Abstract
Objective
To investigate the ability of the Timed Up & Go test to identify patients with Parkinson's disease at risk for a fall.
Design
Cross-sectional cohort study.
Setting
Sixteen participating National Parkinson's Foundation Centers of Excellence.
Participants
A query yielded a total of 2985 records (1828 men and 1157 women). From these, 884 were excluded because of a lack of crucial information (age, diagnosis, presence of deep brain stimulation, disease duration, inability of performing the Timed Up & Go test without assistance) at the time of testing, leaving 2097 patients included in the analysis.
Interventions
Not applicable.
Main Outcome Measures
The primary outcome measure for this study was falls. The chief independent variable was the Timed Up & Go test.
Results
The initial model examined the prediction of falls from the Timed Up & Go test, adjusting for all study covariates. The estimated models in the imputed data sets represented a significant improvement above chance (χ2 range [df=17], 531.29–542.39, P<.001), suggesting that 74% of participants were accurately classified as a faller or nonfaller. The secondary model in which the question of whether the effect of Timed Up & Go test was invariant across disease severity demonstrated 75% of participants were accurately classified as a faller or nonfaller. Additional analysis revealed a proposed cut score of 11.5 seconds for discrimination of those who did or did not fall.
Conclusions
The findings suggest that the Timed Up & Go test may be an accurate assessment tool to identify those at risk for falls.
Keywords: Accidental falls, Gait, Nervous system diseases, Rehabilitation
It is estimated that 70% to 87% of individuals with Parkinson's disease (PD) fall at some point during the course of their disease.1 and 2 Despite these high fall rates, clinicians do not currently have an efficacious and reliable means to fully characterize fall risk. To date, the best predictor of a fall in PD patients is the occurrence of a fall in the preceding year.3 As such, clinicians rely on historical recall during clinic assessments in order to quantify fall risk (question 13 on the Unified Parkinson's Disease Rating Scale [UPDRS]). Unfortunately, there are shortcomings with self-reported fall histories used to predict future falls. Further, fall histories do not inform about potential increased risk of a first fall because of disease progression and/or medical comorbidities.
Of equal importance, the UPDRS includes only 1 physical assessment focused on postural stability (item 30: the retropulsion or pull test). The retropulsion test is not highly associated with postural stability, as measured by the more objective and valid measures of dynamic posturography/balance.4 Unfortunately, the more reliable dynamic posturography is usually not feasible in a clinical setting. As such, an accurate and feasible measure to identify PD patients at risk for a fall is critically needed.
The Timed Up & Go (TUG) test is a physical performance measure in which the ability to rise up from a seated chair position, walk 3m, turn, walk back, and sit down is timed. This measure is useful in an outpatient setting, because it requires only a few minutes, is easy to administer, and requires little equipment. Importantly, the TUG test is highly correlated with functional mobility, gait speed, and falls in older adults.5 Specific to PD, longer TUG test times are associated with decreased mobility and may more accurately predict falls than the pull test of the UPDRS.6 and 7 The TUG test is also demonstrated to have a high test-retest reliability and interrater reliability in PD populations.8 The objective of this study was to investigate the TUG test's predictive ability to identify those with PD at increased risk of a fall during the course of their disease.
Methods
Participants
A cross-sectional study design was used from the National Parkinson Foundation's Quality Improvement Initiative Registry (NPF-QII). The data were obtained from 16 participating National Parkinson Foundation Centers of Excellence from within the United States. All participants signed informed consent.
All evaluations were done in the on medication state. Included were all patients registered in the NPF-QII between 2009 and 2010. The database query yielded a total of 2985 records available (1828 men and 1157 women). From these 2985 cases, 884 were excluded because of a lack of crucial information (age, diagnosis, presence of deep brain stimulation, disease duration, inability of performing the TUG test without assistance) at the time of testing. Demographic information of those used in the analysis can be found in table 1.
Table 1.
Sample demographics
| Demographics | Total Sample | Nonfallers | Fallers | |||
|---|---|---|---|---|---|---|
| n | Mean ± SD | n | Mean ± SD | n | Mean ± SD | |
| Age (y) | 2097 | 66.81±9.99 | 1326 | 65.89±9.65 | 771 | 68.39±10.36 |
| Mass (Kg) | 2044 | 175.85±39.65 | 1298 | 177.31±38.91 | 746 | 173.30±40.80 |
| Height (cm) | 2009 | 67.24±4.16 | 1294 | 67.52±4.04 | 715 | 66.73±4.32 |
| BMI | 1987 | 27.26±5.40 | 1280 | 27.25±5.15 | 707 | 27.28±5.82 |
| Disease duration | 1990 | 7.36±5.38 | 1243 | 6.21±4.64 | 747 | 9.28±5.96 |
| Falls | 2101 | 0.61±0.98 | 1330 | 0.00±0.00 | 771 | 1.66±0.93 |
| TUG test | 2101 | 13.79±6.74 | 1330 | 12.09±5.41 | 771 | 16.72±7.73 |
| Arthritis | 2090 | 0.74±0.96 | 1324 | 0.65±0.89 | 766 | 0.89±1.05 |
| H&Y | 2019 | 2.37±1.01 | 1280 | 2.14±0.89 | 739 | 2.78±1.06 |
| PDQ-39 mobility | 2083 | 11.90±10.67 | 1321 | 8.48±8.96 | 762 | 17.82±10.80 |
| PDQ-39 activities of daily living | 2081 | 7.08±5.70 | 1320 | 5.64±4.96 | 761 | 9.60±6.03 |
| PDQ-39 emotional well-being | 2079 | 5.66±4.67 | 1319 | 4.85±4.30 | 760 | 7.07±4.95 |
| PDQ-39 stigma | 2082 | 2.84±3.23 | 1319 | 2.63±3.12 | 763 | 3.21±3.38 |
| PDQ-39 social support | 2078 | 1.28±1.86 | 1317 | 1.04±1.71 | 761 | 1.69±2.03 |
| PDQ-39 cognition | 2081 | 4.07±3.15 | 1320 | 3.47±2.87 | 761 | 5.12±3.33 |
| PDQ-39 communication | 2081 | 2.72±2.60 | 1320 | 2.12±2.23 | 761 | 3.78±2.86 |
| PDQ-39 pain | 2080 | 3.97±2.84 | 1318 | 3.45±2.66 | 762 | 4.85±2.93 |
| 5 word | 2090 | 4.37±0.95 | 1324 | 4.48±0.84 | 766 | 4.17±1.09 |
| Verbal fluency | 2082 | 18.31±6.65 | 1319 | 19.40±6.61 | 763 | 16.43±6.28 |
| Delayed recall | 2082 | 2.93±1.39 | 1320 | 3.06±1.34 | 762 | 2.70±1.45 |
Measurements
The primary outcome measure for this study, falls, was collected via a self-reported history (over the previous 3mo) from each participant. Scores were reported by frequency as follows: 0 (no falls), 1 (<1 a month), 2 (1–3 falls a month), 3 (1–6 falls a week), and 4 (≥1 a day). As subsequently detailed, in predictive analyses, falls were dichotomized into 0 (no falls) and 1 (any fall) (collapsing original categories 1–4).
For the chief independent variable, the TUG test, patients were instructed to stand up from a chair and walk forward at their normative speed for 3m, then turn around and walk back to the chair and sit down. The whole procedure was timed in seconds from the command to go until the participant made contact sitting in the chair. If the patient could not perform the task without using their hands to push off, they were allowed to do it a second time while using their hands to push off on the chair. Use of assistant devices was not allowed.
Collection of covariates
The following covariates from data routinely collected in the registry including age, body mass index (BMI), disease duration and severity, quality of life, executive function, and presence of arthritis were added to the analysis based on their impact on falls occurrence and/or their potential to limit mobility.9, 10 and 11 In all, including subdomains of the subsequent tests, there were 17 covariates used in the model.
Disease duration and severity
Participants underwent a neurologic examination by a site neurologist, and disease severity was rated using standard Hoehn and Yahr (H&Y) staging. In the analyses, H&Y was dichotomized into those with scores of <2.5 versus >2.5. Disease duration was determined from the date of a diagnosis of idiopathic PD until the date of the study physical exam.
Quality of life
Quality of life was evaluated for each patient during the office visit using the Parkinson's Disease Questionnaire-39 (PDQ-39). The PDQ-39 measures quality of life in 8 discrete domains (mobility, activities of daily living, emotional well-being, stigma, social support, cognition, communication, and pain). The PDQ-39 was administered to each patient during the office visit. Scores for each domain were expressed as a percentage (100 indicating greater disruption and dissatisfaction within a domain). The PDQ-39 summary index score was computed by summing the 8 domain scores and standardizing the score on a 0 to 100 scale.
Executive function abilities
Executive function abilities were evaluated using immediate and delayed word recall and verbal fluency. For immediate word recall, patients were instructed to remember the 5 following words that were provided to them slowly and distinctly 1 time: face, velvet, church, daisy, and red. The patient was then asked to repeat the 5 words, and the number of correct responses was recorded. After at least 1.5 minutes and after performing a distracting task (TUG test), the participants were asked again to produce the same 5 words (delayed recall). The participants were not prompted, and the number of correct responses was recorded. To evaluate verbal fluency, patients were asked to name as many animals as possible in 1 minute. All living creatures that were not plants were counted and recorded.
Presence and severity of arthritis
The presence and severity of arthritis was scored as 0 (absent), 1 (asymptomatic/minimal), 2 (moderate), 3 (severe), and 4 (very severe).
Analysis
Prior to analysis, to ensure that the full sample was employed, multiple imputation using SPSSa missing values procedure was conducted. A total of 50 imputations were employed, and analyses subsequently described were pooled across the 50 imputations.12 Where a pooling approach has not yet been defined, the range of values provided across imputations is shown. The data were assumed to be missing at random following inclusion of covariates, although Schafer and Graham13 suggest that imputation is a superior approach to older methods (ie, listwise deletion), even when some missingness is nonrandom. Of the 17 covariates, 15 had missing data (BMI, disease duration, arthritis, H&Y, verbal fluency, delayed recall, immediate recall, and PDQ-39 subscales including activities of daily living, emotion, stigma, social support, cognition, communication, pain, and mobility). In all, 1.45% (517 missing points out of 35,649 possible values) of all data values were imputed.
Additionally, for control of multicollinearity, both tolerance and variance inflation factors were examined, and neither defied the threshold (<0.10 and >10, respectively).14 Thus, multicollinearity was not judged to be a substantial influence in this model.
The analyses were conducted in 2 steps. First, a binary logistic regression was conducted, with falls (0, no falls; 1, ≥1 falls) as the dependent variable. The TUG test was used as the principal predictor, but the odds ratio for the TUG test was conditional, and adjusted for the covariates previously listed. Second, we reran the analyses, adding an interaction term (TUG × H&Y stage) to determine whether the effect of the TUG test was consistent across PD severity. For the logistic regression, model adequacy was determined on the basis of significance (ie, deviance lower than a null model), measures of association (Cox and Snell, as well as Nagelkerke pseudo-R2 statistics), and classification accuracy (percentage of sample correctly classified; this should exceed chance classification by at least 25%). The adjusted conditional odds ratios for the TUG test and the TUG test × H&Y interaction were the terms of greatest importance and are subsequently detailed.
Given that the first 2 analytic steps supported the utility of the TUG test as a predictor of falls across the PD spectrum, we conducted a receiver operating characteristic (ROC) analysis to identify the cut score of the TUG test that optimally discriminated between fallers and nonfallers. The ROC identifies the cut point on a continuous scale (TUG test in seconds) that produces optimal sensitivity and specificity for the separation of binary groups (fallers and nonfallers). The model is evaluated on 3 criteria: (1) an area under the curve (AUC) greater than chance (ie, >0.5), (2) the AUC is significantly greater than chance, and (3) the AUC is close to or greater than the conventional value of good fit (0.7).
Results
Model 1 examined the prediction of falls from the TUG test, adjusting for all study covariates. The estimated models in the imputed data sets each represented a significant improvement above chance (χ2 range [df=17], 531.29–542.39, P<.001). Measures of association were generally low to moderate (Cox and Snell R2 range, .22–.23; Nagelkerke R2 range, .30–.31). In each of the 5 models, 74% of participants were accurately classified (chance classification =54%; thus, the obtained classification represented a 37% improvement over chance). Table 2 shows the pooled results of the 5 regressions including all covariates, while table 3 demonstrates the average sensitivities and specificities calculations for each of the 50 imputations. Focusing on the TUG test, each 1-second increase in the TUG test was associated with a 2.3% increase in the odds of reporting a fall. Using the b-weight, this means that a 10-second increase in the TUG test was associated with a 25.9% increase in the odds of a fall.
Table 2.
Pooled logistic models for the prediction of falls
| Model 1 | Model 1 | Model 2 | ||||||
|---|---|---|---|---|---|---|---|---|
| B | SE | P | Exp(B) | B | SE | P | Exp(B) | |
| TUG test × H&Y | −0.047 | .018 | .011 | 0.954 | ||||
| TUG test | 0.022 | .010 | .034 | 1.022 | 0.052 | .016 | .001 | 1.054 |
| Age | −0.004 | .066 | .505 | 0.996 | −0.005 | .006 | .392 | 0.995 |
| BMI | −0.006 | .010 | .539 | 0.994 | −0.009 | .010 | .387 | 0.991 |
| Duration of diagnosis | 0.057 | .011 | .000 | 1.058 | 0.056 | .011 | .000 | 1.058 |
| Arthritis | 0.007 | .058 | .903 | 1.007 | 0.006 | .058 | .922 | 1.006 |
| H&Y | 0.644 | .127 | .000 | 1.905 | 1.301 | .288 | .000 | 3.673 |
| PDQ-39 mobility | 0.043 | .009 | .000 | 1.043 | 0.043 | .009 | .000 | 1.044 |
| PDQ-39 activities of daily living | 0.005 | .014 | .708 | 1.005 | 0.007 | 0.14 | .621 | 1.007 |
| PDQ-39 emotion | 0.012 | .017 | .492 | 1.012 | 0.012 | .017 | .467 | 1.012 |
| PDQ-39 stigma | −0.072 | .021 | .001 | 0.931 | −0.073 | 0.21 | .000 | 0.929 |
| PDQ-39 social | 0.019 | .033 | .568 | 1.019 | 0.019 | .033 | .566 | 1.019 |
| PDQ-39 cognitive | 0.004 | .023 | .869 | 1.004 | 0.003 | .023 | .898 | 1.003 |
| PDQ-39 communication | 0.087 | .026 | .001 | 1.090 | 0.085 | .026 | .001 | 1.089 |
| PDQ-39 pain | 0.064 | .023 | .006 | 1.066 | 0.062 | .023 | .008 | 1.064 |
| Immediate recall | 0.007 | .064 | .912 | 1.007 | 0.003 | .064 | .964 | 1.003 |
| Verbal fluency | −0.027 | .010 | .005 | 0.973 | −0.026 | .010 | .006 | 0.974 |
| Delayed recall | 0.011 | .044 | .800 | 1.011 | 0.013 | .044 | .760 | 1.013 |
| Constant | −1.639 | .670 | .014 | 0.194 | −1.850 | .676 | .006 | 0.157 |
NOTE. Results are pooled across 5 stochastic imputations of the data set.
Abbreviations: B, logistic parameter estimate; Exp(B), conditional proportional odds ratio associated with a unit change in the predictor.
Table 3.
Average sensitivities and specificities calculations across 50 data imputations
| Model 2 | Model 1 | Model 2 | ||||
|---|---|---|---|---|---|---|
| Average | Maximum | Minimum | Average | Maximum | Minimum | |
| Specificity | 85.3 | 85.7 | 84.8 | 85.6 | 86.2 | 85.2 |
| Sensitivity | 54.3 | 55.4 | 53.6 | 54.6 | 55.4 | 54.1 |
| Overall correct classification | 73.9 | 74.2 | 73.5 | 74.8 | 74.9 | 74.5 |
NOTE. Model 1 examined the prediction of falls from the TUG test, adjusting for all study covariates, whereas model 2 examined whether the effect of the TUG test was invariant across H&Y levels (ie, an H&Y × TUG interaction was also included). The overall correct classification represents the percentage of the sample whose fall status was correctly classified.
Abbreviations: Sensitivity, percentage of fallers who were correctly identified; specificity, percentage of nonfallers who were correctly identified.
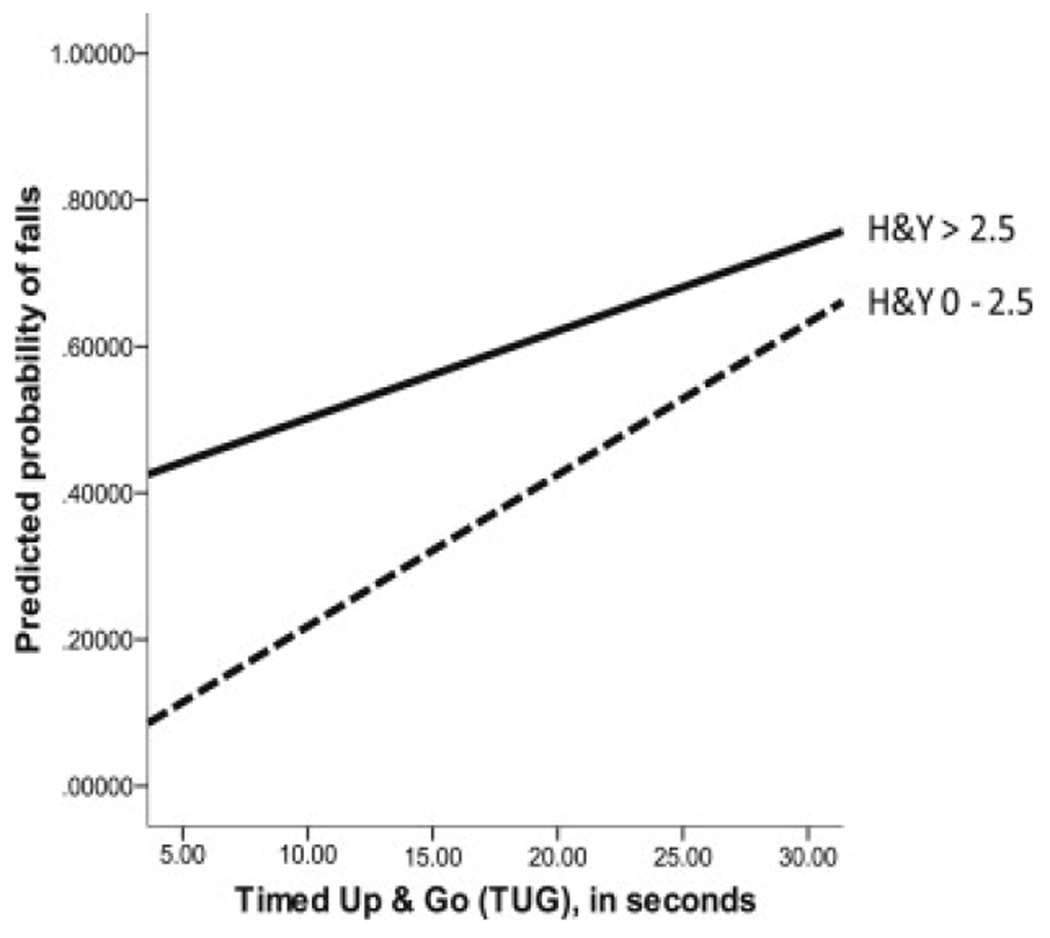
Model 2 examined the additional question of moderation; specifically, whether the effect of the TUG test was invariant across H&Y levels. Results are also shown in table 2. The estimated models in the imputed data sets each represented a significant improvement above the preceding model (χ2 range [df=1], 5.25–7.33, all P<.05). Improvement in measures of association was generally small (.01 improvement in the range for both Cox & Snell R2 and Nagelkerke R2). In each of the 5 models, 75% of participants were accurately classified, representing a 1% improvement over the preceding model. Table 2 also shows the results of this second set of regressions, including all covariates. Focusing on the TUG test, each 1-second increase in the TUG test was associated with a 5.4% increase in the odds of reporting a fall, but was qualified by a significant H&Y × TUG interaction (β=−.47, P=.011). Figure 1 shows the predicted probabilities of falls by the TUG score, separately for the 2 H&Y groups. As figure 1 shows, both H&Y groups experienced increased fall risk with slower TUG test times, but the incremental risk associated with slow TUG test times was more shallow for those in the higher H&Y group.
Fig 1.
Association of the TUG test with predicted probability of falls by H&Y group.
Having shown that the TUG test demonstrates a good association with occurrence of falls, even after statistical controls for relevant covariates, we examined whether we could identify a cut score on the TUG test that provided a good discrimination of those who did or did not fall. Because the data set was defined as persons having no missing TUG test or fall information, the original data set was used for this analysis. The AUC was .69 (well above chance 0.5; SE, .12; P<.001). Inspection of the sensitivity and specificity for univariate correctness of the TUG test in predicting falls revealed a proposed cut score of 11.5 (corresponding to a sensitivity of .66 and a specificity of .62).
Discussion
This study examined the use of TUG scores for predicting fall risk in patients with PD in the clinical setting. We hypothesized that the TUG test may be a viable and reliable clinical assessments tool in patients with PD and may predict those at increased risk of a fall during the course of their disease. Our results suggest that when adjusting for covariates including disease severity, quality of life, cognitive abilities, and arthritis, the TUG test correctly classified over 70% of the sample (faller vs nonfaller). In addition, when adjusting for H&Y disease severity, the model demonstrated that each 1-second increase in the TUG test was associated with a 5.4% increase in the odds of reporting a fall. Finally, of clinical significance, a proposed cut score of 11.5 seconds was identified as representing the highest specificity and sensitivity to discriminate fallers from nonfallers.
From a clinical perspective, identification of those at risk for a fall beyond disease severity and self-report is critically needed. As noted, current clinical assessments often rely heavily on self-report. This reliance has limits in terms of both objectivity and ability of the patient and/or caregiver to accurately recall fall events. Further, relying on disease severity to progress for fall risk awareness implementation does nothing to aid those in the early stages of disease whom are increasingly at risk for a first fall. Interestingly, the results of this study demonstrated that the TUG had a higher predictive value in those with a lower H&Y when compared with those above an H&Y of 2.5 (see fig 1). This finding may be because of other fall risk factors in those with a higher H&Y (ie, higher disease burden) that were not completely accounted for (ie, greater interaction of medication types and dosages). Similarly, those in the later stages of PD may be wheelchair bound and/or self-limited in their mobility, which may lead to a lessened fall rate.
Clinical importance of this study is demonstrated in the identification of a cut point that can serve as an indicator of fall risk. Information about future fall risk allows for the implementation of rehabilitation programs demonstrated to reduce fall risk and possibly prevent falls in patients with PD. This is critical, because some of the major fall risk factors are potentially modifiable.15 Thus, identification of fall clues are important to identify; therefore, a fall risk reduction strategy and education program can begin at a relevant time. This is of utmost importance, because those with PD and their caregivers may not be fully aware of fall risks or situations that may increase risk. For example, Sadowski et al16 examined awareness of risk factors associated with falling among a group of community-dwelling PD patients using the Falls Risk Awareness Questionnaire. This cohort recognized their increased probability of a fall; however, it was unaware of specific risk factors that may increase the chances of a fall (eg, medication use/interactions and/or multitasking).
In a previous study by Viccaro et al,5 the researchers sought to compare the TUG test and gait speed in their ability to predict health, function, and falls in older adults aged ≥65 years. Using a prospective cohort design and a sample of 457 older adults, the researchers demonstrated that gait speed and the TUG test each alone predicted decline in global health, new activities of daily living difficulty, and falls. It is noteworthy to point out that when the TUG test and gait speed were combined in the Viccaro analysis, there was no added predictive value. While our results in PD are in line with the predictive ability of the TUG test in older adults, future research is needed to confirm whether gait speed alone or in conjunction with the TUG test can provide added value when examining fall risk in PD.
Foreman et al6 examined the predictive validity for fall events from a functional gait assessment, the pull test, and the TUG test during on and off mediation states in 36 patients with PD. Their results demonstrated that the pull test was a less accurate predictor of falls than both the functional gait assessment and the TUG test. Interestingly, the functional gait assessment was able to more accurately predict falls than both the TUG test and the pull test during both on and off medication testing. The results of our study suggest that the TUG test (in the on state) may also provide valuable information to a fall evaluation risk profile. This is critical, because the burden of off medication testing and evaluation in both a clinical and research setting may limit the utility of an assessment tool.
Another important factor that needs to be addressed when describing fall risk is the cognitive disturbance that is commonly demonstrated in PD. The postural instability/gait disturbance subtype of PD is characterized by greater fall risk as well as greater cognitive impairment and a more rapidly progressive disease course.17 As such, those patients with the postural instability/gait disturbance subtype whom already exhibited greater instability and fall risk because of their disease phenotype may (1) lack the cognitive faculties to recognize their fall risk adequately and (2) might have a more rapidly progressing disease that increases postural instability faster than strategies and environmental modification can be adapted. As such, it is critical that patients and caregivers be made aware of increasing fall risks and understand how these risks can be minimized.
Study limitations
This study does have its limitations. Most notably, the use of self-reported (3mo recall) fall history may have potentially limited the accuracy of fall rates. Future research using in-home tracking software or validation of falls by a caregiver and/or spouse might improve fall reports. Further, unless notified in advance, most patients with PD report to the clinic with their medication effectiveness maximized. Evaluation of TUG performance in the off-medication state, although burdensome, may add to the predictive value of those at increased risk of a fall. At the same time, it may make sense to screen for falls in the typical on state, because this is the condition in which an individual typically ambulates and faces the opportunity to fall during their normative waking hours. While we tried to control for significant fall risk factors, some important mediating factors could not be controlled for in this analysis, including presence of freezing of gait, loss of postural reflexes, cardiovascular disorders, symptomatic orthostatic hypertension, and the interaction(s) of medication type(s) and dosages. Additionally, the results are only relevant to community-dwelling older adults with PD in a clinical setting, and therefore may not be generalizable to the entire population of patients with PD. Also, these patients were all at National Parkinson Foundation Centers of Excellence, and thus may have had greater optimization of treatment than some community-dwelling patients. Lastly, demonstration of how well future falls are predicted for individuals not already in the sample is lacking, and future research should address this shortcoming. However, the strengths of the study can also be noted. The large sample was obtained from 16 participating centers across the U.S. and included a wide variety of ages and disease durations as well as educational levels. As such, the generalizability of the finding is increased.
Conclusions
The limitations in identifying those with PD at risk for a fall are noteworthy. The findings of this study suggest that the TUG test may be an accurate assessment tool to identity those at risk for a fall. In a clinical setting, where time is critical, a quick and readily available evaluation tool, such as the TUG test, is warranted to provide fall risk insight and hopefully limit adverse outcomes. Using a cut point based on the TUG test may serve as a means to implement a fall prevention plan.
Acknowledgments
Supported by the National Parkinson Foundation.
List of abbreviations
- AUC
area under the curve
- BMI
body mass index
- H&Y
Hoehn and Yahr
- NPF-QII
National Parkinson Foundation's Quality Improvement Initiative Registry
- PD
Parkinson's disease
- PDQ-39
Parkinson's Disease Questionnaire-39
- ROC
receiver operating characteristic
- TUG
Timed Up & Go
- UPDRS
Unified Parkinson's Disease Rating Scale
Footnotes
No commercial party having a direct financial interest in the results of the research supporting this article has or will confer a benefit on the authors or on any organization with which the authors are associated.
References
- 1.Hely MA, Reid WG, Adena MA, Halliday GM, Morris JG. The Sydney multicenter study of Parkinson’s disease: the inevitability of dementia at 20 years. Mov Disord. 2008;23:837–844. doi: 10.1002/mds.21956. [DOI] [PubMed] [Google Scholar]
- 2.Bloem BR, Hausdorff JM, Visser JE, Giladi N. Falls and freezing of gait in Parkinson’s disease: a review of two interconnected, episodic phenomena. Mov Disord. 2004;19:871–884. doi: 10.1002/mds.20115. [DOI] [PubMed] [Google Scholar]
- 3.Pickering RM, Grimbergen YA, Rigney U, et al. A meta-analysis of six prospective studies of falling in Parkinson’s disease. Mov Disord. 2007;22:1892–1900. doi: 10.1002/mds.21598. [DOI] [PubMed] [Google Scholar]
- 4.Bloem BR, Beckley DJ, van Hilten BJ, Roos RA. Clinimetrics of postural instability in Parkinson’s disease. J Neurol. 1998;245:669–673. doi: 10.1007/s004150050265. [DOI] [PubMed] [Google Scholar]
- 5.Viccaro LJ, Perera S, Studenski SA. Is timed up and go better than gait speed in predicting health, function, and falls in older adults? J Am Geriatr Soc. 2011;59:887–892. doi: 10.1111/j.1532-5415.2011.03336.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Foreman KB, Addison O, Kim HS, Dibble LE. Testing balance and fall risk in persons with Parkinson disease, an argument for ecologically valid testing. Parkinsonism Relat Disord. 2011;17:166–171. doi: 10.1016/j.parkreldis.2010.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Falvo MJ, Earhart GM. Six-minute walk distance in persons with Parkinson disease: a hierarchical regression model. Arch Phys Med Rehabil. 2009;90:1004–1008. doi: 10.1016/j.apmr.2008.12.018. [DOI] [PubMed] [Google Scholar]
- 8.Huang SL, Hsieh CL, Wu RM, Tai CH, Lin CH, Lu WS. Minimal detectable change of the timed “up & go” test and the dynamic gait index in people with Parkinson disease. Phys Ther. 2011;91:114–121. doi: 10.2522/ptj.20090126. [DOI] [PubMed] [Google Scholar]
- 9.Mak MK, Pang MY. Fear of falling is independently associated with recurrent falls in patients with Parkinson’s disease: a 1-year prospective study. J Neurol. 2009;256:1689–1695. doi: 10.1007/s00415-009-5184-5. [DOI] [PubMed] [Google Scholar]
- 10.Snijders AH, Nonnekes J, Bloem BR. Recent advances in the assessment and treatment of falls in Parkinson’s disease. F1000 Med Rep. 2010;2:76. doi: 10.3410/M2-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yogev-Seligmann G, Hausdorff JM, Giladi N. The role of executive function and attention in gait. Mov Disord. 2008;23:329–342. doi: 10.1002/mds.21720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Graham JW, Olchowski AE, Gilreath TD. How many imputations are really needed? Some practical clarifications of multiple imputation theory. Prev Sci. 2007;8:206–213. doi: 10.1007/s11121-007-0070-9. [DOI] [PubMed] [Google Scholar]
- 13.Schafer JL, Graham JW. Missing data: our view of the state of the art. Psychol Methods. 2002;7:147–177. [PubMed] [Google Scholar]
- 14.O’Brien RM. A caution regarding rules of thumb for variance inflation factors. Qual Quant. 2007;41:673–690. [Google Scholar]
- 15.Wood BH, Bilclough JA, Bowron A, Walker RW. Incidence and prediction of falls in Parkinson’s disease: a prospective multidisciplinary study. J Neurol Neurosurg Psychiatry. 2002;72:721–725. doi: 10.1136/jnnp.72.6.721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sadowski CA, Jones CA, Gordon B, Feeny DH. Knowledge of risk factors for falling reported by patients with Parkinson disease. J Neurosci Nurs. 2007;39:336–341. doi: 10.1097/01376517-200712000-00004. [DOI] [PubMed] [Google Scholar]
- 17.Alves G, Larsen JP, Emre M, Wentzel-Larsen T, Aarsland D. Changes in motor subtype and risk for incident dementia in Parkinson’s disease. Mov Disord. 2006;21:1123–1130. doi: 10.1002/mds.20897. [DOI] [PubMed] [Google Scholar]