Abstract
Glomerulonephritis (GN) is an immunological phenomenon in bacterial endocarditis. These may be pauci-immune/vasculitic GN, post-infective GN, and sub-endothelial membranoproliferative glomerulonephritis. Each type of glomerulonephritis usually occurs in isolation. We report a case of infective endocarditis with dual existence of pauci-immune/vasculitic GN and post infective type of GN at the same time.
Keywords: ANCA, glomerulonephritis, infective endocarditis
Case Description
A 40-year-old male, a daily laborer, was referred from a tertiary care hospital with complaints of fever for 2 months, along with progressive breathlessness, decreased urine output, and generalized swelling for 1½ months. He was being treated with intravenous antibiotics and diuretics without any improvement. He was not a diabetic or hypertensive. He denied any history of alcohol intake, intravenous drug abuse, and smoking. There was no history suggestive of rheumatic fever. At the time of presentation, his pulse was regular at a rate of 104/min. His blood pressure was 100/70 mm Hg and respiratory rate was 28/min. He was pale, with swelling all over the body. Jugular Venous Pulse was raised. Peripheral stigmata of infective endocarditis were absent. Cardiovascular examination showed left-sided precordial bulge and hyperdynamic type of apical impulse in left sixth intercoastal space lateral to mid clavicular line. A grade 4/6 (Levine grading) pan systolic murmur was heard all over the precordium, but it was best heard over the third intercostals space in the left parasternal region. Abdominal examination revealed ascites and tender hepatomegaly. A provisional diagnosis of ventricular septal defect (VSD) with infective endocarditis and congestive heart failure with associated acute renal failure was made. Initial blood investigations showed hemoglobin of 10.8gm/dl. Total leukocyte count was 9500/mm3, blood urea was 224 mg/dl, and serum creatinine was 16.6 mg/dl. Urine examination revealed urinary protein loss of 2 gm in 24 hours, with presence of dysmorphic red blood cells. 2D-echocardiography done in emergency (Figure 1) showed large perimembranous VSD of 1.1 cm diameter with a vegetation of 1.1 x 0.4 cm attached to the right side of the inter ventricular septum. After obtaining samples for blood cultures, injection ceftriaxone was started with intravenous diuretics. The patient was taken for emergency hemodialysis. Further evaluation revealed normal size and echo texture of both kidneys on ultrasound. His C-reactive protein (CRP) level was elevated, and rheumatoid factor and cytoplasmic antineutrophil cytoplasmic antibody (c-ANCA) was positive. Serum levels of complements, both C3 (37.7mg/dl) and C4 (15.8mg/dl), were decreased. Kidney biopsy was done. The patient underwent six cycles of hemodialysis, and injection gentamicin was given after each hemodialysis. The patient’s condition improved, but his renal functions did not normalize. Blood and urine were sterile on aerobic culture. Renal biopsy revealed fibrocellular crescents in all glomeruli (Figure 3) and diffuse endocapillary hyperplasia with compression of Bowman’s capsule and infiltration by neutrophils (Figure 4). Tubules showed focal atrophy hyaline and RBC casts. Vessels showed medial hypertrophy, and interstitium showed collection of inflammatory infiltrate comprised of lymphocytes, plasma cells, and occasional eosinophils. On immunofluroscent microscopy, non-linear deposits on glomerular basement membrane were positive for IgG, IgM, and C3. Based upon renal biopsy report, pulse therapy of methylprednisolone was given for 3 days and then oral prednisone 1 mg/kg body weight was started. The patient responded, and his renal functions improved. Repeat echocardiography (Figure 2) also revealed large perimembranous VSD as previously described with pulmonary systolic gradient around 150 mm hg. Subsequently, intravenous antibiotics were given for a total duration of 6 weeks. Gradually, the patient improved clinically and renal parameters normalized (Figure 5). Repeat 2D-echocardiography at end of therapy revealed healed vegetation with reduced size. The patient was discharged and referred to cardiothoracic department for correction of VSD.
Figure 1.
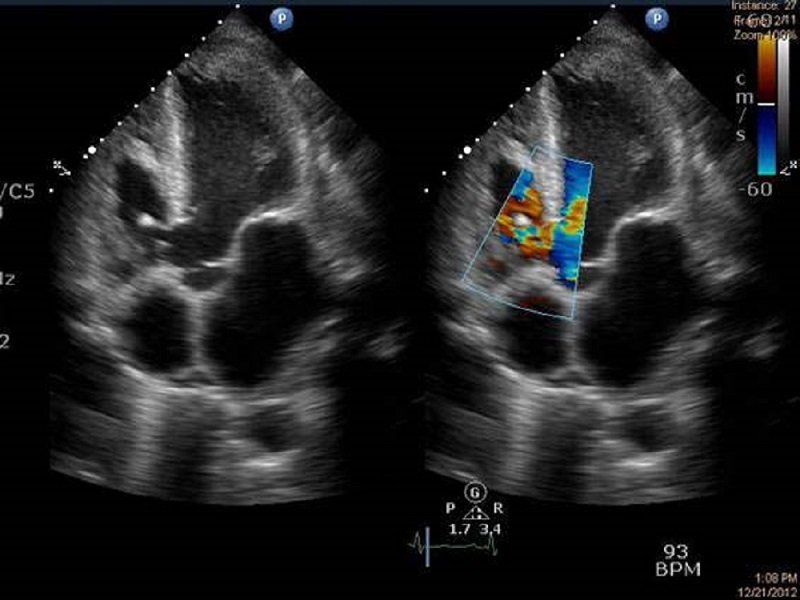
Transthoracic echocardiography showing perimembranous ventricular septal defect with a vegetation on the right ventricular side of septum.
Figure 3.
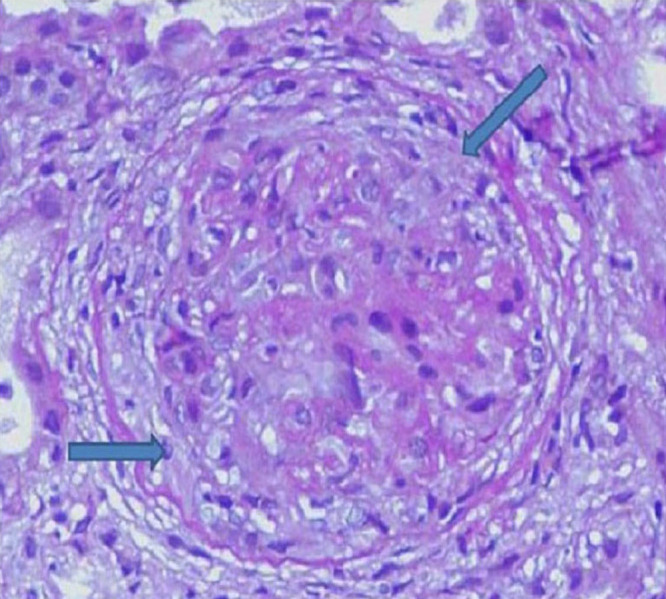
Photomicrograph showing glomerulus with crescents formation (H & E,200X).
Figure 4.
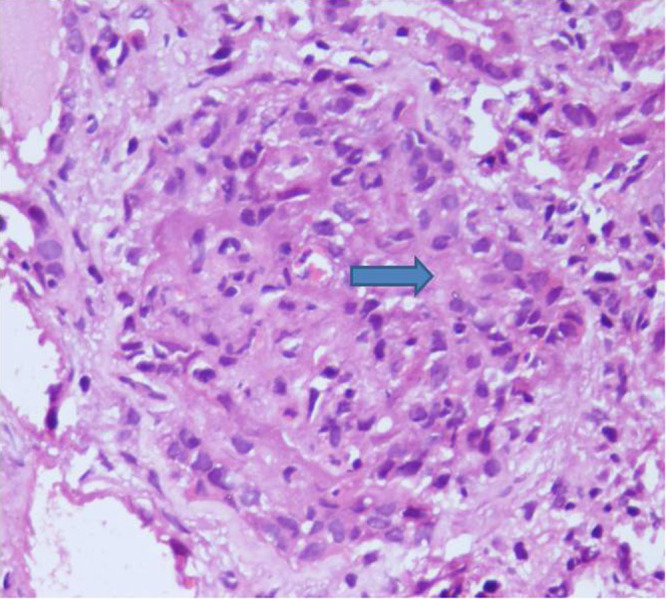
Photomicrograph of glomerulous showing endocapillary hyperplasia with compressed bowman’s space (H & E,400X).
Figure 2.
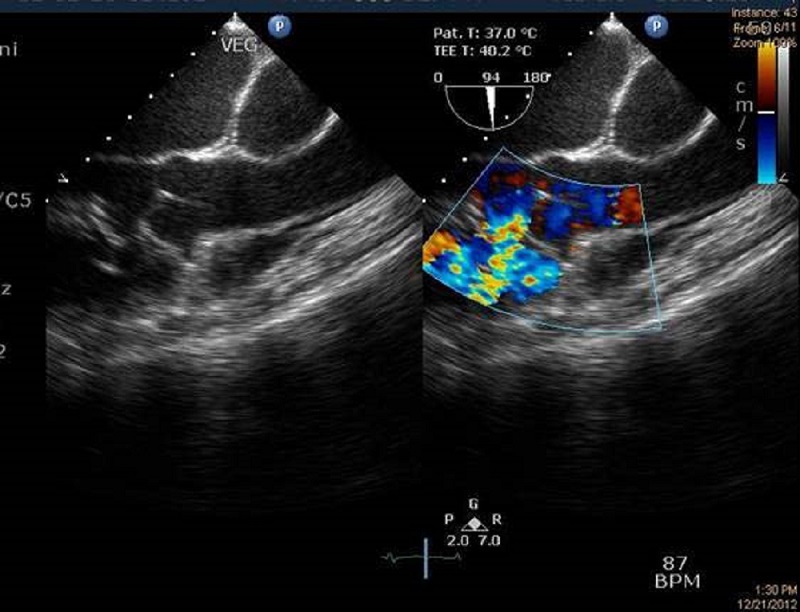
Transesophageal echocardiography showing perimembranous ventricular septal defect. Dopplar echocardiography showing jet from left to right side of heart chamber.
Figure 5.
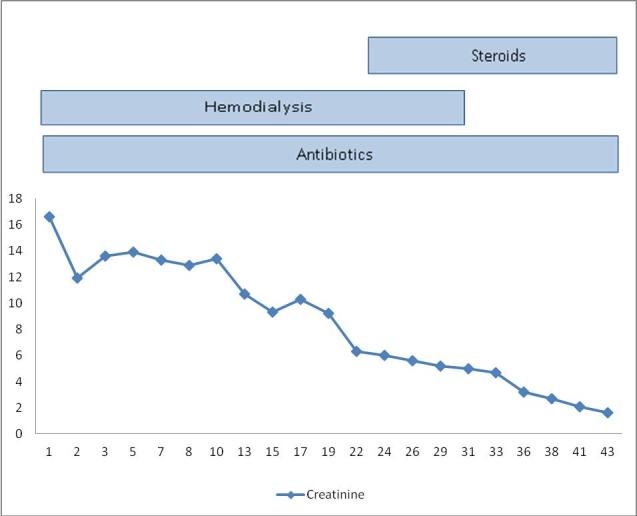
Showing gradual fall of serum creatinine levels with treatment and marked fall in the level after addition of steroid.
Discussion
Three types of glomerulonephritis are reported in patients of subacute bacterial endocarditis. These are pauci immune/vasculitic GN, post infective GN, and sub-endothelial membranoproliferative glomerulonephritis [1]. In post-infective GN, glomeruli diffuse hypercellularity, due to endothelial, mesangial cell increase, and a large number of polymorphonuclear cells is seen. Crescent may occasionally be seen, and it may rupture with lymphoplasmacytoid infiltration in interstitium. The most prominent feature on light microscopy in pauci immune glomerulonephritis is cellular or fibrous crescents and fibrinod necrosis. Tubular, interstitial, and vascular changes are also present [2]. Tubules may show acute changes such as simplification or chronic changes such as atrophy. Interstitial leucocytic infiltrate and interstitial edema are seen, and vessels other than glomerular may show small vessel vasculitis [2]. On immunofluroscent microscopy, granular deposition of IgG with complement and heavy deposition of IgM with complement is seen in post-infective and membranoproliferative GN respectively, whereas vasculitic type does not have significant depositions in glomeruli. Serum complement levels are normal in vasculitic type GN. Vasculitic type has crescents and has poor prognosis if left untreated [1].
In our case, the patient was c-ANCA positive, and renal biopsy showed features of crescentic glomerulonephritis and diffuse endocapillary hyperplasia with compression of Bowman’s capsule that was filled with polymorphonuclear cell infiltrate. Interstitial edema and infiltration with mixed inflammatory infiltrate comprising of lymphocyte, plasma cell, and occasional eosinophil were present. Tubule showed atrophy. Immunofluoroscent microscopy was positive for granular deposits of IgG, IgM, and C3. Points in favor of post-infective GN were presence of immune deposits and low complement levels and diffuse endocapillary hyperplasia with compression of Bowman’s capsule filled with predominantly polymorph nuclear cell infiltrate. However, the failure to respond to antibiotics and hemodialysis regimen, positive c-ANCA, the presence of crescents, interstitial mixed leucocytic infiltration, tubular atrophy, and gratifying response to methylprednisolone were points in favor of vasculitis type of GN.
The implication of the presence of c-ANCA in bacterial endocarditis remains unclear. However, the infectious process may induce the production of c-ANCA, possibly through polyclonal B-cell activation. Persistent vascular injury by a bacterial antigen may activate endothelial cells, induce the expression of cytoplasmic enzymes by polymorphonuclear cells, and result in the expression of autoantibodies [3]. Our patient had an infective illness for around 8 weeks, so the prolonged inflammatory process might have resulted in c-ANCA positivity. About 30 percent of bacterial endocarditis is complicated by renal failure, and renal failure is a significant predictor of mortality [4].
ANCA can be formed following environmental exposure (silica), use of drugs, or during the course of various disease processes. Drugs that most frequently lead to ANCA formation are hydralazine, propylthiouracil, penicillamine, allopurinol, and sulfasalazine. The list of medical conditions associated with ANCA formation during their course is expanding, like the list of drugs. These conditions are classified into one of three categories: a) chronic inflammatory processes; b) neoplasms, especially solid tumors; and c) infections. Evidence suggests that infections play a central role in the formation of ANCA. Glomerulonephritis developing in patients who become positive for ANCA during the course of infectious episodes is associated with increased morbidity and mortality, regardless of its specific histological picture [5].
Chronic infections may mimic ANCA-associated vasculitides (AAV). In patients with ANCA-positive vasculitis, subacute bacterial endocarditis and hepatitis C infection should be ruled out. In patients with infections and concomitant presence of ANCA, cryoglobulins and hypocomplementemia were associated with severe glomerulonephritis [6].
Another entity to be ruled out in cases of ANCA-positive glomerulonephritis is Goodpasture’s syndrome, which is a triad of alveolar hemorrhage, glomerulonephritis, and circulating anti-glomerular basement membrane antibodies. Twenty-five percent of cases of Goodpasture’s syndrome test positive for ANCA antibodies, and this association is known as double positive disease [7]. In most of these “double positive” cases, ANCA is specific for myeloperoxidase (p-ANCA), although rare instances of c-ANCA-associated “double positive” disease has also been reported in literature [8]. A suggested pathophysiology of this association is the damage to glomerular basement membrane that occurs as part of ANCA-associated vasculitis involving the glomerular capillaries leading to uncovering “hidden antigens” from the membrane, inducing the formation of antibodies. In other words, the underlying etiology is ANCA vasculitis, and the production of anti-GBM is a secondary phenomenon [7]. Our patient did not have any history of hemoptysis, his chest X-ray was unremarkable except for cardiomegaly, and all his urinary symptoms started after the cardiac events. Around 40 percent of patients who have Goodpasture’s may have only renal involvement. Our patient, however, was c-ANCA positive, and his renal biopsy revealed features of both crescents formation and endocapillary hyperplasia with immunofluorescence study showing non-linear deposits of IgG, IgM, and C3. The pathgnomonic finding in Goodpasture’s is linear deposition of IgG. Thus, we ruled out anti-GBM disease as the diagnosis. Though our patient had echocardiography-proven vegetation on the right ventricular side of interventricular septal defect, his culture was repeatedly negative, probably because of antibiotic use prior to admission at our institute. Thus, our patient probably had dual glomerulopathy associated with bacterial endocarditis, as he had evidence of both post-infective as well as vasculitic glomerulonephritis.
The treatment of bacterial endocarditis-associated glomerulonephritis usually leads to recovery of renal function, but irreversible renal failure can also occur if treatment is delayed [9]. Therapy with a bactericidal antimicrobial agent or combination of agents is usually effective [10]. But in some cases, antibiotic therapy fails, resulting in end-stage renal failure requiring dialysis therapy. In our case, the patient was initially treated with antibiotics and hemodialysis, but response to this treatment was sub-optimal. When we added corticosteroid pulse therapy followed by maintenance dose corticosteroid for 4 weeks to the above regimen, dramatic improvement occurred in the patient’s clinical and biochemical parameters. The patient recovered, and the renal functions became normal. Similar response has been documented in other case reports [11,12]. Earlier, a dual-type glomerulopathy has been postulated in cases of infective endocarditis [13]. Thus, our patient probably had dual glomerulopathy as he had evidence of both post-infective as well as vasculitic glomerulonephritis. On initial treatment with antibiotics and hemodialysis, the response was suboptimal, as only the post-infective component responded, but as soon as corticosteroid was added to the treatment regimen, the vasculitis component also responded and the patient had dramatic improvement. Though the recommended treatment [14] of ANCA-associated vasculitis is cyclophosphamide pulse along with glucocorticoid followed by oral glucocorticoid and azathioprine, we initiated the patient on glucocorticoids only as our patient had both post-infectious and vasculitic glomerulonephritis. We had no set guidelines for this kind of entity, as post-infectious GN needs treatment with antibiotics alone.
In conclusion, it is difficult to rule out ANCA-associated small vessel vasculitis in a patient with sub-acute infective endocarditis-associated glomerulonephritis with positive c-ANCA, and renal biopsy may have a crucial role in clinching the diagnosis. Secondly, corticosteroid may have a role in the treatment of patients with infective endocarditis-associated glomerulonephritis when it is not responding to intravenous antibiotics alone.
Abbreviations
- VSD
ventricular septal defect
- GN
glomerulonephritis
- c-ANCA
cytoplasmic antineutrophil cytoplasmic antibody
- CRP
C-reactive protein
- AAV
ANCA-associated vasculitides
- p-ANCA
myeloperoxidase
References
- Majumdar A, Chowdhary S, Ferreira MA. et al. Renal pathological findings in infective endocarditis. Nephrol Dial Transplant. 2000;15(11):1782–1787. doi: 10.1093/ndt/15.11.1782. [DOI] [PubMed] [Google Scholar]
- de Lind van Wijngaarden RA, Hauer HA, Wolterbeek R. et al. Clinical and Histologic Determinants of Renal Outcome in ANCA-Associated Vasculitis: A Prospective Analysis of 100 Patients with Severe Renal Involvement. J Am Soc Nephrol. 2006;17(8):2264–2274. doi: 10.1681/ASN.2005080870. [DOI] [PubMed] [Google Scholar]
- Wagner J, Andrassy K, Ritz E. Is vasculitis in subacute bacterial endocarditis associated with ANCA? Lancet. 1991;337:799–800. doi: 10.1016/0140-6736(91)91427-v. [DOI] [PubMed] [Google Scholar]
- Gagliardi JP, Nettles RE, McCarty DE. et al. Native valve infective endocarditis in elderly and younger adult patients: comparison of clinical features and outcomes with use of the Duke criteria and the Duke Endocarditis Database. Clin Infect Dis. 1998;26:1165–1168. doi: 10.1086/520304. [DOI] [PubMed] [Google Scholar]
- Konstantinov KN, Emil SN, Barry M. et al. Glomerular Disease in Patients with Infectious Processes Developing Antineutrophil Cytoplasmic Antibodies. ISRN Nephrology. 2013 doi: 10.5402/2013/324315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonaci-Nikolic B, Andrejevic S, Pavlovic M. et al. Prolonged infections associated with antineutrophil cytoplasmic antibodies specific to proteinase 3 and myeloperoxidase: diagnostic and therapeutic challenges. Clin Rheomatol. 2010;29(8):893–904. doi: 10.1007/s10067-010-1424-4. [DOI] [PubMed] [Google Scholar]
- Almouradi T, Hart P, Muram-Zborovski T. An 80-year-old female with double positive disease: Case report and brief review of literature. Am J Case Rep. 2013;14:30–33. doi: 10.12659/AJCR.883761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kashif W, Yaqub S, Mahmood SF, Patel J. Double positive Goodpasture’s Syndrome with Concomitant active pulmonary tuberculosis. Saudi J Kidney Dis Transpl. 2013;24(4):783–788. doi: 10.4103/1319-2442.113886. [DOI] [PubMed] [Google Scholar]
- Neugarten J, Neugarten DS. Glomerulonephritis in bacterial endocarditis. Am J Med. 1984;77:297–304. doi: 10.1016/0002-9343(84)90706-x. [DOI] [PubMed] [Google Scholar]
- Mylonakis E, Calderwood SB. Infective endocarditis in adults. N Engl J Med. 2001;345:1318–1330. doi: 10.1056/NEJMra010082. [DOI] [PubMed] [Google Scholar]
- Koya D, Shibuya K, Kikkawa R, Haneda M. Successful recovery of infective endocarditis-induced rapidly progressive glomerulonephritis by steroid therapy combined with antibiotics: a case report. BMC Nephrology. 2004;5:18. doi: 10.1186/1471-2369-5-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Moing V, Lacassin F, Delahousse M. et al. Use of Corticosteroids in Glomerulonephritis Related to Infective Endocarditis: Three Cases and Review. Clin Infect Dis. 1999;28(5):1057–1061. doi: 10.1086/514734. [DOI] [PubMed] [Google Scholar]
- Haas M, Eustace JA. Immune complex deposits in ANCA-associated crescentic glomerulonephritis: a study of 126 cases. Kidney Int. 2004;65(6):2145–2152. doi: 10.1111/j.1523-1755.2004.00632.x. [DOI] [PubMed] [Google Scholar]
- Galesic K, Ljubanovic D, Horvatic I. J Nephropathology. 2013;2:6–19. doi: 10.5812/nephropathol.8971. [DOI] [PMC free article] [PubMed] [Google Scholar]


