Abstract
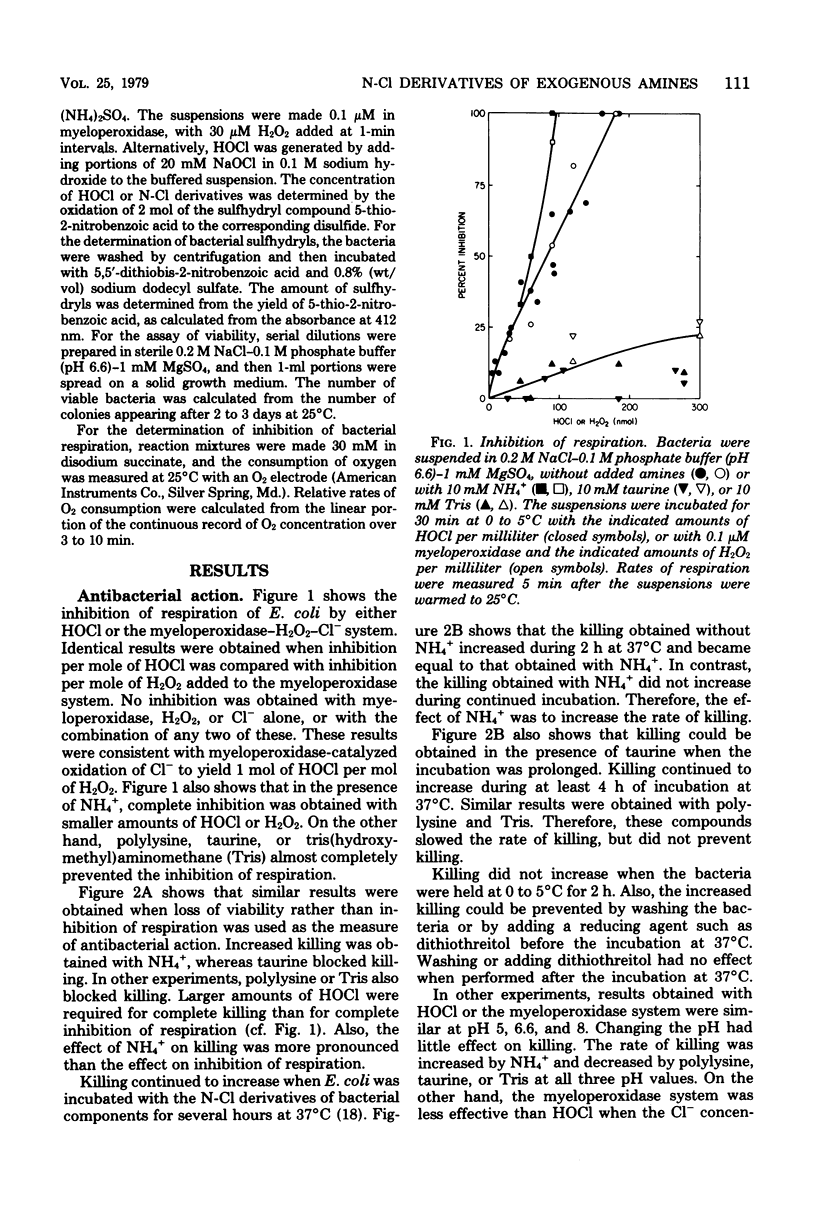
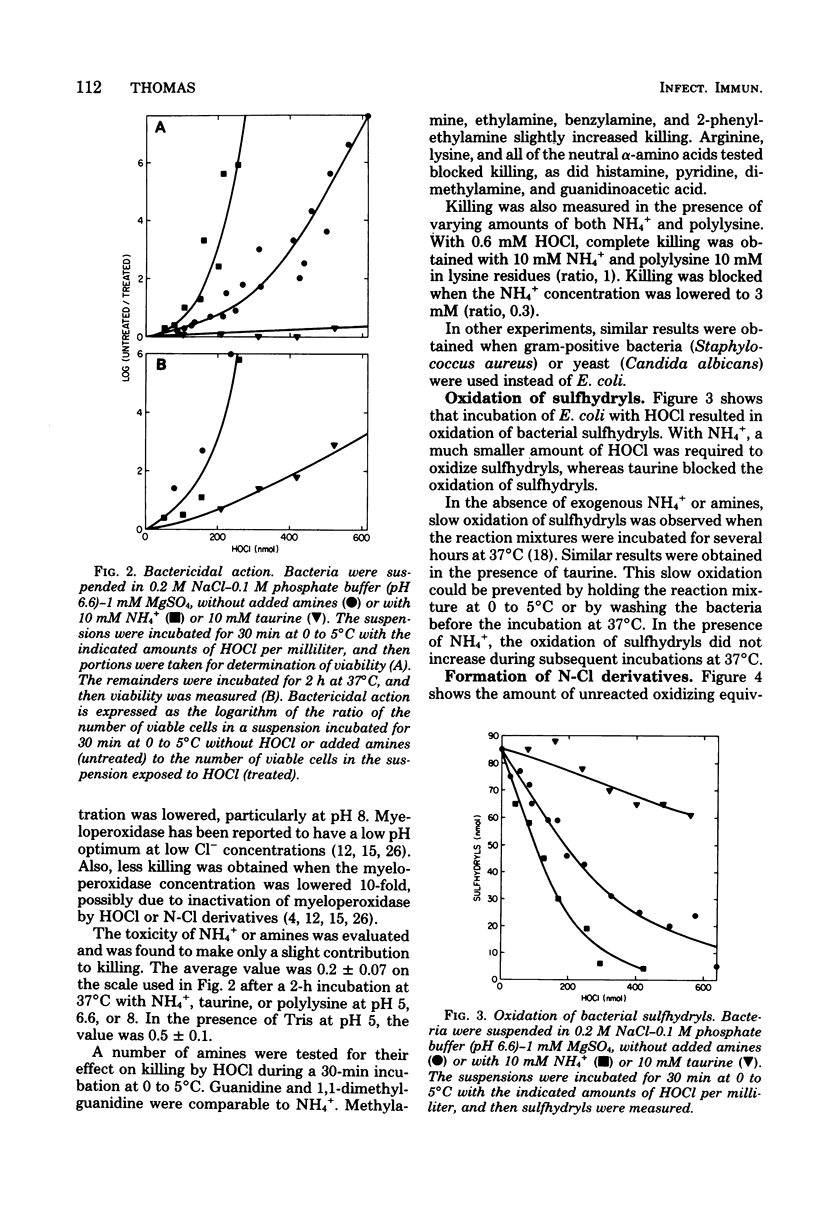
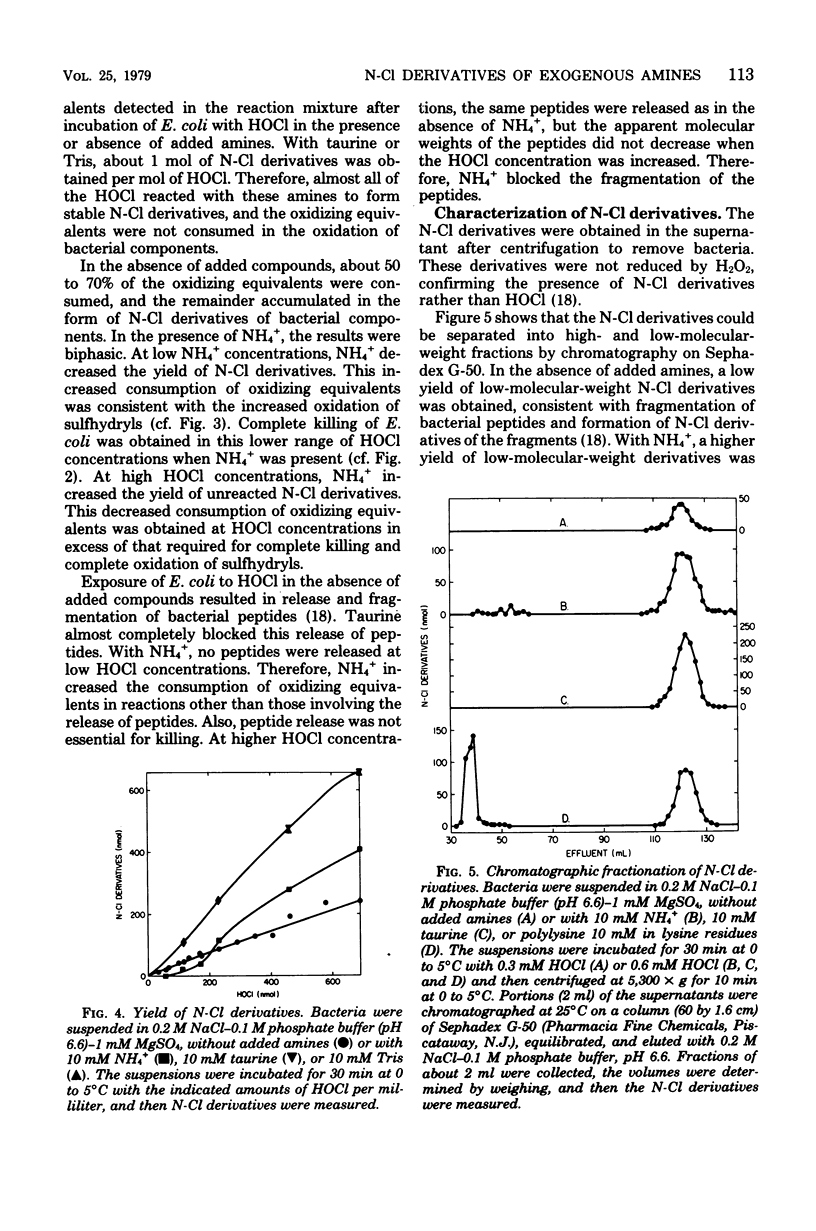
Exogenous ammonium ions (NH4+) and amine compounds had a profound influence on the antibacterial activity of the myeloperoxidase-hydrogen peroxide-chloride system against Escherichia coli. The rate of killing increased in the presence of NH4+ and certain guanidino compounds and decreased in the presence of α-amino acids, polylysine, taurine, or tris (hydroxymethyl) aminomethane. Myeloperoxidase catalyzed the oxidation of chloride to hypochlorous acid, which reacted either with bacterial amine or amide components or both or with the exogenous compounds to yield chloramine or chloramide derivatives or both. These nitrogen-chlorine derivatives could oxidize bacterial components. Killing was correlated with oxidation of bacterial components. The rate of oxidation of bacterial sulfhydryls increased in the presence of the compounds that increased the rate of killing and decreased in the presence of the other compounds. The reaction of HOCl with NH4+ yielded monochloramine (NH2Cl), which could be extracted into organic solvents. The N-Cl derivatives of bacterial components or of polylysine, taurine, or tris(hydroxymethyl)aminomethane could not be extracted. The effect of NH4+ on killing is attributed to the ability of NH2Cl to penetrate the hydrophobic cell membrane and thus to oxidize intracellular components. Polylysine, taurine, and tris(hydroxymethyl)aminomethane formed high-molecular-weight, charged, or polar N-Cl derivatives that would be unable to penetrate the cell membrane. These results suggest an important role for leukocyte amine components in myeloperoxidase-catalyzed antimicrobial activity in vivo.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baehner R. L. Molecular basis for functional disorders of phagocytes. J Pediatr. 1974 Mar;84(3):317–327. doi: 10.1016/s0022-3476(74)80711-0. [DOI] [PubMed] [Google Scholar]
- Harrison J. E., Schultz J. Studies on the chlorinating activity of myeloperoxidase. J Biol Chem. 1976 Mar 10;251(5):1371–1374. [PubMed] [Google Scholar]
- Klebanoff S. J. Antimicrobial mechanisms in neutrophilic polymorphonuclear leukocytes. Semin Hematol. 1975 Apr;12(2):117–142. [PubMed] [Google Scholar]
- Lehrer R. I. Antifungal effects of peroxidase systems. J Bacteriol. 1969 Aug;99(2):361–365. doi: 10.1128/jb.99.2.361-365.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Migler R., DeChatelet L. R., Bass D. A. Human eosinophilic peroxidase: role in bactericidal activity. Blood. 1978 Mar;51(3):445–456. [PubMed] [Google Scholar]
- Paul B. B., Strauss R. R., Selvaraj R. J., Sbarra A. J. Peroxidase mediated antimicrobial activities of alveolar macrophage granules. Science. 1973 Aug 31;181(4102):849–850. doi: 10.1126/science.181.4102.849. [DOI] [PubMed] [Google Scholar]
- Quie P. G., Mills E. L., Holmes B. Molecular events during phagocytosis by human neutrophils. Prog Hematol. 1977;10:193–210. [PubMed] [Google Scholar]
- Rest R. F., Spitznagel J. K. Myeloperoxidase-Cl--H2O2 bactericidal system: effect of bacterial membrane structure and growth conditions. Infect Immun. 1978 Mar;19(3):1110–1112. doi: 10.1128/iai.19.3.1110-1112.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sbarra A. J., Selvaraj R. J., Paul B. B., Poskitt P. K., Mitchell G. W., Jr, Louis F., Asbell M. A. Granulocyte biochemistry and a hydrogen peroxide-dependent microbicidal system. Prog Clin Biol Res. 1977;13:29–48. [PubMed] [Google Scholar]
- Selvaraj R. J., Paul B. B., Strauss R. R., Jacobs A. A., Sbarra A. J. Oxidative peptide cleavage and decarboxylation by the MPO-H2O2-Cl- antimicrobial system. Infect Immun. 1974 Feb;9(2):255–260. doi: 10.1128/iai.9.2.255-260.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stelmaszyńska T., Zgliczyński J. M. Myeloperoxidase of human neutrophilic granulocytes as chlorinating enzyme. Eur J Biochem. 1974 Jun 1;45(1):305–312. doi: 10.1111/j.1432-1033.1974.tb03555.x. [DOI] [PubMed] [Google Scholar]
- Strauss R. R., Paul B. B., Jacobs A. A., Sbarra A. J. Role of the Phagocyte in Host-Parasite Interactions XXVII. Myeloperoxidase-H(2)O(2)-Cl-Mediated Aldehyde Formation and Its Relationship to Antimicrobial Activity. Infect Immun. 1971 Apr;3(4):595–602. doi: 10.1128/iai.3.4.595-602.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas E. L., Aune T. M. Cofactor role of iodide in peroxidase antimicrobial action against Escherichia coli. Antimicrob Agents Chemother. 1978 Jun;13(6):1000–1005. doi: 10.1128/aac.13.6.1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas E. L., Aune T. M. Lactoperoxidase, peroxide, thiocyanate antimicrobial system: correlation of sulfhydryl oxidation with antimicrobial action. Infect Immun. 1978 May;20(2):456–463. doi: 10.1128/iai.20.2.456-463.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas E. L., Aune T. M. Oxidation of Escherichia coli sulfhydryl components by the peroxidase-hydrogen peroxide-iodide antimicrobial system. Antimicrob Agents Chemother. 1978 Jun;13(6):1006–1010. doi: 10.1128/aac.13.6.1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas E. L., Aune T. M. Susceptibility of Escherichia coli to bactericidal action of lactoperoxidase, peroxide, and iodide or thiocyanate. Antimicrob Agents Chemother. 1978 Feb;13(2):261–265. doi: 10.1128/aac.13.2.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas E. L. Myeloperoxidase, hydrogen peroxide, chloride antimicrobial system: nitrogen-chlorine derivatives of bacterial components in bactericidal action against Escherichia coli. Infect Immun. 1979 Feb;23(2):522–531. doi: 10.1128/iai.23.2.522-531.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkelstein J. A., Drachman R. H. Phagocytosis: The normal process and its clinically significant abnormalities. Pediatr Clin North Am. 1974 Aug;21(3):551–569. doi: 10.1016/s0031-3955(16)33024-3. [DOI] [PubMed] [Google Scholar]
- Zeya H. I., Spitznagel J. K. Cationic proteins of polymorphonuclear leukocyte lysosomes. I. Resolution of antibacterial and enzymatic activities. J Bacteriol. 1966 Feb;91(2):750–754. doi: 10.1128/jb.91.2.750-754.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeya H. I., Spitznagel J. K. Cationic proteins of polymorphonuclear leukocyte lysosomes. II. Composition, properties, and mechanism of antibacterial action. J Bacteriol. 1966 Feb;91(2):755–762. doi: 10.1128/jb.91.2.755-762.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zgliczynski J. M., Selvaraj R. J., Paul B. B., Stelmaszynska T., Poskitt P. K., Sbarra A. J. Chlorination by the myeloperoxidase-H2O2-Cl- antimicrobial system at acid and neutral pH. Proc Soc Exp Biol Med. 1977 Mar;154(3):418–422. doi: 10.3181/00379727-154-39684. [DOI] [PubMed] [Google Scholar]
- Zgliczyński J. M., Stelmaszyńska T., Domański J., Ostrowski W. Chloramines as intermediates of oxidation reaction of amino acids by myeloperoxidase. Biochim Biophys Acta. 1971 Jun 16;235(3):419–424. doi: 10.1016/0005-2744(71)90281-6. [DOI] [PubMed] [Google Scholar]
- Zgliczyński J. M., Stelmaszyńska T., Ostrowiski W., Naskalski J., Sznajd J. Myeloperoxidase of human leukaemic leucocytes. Oxidation of amino acids in the presence of hydrogen peroxide. Eur J Biochem. 1968 May;4(4):540–547. doi: 10.1111/j.1432-1033.1968.tb00246.x. [DOI] [PubMed] [Google Scholar]



