Abstract
Vein graft adaptation to the arterial environment is characterized by loss of venous identity, with reduced Ephrin type-B receptor 4 (Eph-B4) expression but without increased Ephrin-B2 expression. We examined changes of vessel identity of human saphenous veins in a flow circuit in which shear stress could be precisely controlled. Medium circulated at arterial or venous magnitudes of laminar shear stress for 24 hours; histologic, protein, and RNA analyses of vein segments were performed. Vein endothelium remained viable and functional, with platelet endothelial cell adhesion molecule (PECAM)-expressing cells on the luminal surface. Venous Eph-B4 expression diminished (p = .002), Ephrin-B2 expression was not induced (p = .268), and expression of osteopontin (p = .002) was increased with exposure to arterial magnitudes of shear stress. Similar changes were not found in veins placed under venous flow or static conditions. These data show that human saphenous veins remain viable during ex vivo application of shear stress in a bioreactor, without loss of the venous endothelium. Arterial magnitudes of shear stress cause loss of venous identity without gain of arterial identity in human veins perfused ex vivo. Shear stress alone, without immunologic or hormonal influence, is capable of inducing changes in vessel identity and, specifically, loss of venous identity.
Keywords: vein graft adaptation, EphB4, Ephrin-B2, osteopontin, shear stress, bioreactor, Saphenous vein
Introduction
While the arterial autograft is the gold standard for both cardiac and peripheral vascular bypass, it is not always possible to use arterial conduits, for example in lower extremity vascular reconstruction. As such, the autogenous saphenous vein remains the best conduit for arterial reconstruction in the peripheral system [1]. Vein graft adaptation to the arterial environment, commonly known as “arterialization,” is characterized by vessel wall thickening with deposition of smooth muscle cells and extracellular matrix in all layers of the vessel [2,3]. This remodeling process is thought to be an essential response to the different arterial environment, including increased wall shear stress, stretch force, transmural pressure, and oxygen tension [4-6]. Despite this physiologic adaptation, 30 to 50 percent of vein grafts eventually fail with significant clinical morbidity and mortality for patients [7,8]. The failure of the PREVENT III and IV trials shows that our understanding of the biology of venous remodeling is incomplete and needs additional approaches for this important clinical need [9,10].
The Eph receptors ― and their ligands the Ephrins — are differentially expressed on arteries and veins [11]. Ephrin-B2 is a determinant of arterial fate during embryologic development and persists as an arterial marker on adult vessels; alternatively, Eph-B4 is a determinant of venous fate in development and persists as a marker of venous identity on adult veins [11,12]. We have previously reported that Eph-B4 expression is reduced, but Ephrin-B2 expression is not induced, during human and rat vein graft adaptation [13], i.e., venous identity is lost without development of arterial identity.
Previous studies have examined the effects of shear stress on isolated cells, including endothelial cells, progenitor cells, stem cells, or cells in tissue-engineered grafts [14-18]. We have previously shown that bioreactors can control shear stress to alter the thickness of endothelial-lined prosthetic grafts, as well as enhance the culture of tissue-engineered vessels with pulsatile stimulation in vitro [19-21]. However, it is not clear whether these studies are adequate models of saphenous vein segments used by vascular surgeons for bypass in adult humans. We hypothesized that, using a bioreactor that can precisely control the magnitude of shear stress, we can test whether changes in magnitudes of shear stress can induce changes in vessel identity, i.e., changes in expression of Eph-B4 or Ephrin-B2 in adult human saphenous vein segments ex vivo. Therefore, we examined whether a bioreactor, with a flow circuit that reproduces arterial or venous magnitudes of shear stress while minimizing the effects of variations and high magnitudes of pressure, can support short-term viability in an explanted adult human saphenous vein segment and whether changes in vessel identity occur during this period [20].
Materials and Methods
Flow Model
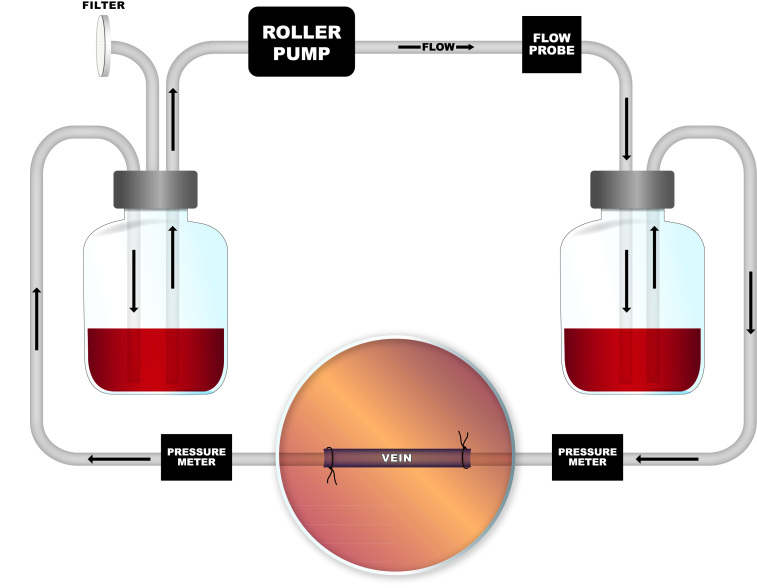
Segments of adult human saphenous veins that were surgically harvested but not used for surgical bypass were removed from the operating room (HIC Protocol #9908011041, Yale University Human Investigation Committee). Vein remnants were then transported in Dulbecco’s Modified Eagle Medium (DMEM)-based media (11995-065; Invitrogen, Grand Island, NY) and brought immediately to the laboratory. Veins were inspected for feasibility of incorporation into the bioreactor, and structural parameters were measured. The vein segment was interposed in a reversed fashion to avoid native valve obstruction and secured on each end with silk suture between custom glass cannulae of various internal and external diameters depending on the vein sample and placed within the glass bioreactor (Figure 1).
Figure 1.
Bioreactor schematic. Digital programmable roller pump pushes fluid through system in a simple pulsatile fashion, set at desired rate for arterial or venous shear.
The flow system consists of a 300+ mL capacity glass chamber with inflow and outflow ports with two equally sized compliance vessels on either side of the bioreactor to equalize pressure in the system and an intake 0.22µm air filter on the distal chamber. These were all connected with platinum-cured silicone tubing (MasterFlex, Cole-Palmer, Vernon Hills, IL). A digital programmable, peristaltic roller-pump (MasterFlex) was used to push media along the system, which was placed in an incubator (37°C; 21% O2, 5% CO2).
Flow rates, pressure, and pressure-drop across the system were verified in the bioreactor with interposition of a flow meter and pressure gauges, with data acquired via PowerLab 26T LTS (ML4856) system and LabChart (MLU60/8) software (AD Instruments, Colorado Springs, CO). The flow meter was interposed directly after the tubing exit from roller pump. Pressure meters were placed before and after the venous segment, allowing measurement of both the upstream pressure as well as the pressure change across the vein (Figure 1). A thin-walled, distensible silicone tube with a 4mm internal diameter was used as a sample for calibration of the Powerlab system. Flow rates for each specimen were estimated to achieve desired wall shear stress (WSS) via the Hagen-Poiseuille formula
τmean = 4µQ/πR3 [1]
where τ = wall shear stress, Q = volume flow rate, µ = viscosity of fluid, and R = inner radius of cylindrical tube. The arterial environment was set at a wall shear stress of 20 dynes/cm2, and the venous environment was set at a wall shear stress of 3 dynes/cm2, independently of pressure [22]. For determination of the flow rate for each environment, a derivation of this formula was used to approximate the shear stress:
ΔQ = πPd4/128µl [2]
where P = pressure difference, d = diameter of vein, µ = viscosity of fluid, and l = length of vein. Using a given vein’s radius, a standard length of 10 cm, constant viscosity of 3.8cP, and the measured pressure-drop across the system, the flow rate for the desired WSS was calculated. Vein tautness was adjusted to prevent noticeable vein distention or bowing during various flow rates. Finally, venous segments were applied to the bioreactor in “static” conditions for additional control comparison; to prevent vessel death, media was circulated at a minimal rate (1 mL/min) to maintain intraluminal exposure to the flow media and oxygenation as well as prevention of desiccation that occurs with 0 mL/min.
Endothelial cell basal media (CC-3156, Lonza, Ltd.) with 150mL Fetal Bovine Serum (FBS) (HyClone, SV3001403, Thermo Scientific, Wilmington, DE) per 1000mL media and Penicillin & Streptomycin (P&S) (10,000 U/mL, Gibco, 15140-122) was used for internal circulation. Xanthum Gum (XG; Sigma-Aldrich Co., LLC) was sterilized and added as a thickening agent for the internal media to the desired viscosity of 3.8cP to approximate human blood and was tested with a glass capillary viscometer. Medium was circulated for 24 hours at calculated flow rates. The external media bathing the vein within the bioreactor chamber consisted of a DMEM solution with FBS and P&S. Specimens were removed from the bioreactor, excluding approximately 1 cm of vein from each attachment site, in order to avoid effects from the connections.
Histology
Vein segments were fixed in 30 percent sucrose solution overnight for dehydration and then 4 percent formaldehyde solution. Specimens were subsequently analyzed with H&E, Van Gieson, and TUNEL staining per standard protocols of the Yale Histology Service at the Yale School of Medicine.
Western Blot Analysis
Equal amounts of protein initially isolated from each specimen and controls with a lysis buffer were loaded and run in SDS-PAGE, then probed with antibodies (antibodies for PECAM-1, ClvCasp3, and ClvPARP; Cell Signaling Technology, Inc., Danvers, MA, Catalog # - 3528, 9661, and 9541, respectively). Membrane signals were detected using ECL detection reagent (GE Healthcare, Denville scientific). As a positive control for apoptosis, staurosporine (1µm) was applied to human umbilical endothelial cells (HUVEC) for 5 minutes.
Immunofluorescence Analysis
Specimens were fixed in formaldehyde as above. Unstained and sectioned samples were de-paraffinized and dehydrated with three xylene washes of 5 minutes each, two 100 percent ethanol washes of 10 minutes each, and two 70 percent ethanol washes of 10 minutes each. Antigen unmasking was achieved with sodium citrate buffer. Primary antibody treatment was performed according to the manufacturer’s instructions, and concentrations optimized when needed (antibody for EphB4, ABCAM, Cambridge, MA, Catalog # - ab64820). Secondary detection was performed using Donkey Anti-Rabbit IgG (H&L) Alexa Fluor 568 secondary antibody (A-21206, Invitrogen) and counterstained with DAPI. Images were acquired with an AxioImager A1 (Carl Zeiss, Inc., Thornwood, NY).
Relative quantification of immunofluorescence images was performed (MetaMorph, Molecular Devices, LLC, Sunnyvale, CA). Each sample was compared to static conditions.
RT-PCR Analysis
RNA was isolated from cells or tissue using TRIzol Reagent (Invitrogen), and RNA was cleaned using the RNeasy Mini kit (Qiagen, Germantown, MD). Total RNA quantification quality was measured with a spectrophotometer (Nanodrop, Thermo Scientific). RT was performed using the SuperScript III First-Strand Synthesis Supermix (Invitrogen) according to the manufacturer’s instructions. Real-time quantitative PCR was performed using SYBR Green Supermix (Bio-Rad Laboratories, Hercules, CA) and amplified for 40 cycles using the iQ5 Real-Time PCR Detection system (Bio-Rad Laboratories). Correct target amplification and exclusion of nonspecific amplification was confirmed by 2 percent agarose gel electrophoresis, and primer efficiencies were determined by melting curve analysis. All samples were normalized by GAPDH amplification. Primers are listed in Table 1.
Table 1. Primers used in PCR analysis.
| Primer | Sequence |
| Eph-B4 | 5'-GTCTGACTTTGGCCTTTCCC-3'/5'-TGACATCACCTCCCACATCA-3' |
| Ephrin-B2 | 5'-CTGCTGGATCAACCAGGAAT-3'/5'-GGGTCCCTCTCAGACCTTGT-3' |
| GAPDH | 5'-CCAGGCGCCCAATACGA-3'/5'-GCCAGCCGAGCCACATC-3' |
| 28S | 5'-GGTGGAATGCGAGTGCCTAGT-3'/5'-AGTTGATTCGGCAGGTGAGTT-3' |
| Osteopontin | 5’-TTGCAGTGATTTTGCTTTTGC-3’/5’-GCCACAGCATCTGGGTATT-3’ |
Statistical Analysis
Statistical analysis with paired t-tests was performed with Sigma Plot 11.0 software (Systat Software Inc., San Jose, CA). P < 0.05 was considered significant.
Results
Flow Model and Vein Samples Configured to Analyze Shear Stress Independent of Pressure
All vein segments were trimmed to as close to 10 cm as possible, and the diameter at each end measured. The flow rate was calculated based on the measured diameter, vein length, constant viscosity, and measured pressure drop across the system according to Equation 2. Calculated examples of flow rates for venous (3 dynes/cm2) and arterial (20 dynes/cm2) shear are listed in Table 2. The mean flow rate was 24.8 mL/min for venous shear conditions, and the mean flow rate was 200.6 mL/min for arterial shear conditions. Although the bioreactor can be adjusted to near physiologic arterial pressures (~100/85mmHg) with post-vein clamping, pressures were held near constant and minimal to prevent confounding on the effects of shear stress.
Table 2. Parameters of vein and flow conditions.
| Parameter | |||
| Radius (mm) | Mean | Min | Max |
| 1.8 ± 0.5 | 1 | 2.5 | |
|
| |||
| Pressure, mean (mmHg) | Upstream | Post-vein | Drop |
| Venous shear (3 dynes/cm2) | 5.4 | 3.1 | 2.3 |
| Arterial shear (20 dynes/cm2) | 37.6 | 7.8 | 29.8 |
|
| |||
| Flow (mL/min) | Mean | Min | Max |
| Venous shear (3 dynes/cm2) | 24.8 ± 20.9 | 3.7 | 58.1 |
| Arterial shear (20 dynes/cm2) | 200.6 ± 133.2 | 83.7 | 387.5 |
Flow rate calculated based on measured radius, measured pressure change, and approximate length of 10cm using Equation 2.
Retention of Endothelial Cells without Apoptosis after Venous and Arterial Shear Stress
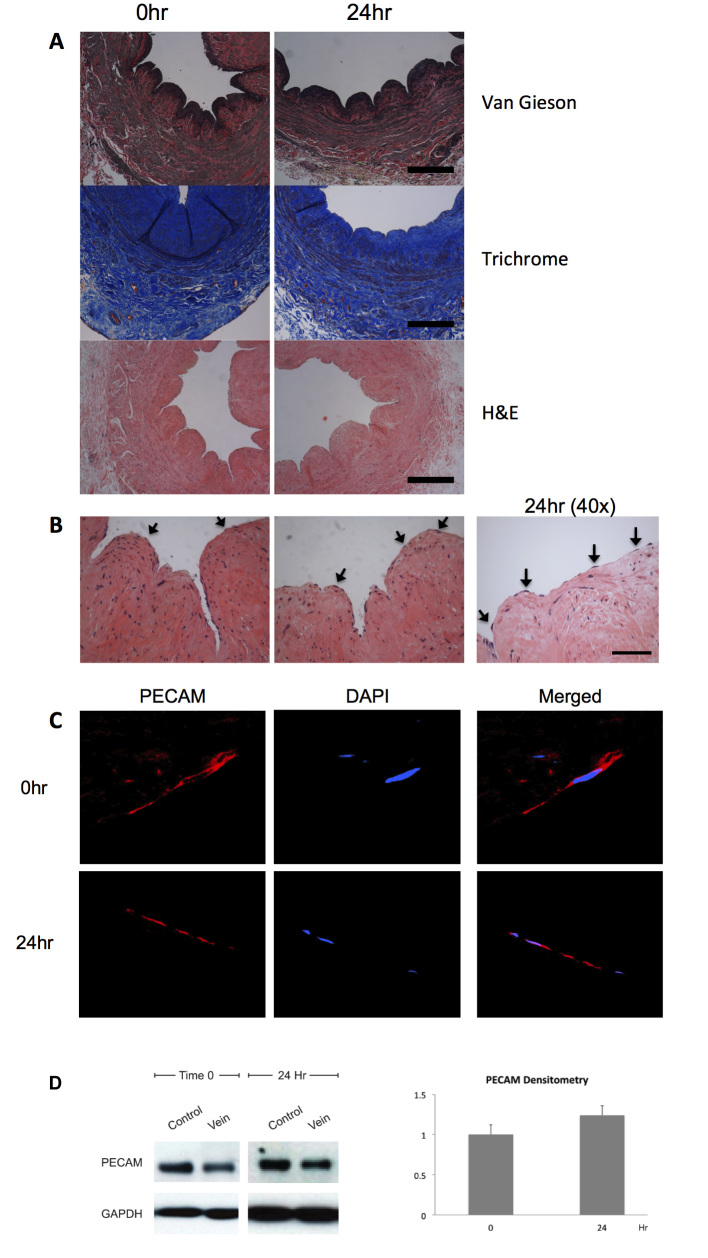
The effect of arterial magnitudes of shear stress (20 dynes/cm2) on venous structure and endothelium was examined (n = 6). General architecture was preserved in veins at baseline and after 24 hours of arterial flow in the bioreactor, with thin elastic laminae present at both time points (Figure 2A). Multiple endothelial cells were easily identifiable on the luminal surface of the veins, at both 0 and 24 hours after exposure to arterial flow (Figure 2B). To confirm that the cells were endothelial cells, immunofluorescence staining for PECAM was performed; PECAM-reactive cells were seen at both 0 and 24 hours after exposure to arterial flow, consistent with endothelial identity (Figure 2C). Similar to the maintained PECAM immunoreactivity between 0 and 24 hours of arterial shear stress, PECAM protein was also detected via Western blot after 24 hours of arterial shear stress (n = 4, Figure 2D). Densitometry showed similar PECAM protein amounts after 24 hours of arterial magnitudes of shear stress. These results are consistent with retention of the endothelial cells within the saphenous vein segment under arterial and venous magnitudes of shear stress in the bioreactor.
Figure 2.
Vein structural and endothelial integrity after arterial shear stress. A. Photomicrographs of segments of adult human saphenous vein, 0 or 24 hours after shear stress treatment in the bioreactor. Staining with either Van Gieson, trichrome, or H&E stains. Scale bar represents 200 μm. B. Photomicrographs of saphenous vein segments, high power, stained with H&E. Arrows show endothelial cells on the luminal surface. Scale bar represents 50 μm. C. Immunofluourescence of the luminal surface of a vein segment, 0 or 24 hours of arterial shear, focused on individual endothelial cells. Red, PECAM; Blue, DAPI. N = 6, representative sample shown. D. Left panel, representative Western blot of PECAM and GAPDH in vein samples treated with 0 or 24 hours of arterial shear stress. Control Lane is HUVEC. N = 4. Right panel, bar graph shows mean densitometry of PECAM normalized to GAPDH, with time 0 as the reference; y-axis is in arbitrary units.
Vein Viability and Function after Venous and Arterial Shear Stress
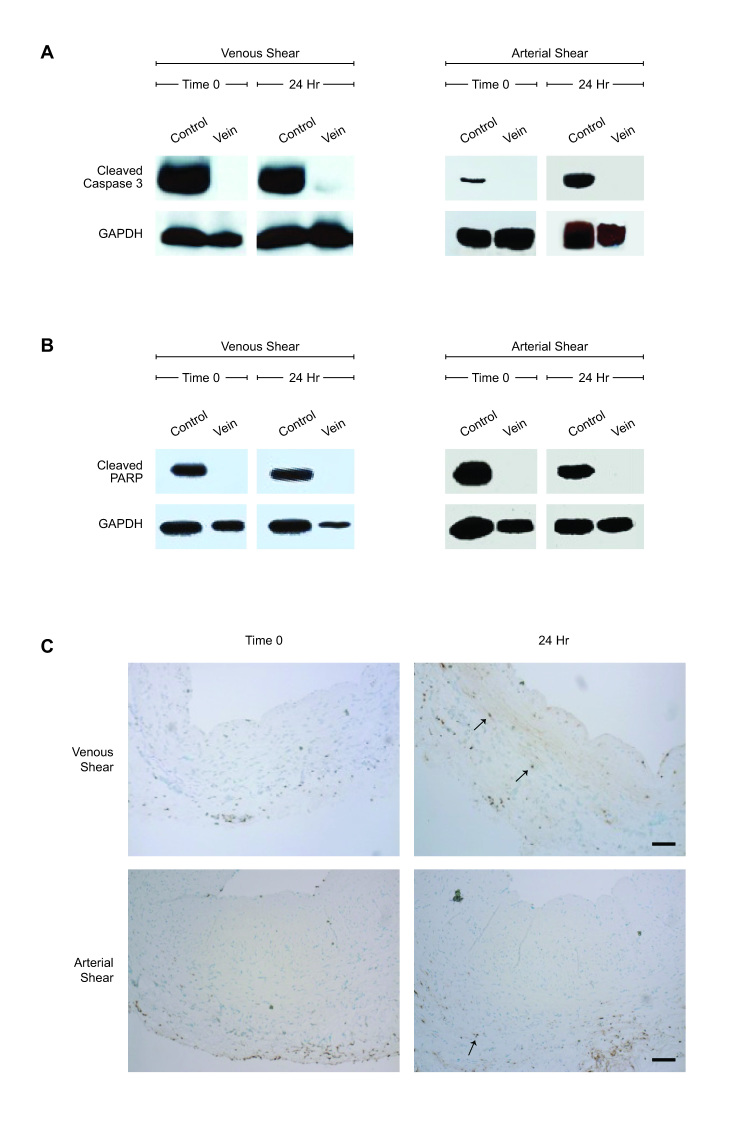
The effect of arterial shear stress on vein viability was also examined. Western blots for cleaved caspase-3 and cleaved PARP were performed on paired (0 and 24 hours) individual vein samples to assess apoptosis; no apoptosis was detectable at either baseline or after 24 hours of arterial shear stress (n = 6, Figures 3A,B).
Figure 3.
Vein viability after venous or arterial shear stress. A. Western blot for cleaved caspase-3 or GAPDH in representative vein samples at 0 or 24 hours of venous or arterial shear stress. Positive control; staurosporine-killed HUVEC. N = 6. B. Western blot for cleaved PARP or GAPDH in representative vein samples at 0 or 24 hours of venous and arterial shear stress. Positive control; staurosporine-killed HUVEC. N = 6. C. Photomicrographs showing representative TUNEL staining of matched vein samples at 0 or 24 hours of arterial (upper panels) or venous (lower panels) shear stress. Arrows indicate TUNEL positive cells. Scale bar represents 200 μm. N = 8.
To determine whether venous magnitudes of shear stress were associated with apoptosis in the vein, veins were examined with TUNEL at baseline and after 24 hours of shear stress. Very few TUNEL positive cells were seen, with only a minimal increase in adventitial staining at both 0 and 24 hours (n = 8, Figure 3C). These results are consistent with lack of apoptosis with exposure to either venous or arterial magnitudes of shear stress in the bioreactor.
Metabolic health of the saphenous vein segment was assessed using immunoreactivity of mTOR, a synthetic protein reflecting global cellular metabolism [23], at baseline and after 24 hours of arterial shear within the same vessel. Although mTOR immunoreactivity was variable between the samples, there was a trend (p = 0.08) toward increased mTOR immunoreactivity after 24 hours of arterial shear (n = 8, Figure 4A).
Figure 4.
Vein function and metabolic activity after arterial shear stress. A. Western blot and densitometry for mTOR or GAPDH in individual vein samples at 0 or 24 hours of arterial shear. Control, HUVECs. N = 8. B. Western blot and densitometry for eNOS or GAPDH in representative vein samples at 0 and 24 hours of arterial shear stress. Control, HUVECs. N = 8 C. Western blot and densitometry for phospho-eNOS or GAPDH in representative vein samples at 0 and 24 hours of arterial shear stress. Control, HUVECs. N = 8.
Since mTOR may reflect metabolic activity of both the endothelial cells and the smooth muscle cells in the vein, we assessed endothelial cell metabolic health using eNOS immunoreactivity [24], at baseline and after 24 hours of arterial shear within the same vessel. Western blots for 8 paired samples at 0 and 24 hours of arterial shear showed no difference in eNOS immunoreactivity at 0 and 24 hours of arterial shear stress (Figure 4B). There was a decrease in phosphorylated eNOS immunoreactivity at 0 and 24 hours of arterial shear stress (n = 8, Figure 4C).
Reduced EphB4 Expression with Arterial but not Venous Shear Stress
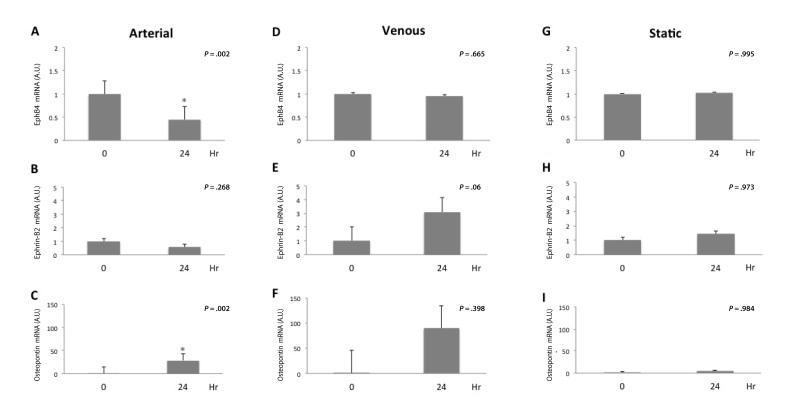
To determine the effect of shear stress on markers of cellular identity, segments of the same saphenous vein were exposed to either arterial or venous magnitudes of shear stress and then PCR was performed to assess cell identity; we also assessed the effect on osteopontin expression, a marker of vein graft adaptation [25]. Exposure to an arterial magnitude of shear stress for 24 hours was associated with decreased number of Eph-B4 mRNA transcripts (n = 10, P = .002, Figure 5A), no change in Ephrin-B2 mRNA transcripts (n = 9, P = .268, Figure 5B), and increased number of osteopontin mRNA transcripts (n = 7, P = 002, Figure 5C). Exposure to a venous magnitude of shear stress did not result in decreased number of Eph-B4 mRNA transcripts (n = 4, P = .665), and there was a trend toward increased number of both Ephrin-B2 (n = 6, P = .06) and osteopontin (n = 4, P = .398) transcripts (Figure 5D-F). Segments exposed to static conditions demonstrated no significant change in Eph-B4 (n = 3, P = .995), ephrin-B2 (n = 3, P = .973), or osteopontin (n = 3, P = .984) expression at 24 hours compared to time 0 (Figure 5G-I); the lack of change in osteopontin expression confirms the static condition as a control.
Figure 5.
Eph-B4 expression is reduced with arterial but not venous shear stress treatment. A. Bar graph shows Eph-B4 mRNA transcript number, normalized to GAPDH, at 0 or 24 hours of arterial shear stress. n = 10; *, P = .002. B. Bar graph shows Ephrin-B2 mRNA transcript number, normalized to GAPDH, at 0 or 24 hours of arterial shear stress. n = 9; P = .268. C. Bar graph shows osteopontin mRNA transcript number, normalized to GAPDH, at 0 or 24 hours of arterial shear stress. n = 7; *, P =.002. D. Bar graph shows Eph-B4 mRNA transcript number, normalized to GAPDH, at 0 or 24 hours of venous shear stress. n = 4; P = .665. E. Bar graph shows Ephrin-B2 mRNA transcript number, normalized to GAPDH, at 0 or 24 hours of venous shear stress. n = 6; P = .06. F. Bar graph shows osteopontin mRNA transcript number, normalized to GAPDH, at 0 or 24 hours of venous shear stress. n = 4; P = .398. G. Bar graph shows Eph-B4 mRNA transcript number, normalized to GAPDH, at 0 or 24 hours of static conditions. n = 3, P = .995. H. Bar graph shows Ephrin-B2 mRNA transcript number, normalized to GAPDH, at 0 or 24 hours of static conditions. n = 3, P = .973. I. Bar graph shows osteopontin mRNA transcript number, normalized to GAPDH, at 0 or 24 hours of static conditions. n = 3, P = .984.
Immunofluorescence examining Eph-B4 also showed diminished Eph-B4 immunoreactivity after 24 hours of arterial compared with venous magnitudes of shear stress (n = 3, Figure 6). Relative quantification of immunofluorescence images demonstrated a trend toward loss of Eph-B4 protein in the arterial samples compared to static, although not statistically significant.
Figure 6.
Eph-B4 immunofluorescence is reduced after 24 hours of arterial shear stress but not static conditions. Bar graphs demonstrate relative immunoreactivity quantification of arterial and venous conditions normalized to static conditions at both time 0 (P = .98) and 24 hours (P = .53).
Discussion
We show that human saphenous veins treated with arterial shear stress for 24 hours retain their endothelial monolayer without apoptosis. In addition, saphenous veins exposed to arterial, but not venous, magnitudes of shear stress have reduced Eph-B4 expression, no change in Ephrin-B2 expression, and increased osteopontin expression. These changes recapitulate the previously described changes in vessel identity that occur during venous adaptation to the arterial environment [3,13] and confirm that shear stress is capable of inducing these changes.
The endothelium plays a critical role in the prevention of vein graft neointimal hyperplasia and thrombosis, with functions that include sensing of shear stress, regulation of inflammation, and prevention of platelet adherence, thrombosis, and plaque formation [26-29]. Surgical manipulation of the vein is thought to be an important component of endothelial injury that leads to neointimal hyperplasia and ultimately vein graft failure [30,31]. There are several potential mechanisms of venous injury during the surgical harvest and implantation, including devascularization of the adventitia leading to ischemia; storage in non-physiologic solutions; high-pressure distention resulting in endothelial damage, dissection, and cell loss; and possibly tissue crushing with poor instrumentation or suturing techniques [32-36]. As such, the viability of the vein ― and especially the endothelium — has been a persistent question in vascular surgery. We show that endothelial cells are present, without apoptosis, after routine surgical vein harvest. We also confirm the presence of endothelial cells with molecular markers (Figure 2). It is known that some endothelial cells are lost during surgical harvest. Repopulation in vivo may be from the remaining endothelial cells, or potentially from circulating endothelial progenitor cells after reimplantation, or even pannus ingrowth in young patients [15,37-39]. Nonetheless, while it is possible that the endothelium is lost upon implantation of the vein into the arterial system in vivo, our results show that at least some endothelial cells can survive the perioperative harvest period and persist after exposure to arterial magnitudes of shear stress.
Our finding that venous Eph-B4 is reduced without induction of Ephrin-B2 under conditions of arterial shear stress (Figure 5) is consistent with previous reports showing loss of venous identity without gain of arterial identity in both human, rat, and mouse vein grafts exposed to the arterial environment [13,40]. Since we examined the response in whole veins, and Eph-B4 is found in both endothelial cells as well as smooth muscle cells [11], we have not identified the putative cell source of these changes. Previous work with endothelial and endothelial progenitor cells (EPC) have shown that shear stress can alter cell identity [19,41]. However, our study used whole saphenous veins taken from adult patients, with cardiovascular disease; as such, differences between our findings and these reports using cultured, presumably healthy cells of either venous or arterial origin, may be expected. Moreover, our finding that Ephrin-B2 expression does not increase with arterial shear stress may reflect our examination of adult vessels [13], as adult vessels may be deficient in delta-notch signaling upstream of Ephrin-B2, as reported by Kondo et al. in a vein graft model using aged rats [42]. Nevertheless, our findings mirror those described by Kudo et al. in adult human and aged rat vein grafts [13,40], suggesting the utility of our bioreactor model to recapitulate the adaptive response seen in vivo during vein graft adaptation to arterial shear stress. Interestingly, we show that osteopontin expression is induced with arterial magnitudes of shear stress (Figure 5C), showing the viability of these specimens in the bioreactor. However, the lack of increased osteopontin expression under venous shear stress conditions (Figure 5F) may reflect the variability and small number of human specimens, or the possibility that venous magnitudes of shear stress do not induce vein graft adaptation.
Our finding that a venous magnitude of shear stress was not associated with significantly diminished Eph-B4 expression (Figure 5B) is consistent with the presence of venous identity in adult veins. Laminar shear stress is considered atheroprotective [42,43], whereas disturbed or turbulent shear stress is associated with atherosclerosis as well as vein graft neointimal hyperplasia [44]. Veins are exposed to low magnitudes of laminar shear stress in vivo, suggesting that the laminar character of shear stress is important for normal venous endothelial homeostasis. However, the failing vein graft in vivo is also associated with low magnitudes of arterial shear stress [45]; these findings suggest that the magnitude of the shear stress changes may be more important than the frequency in the regulation of vein graft identity. Diminished venous endothelial Eph-B4 expression is associated with an angiogenic and mitogenic phenotype characterized by increased secretion of smooth muscle cell mitogens and reduced nitric oxide production [46], suggesting that vein graft adaptation to the arterial environment may be mediated directly by the effects of changes in shear stress magnitude on Eph-B4.
There are several limitations to our in vitro experiments. Firstly, the artificial in vitro environment cannot completely model all aspects of the in vivo system, including lack of an immune system, as well as the circulating elements such as platelets or EPCs. Secondly, although we have examined shear stress as a variable, the roles of pressure and oxygen tension have not been examined. Finally, the extended viability of the veins in the flow system has not yet been examined beyond 24 hours. Nonetheless, acute shear stress has been shown to elicit numerous changes in cell structure and function, including cytoskeletal remodeling and activation of signaling cascades that are different with exposure to chronic shear stress [47]. Whether the cells of intact vessels experience the same acute changes remains unclear.
Many other investigators have examined veins in ex vivo perfusion circuits. For example, Hoenicka et al. [48] have reported a similar bioreactor system to characterize the metabolic changes that occur during vessel perfusion. Importantly, this study examined the larger bovine saphenous vein and did not assess effects on vessel identity; nevertheless, their demonstration of increased endothelial survival in the presence of shear stress is consistent with our data. Similarly, Gusic et al. [49] showed that shear stress regulates intimal hyperplasia, whereas transmural pressure regulates medial hypertrophy in perfused veins, but they also did not report effects on vessel identity. Recently, Berard et al. [50] treated human saphenous veins in a perfusion system similar to ours for 7 days and showed that both Eph-B4 and Ephrin-B2 expression decrease in response to arterial magnitudes of shear stress but not pressure. Our data is complementary to this study, showing that arterial magnitudes of shear stress increases osteopontin expression and that these expression changes do not occur with static controls (Figure 5). In addition, we show lack of apoptosis in the perfused veins (Figure 3). Importantly, we also show preservation of eNOS and mTOR (Figure 4), downstream effectors of the Eph-B4 pathway. Lastly, multiple adjustable aspects of our system allow for the close approximation of laminar flow without high pressure gradients, compression effects, pinch shear or distention, preventing confounding effects on vessel structure or function.
Our finding that Eph-B4 expression was diminished with treatment using an arterial but not a venous magnitude of shear stress shows the importance of shear stress in the upstream regulation of Eph-B4 expression as well as in the regulation of vein graft adaptation. It is not currently clear whether shear stress directly induces changes in venous endothelial Eph-B4 signaling, such that Eph-B4 is a direct mechanosensing molecule, or whether Eph-B4 is part of the mechanotransduction cascade.
Abbreviations
- RNA
ribonucleic acid
- PECAM
platelet endothelial cell adhesion molecule
- HIC
human investigation committee
- TUNEL
terminal deoxynucleotidyl transferase dUTP nick end labeling
- H&E
hematoxylin & eosin
- SDS-PAGE
sodium dodecyl sulfate-polyacrylamide gel electrophoresis
- ClvCasp3
cleaved caspase-3
- ClvPARP
cleaved PARP (poly ADP ribose polymerase)
- ECL
enhanced chemiluminescence
- DAPI
4’,6-diamidino-2-phenylindole
- RT-PCR
real time polymerase chain reaction
- GAPDH
glyceraldehyde 3-phosphate dehydrogenase
- EPCs
endothelial progenitor cells
Author contributions
LM: conception and design, analysis and interpretation, data collection, writing the article, critical revision of the article, final approval of the article, statistical analysis; MH: analysis and interpretation, data collection, writing the article, critical revision of the article, final approval of the article, statistical analysis; DW: data collection, critical revision of the article, final approval of the article, statistical analysis; AM, YK. LZ. AF, and CQ: conception and design, analysis and interpretation, final approval of the article; LN: conception and design, analysis and interpretation, critical revision of the article, final approval of the article, statistical analysis; AD: conception and design, analysis and interpretation, critical revision of the article, final approval of the article, statistical analysis. LM and MH are co-first authors.
Author's note
This work was supported by the National Institutes of Health (R01-HL095498 to A.D. and R01-HL083895-06A1 to L.N.); the American Vascular Association William J. von Liebig Award (to A.D.); the Japan Society for the Promotion of Science (JSPS) KAKENHI grant number 24390299 (to A.M.); as well as with the resources and the use of facilities at the VA Connecticut Healthcare System, West Haven, CT.
References
- Bradbury AW, Adam DJ, Bell J, Forbes JF, Fowkes FGR, Gillespie I. et al. Bypass versus Angioplasty in Severe Ischaemia of the Leg (BASIL) trial: A survival prediction model to facilitate clinical decision making. J Vasc Surg. 2010;51(5):52S–68S. doi: 10.1016/j.jvs.2010.01.077. [DOI] [PubMed] [Google Scholar]
- Kocher O, Gabbiani G. Cytoskeletal features of normal and atheromatous human arterial smooth muscle cells. Hum Pathol. 1986;17(9):875–880. doi: 10.1016/s0046-8177(86)80637-2. [DOI] [PubMed] [Google Scholar]
- Muto A, Model L, Ziegler K, Eghbalieh SDD, Dardik A. Mechanisms of Vein Graft Adaptation to the Arterial Circulation. Circ J. 2010;74(8):1501–1512. doi: 10.1253/circj.cj-10-0495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard AB, Alexander RW, Nerem RM, Griendling KK, Taylor WR. Cyclic strain induces an oxidative stress in endothelial cells. Am J Physiol. 1997;272(2 Pt 1):C421–C427. doi: 10.1152/ajpcell.1997.272.2.C421. [DOI] [PubMed] [Google Scholar]
- Schwartz LB, O’Donohoe MK, Purut CM, Mikat EM, Hagen PO, McCann RL. Myointimal thickening in experimental vein grafts is dependent on wall tension. J Vasc Surg. 1997;15(1):176–186. doi: 10.1067/mva.1992.33805. [DOI] [PubMed] [Google Scholar]
- Sumpio BE, Banes AJ. Response of porcine aortic smooth muscle cells to cyclic tensional deformation in culture. J Surg Res. 1988;44(6):696–701. doi: 10.1016/0022-4804(88)90103-5. [DOI] [PubMed] [Google Scholar]
- Fitzgibbon GM, Kafka HP, Leach AJ, Keon WJ, Hooper GD, Burton JR. Coronary bypass graft fate and patient outcome: angiographic follow-up of 5,065 grafts related to survival and reoperation in 1,388 patients during 25 years. J Am Coll Cardiol. 1996;28(3):616–626. doi: 10.1016/0735-1097(96)00206-9. [DOI] [PubMed] [Google Scholar]
- Veith FJ, Gupta SK, Ascer E, White-Flores S, Samson RH, Scher LA. et al. Six-year prospective multicenter randomized comparison of autologous saphenous vein and expanded polytetrafluoroethylene grafts in infrainguinal arterial reconstructions. J Vasc Surg. 1986;3(1):104–114. doi: 10.1067/mva.1986.avs0030104. [DOI] [PubMed] [Google Scholar]
- Conte MS, Bandyk DF, Clowes AW, Moneta GL, Seely L, Lorenz TJ. et al. Results of PREVENT III: A multicenter, randomized trial of edifoligide for the prevention of vein graft failure in lower extremity bypass surgery. J Vasc Surg. 2006;43(4):742–751.e1. doi: 10.1016/j.jvs.2005.12.058. [DOI] [PubMed] [Google Scholar]
- Alexander JH, Hafley G, Harrington RA, Peterson ED, Ferguson TB, Lorenz TJ. et al. Efficacy and safety of edifoligide, an E2F transcription factor decoy, for prevention of vein graft failure following coronary artery bypass graft surgery: PREVENT IV: a randomized controlled trial. JAMA. 2005;294(19):2446–2454. doi: 10.1001/jama.294.19.2446. [DOI] [PubMed] [Google Scholar]
- Wang HU, Chen ZF, Anderson DJ. Molecular distinction and angiogenic interaction between embryonic arteries and veins revealed by ephrin-B2 and its receptor Eph-B4. Cell. 1998;93(5):741–753. doi: 10.1016/s0092-8674(00)81436-1. [DOI] [PubMed] [Google Scholar]
- Gale NW, Baluk P, Pan L, Kwan M, Holash J, DeChiara TM. et al. Ephrin-B2 Selectively Marks Arterial Vessels and Neovascularization Sites in the Adult, with Expression in Both Endothelial and Smooth-Muscle Cells. Dev Biol. 2001;230(2):151–160. doi: 10.1006/dbio.2000.0112. [DOI] [PubMed] [Google Scholar]
- Kudo FA, Muto A, Maloney SP, Pimiento JM, Bergaya S, Fitzgerald TN. et al. Venous Identity Is Lost but Arterial Identity Is Not Gained During Vein Graft Adaptation. Arterioscler Thromb Vasc Biol. 2007;27(7):1562–1571. doi: 10.1161/ATVBAHA.107.143032. [DOI] [PubMed] [Google Scholar]
- Barron V, Lyons E, Stenson-Cox C, McHugh P, Pandit A. Bioreactors for cardiovascular cell and tissue growth: a review. Ann Biomed Eng. 2003;31(9):1017–1030. doi: 10.1114/1.1603260. [DOI] [PubMed] [Google Scholar]
- Brown MA, Zhang L, Levering VW, Wu JH, Satterwhite LL, Brian L. et al. Human Umbilical Cord Blood-Derived Endothelial Cells Reendothelialize Vein Grafts and Prevent Thrombosis. Arterioscler Thromb Vasc Biol. 2010;30(11):2150–2155. doi: 10.1161/ATVBAHA.110.207076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goettsch W, Augustin HG, Morawietz H. Down-regulation of endothelial ephrinB2 expression by laminar shear stress. Endothelium. 2004;11(5-6):259–265. doi: 10.1080/10623320490904151. [DOI] [PubMed] [Google Scholar]
- Masumura T, Yamamoto K, Shimizu N, Obi S, Ando J. Shear Stress Increases Expression of the Arterial Endothelial Marker EphrinB2 in Murine ES Cells via the VEGF-Notch Signaling Pathways. Arterioscler Thromb Vasc Biol. 2009;29(12):2125–2131. doi: 10.1161/ATVBAHA.109.193185. [DOI] [PubMed] [Google Scholar]
- Obi S, Yamamoto K, Shimizu N, Kumagaya S, Masumura T, Sokabe T. et al. Fluid shear stress induces arterial differentiation of endothelial progenitor cells. J Appl Physiol. 2009;106(1):203–211. doi: 10.1152/japplphysiol.00197.2008. [DOI] [PubMed] [Google Scholar]
- Dardik A, Liu A, Ballermann BJ. Chronic in vitro shear stress stimulates endothelial cell retention on prosthetic vascular grafts and reduces subsequent in vivo neointimal thickness. J Vasc Surg. 1999;29(1):157–167. doi: 10.1016/s0741-5214(99)70357-5. [DOI] [PubMed] [Google Scholar]
- Huang AH, Niklason LE. Engineering biological-based vascular grafts using a pulsatile bioreactor. J Vis Exp. 2011;(52) doi: 10.3791/2646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niklason LE. Functional Arteries Grown in Vitro. Science. 1999;284(5413):489–493. doi: 10.1126/science.284.5413.489. [DOI] [PubMed] [Google Scholar]
- Zarins CK, Giddens DP, Bharadvaj BK, Sottiurai VS, Mabon RF, Glagov S. Carotid bifurcation atherosclerosis. Quantitative correlation of plaque localization with flow velocity profiles and wall shear stress. Circ Res. 1983;53(4):502–514. doi: 10.1161/01.res.53.4.502. [DOI] [PubMed] [Google Scholar]
- Laplante M, Sabatini DM. mTOR Signaling in Growth Control and Disease. Cell. 2012;149(2):274–293. doi: 10.1016/j.cell.2012.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higashi Y, Noma K, Yoshizumi M, Kihara Y. Endothelial function and oxidative stress in cardiovascular diseases. Circ J. 2009;73(3):411–418. doi: 10.1253/circj.cj-08-1102. [DOI] [PubMed] [Google Scholar]
- Kang N, Ng CSH, Hu J, Qiu ZB, Underwood MJ, Jeremy JY. et al. Role of osteopontin in the development of neointimal hyperplasia in vein grafts. Eur J Cardiothorac Surg. 2012;41(6):1384–1389. doi: 10.1093/ejcts/ezr200. [DOI] [PubMed] [Google Scholar]
- Davies PF. Mechanical sensing mechanisms: shear stress and endothelial cells. J Vasc Surg. 1991;13(5):729–731. doi: 10.1016/0741-5214(91)90364-z. [DOI] [PubMed] [Google Scholar]
- Hartmannsgruber V, Heyken WT, Kacik M, Kaistha A, Grgic I, Harteneck C. et al. Arterial Response to Shear Stress Critically Depends on Endothelial TRPV4 Expression. PLoS ONE. 2007;2(9):e827. doi: 10.1371/journal.pone.0000827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong D. Enhanced Inhibition of Neointimal Hyperplasia by Genetically Engineered Endothelial Progenitor Cells. Circulation. 2004;109(14):1769–1775. doi: 10.1161/01.CIR.0000121732.85572.6F. [DOI] [PubMed] [Google Scholar]
- Pober JS, Cotran RS. Cytokines and endothelial cell biology. Physiological Reviews. Physiol Rev. 1990;70(2):427–451. doi: 10.1152/physrev.1990.70.2.427. [DOI] [PubMed] [Google Scholar]
- Dregelid E, Svendsen E. Endothelial cell injury in human saphenous veins after manipulation and tweezer grasping. J Cardiovasc Surg (Torino) 1988;29(4):464–469. [PubMed] [Google Scholar]
- Soyombo AA, Angelini GD, Bryan AJ, Newby AC. Surgical preparation induces injury and promotes smooth muscle cell proliferation in a culture of human saphenous vein. Cardiovasc Res. 1993;27(11):1961–1967. doi: 10.1093/cvr/27.11.1961. [DOI] [PubMed] [Google Scholar]
- Eagle S, Brophy CM, Komalavilas P, Hocking K, Putumbaka G, Osgood M. et al. Surgical skin markers impair human saphenous vein graft smooth muscle and endothelial function. Am Surg. 2011;77(7):922–928. [PMC free article] [PubMed] [Google Scholar]
- Hocking KM, Brophy C, Rizvi SZ, Komalavilas P, Eagle S, Leacche M. et al. Detrimental effects of mechanical stretch on smooth muscle function in saphenous veins. J Vasc Surg. 2011;53(2):454–460. doi: 10.1016/j.jvs.2010.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakaguchi T, Asai T, Belov D, Okada M, Pinsky DJ, Schmidt AM. et al. Influence of ischemic injury on vein graft remodeling: Role of cyclic adenosine monophosphate second messenger pathway in enhanced vein graft preservation. J Thorac Cardiovasc Surg. 2005;129(1):129–137. doi: 10.1016/j.jtcvs.2004.04.014. [DOI] [PubMed] [Google Scholar]
- Sepehripour AH, Jarral OA, Shipolini AR, McCormack DJ. Does a “no-touch” technique result in better vein patency? Interact Cardiovasc Thorac Surg. 2011;13(6):626–630. doi: 10.1510/icvts.2011.281998. [DOI] [PubMed] [Google Scholar]
- Yu H, Kumar SR, Tang L, Terramani TT, Rowe VL, Wang Y. et al. Injury induced neointima formation and its inhibition by retrovirus-mediated transfer of nitride oxide synthase gene in an in-vitro human saphenous vein culture model. Atherosclerosis. 2002;161(1):113–122. doi: 10.1016/s0021-9150(01)00625-6. [DOI] [PubMed] [Google Scholar]
- Asahara T. Isolation of Putative Progenitor Endothelial Cells for Angiogenesis. Science. 1997;275(5302):964–966. doi: 10.1126/science.275.5302.964. [DOI] [PubMed] [Google Scholar]
- Jiang S, Walker L, Afentoulis M, Anderson DA, Jauron-Mills L, Corless CL. et al. Transplanted human bone marrow contributes to vascular endothelium. Proc Natl Acad Sci USA. 2004;101(48):16891–16896. doi: 10.1073/pnas.0404398101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Q, Rafii S, Hong-De Wu M, Wijelath ES, Yu C, Ishida A. et al. Evidence for circulating bone marrow-derived endothelial cells. Blood. 1998;92(2):362–367. [PubMed] [Google Scholar]
- Muto A, Yi T, Harrison KD, Davalos A, Fancher TT, Ziegler KR. et al. Eph-B4 prevents venous adaptive remodeling in the adult arterial environment. J Exp Med. 2011;208(3):561–575. doi: 10.1084/jem.20101854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C-Y, Bertozzi C, Zou H, Yuan L, Lee JS, Lu M. et al. Blood flow reprograms lymphatic vessels to blood vessels. J Clin Invest. 2012;122(6):2006–2017. doi: 10.1172/JCI57513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondo Y, Muto A, Kudo FA, Model L, Eghbalieh S, Chowdhary P. et al. Age-Related Notch-4 Quiescence is Associated with Altered Wall Remodeling During Vein Graft Adaptation. J Surg Res. 2011;171(1):e149–e160. doi: 10.1016/j.jss.2011.06.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dardik A, Chen L, Frattini J, Asada H, Aziz F, Kudo FA. et al. Differential effects of orbital and laminar shear stress on endothelial cells. J Vasc Surg. 2005;41(5):869–880. doi: 10.1016/j.jvs.2005.01.020. [DOI] [PubMed] [Google Scholar]
- Paszkowiak JJ, Dardik A. Arterial Wall Shear Stress: Observations from the Bench to the Bedside. Vasc Endovascular Surg. 2003;37(1):47–57. doi: 10.1177/153857440303700107. [DOI] [PubMed] [Google Scholar]
- Meyerson SL, Skelly CL, Curi MA, Shakur UM, Vosicky JE, Glagov S. et al. The effects of extremely low shear stress on cellular proliferation and neointimal thickening in the failing bypass graft. J Vasc Surg. 2001;34(1):90–97. doi: 10.1067/mva.2001.114819. [DOI] [PubMed] [Google Scholar]
- Jadlowiec CC, Feigel A, Yang C, Feinstein AJ, Kim ST, Collins MJ. et al. Reduced adult endothelial cell EphB4 function promotes venous remodeling. Am J Physiol Cell Physiol. 2013;304(7):C627–C630. doi: 10.1152/ajpcell.00333.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballermann BJ, Dardik A, Eng E, Liu A. Shear stress and the endothelium. Kidney Int Suppl. 1998;67:S100–S108. doi: 10.1046/j.1523-1755.1998.06720.x. [DOI] [PubMed] [Google Scholar]
- Hoenicka M, Wiedemann L, Puehler T, Hirt S, Birnbaum DE, Schmid C. Effects of Shear Forces and Pressure on Blood Vessel Function and Metabolism in a Perfusion Bioreactor. Ann Biomed Eng. 2010;38(12):3706–3723. doi: 10.1007/s10439-010-0116-1. [DOI] [PubMed] [Google Scholar]
- Gusic RJ, Myung R, Petko M, Gaynor JW, Gooch KJ. Shear stress and pressure modulate saphenous vein remodeling ex vivo. J Biomech. 2005;38(9):1760–1769. doi: 10.1016/j.jbiomech.2004.10.030. [DOI] [PubMed] [Google Scholar]
- Berard X, Déglise S, Alonso F, Saucy F, Meda P, Bordenave L. et al. Role of hemodynamic forces in the ex vivo arterialization of human saphenous veins. J Vasc Surg. 2013;57(5):1371–1382. doi: 10.1016/j.jvs.2012.09.041. [DOI] [PubMed] [Google Scholar]