PubMed] [Google Scholar]
- Lewis DA, Hashimoto T, Volk DW. Cortical inhibitory neurons and schizophrenia. Nat. Rev. Neurosci. 2005;6:312–324. doi: 10.1038/nrn1648. [DOI] [PubMed] [Google Scholar]
- Li MD. Grand challenges and opportunities for molecular psychiatry research: a perspective. Front. Psychiatry. 2010;1(2):1–3. doi: 10.3389/fpsyt.2010.00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipska BK, Peters T, Hyde TM, Halim N, Horowitz C, Mitkus S. Expression of DISC1 binding partners is reduced in schizophrenia and is associated with DSC1 SNPs. Hum. Mol Genet. 2006;15:1245–1258. doi: 10.1093/hmg/ddl040. [DOI] [PubMed] [Google Scholar]
- Lisman JE, Coyle JT, Green RW, Javitt DC, Benes FM, Heckers S, Grace AA. Circuit-based framework for understanding neurotransmitter and risk gene interactions in schizophrenia Trends. Neurosci. 2008;31:234–242. doi: 10.1016/j.tins.2008.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loscher W. Valproate: a reappraisal of its pharmacodynamic properties and mechanisms of action. Prog. Neurobiol. 1999;58:31–59. doi: 10.1016/s0301-0082(98)00075-6. [DOI] [PubMed] [Google Scholar]
- Ma DK, Jang MH, Guo JU, Kitabatake Y, Chang ML, Pow-Anpongkul N, Flavell RA, Lu B, Ming GL, Song H. Neuronal activity-induced Gadd45b promotes epigenetic DNA demethylation and adult neurogenesis. Science. 2009;323:1074–1077. doi: 10.1126/science.1166859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAllister AK, Katz LC, Lo DC. Neurotrophin regulation of cortical dendritic growth requires activity. Neuron. 1996;17:1057–1064. doi: 10.1016/s0896-6273(00)80239-1. [DOI] [PubMed] [Google Scholar]
- Mill J, Tang T, Kaminsky Z, Khare T, Yazdanpanah S, Bouchard L, Jia P, Assadzadeh A, Flanagan J, Schumacher A, Wang SC, Petronis A. Epigenomic profiling reveals DNA-methylation changes associated with major psychosis. Am. J. Hum. Genet. 2008;82:696–711. doi: 10.1016/j.ajhg.2008.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller G. Is Pharma Running out of Brainy Ideas? Science. 2010a;329:502–504. doi: 10.1126/science.329.5991.502. [DOI] [PubMed] [Google Scholar]
- Miller CA, Gavin CF, White JA, Parrish RR, Honasoge A, Yancey CR, Rivera IM, Rubio MD, Rumbaugh G, Sweatt JD. Cortical DNA methylation maintains remote memory. Nat. Neurosci. 2010b;13:664–666. doi: 10.1038/nn.2560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller CA, Sweatt JD. Covalent modification of DNA regulates memory formation. Neuron. 2007;1533:857–869. doi: 10.1016/j.neuron.2007.02.022. [DOI] [PubMed] [Google Scholar]
- Mitchell CP, Chen Y, Kundakovic M, Costa E, Grayson DR. Histone deacetylase inhibitors decrease reelin promoter methylation in vitro. J. Neurochem. 2005;93:483–492. doi: 10.1111/j.1471-4159.2005.03040.x. [DOI] [PubMed] [Google Scholar]
- Moghaddam B. Bringing order to the glutamate chaos in schizophrenia. Neuron. 2003;20:881–884. doi: 10.1016/s0896-6273(03)00757-8. [DOI] [PubMed] [Google Scholar]
- Noh JS, Sharma RP, Veldic M, Salvacion AA, Jia X, Chen Y, Costa E, Guidotti A. DNA methyltransferase 1 regulates reelin mRNA expression in mouse primary cortical cultures. Proc. Natl. Acad. Sci. U.S.A. 2005;102:1749–1754. doi: 10.1073/pnas.0409648102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ooi SKT, Bestor TH. The colorful history of active DNA demethylation. Cell. 2008;133:145–148. doi: 10.1016/j.cell.2008.06.009. [DOI] [PubMed] [Google Scholar]
- Peleg S, Sananbenesi F, Zovoilis A, Burkhardt S, Bahari-Javan S, Agis-Balboa RC, Cota P, Wittnam JL, Gogol-Doering A, Opitz L, Salinas-Riester G, Dettenhofer M, Kang H, Farinelli L, Chen W, Fischer A. Altered histone acetylation is associated with age-dependent memory impairment in mice. Science. 2010;328:753–756. doi: 10.1126/science.1186088. [DOI] [PubMed] [Google Scholar]
- Ptak C, Petronis A. Epigenetics and complex disease: from etiology to new therapeutics. Ann. Rev. Pharmacol. Toxicol. 2008;48:257–276. doi: 10.1146/annurev.pharmtox.48.113006.094731. [DOI] [PubMed] [Google Scholar]
- Pujadas L, Gruart A, Bosch C, Delgado L, Teixeira C, Rossi D, deLecea L, Martinez A, Delgado-Garcia JM, Soriano E. Reelin regulates postnatal neurogenesis and enhances spine hypertrophy and long-term potentiation. J. Neurosci. 2010;30:4636–4649. doi: 10.1523/JNEUROSCI.5284-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Razin A, Shemer R. DNA methylation in early development. Hum. Mol. Genet. 1995;4:1751–1755. doi: 10.1093/hmg/4.suppl_1.1751. [DOI] [PubMed] [Google Scholar]
- Roth TL, Lubin FD, Sodhi M, Kleinman JE. Epigenetic mechanisms in schizophrenia. Biochem. Biophys. Acta. 2009;1790:869–877. doi: 10.1016/j.bbagen.2009.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth BL, Sheffler DJ, Kroeze WK. Magic shotguns versus magic bullets: selectively non-selective drugs for mood disorders and schizophrenia. Nat. Rev. Drug Discov. 2004;3:353–359. doi: 10.1038/nrd1346. [DOI] [PubMed] [Google Scholar]
- Ruzicka WB, Zhubi A, Veldic M, Grayson DR, Costa E, Guidotti A. Selective epigenetic alteration of layer I GABAergic neurons isolated from prefrontal cortex of schizophrenia patients using laser-assisted microdissection. Mol. Psychiatry. 2007;12:385–397. doi: 10.1038/sj.mp.4001954. [DOI] [PubMed] [Google Scholar]
- Satta R, Maloku E, Zhubi A, Pibiri F, Hajos M, Costa E, Guidotti A. Nicotine targets the epigenetic mechanisms in selected populations of mouse telencephalic GABAergic neurons. Proc. Natl. Acad. Sci. U.S.A. 2008;105:16356–16361. doi: 10.1073/pnas.0808699105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma RP, Grayson DR, Gavin DP. Histone deacetylase 1 expression is increased in the prefrontal cortex of schizophrenia subjects: analysis of the National Brain Databank microarray collection. Schizophr. Res. 2008;98:111–117. doi: 10.1016/j.schres.2007.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shifman S, Johannesson M, Bronstein M, Chen SX, Collier DA, Craddock NJ, Kendler KS, Li T, O’Donovan M, O’Neill FA, Owen MJ, Walsh D, Weinberger Dr, Sun C, Flint J, Darvasi A. Genome-wide association identifies a common variant in the reelin gene that increases the risk of schizophrenia only in women. PLoS. Genet. 2008;4:e28. doi: 10.1371/journal.pgen.0040028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simonini MV, Camargo LM, Dong E, Maloku E, Veldic M, Costa E, Guidotti A. The benzamide MS-275 is a potent long-lasting brain region-selective inhibitor of histone deacetylases. Proc. Natl. Acad. Sci. U.S.A. 2006;103:1587–1592. doi: 10.1073/pnas.0510341103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straub RE, Lipska BK, Egan MF. Allelic variation in GAD1 (GAD67) is associated with schizophrenia and influences cortical function and gene expression. Mol. Psychiatry. 2007;12:854–869. doi: 10.1038/sj.mp.4001988. [DOI] [PubMed] [Google Scholar]
- Sullivan PF, Lin D, Tzeng JY, van den Oord E, Perkins D, Stroup TS, Wagner M, Lee S, Wright FA, Zou F, Liu W, Downing AM, Lieberman J, Close SL. Genomewide association for schizophrenia in the CATIE study: results of stage 1. Mol. Psychiatry. 2008;13:570–584. doi: 10.1038/mp.2008.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szyf M. DNA methylation and demethylation as targets for anticancer therapy. Biochemistry (Moscow) 2005;70:533–544. doi: 10.1007/s10541-005-0147-7. [DOI] [PubMed] [Google Scholar]
- Tremolizzo L, Carboni G, Ruzicka WB, Mitchell CP, Sugaya I, Tueting P, Sharma R, Grayson DR, Costa E, Guidotti A. An epigenetic mouse model for molecular and behavioral neuropathologies related to schizophrenia vulnerability. Proc. Natl. Acad. Sci. U.S.A. 2002;99:17095–17100. doi: 10.1073/pnas.262658999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tremolizzo L, Doueiri M-S, Dong E, Grayson DR, Davis J, Pinna G, Tueting P, Rodriguez-Menendez V, Costa E, Guidotti A. Valproate corrects the schizophrenia-like epigenetic behavioral modifications induced by methionine in mice. Biol. Psychiatry. 2005;57:500–509. doi: 10.1016/j.biopsych.2004.11.046. [DOI] [PubMed] [Google Scholar]
- Tueting P, Costa E, Dwivedi Y, Guidotti A, Impagnatiello F, Manev R, Pesold C. The phenotypic characteristics of heterozygous reeler mouse. Neuroreport. 1999;10:1329–1334. doi: 10.1097/00001756-199904260-00032. [DOI] [PubMed] [Google Scholar]
- Tueting P, Davis JM, Veldic M, Pibiri F, Kadriu B, Guidotti A, Costa E. Valproate: a reappraisal of its pharmacodynamic properties and mechanisms of action. Neuroreport. 2010;21:543–548. doi: 10.1097/WNR.0b013e3283373126. 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Emburgh BO, Robertson KD. DNA methyltransferases and methyl CpG binding proteins as multifunctional regulators of chromatin structure and development in mammalian cells. In: Tost J, editor. Epigenetics. Caister. Norwich, UIK: Academic Press; 2008. pp. 23–62. [Google Scholar]
- Veldic M, Caruncho JH, Liu WS, Davis J, Satta R, Grayson DR, Guidotti A, Costa E. DNA methyltransferase-1 (DNMT1) is selectively overexpressed in telencephalic GABAergic interneurons of schizophrenia brains. Proc. Natl. Acad. Sci. U.S.A. 2004;101:348–353. doi: 10.1073/pnas.2637013100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veldic M, Guidotti A, Maloku E, Davis JM, Costa E. In psychosis, cortical interneurons overexpress DNA-methyltransferase 1. Proc. Natl. Acad. Sci. U.S.A. 2005;102:2152–2157. doi: 10.1073/pnas.0409665102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veldic M, Kadriu B, Maloku E, Agis-Balboa RC, Guidotti A, Davis JM, Costa E. Epigenetic mechanisms expressed in basal ganglia GABAergic neurons differentiate schizophrenia from bipolar disorder. Schizophr. Res. 2007;91:51–61. doi: 10.1016/j.schres.2006.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wassef AA, Dott SG, Harris A, Brown A, O’Boyle M, Meyer WJ, 3rd, Rose RM. Randomized, placebo-controlled pilot study of divalproex sodium in the treatment of acute exacerbations of chronic schizophrenia. J. Clin. Psychopharmacol. 2000;20:357–361. doi: 10.1097/00004714-200006000-00011. [DOI] [PubMed] [Google Scholar]
- Weaver IC, Meaney MJ, Szyf M. Maternal care effects on the hippocampal transcriptome and anxiety mediated behaviors in the offspring that are reversible in the adulthood. Proc. Natl. Acad. Sci. U.S.A. 2006;103:3480–3485. doi: 10.1073/pnas.0507526103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wedenoja J, Loukola A, Tuulio-Henriksson A, Paunio T, Ekelund J, Silander K, Varilo T, Heikkilä K, Suvisaari J, Partonen T, Lönnqvist J, Peltonen L. Replication of linkage on chromosome 7q22 and association of the regional Reelin gene with working memory in schizophrenia families. Mol. Psychiatry. 2008;13:673–684. doi: 10.1038/sj.mp.4002047. [DOI] [PubMed] [Google Scholar]
- Woo T-U, Shrestha K, Lamb D, Minns MM, Benes FM. N-methyl-d-aspartate receptor and calbindin-containing neurons in the anterior cingulate cortex in schizophrenia and bipolar disorder. Biol. Psychiatry. 2008;64:803–809. doi: 10.1016/j.biopsych.2008.04.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woo T-U, Walsh JP, Benes FM. Density of glutamic acid decarboxylase67 messenger RNA-containing neurons that express n-methyl-d-aspartate receptor subunit NR2Ain the anterior cingulate cortex in schizophrenia and bipolar disorder. Arch. Gen. Psychiatry. 2004;61:649–657. doi: 10.1001/archpsyc.61.7.649. [DOI] [PubMed] [Google Scholar]
- Wyatt RJ, Termini BA, Davis J. Biochemical and sleep studies of schizophrenia. A review of the literature 1960–1970. Schiz. Bull. 1971;4:10–44. [Google Scholar]
- Zhu JK. Active DNA demethylation mediated by DNA glycosylases. Ann. Rev. Genetics. 2009;43:143–166. doi: 10.1146/annurev-genet-102108-134205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhubi A, Veldic M, Puri NV, Kadriu B, Caruncho H, Loza I, Sershen H, Lajtah A, Smith RC, Guidotti A, Davis JM, Costa E. An upregulation of DNA-methyltransferase 1 and 3a expressed in telencephalic GABAergic neurons of schizophrenia patients is also detected in peripheral blood lymphocytes. Schiz. Res. 2009;111:115–122. doi: 10.1016/j.schres.2009.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]