Abstract
Background:
The U.S. Food and Drug Administration reported a higher incidence of cancer in patients who had spinal arthrodesis and were exposed to a high dose of recombinant human bone morphogenetic protein-2 (rhBMP-2) compared with the control group in a randomized controlled trial. The purpose of this study was to determine the risk of cancer after spinal arthrodesis with BMP.
Methods:
We retrospectively analyzed the incidence of cancer in 467,916 Medicare patients undergoing spinal arthrodesis from 2005 to 2010. Patients with a preexisting diagnosis of cancer were excluded. The average follow-up duration was 2.85 years for the BMP group and 2.94 years for the control group. The main outcome measure was the relative risk of developing new malignant lesions after spinal arthrodesis with or without exposure to BMP.
Results:
The relative risk of developing cancer after BMP exposure was 0.938 (95% confidence interval [95% CI]: 0.913 to 0.964), which was significant. In the BMP group, 5.9% of the patients developed an invasive cancer compared with 6.5% of the patients in the control group. The relative risk of developing cancer after BMP exposure was 0.98 in males (95% CI: 0.94 to 1.02) and 0.93 (95% CI: 0.90 to 0.97) in females. The control group showed a higher incidence of each type of cancer except pancreatic cancer.
Conclusions:
Recent clinical use of BMP was not associated with a detectable increase in the risk of cancer within a mean 2.9-year time window.
Level of Evidence:
Therapeutic Level III. See Instructions for Authors for a complete description of levels of evidence.
Bone morphogenetic proteins (BMPs) are members of the transforming growth factor-beta (TGF-β) superfamily and are essential cytokines involved in the development, homeostasis, and repair of the musculoskeletal system as well as other tissues. They are potent biological agents that can induce bone formation and eliminate morbidity associated with autologous bone-grafting. The U.S. Food and Drug Administration (FDA) approved recombinant human bone morphogenetic protein-2 (rhBMP-2) (INFUSE; Medtronic, Memphis, Tennessee) for use in single-level anterior lumbar interbody fusions in 2002, open tibial fractures in 2004, and sinus and alveolar ridge augmentations associated with extraction sockets in 2007.
Because growth factors such as BMP stimulate proliferation of some cell types, the potential stimulation or progression of neoplasms is of concern. In a randomized controlled trial studying posterior lumbar spine arthrodesis, AMPLIFY (Medtronic), a high-dose (20-mg/side) formulation of rhBMP-2 with a compression matrix ceramic carrier, led to an increased incidence of cancer; however, the increase was not significant1. Similarly, the FDA Executive Summary for AMPLIFY noted a higher rate of neoplasms in the rhBMP-2 group than in the controls at the time of twenty-four and sixty-month follow-up2. Consequently, AMPLIFY was not approved by the FDA, stating a possible increased risk of cancer as the primary safety concern2. A recent critical review of rhBMP-2 clinical trials and FDA safety data highlighted the possible cancer risk and questioned the statistical methods used to analyze an infrequent, but serious, adverse event3.
Population-based studies can provide insight into the true prevalence of rare events. Because low risks are difficult to detect in a small sample size such as that in a phase-3 controlled trial of a medical device, the effects on carcinogenesis may be detectable only in a large population. The aim of this study was to compare the incidence of new malignant neoplasms after spinal arthrodesis with and without exposure to BMP, using a large administrative database. We estimated the relative risk of cancer in the population exposed to BMP in clinical use relative to that in the population without such exposure.
Materials and Methods
Database Cohorts
Medicare patient data from January 1, 2005, to December 31, 2010, were reviewed using the PearlDiver Technologies database (Warsaw, Indiana). The database contains the Medicare Standard Analytical Files, which represent 100% of Medicare inpatient and outpatient facility data. International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) procedural codes identified patients who underwent cervical or thoracolumbar spinal arthrodesis (see Appendix).
The ICD-9-CM code 84.52 detected patients who underwent spinal arthrodesis with BMP, while the absence of the code determined the control group (spinal arthrodesis without the use of BMP). Inclusion criteria were Medicare patients who underwent a spinal arthrodesis. We excluded patients with a preexisting cancer diagnosis at the time of surgery. The University of Wisconsin institutional review board approved an exemption for this project.
Cancer Types
Twenty-four invasive cancer types used in the National Cancer Institute’s Surveillance, Epidemiology and End Results (SEER) Program were defined as the outcomes of interest in the FDA executive summary of AMPLIFY2 as well as in this study (see Appendix). We searched the database using ICD-9-CM diagnosis codes to identify new diagnoses of malignant neoplasms after spinal arthrodesis with or without BMP.
Cancer Incidence
Patients having spinal arthrodesis from 2005 to 2009 were followed from the date of surgery until that date in 2010. Therefore, the follow-up period was exactly five years, four years, three years, two years, and one year for patients who underwent surgery in 2005, 2006, 2007, 2008, and 2009, respectively. Patients dying or lost to follow-up without a diagnosis of a new malignancy were counted as cancer free, and therefore the cancer incidence reported in the present study should be viewed as a minimum value.
Statistical Methods
The incidence of cancer per 100,000 patient-years at risk was calculated for the treatment and control groups. Important covariates were sex, five-year age intervals, and sex-specific five-year age intervals. A sensitivity analysis, in which patients who had a cancer diagnosis within one year and within two years were excluded, was performed. Lastly, we compared these results with predicted age and sex SEER rates by the National Cancer Institute4. The relative risk and 95% confidence interval (95% CI) were determined for the association of cancer with the use of BMP in comparison with controls. The standard error (s.e.) of the logarithm of the relative risk (RR) in the two groups with the observed number of incident cancer cases, r1 and r2, was approximated by the following formula:
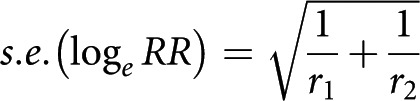 |
That is, it ignored, conservatively, the uncertainty arising from the total number of patients in the two groups. A 95% CI for the logarithm of RR was calculated as ±1.96 s.e. and was transformed back to a confidence interval for RR by taking the exponential function of the lower and upper confidence limits for loge RR. Significance was defined as a type-I error (α) of <0.05.
Source of Funding
This study was funded by the Education and Research Grant from the Department of Orthopedics and Rehabilitation at the University of Wisconsin. This project was supported in part by the University of Wisconsin Institute for Clinical and Translational Research, funded through a National Institutes of Health Clinical and Translational Science Award (grant number 1 UL1 TR000427, NCATS). The funding sponsors did not play a role in this investigation.
Results
Patient Demographics
From 2005 to 2009, 467,916 Medicare patients who had spinal arthrodesis were identified. BMP was used in 110,808 patients (23.7%) (Table I). The average duration of follow-up was 2.85 years in the BMP group and 2.94 years in the control group. Females composed 62.5% of the BMP group compared with 58.4% of the control group (p < 0.001). A chi-square test to compare age groups also showed a significant difference (p < 0.001). The patient distributions of sex and age were generally uniform between groups despite the significant differences due to large sample size (Table I). The greatest number of patients was in the age group of under sixty-five years, and the number of patients decreased in each successive age group (Table I).
TABLE I.
Patient Demographics
| Control | BMP | |
| Total no. of patients | 357,108 | 110,808 |
| Male (%) | 41.6 | 37.5 |
| Female (%) | 58.4 | 62.5 |
| Age group (%) | ||
| <65 yr | 29.8 | 27.2 |
| 65-69 yr | 25.7 | 26.5 |
| 70-74 yr | 20.1 | 21.4 |
| 75-79 yr | 14.6 | 15.5 |
| 80-84 yr | 7.3 | 7.2 |
| ≥85 yr | 2.5 | 2.2 |
Cancer Relative Risk
In the BMP group, 6557 patients (5.9%) developed a SEER cancer compared with 23,232 patients (6.5%) in the control group after spinal arthrodesis (Table II). The cancer incidence per 100,000 patient-years was 2076 in the BMP group and 2212 in the control group. The relative risk of developing cancer in the BMP group compared with the control group was 0.938 (95% CI: 0.913 to 0.964) (Table II). The difference was significant (p < 0.001), indicating a lower risk in the BMP group. The relative risk reduction in the BMP group was 6.2%. The relative risk of cancer after excluding incident cancers within one year was 1.013 (95% CI: 0.977 to 1.050) overall, 1.073 (95% CI: 1.018 to 1.131) for men, and 1.000 (95% CI: 0.947 to 1.047) for women. The relative risk of cancer after excluding incident cancers within two years was 0.993 (95% CI: 0.946 to 1.042) overall, 1.027 (95% CI: 0.957 to 1.103) for men, and 0.996 (95% CI: 0.932 to 1.065) for women.
TABLE II.
Cancer Risk
| Percent with Diagnosis |
Incidence per 100,000 Patient-Years* |
|||||
| BMP | Control | BMP | Control | SEER Predicted | RR (95% CI) | |
| Overall | 5.9 | 6.5 | 2076 | 2212 | — | 0.938 (0.913, 0.964) |
| Male | 7.5 | 8.0 | 2674 | 2722 | — | 0.982 (0.944, 1.022) |
| Female | 4.9 | 5.4 | 1699 | 1823 | — | 0.932 (0.896, 0.968) |
| Males by age | ||||||
| <65 yr | 1.1 | 1.2 | 1269 | 1355 | — | 0.94 (0.84-1.04) |
| 65-69 yr | 2.1 | 2.1 | 2856 | 2868 | 2221 | 1.00 (0.92-1.07) |
| 70-74 yr | 1.8 | 2.0 | 3085 | 3368 | 2687 | 0.92 (0.85-0.99)† |
| 75-79 yr | 1.6 | 1.6 | 3665 | 3807 | 3042 | 0.96 (0.88-1.05) |
| 80-84 yr | 0.8 | 0.8 | 3980 | 3906 | 3158 | 1.02 (0.90-1.15) |
| ≥85 yr | 0.3 | 0.3 | 4542 | 3630 | 3110 | 1.25 (1.01-1.55)† |
| Females by age | ||||||
| <65 yr | 0.8 | 1.0 | 1126 | 1200 | — | 0.94 (0.86-1.03) |
| 65-69 yr | 1.3 | 1.5 | 1720 | 1933 | 1366 | 0.89 (0.83-0.96)† |
| 70-74 yr | 1.1 | 1.3 | 1829 | 2102 | 1606 | 0.87 (0.80-0.94)† |
| 75-79 yr | 1.0 | 1.0 | 2155 | 2191 | 1823 | 0.98 (0.90-1.07) |
| 80-84 yr | 0.5 | 0.5 | 2149 | 2206 | 1948 | 0.97 (0.86-1.10) |
| ≥85 yr | 0.1 | 0.1 | 2003 | 2009 | 1831 | 1.00 (0.79-1.29) |
SEER = Surveillance Epidemiology and End Results, and RR = relative risk.
The difference was significant (p < 0.05).
Effect of Sex
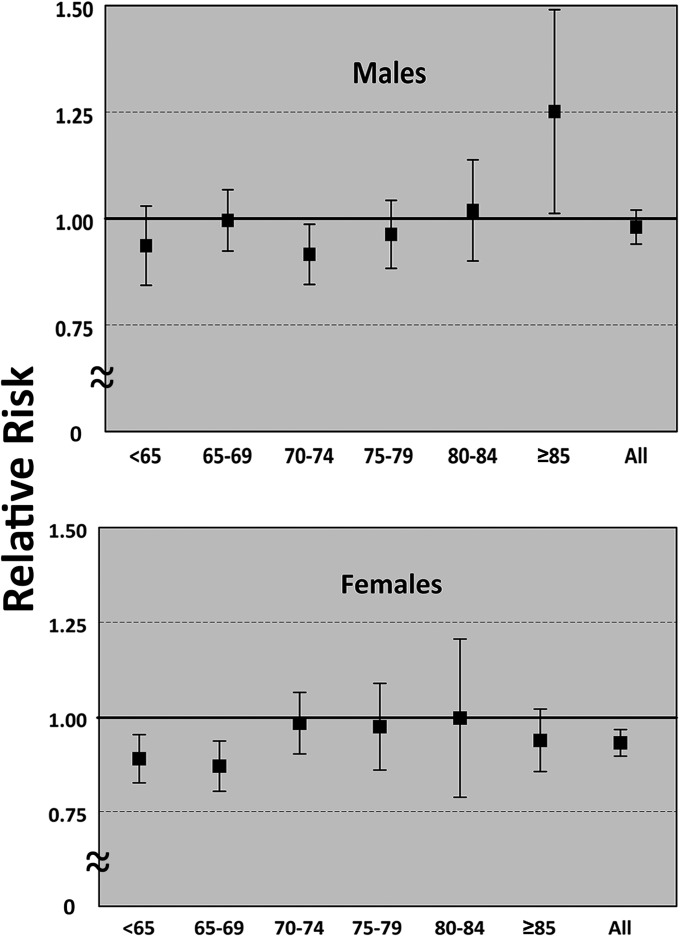
The incidence of cancer was significantly less in women than in men in both treatment groups. The relative risk of cancer in women was 0.93 (95% CI: 0.90 to 0.97; p < 0.001) (Table II). There was a significant decrease in relative risk with BMP exposure in women between sixty-five and seventy-four years old (Fig. 1, Table II). The relative risk of developing cancer in men was 0.98 (95% CI: 0.94 to 1.02), which was not significant (Table II). However, a multiple-comparison correction of the p value was not applied.
Fig. 1.
Relative risk of developing cancer. Values represent patients exposed to bone morphogenetic protein (BMP) relative to controls by age group (in years) for males and females. Error bars represent 95% confidence intervals.
Effect of Age
As expected and as predicted by the National Cancer Institute, cancer risk in both sexes increased with increasing age for both treatment groups (Table II). However, the relative risk between groups for both sexes was consistent and mirrored the overall results with ratios close to unity, with only a few exceptions. For men, there was a significant decrease in relative risk for those from seventy to seventy-four years old and a significant increase in relative risk for patients who are eighty-five years and older (Table II). For women, there was a significant decrease in relative risk for those between sixty-five and seventy-four years old. The age and sex-adjusted relative risk was 0.932 (95% CI: 0.857 to 1.030).
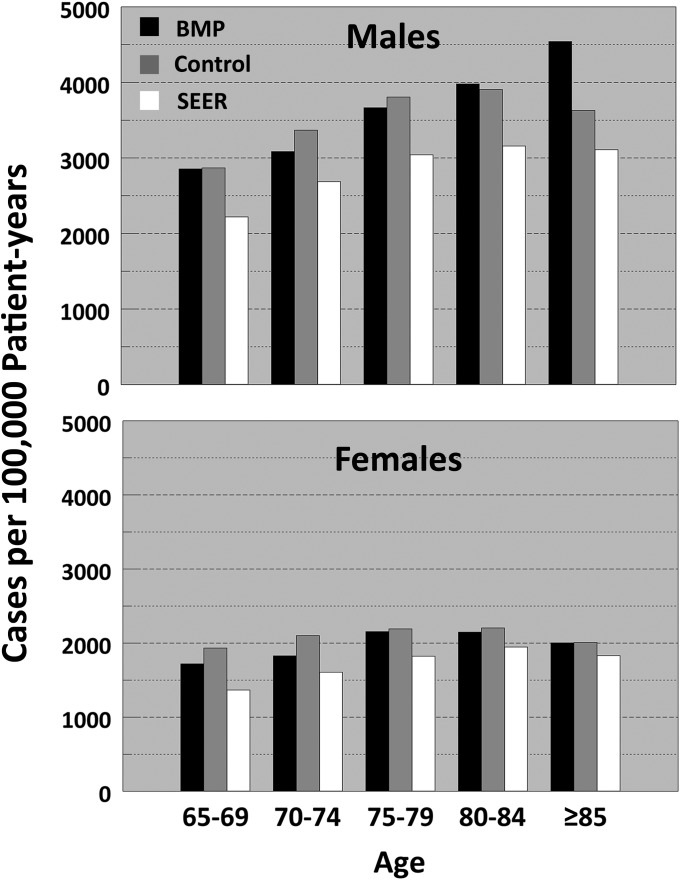
SEER-Predicted Values
The results of this study in patients sixty-five years of age and older were compared with the predicted SEER overall incidence rates from 2005 to 20094. We excluded patients less than sixty-five years of age since this group included an undefined age distribution, which was not likely similar to the SEER data (Table II). The incidences per 100,000 patient-years of exposure were consistently greater than SEER predictions by 21% to 24% in both treatment groups across all categories matched for age and sex (Fig. 2).
Fig. 2.
Incidence rates of new cancer per 100,000 patient-years compared with Surveillance Epidemiology and End Results (SEER)-predicted values. BMP = bone morphogenetic protein.
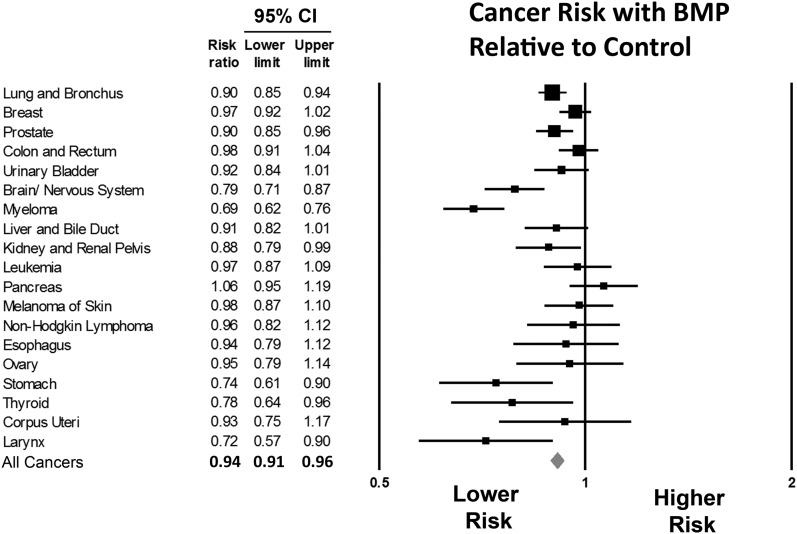
Cancer Types
We calculated the relative risk of new neoplasms of nineteen of the twenty-four most common SEER cancer types (Fig. 3). Relative risks were not calculated for Hodgkin lymphoma, cervix uteri, oral cavity and pharynx, Kaposi sarcoma, and testis cancer because of the small numbers of diagnoses. The relative risk ranged from 0.69 to 1.06. Lung and bronchus, breast, and prostate cancer were most common in both groups. The relative risk was <1 (indicating a lower risk in the BMP group than in the control group) for each cancer type, except pancreatic cancer. However, this was not significant (Fig. 3).
Fig. 3.
Relative risk of types of cancer. Error bars represent 95% confidence intervals. A value of <1 signifies a lower risk with bone morphogenetic protein (BMP) exposure.
Discussion
This study utilized a large Medicare database and analyzed the relative risk of developing new cancers after spinal surgery. We found that exposure to BMP was not only not associated with increased cancer, but the opposite was seen; the relative risk reduction in the BMP group was 6.2%. Although this association was present, we cannot identify a biologic explanation from our study for this effect.
The distribution of age and sex between the groups was generally uniform, although differences were significant because of the large sample size. To control for this, we stratified the results by sex and age groups. BMP exposure was associated with a significantly decreased risk of cancer in only one of the six age groups in men (seventy to seventy-four years) and two of the six age groups in women (sixty-five to seventy-four years). In addition to stratification, we calculated the relative risk after adjusting for age and sex differences between the groups, with a similar relative risk of 0.932 (95% CI: 0.857 to 1.030).
The incidence of cancer in this study was higher than SEER-predicted incidence values across all age intervals for each sex. These differences are expected and reassure us that we did not fail to capture many cancer diagnoses. The increased incidence overpredicted in this study may be explained by the differences in patient populations. SEER incidence values are predicted across the general population, while this study involved Medicare patients who underwent spinal arthrodesis.
The malignancies seen in the AMPLIFY study were diverse, including solid tumors, skin cancer, and hematologic malignancies5. This diversity is consistent with in vitro studies showing that many cancers types have cell membrane receptors to BMP that can be stimulated to promote growth and metastasis5. However, we are aware of no preclinical data showing that BMPs can induce cancer5,6. In our study, the specific types of cancer in each group were diverse as well. Pancreatic cancer was the only neoplasm that had a higher incidence in patients exposed to BMP. A recent study found a significant increase in cancer events after BMP exposure7. Of note, nine of the twenty cancer events in the BMP group were basal cell carcinoma or squamous cell carcinoma, which are non-SEER cancer types and frequently affect multiple sites. Our study found no association between BMP and cancer when analyzing SEER malignancies in distinct patients.
As follow-up data on cancer after exposure to BMP are available only since its recent approval by the FDA in 2002, we cannot accurately predict a lifetime risk of cancer. However, a mean follow-up period of 2.9 years is adequate to capture the incidence of cancer from exposure to a cancer promoter. This follow-up period is consistent with the AMPLIFY study, which noted an increased risk of cancer2. Furthermore, an unknown latent period exists between carcinogenic exposure and cancer outcome. A sensitivity analysis produced consistent results of relative risk of 1.013 (95% CI: 0.977 to 1.050) and 0.993 (95% CI: 0.946 to 1.042) after excluding incident cancers within one year and within two years, respectively.
Two institutions performed systematic reviews analyzing individual patient data from thirteen randomized clinical trials, regulatory documents, and confidential reports8,9. The results between studies were contradictory, with Fu et al. reporting significantly greater cancer risk at twenty-four months8, while the results reported by Simmonds et al. were not significant9. The difference between studies resulted from the inclusion of a single study, which led to divergent outcomes. At forty-eight months, the significance was no longer present in the study by Fu et al. We performed sensitivity analysis by single study elimination that rejected the significance as reported in the study by Fu et al. Additionally, the study by Fu et al. did not include seven Medtronic-sponsored randomized controlled trials and the study by Simmonds et al. did not include six Medtronic-sponsored randomized controlled trials, in which neither group developed cancer. If included, the addition of these studies would have decreased the risk ratio toward unity8,9.
Limitations of the present study include the use of institutional databases, which involve potential confounding variables that could not be analyzed, such as comorbidities, surgical techniques, and BMP carriers. Another limitation is surgical selection bias. A possible explanation for the observed differences between groups is the variable indications for BMP use that introduced bias in terms of demographics and medical comorbidities, which are important determinants of cancer risk. Smoking has a known negative influence on arthrodesis success and is a common indication for the use of BMP that could have biased results toward higher relative risk with BMP; however, this was not observed. We initially planned to examine smoking history as a covariate, but because of poor reporting, the data did not have sufficient accuracy.
A further limitation of this study is that dosage of BMP was not available. A recent systematic review of BMP and cancer suggests a possible dose-dependent relationship10. In clinical use, it is uncertain how much rhBMP-2 was given, as multiple kits may have been used. In the INFUSE clinical trial, the cancer rate was similar (0.7%) in both the rhBMP-2 group and the control group after exposure to 4.2 to 8.4 mg of rhBMP-211,12. However, the total dose in the AMPLIFY trial was 40 mg for each patient1. The AMPLIFY trial involved a higher dose and concentration; therefore, a dose-dependent relationship could be the result of systemic dosage or local concentration.
In conclusion, this study did not find an association between BMP and cancer within a mean follow-up period of 2.9 years.
Appendix
Tables showing the ICD-9-CM procedural codes and the National Cancer Institute’s Surveillance, Epidemiology and End Results invasive cancer types are available with the online version of this article as a data supplement at jbjs.org.
Acknowledgments
Note: The authors thank the University of Wisconsin Department of Orthopedics and Rehabilitation coding staff for their guidance and support. In addition, they thank Scott Hetzel, MS, of the Department of Biostatistics and Medical Informatics at the University of Wisconsin for his statistical advice.
Footnotes
Investigation performed at the Department of Orthopedics and Rehabilitation, University of Wisconsin, Madison, Wisconsin
Disclosure: One or more of the authors received payments or services, either directly or indirectly (i.e., via his or her institution), from a third party in support of an aspect of this work. In addition, one or more of the authors, or his or her institution, has had a financial relationship, in the thirty-six months prior to submission of this work, with an entity in the biomedical arena that could be perceived to influence or have the potential to influence what is written in this work. No author has had any other relationships, or has engaged in any other activities, that could be perceived to influence or have the potential to influence what is written in this work. The complete Disclosures of Potential Conflicts of Interest submitted by authors are always provided with the online version of the article.
References
- 1.Dimar JR 2nd, Glassman SD, Burkus JK, Pryor PW, Hardacker JW, Carreon LY. Clinical and radiographic analysis of an optimized rhBMP-2 formulation as an autograft replacement in posterolateral lumbar spine arthrodesis. J Bone Joint Surg Am. 2009June;91(6):1377-86 [DOI] [PubMed] [Google Scholar]
- 2.Food and Drug Administration. Executive summary for P050036 Medtronic’s AMPLIFY rhBMP-2 matrix. 2010 Jul 27. http://www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeeting%20Materials/MedicalDevices/MedicalDevicesAdvisoryCommittee/OrthopaedicandRehabilitationDevicesPanel/UCM220079.pdf. Accessed 2013 Jan 25
- 3.Carragee EJ, Hurwitz EL, Weiner BK. A critical review of recombinant human bone morphogenetic protein-2 trials in spinal surgery: emerging safety concerns and lessons learned. Spine J. 2011June;11(6):471-91 [DOI] [PubMed] [Google Scholar]
- 4.Howlader NNA, Krapcho M, Neyman N, Aminou R, Altekruse SF, Kosary CL, Ruhl J, Tatalovich Z, Cho H, Mariotto A, Eisner MP, Lewis DR, Chen HS, Feuer EJ, Cronin KA SEER Cancer statistics review, 1975-2009. National Cancer Institute. http://seer.cancer.gov/csr/1975_2009_pops09/. Accessed 2013 Jan 7.
- 5.Thawani JP, Wang AC, Than KD, Lin CY, La Marca F, Park P. Bone morphogenetic proteins and cancer: review of the literature. Neurosurgery. 2010February;66(2):233-46; discussion 246 [DOI] [PubMed] [Google Scholar]
- 6.Benglis D, Wang MY, Levi AD. A comprehensive review of the safety profile of bone morphogenetic protein in spine surgery. Neurosurgery. 2008May;62(5)(Suppl 2):ONS423-31; discussion ONS431 [DOI] [PubMed] [Google Scholar]
- 7.Carragee EJ, Chu G, Rohatgi R, Hurwitz EL, Weiner BK, Yoon ST, Comer G, Kopjar B. Cancer risk after use of recombinant bone morphogenetic protein-2 for spinal arthrodesis. J Bone Joint Surg Am. 2013September4;95(17):1537-45 [DOI] [PubMed] [Google Scholar]
- 8.Fu R, Selph S, McDonagh M, Peterson K, Tiwari A, Chou R, Helfand M. Effectiveness and harms of recombinant human bone morphogenetic protein-2 in spine fusion: a systematic review and meta-analysis. Ann Intern Med. 2013June18;158(12):890-902 [DOI] [PubMed] [Google Scholar]
- 9.Simmonds MC, Brown JV, Heirs MK, Higgins JP, Mannion RJ, Rodgers MA, Stewart LA. Safety and effectiveness of recombinant human bone morphogenetic protein-2 for spinal fusion: a meta-analysis of individual-participant data. Ann Intern Med. 2013June18;158(12):877-89 [DOI] [PubMed] [Google Scholar]
- 10.Devine JG, Dettori JR, France JC, Brodt E, McGuire RA. The use of rhBMP in spine surgery: is there a cancer risk? Evid Based Spine Care J. 2012May;3(2):35-41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Burkus JK, Gornet MF, Dickman CA, Zdeblick TA. Anterior lumbar interbody fusion using rhBMP-2 with tapered interbody cages. J Spinal Disord Tech. 2002October;15(5):337-49 [DOI] [PubMed] [Google Scholar]
- 12.Food and Drug Administration. Summary of safety and effectiveness data (SSED) for P000058 Medtronic’s InFUSE Bone Graft/LT-CAGE Lumbar Tapered Fusion Device. 2002. http://www.accessdata.fda.gov/cdrh_docs/pdf/P000058b.pdf. Accessed 2012 Aug 14