Abstract
Converging evidence suggests that addiction can be considered a disease of aberrant learning and memory with impulsive decision-making. In the past decades, numerous studies have demonstrated that drug addiction is involved in multiple memory systems such as classical conditioned drug memory, instrumental learning memory and the habitual learning memory. However, most of these studies have focused on the contributions of non-declarative memory, and declarative memory has largely been neglected in the research of addiction.
Based on a recent finding that hippocampus, as a core functioning region of declarative memory, was proved biased the decision-making process based on past experiences by spreading associated reward values throughout memory. Our present study focused on the hippocampus. By utilizing seed-based network analysis on the resting-state functional MRI datasets with the seed hippocampus we tested how the intrinsic hippocampal memory network altered towards drug addiction, and examined how the functional connectivity strength within the altered hippocampal network correlated with behavioral index ‘impulsivity’.
Our results demonstrated that HD group showed enhanced coherence between hippocampus which represents declarative memory system and non-declarative rewardguided learning memory system, and also showed attenuated intrinsic functional link between hippocampus and top-down control system, compared to the CN group. This alteration was furthered found to have behavioral significance over the behavioral index ‘impulsivity’ measured with Barratt Impulsiveness Scale (BIS). These results provide insights into the mechanism of declarative memory underlying the impulsive behavior in drug addiction.
Keywords: drug addiction, hippocampus, declarative memory, functional connectivity, impulsivity, decision-making
1. Introduction
One of the defining clinical characteristics of drug dependence is uncontrolled drug use in the face of adverse consequences, making the “loss of impulsive control” the hallmark of drug addiction [1–5]. Individuals with addiction are unable to base their decision-making on the long-term outcome of their choices and exhibit uncontrolled impulsive behavior [6, 7]. Converging evidence from cellular, behavioral and pharmacological studies suggests that the abnormality in decision-making of addicted subjects can be considered a disease of aberrant learning and memory [8–11]. In the past decades, studies from animal models to human subjects have demonstrated that drug addiction is involved in multiple memory systems. For example, the mechanisms involved in classical Pavlovian conditioned drug memory and instrumental conditioned incentive learning memory, mediated in part by nucleus accumbens (NAcc), amygdala, and the orbitofrontal cortex (OFC) could be responsible for the drug reinforcement and motivational drive, resulting in executive control failure [5, 6, 12–15]. The procedural (habitual) learning disorder, mediated in part by the caudate and the putamen, represents a transition at the neural level from prefrontal cortical to striatal control over drug seeking and drug-taking behavior [16]. Recent resting-state functional studies supported these findings and illustrated abnormalities in these abovementioned brain regions involved in multiple memory systems in addiction [17–20].
Despite having established a significant role for learning and memory in addiction, most of these studies have focused on the contributions of non-declarative memory. Nevertheless, memory is not a unitary process but rather consists of multiple processes that engage distinct neural substrates. For drug memory, a major dissociation is between declarative drug memory and non-declarative drug memory [21– 23]. Distinguished from our understanding of mechanisms of non-declarative memory such as conditioned incentive learning memory and habitual learning memory, mechanisms of declarative memories have largely been neglected in research of addiction [22, 23]. Declarative memory consists of knowledge of facts, events and biographical memory of the personal experiences that can be explicitly stated and relies crucially on the integrity of hippocampus and surrounding medial temporal lobes (MLT) [22–27]. In addiction for example, declarative drug memory includes places, people or paraphernalia associated with past drug-using experiences [28].
Understanding the contributions of the declarative memory encoded by the hippocampus may present new direction in decision-making research and highlight how humans flexibly use previous memorized information to guide future choices in novel context, especially those whose decision-making is adversely affected by disease such as addiction [22]. Recently, it was reported that as a core functioning region of declarative memory, hippocampus was activated to dynamically modulate value representations, spreading reward values across associated item pairs, thereby creating biased decisions based on past experience [29]. With this new mechanism in decision-making, the present study focuses on the hippocampus [22, 23]. We tested how the intrinsic hippocampal memory network altered towards drug addiction, and examined how the functional connectivity strength within the altered hippocampal network correlated with behavioral index ‘impulsivity’ measured with Barratt Impulsiveness Scale (BIS) in abstinent heroin-dependent subjects.
2. Material and Methods
2.1. Participants
Thirty abstinent heroin-dependent (HD) subjects were recruited from Beijing Ankang Hospital (Beijing, China), and 20 healthy control nondrug users (CN) also participated in this study. Both groups of participants were comprised of right-handed males with normal intelligence and were well matched for age and years of education. Detailed inclusion and exclusion criteria for heroin abusers and control subjects were described in our previously publications [17, 30, 31]. Briefly, the HD subjects met DSM-IV criteria for heroin-dependence, with more than two years of heroin use, and were abstinent for at least four weeks. They were also tested negative for morphine in the urinalysis and negative for human HIV in blood test. None of the subjects had a history of neurological and psychiatric diseases, seizures, head injury or abnormalities demonstrated by an anatomical MRI scan. The inclusion and exclusion were assessed by two experienced psychiatric physicians in accordance with the Structured Clinical Interview for DSM-IV (SCID) (for demographic information see Table 1). The study was approved by the Research Ethics Committee of Beijing Ankang Hospital and the Beijing Institute of Basic Medical Science and conducted in accordance with the Declaration of Helsinki. Written informed consent was obtained from all individual subjects prior to the study. Eight HD and five CN subjects were excluded from this study due to motion artifact (i.e., translational movement exceeded 1mm or more than 1° rotational movement), thus leaving 22 and 15 subjects in the HD and CN groups for further analysis, respectively.
Table 1.
Demographic information and the behavioral measurement.
| HD (n=22) | CN (n=15) |
p value |
|||
|---|---|---|---|---|---|
| Mean | SD | Mean | SD | ||
| Age (years) | 33.05 | 6.04 | 28.87 | 8.12 | 0.10 |
| Education (years) | 10.86 | 2.40 | 9.60 | 2.16 | 0.10 |
| Duration of heroin use (years) | 6.59 | 3.72 | N/A | N/A | N/A |
| Dosage of heroin use (g/day) | 0.96 | 1.26 | N/A | N/A | N/A |
| Abstinent period (days) | 139 | 17.69 | N/A | N/A | N/A |
| BIS total score | 66.45 | 10.07 | 59.33 | 6.51 | 0.01 |
| Gray matter volume (mm3) | 600.7 | 57.94 | 668.28 | 89.04 | 0.02 |
Abbreviations: HD, heroin dependent subjects; CN, control non-drug users; SD, standard deviation; BIS, Barat impulsive scale.
2.2. Behavioral measurement
We used impulsivity as behavioral marker of heroin addiction. Impulsivity was assessed by using the Barratt Impulsiveness Scale ver.11 (BIS-11, Chinese revised edition) [32], which constitutes 30 4-point Likert-type items. Higher scores signify higher impulsivity, and vice versa. All participants completed the questionnaire.
2.3. MRI acquisition
MR-Images were acquired by using a 3T Signa GE scanner with a standard quadrature transmit-receive head coil. The whole-brain resting-state fMRI data was acquired with a single-shot gradient-recalled echo planar imaging (EPI) sequence, and the scanning parameters were as follows: TE/TR=25/2000ms, flip angle=90°, slice=20, slice thickness=5mm (with an additional 1-mm gap space), matrix size=64 × 64, FOV=24cm × 24cm. The total 180 time points of images were collected. During the 6- minute resting scans, all the subjects were instructed to rest, keep their heads still, eyes closed and not to fall asleep. We also obtained high resolution anatomical images of each individual using three dimensional T1-weighted spoiled gradient-recalled echo (SPGR) scans with the following scanning parameters: TE/TR=4.8/10.4ms, flip angle=15°, slice=140, slice thickness=1mm, matrix size=256 × 256, FOV=24cm × 24cm. Besides, we also recorded cardiac activity and respiratory activity of each subject for further use in physiological motion correction for fMRI signals.
2.4. Image preprocessing
All imaging data preprocessing and functional synchrony analyses were conducted using the Analysis of Functional NeuroImages software (AFNI, http://afni.nimh.nih.gov/afni/), Statistical Parametric Mapping (SPM8, http://www.fil.ion.ucl.ac.uk/spm/software/spm8/) on Matlab platform. For imaging preprocessing, the first five volumes of the datasets were discarded due to the T1 nonequilibrium effect. This was followed by slice timing correction and volume registration (3dTshift, 3dvolreg, AFNI), linear de-trend (3dDetrend, AFNI) and physiological motion correction (respiratory and heart rate, 3dretroicor, 3dDeconvolve, AFNI). Possible contamination from noise including signal from white matter, cerebral spinal fluid (CSF), global signal, and six-way motion vectors (as well as their 1st order derivatives) were regressed out (3dDeconvolve, AFNI). A band-pass filter was then applied to keep the low-frequency fluctuations between 0.015Hz and 0.1 Hz (3dFourier, AFNI) [33, 34].
2.5. Structural image analysis
Optimized Voxel-Based Morphometric (o-VBM) analysis was conducted using SPM8 (http://www.fil.ion.ucl.ac.uk/spm/software/spm8) [35] to identify the gray matter (GM) volume of each subject in the two groups. The individual T1-weighted images for all subjects were first segmented into three parts: GM, white matter (WM) and cerebral spinal fluid (CSF). The segmented GM was then normalized into MNI space. In the mean time, the anatomical images were also normalized into MNI space using the deformation field generated by the normalization of GM. The normalized anatomical images were then segmented for the second time into GM, WM and CSF. The o-VBM procedure was finished and the current segmented (2nd time) GM (modulated images) could be extracted using a cluster detection method to determine the GM volume for each individual (3dclust, AFNI, cluster detection size set as voxel size = 2mm×2mm×2mm). The GM volume of each individual underwent 2-sample t-test to see if they have significant group difference in GM volume. If yes, the GM volume of each individual would be further used as controlled covariate of no interest to control the GM atrophy effects when conducting the ANCOVA test, as described below.
2.6. Intrinsic hippocampus functional network analysis with predefined seed regions
Based on the recently demonstrated new role of the hippocampus in value-based decision-making through dynamic modulating value representations in reward associated declarative memory, seed regions of interest (ROIs) from the bilateral hippocampus (spherical ROI centered at [26, -34, -12] in MNI space or [26, -33, -8] in Talairach space, with 4-mm radius, along with its opposite side: [-26, -34, -12] in MNI space or [-26, -33, -8] in Talairach space) were selected [29]. These predefined generic seed ROIs were further resampled and transformed into the original space of each individual in reference with their anatomical images (3dfractionize, AFNI). Only voxels in EPI functional images that occupied more than 70% of the masked anatomical images in the resampled ROI masks were included in the voxel-wise analysis [31]. For each individual, the averaged time courses within the seed ROIs were then extracted from the preprocessed EPI functional images and correlated with the time courses of all voxels within the whole brain by using Pearson Cross-Correlation Coefficient (CC). Next, the connectivity maps (CC map) were spatially normalized to the Talairach space and resampled to 2-mm isotropic voxels. After applying the Fisher r-to-z transformation [z = 0.5 × log(1+r)/(1-r)] and the smoothing procedure with a Gaussian kernel (4-mm FWHM), the intrinsic hippocampus functional network map (z-map, hereafter referred to as ‘m-map’ or ‘m-value’ to avoid confusion between ‘Fisher z’ and the normalized statistical ‘z-value’ ) for each individual was generated and ready for our next step group comparison.
2.7. Statistical analysis
To identify the significant patterns of group-level intrinsic hippocampus functional network for each of the two groups (results shown in Fig. 1), we conducted a voxel-wise one-sample t test with the m-map of each individual (3dttest++, AFNI). The results were further corrected for multiple comparisons (3dClustSim, AFNI, α=0.05, p<0.05, cluster size > 2630mm3).
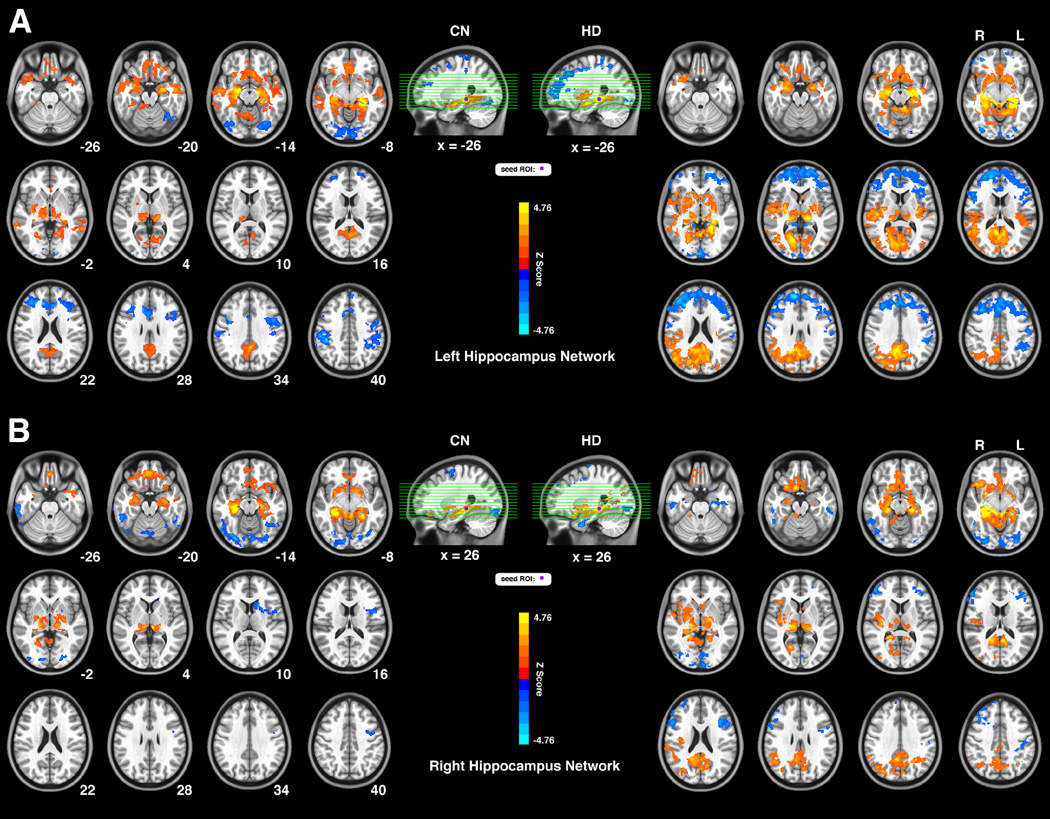
Fig. 1.
Connectivity patterns of the intrinsic hippocampus functional network for two groups both seeded in the left (Fig. 1A) and right (Fig. 1B) hippocampus. The bright color marked brain regions with positive functional connectivity and the blue color show anticorrelated connectivity. The left part of the Fig. 1A and Fig. 1B indicates connectivity patterns for the CN group, and the right part for the HD group. The letters ‘R’ and ‘L’ on top of the axial images show the side of the hemisphere; the numbers below the axial images indicate the slice position at the z-coordinate. This group-level pattern is obtained via a voxel-wise 1-sample t-test and significance level was set at threshold p < 0.05. The results were corrected for multiple comparisons (3dClustSim, AFNI, α=0.05, p<0.05, cluster size > 2630mm3).
Abbreviations: CN, control non-drug users; HD, heroin dependent subjects.
To find group differences of the intrinsic hippocampus functional network (results shown in Fig. 2 and Table 2), a voxel-wise Analysis of Covariance (ANCOVA) was employed based on the m-map across all subjects (3dRegAna, AFNI). We combined the left- and the right- side of the m-maps together benefitted from their spatial symmetry in pattern with a covariate labeling the ‘side’ and regressed out the ‘side’ covariate to obtain a generalized, non-lateralized intrinsic hippocampus connectivity group difference map. The factors such as age and years of education were also regressed out from the main effects of group as covariates of no interest, and the GM volume was regressed out as well to reduce the effects of grey matter atrophy to functional connectivity difference. The voxel-wise results of group difference were corrected for multiple comparisons (3dClustSim, AFNI, α=0.05, p<0.05, cluster size > 2630 mm3).
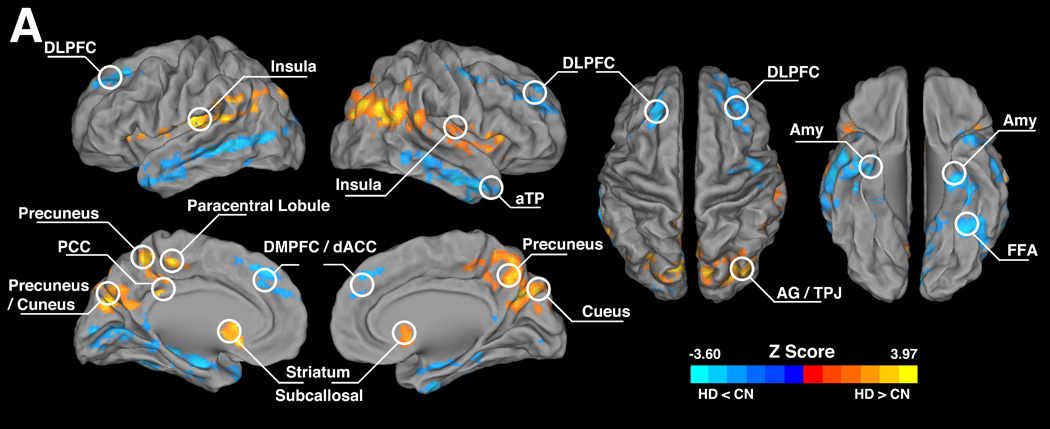
Fig. 2.
Illustration of the group-level difference of the intrinsic hippocampus functional network between the HD and the CN groups. The bright color illustrates brain regions with higher connectivity strength in the HD group while the blue color indicates lower connectivity strength, compared to the CN group. Significance level of this group-level difference was set at threshold p < 0.05, and p value of each voxel was obtained via a voxel-wise analysis of covariance (ANCOVA) with age, years of education and grey matter volume of each individual as covariance of non-interests. The results were corrected for multiple comparisons (3dClustSim, AFNI, α=0.05, p<0.05, cluster size > 2630mm3).
Abbreviations: DLPFC, dorsal lateral prefrontal cortex; Amy, amygdala; aTP, anterior temporal pole; PCC, posterior cingulate cortex; FFA, fusiform face area; DMPFC, dorsal medial prefrontal cortex; AG, angular gyrus; TPJ, temporal-parietal junction; dACC, dorsal anterior cingulate cortex; CN, control non-drug users; HD, heroin dependent subjects.
Table 2.
Group-level difference of the intrinsic hippocampus functional network between the HD and CN groups.
| Brain Region | Side | BA | Cluster Size (mm3) |
TLRC Coordinates (LPI) |
Z Score |
||
|---|---|---|---|---|---|---|---|
| x | y | z | |||||
| Insula | R/L | 13 | 90344 | 42 | −7 | 13 | 4.08 |
| Putamen | L/R | −24 | 10 | 6 | 3.59 | ||
| Caudate | R/L | 17 | 25 | 1 | 3.83 | ||
| SCG/sACC | L/R | 25 | −2 | 17 | −5 | 3.37 | |
| PCC | R/L | 23/31 | 1 | −35 | 35 | 3.17 | |
| Paracentral Lobule | L/R | 31/5 | −4 | −25 | 47 | 3.42 | |
| AG/TPJ | R/L | 39 | 35 | −69 | 28 | 4.81 | |
| Precuneus | R/L | 7 | 8 | −54 | 38 | 3.81 | |
| Cuneus | R/L | 18 | 9 | −76 | 19 | 3.54 | |
| FFA | L | 37 | 22296 | −31 | −47 | −14 | −4.06 |
| MTG | L | 22 | −54 | −47 | −1 | −3.98 | |
| Amygdala | L | −27 | −8 | −19 | −3.54 | ||
| aTP | L | 38 | −49 | 13 | −17 | −2.49 | |
| MTG | R | 21 | 16672 | 65 | −47 | −10 | −4.39 |
| Amygdala | R | 29 | −4 | −19 | −2.62 | ||
| aTP | R | 38 | 45 | 18 | −29 | −2.98 | |
| Parahippocampal | R | 32 | −6 | −30 | −4.07 | ||
| SFG | R | 16328 | 29 | 57 | 22 | −3.80 | |
| DLPFC | L/R | 9 | −27 | 43 | 38 | −3.45 | |
| DMPFC | L/R | 6/9 | −3 | 39 | 39 | −3.56 | |
| dACC | L/R | 32 | −4 | 35 | 29 | −2.91 | |
| Lingual Gyrus | L | 18 | 6240 | −7 | −79 | −5 | −3.54 |
| MOG | L | 2824 | −25 | −93 | 18 | −3.75 | |
Brain regions with positive z scores indicate significantly increased connectivity in the HD group compared to the CN group, while those with negative z scores indicate significantly decreased connectivity.
Abbreviations: BA, Brodmann area; SCG, subcallosal gyrus; sACC, subgenual dorsal anterior cingulate cortex; PCC, posterior cingulate cortex; AG, angular gyrus; TPJ, temporal-parietal junction; FFA, fusiform face area; MTG, middle temporal gyrus; aTP, anterior temporal pole; SFG, superior frontal gyrus; DLPFC, dorsal lateral prefrontal cortex; DMPFC, dorsal medial frontal gyrus; dACC, dorsal anterior cingulate cortex; MOG, middle occipital gyrus.
Further, in order to detect brain regions with behavioral significance of decision-making tendency regarding the behavioral index ‘impulsivity’ (results shown in Fig. 3), we conducted a whole-brain voxel-wise linear regression analysis (3dRegAna, AFNI) between the m-maps and the measured BIS total scores of all subjects within each of the two groups. This analysis was performed separately for the HD and the CN group. Similar to the procedure of group difference analysis, the factors such as ‘side’, age, years of education and GM volume were controlled as covariates of no interest. The behavioral significance maps of the two groups were also corrected for multiple comparisons (3dClustSim, AFNI, α=0.05, p<0.05, cluster size > 2630 mm3).
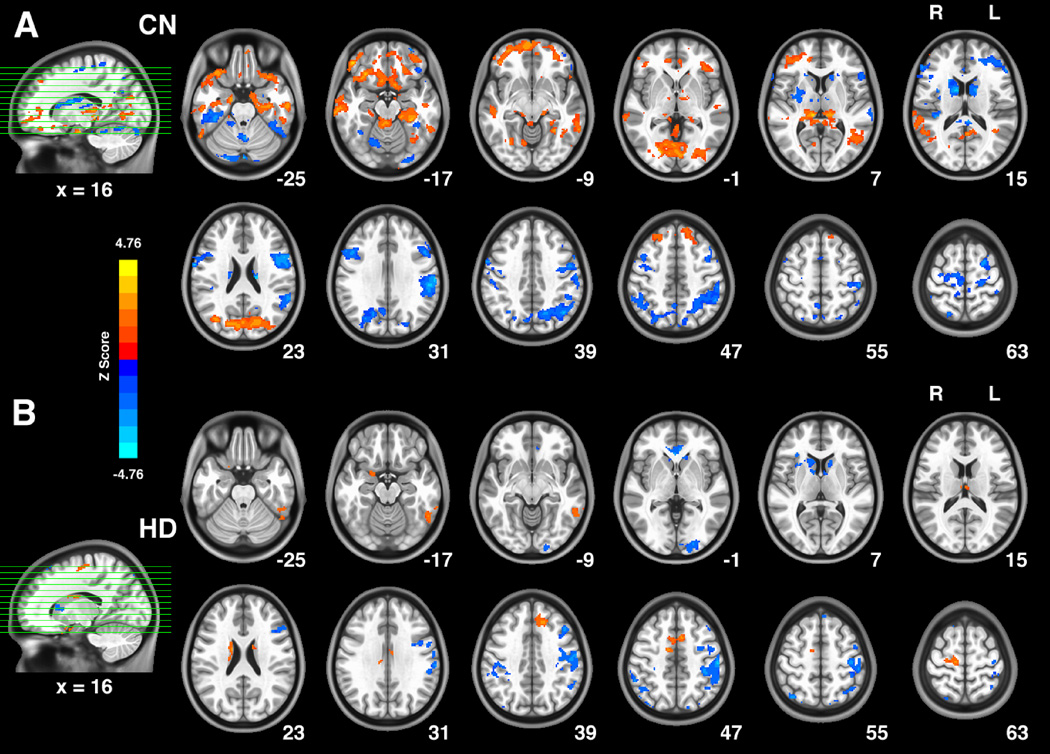
Fig. 3.
The voxel-wise correlation between the intrinsic hippocampus functional network strength (m-value) and the BIS Total score in the HD group (A) and the CN group (B). Bright color in these patterns indicates positive correlation between BIS total score and the connectivity strength, while blue color indicates negative correlation. The age, years of education and gray matter volume of each individual were controlled as covariates of no interests. The significance level of these two distinct behavioral correlation patterns were set at threshold p < 0.05, and the results were corrected for multiple comparisons (3dClustSim, AFNI, α=0.05, p<0.05, cluster size > 2630mm3).
Abbreviations: CN, control non-drug users; HD, heroin dependent subjects.
Finally, through conjunction analyses between the impulsivity behavioral correlation patterns of CN and HD group, we assessed those overlapped regions of the two groups that exhibited behavioral (impulsivity) significance. The regions based on conjunction analysis results are used as ROIs to identify the linear correlation relationship between the averaged m-values within each of the ROIs and the BIS total scores for the CN and the HD groups respectively.
3. Results
3.1. Demographic and psychometric data of the behavioral measurement
The demographic and Clinical Evaluation information in Table 1 showed no significant difference in age and years of education between the HD and the CN group. The BIS total score of the HD group was significantly higher than that of the CN group (p<0.05).
3.2. oVBM results of the HD and CN groups
Our oVBM analysis revealed that the gray matter volume of subjects in HD group was significantly lower than that of the CN group (p<0.05), as shown in Table 1. The GM volume obtained for each individual was further utilized as controlled covariate in the following ANCOVA test to control the effect of GM atrophy.
3.3. Connectivity patterns of the intrinsic hippocampus functional network for the CN and HD groups
As shown in Fig. 1A (left panel), for the left hippocampus network, in the CN group, the positively correlated regions included the bilateral hippocampus, parahippocampal gyrus, amygdala, middle temporal gyrus (MTG, BA21), anterior temporal pole (aTP, BA38), subcallosal gyrus (BA25), orbital frontal cortex (OFC, BA11), thalamus, posterior cingulate/precuneus (PCC, BA23/31), cuneus (BA23) and lentiform nucleus/putamen, right insula. The anticorrelated regions included the bilateral fusiform gyrus (BA37), lingual gyrus, dorsal anterior cingulate cortex (dACC, BA24/32), dorsal medial prefrontal cortex (DMPFC, BA8/6), dorsal lateral prefrontal cortex (DLPFC, BA9/46), postcentral gyrus (BA2/3/4), inferior parietal lobule (IPL, BA40) and left precentral gyrus (BA6). In the HD group as shown in Fig. 1A (right panel), the positively correlated regions were mainly located in the regions of the temporal-parietal cortices and subcortical regions, including the bilateral hippocampus, precuneus (BA7), PCC (BA23/31), cuneus (17/18/19), orbital frontal cortex (OFC, BA11), amygdala, parahippocampal gyrus, subcallosal gyrus (BA25), thalamus, lentiform nucleus/putamen, caudate, nucleus accumbens (NAcc), insula (BA13), superior temporal gyrus (STG, BA41) and right AG/TPJ (angular gyrus/temporal-parietal junction, BA/39). The anticorrelated regions were mainly located in the frontal system including the bilateral DLPFC (BA9/46), DMPFC (BA9/6), inferior frontal gyrus (IFG, BA44/45), dACC (BA24/32) and the right fusiform gyrus (BA37). Similar patterns of the right hippocampus network for both the CN and the HD groups are illustrated in Fig. 1B.
3.4. Group-level difference of the intrinsic hippocampus functional network between the HD and the CN groups
Several brain regions within the intrinsic hippocampus functional network showed significant differences between the HD group and the CN group via ANCOVA procedure. Among these regions, increased functional connectivity could be seen mainly in the limbic areas and some of the cortical regions, including bilateral insula (BA13), putamen, caudate, subcallosal gyrus/subgenual ACC (SCG/sACC, BA25), PCC (BA23/31), paracentral lobule (BA31/5), precuneus (BA7), cuneus (BA18), and TPJ, while the decreased functional connectivity were largely distributed in the cortices and some subcortical areas, including the bilateral DLPFC (BA9/46), DMPFC (BA6/9), right anterior temporal pole (aTP, BA38), dorsal anterior cingulate cortex (dACC, BA32) left MTG (BA22), amygdala, aTP and fusiform face area (FFA, BA37). These results are illustrated in Fig. 2 and Table 2.
3.5. Neural correlation between the intrinsic hippocampus functional network strength (m-value) and the behavioral index ‘impulsivity’ in the HD and the CN groups
The voxel-wise multivariate regression analysis identified distinct behavioral correlation patterns of the hippocampus network for the two groups. In the CN group (Fig. 3A), the positive correlation pattern mainly consisted of the regions including the bilateral IFG (BA47), ACC (BA32), MTG (BA21), parahippocampal gyrus, ITG, cuneus/lingual gyrus (BA18), ventral lateral thalamus and aTP (BA38), left DMPFC (BA6/8), right AG/TPJ (BA39). The negatively correlated regions of the CN group include the bilateral FFA (BA37), dorsal thalamus, dorsal caudate body, postcentral gyrus (BA3), precentral gyrus (BA4), paracentral lobule (BA6), precuneus (BA7), IPL (BA40), and the right lentiform/putamen. In the HD group (Fig. 3B), positively correlated areas included the bilateral dorsal medial thalamus, MCC (BA24), right dorsal caudate body, left FFA (BA37) and MFG (BA6/8), while the negatively correlated regions of the HD group included the bilateral IPL (BA40), ACC (BA24/32), left DLPFC (BA9), and the right insula/IFG (BA47).
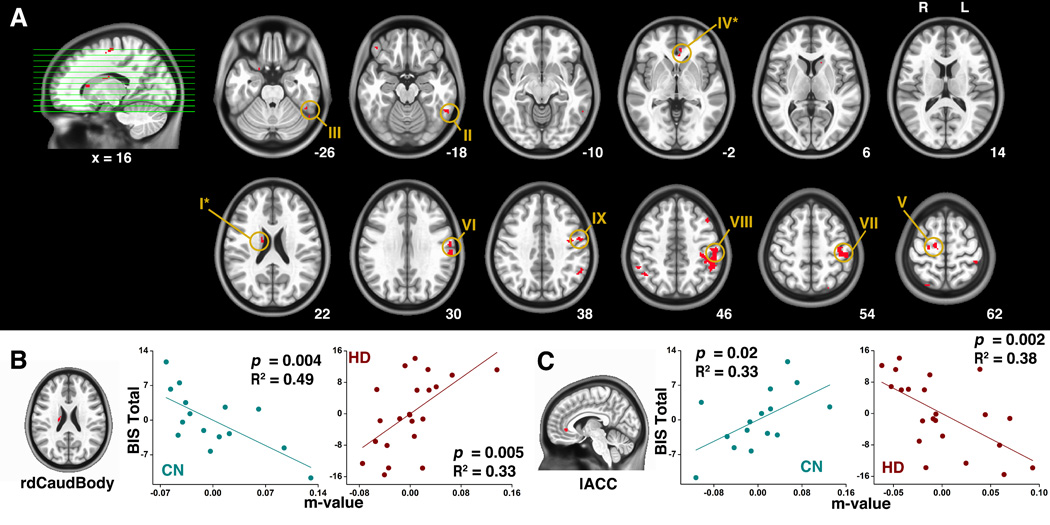
The conjunction analysis identified the overlapped regions between Fig. 3A and Fig. 3B, as shown in Fig. 4A. These regions included the bilateral dorsal thalamus, left ITG (BA20/37), ACC (BA24/32), postcentral gyrus, precentral gyrus, right dorsal caudate body, MFG (BA6), IPL and IFG. From the multivariate regression analysis, as shown by Fig. 4B and Fig. 4C, the CN and the HD group exhibited contrary correlation pattern in the following regions: the right caudate body and the left ACC. For the right caudate body (Fig. 4B), in the CN group, the m-value was negatively correlated with the BIS total score, indicating the stronger connectivity with hippocampus of right caudate body, the less evident the impulsivity. However, in the HD group, such a relationship was the opposite. The stronger the m-value, the severer the impulsivity, the impulsivity in HD group is positively correlated with the connectivity strength of the right caudate body to hippocampus. For the left ACC (Fig. 4C), in the CN group, the m-value was positively correlated with the BIS total score, indicating the stronger the impulsivity, the stronger the connectivity strength with the hippocampus. In the HD group, however, it was the opposite, suggesting impaired controllability.
Fig. 4.
(A) Results from the conjunction analysis between Fig. 3A and Fig. 3B. (B) The significant correlations between the connectivity strength of hippocampus - right dorsal caudate body and the BIS total score in CN subjects (the left panel) and in the HD subjects (the right panel). (C) The significant correlations between the connectivity strength of hippocampus - left ACC and the BIS total score in CN subjects (left panel) and in the HD subjects (right panel).
Abbreviations: rdCaudBody, right dorsal caudate body; lACC, left anterior cingulate cortex.
4. Discussion
Understanding how hippocampus is intrinsically connected to the other part of the brain provides insight into the mechanisms of declarative memory in drug-taking and drug-seeking behaviors in addiction.
Our current study demonstrated a new set of results that in both of the two groups, hippocampus was functionally connected to NAcc, OFC, caudate and putamen. The HD subjects showed stronger functional connectivity between hippocampus and multiple cortical and subcortical regions including putamen, caudate, PCC, precuneus, insula and temporal-parietal junction and relatively weaker connectivity in regions of amygdala and cortical areas including anterior temporal pole, DLPFC, ACC and DMPFC, compared to the CN subjects (Fig. 2 and Table 2). Further from the results of the multivariate regression analysis, we identified distinct behavior correlation of impulsivity in each of the two groups (Fig. 3). We also identified overlapped regions between the impulsivity correlation patterns of the two groups by using conjunction analysis (Fig. 4A). Our main finding of behavioral correlation analysis is that the CN group and HD group revealed contrary impulsivity correlation in the regions of the left ACC and right dorsal caudate body within the hippocampus memory network (Fig. 4B and Fig. 4C). For the right dorsal caudate body, the connectivity strength was positively correlated with impulsivity in HD subjects, whereas in CN subjects, such a correlation was negative. For the left ACC, the connectivity strength was negatively correlated with impulsivity in HD subjects, whereas in CN subjects, such a relationship was positive correlation.
These results corroborate earlier findings and extend them in three ways [11, 36]. First, our findings demonstrate that declarative memory system and non-declarative memory (reward-related learning) system are intrinsically connected. In both the HD and the CN groups, as central node of declarative memory, hippocampus was intrinsically connected to NAcc, OFC, caudate and putamen. NAcc and OFC have been demonstrated to be involved in reward-related learning such as classical and instrumental conditioned-incentive learning and memory, habit/procedure learning and instrumental learning [6, 12–16, 37–39]. The intrinsic connectivity between hippocampus and these regions involved in the reward-related learning suggests that multiple memory systems are functionally associated.
Second, our findings have implications that the declarative drug memory system and non-declarative drug memory (reward-related learning) system have enhanced association in heroin addiction. In our study, the caudate and putamen showed stronger connectivity with hippocampus in the HD group. These regions are not only involved in non-declarative learning memory [22, 23], but also are major parts of the ventral neural network or the 'β-network' in neuroeconomics’ term which mediates immediate rewards [40, 41]. According to the neuroeconomics' theory, reward-related valuation is the central part in the process of making decisions when facing alternative options [42–44]. The stronger coupling between these two memory systems could bias immediate and impulsive behaviors in HD subjects [45]. The present study further supports this notion by demonstrating stronger intrinsic functional coupling between the hippocampus and the dorsal striatum, indicating stronger declarative - non-declarative memory coherence in the HD subjects. Furthermore, this stronger connectivity between the hippocampus and caudate in HD subjects has behavioral significance. The stronger the connectivity, the severer the impulsivity. The nature of this positive correlation provides a biological basis as to why drug-associated declarative memory could trigger impulsive drug use.
Third, our findings not only indicate that the declarative drug memory system is more closely associated with non-declarative drug memory (reward related learning) system in the HD group, as described above, but also has attenuated intrinsic link with the top-down executive control system. In our study, the dACC, DLPFC and DMPFC showed relative weaker connectivity with hippocampus in the HD group. The DLPFC plays an important role in high order executive control such as self-impulse control by integrating sensory and mnemonic information [46, 47]. Various studies have identified that the dACC and DMPFC are crucially functioning in error detection and conflict monitoring for impulsive inhibitory control [48, 49]. Attenuated activity in ACC and decreased connectivity between hippocampus and DMPFC were also detected in subjects with cocaine addiction, nicotine addiction, heroin/opiate addiction, and even subjects with acute heroin administration [30, 50–54]. These regions with decreased hippocampus connectivity are also the major regions of the 'δ network' in neuroeconomics theory and are considered to carry out the executive control function by taking the long-term value into account when facing alternatives in decision-making [40, 41, 45, 55]. Furthermore, the connectivity between the hippocampus and ACC also has behavioral significance. For the CN group, the stronger the connectivity, the higher the impulsivity, indicating strengthened functional coupling between hippocampus and ACC for stronger controllability in individuals with higher impulsive level in CN group. However in HD group, this behavioral correlation was reversed. The weaker the connectivity, the severer the impulsivity, indicating their relativity weaker controllability for impulsive behaviors.
In summary, the strengthened connectivity between the hippocampus and the non-declarative drug memory (reward-related learning) regions could be neural mechanisms underlying the pathologically enhancement of motivational drive toward impulsive drug-related behaviors at a system level in the HD group. While the attenuated connectivity between the hippocampus and the executive control related regions therefore could be neural mechanisms underlying the impaired impulsive control ability over drug-related behaviors at a system level in the HD group. In heroin addiction, these two parts reciprocally form an imbalance between the impetus and the resistance towards drug-related behavior. Based on these discussions, we could further infer that the decision-making processes and the impulsive behaviors are dependent not only on the executive control system for top-down control [3], but also on the non-declarative reward-related learning memory system for bottom-up drive. It is dependent on the balance of the two systems, instead of on one system alone. Such an inference may advance the debate among the single model, dual valuation model and self-control model during the conflict decision-making processes [40, 41, 46, 56].
Digging into the intrinsic connectivity of hippocampus could provide scientific evidence for brain mechanisms of declarative memory, thus shedding light on the process of developing new strategy of treating addiction. New candidate treatment strategy should focus not only on non-declarative drug memory extinction and disruption of declarative drug memory reconsolidation process, but also on shearing the interaction between these two memory systems and reestablish the association between the memory system and the executive control system.
Several limitations of our study should be considered. First, we revealed functional reorganization in the hippocampus declarative memory network in abstinent heroin addiction subjects. However it is not clear whether it is the alteration in functional connectivity lead to addiction or it is the long-term exposure to addictive drugs lead to the alteration in functional connectivity. Future studies could be conducted to probe into this question by exploring the genetic factor in addiction, such as sibling study. Second, this is a cross-sectional study without treatment. Future studies entailing longitudinal studies with detoxification treatment are needed to evaluate the alterations in brain function under treatment and might provide new insight into the treatment strategy of drug addiction.
Highlights.
We revealed brain mechanism of how declarative memory affected addicted brain.
We based memory network on new role of hippocampus in value-based decision-making.
We utilized R-fMRI combined with temporal binding model using the seed hippocampus.
Non-declarative learning and executive control regions are imbalanced link with seed.
Dorsal caudate body and ACC have reversed impulsivity correlation between two groups.
Acknowledgments
The authors would like to thank Ms. Carrie M. O’Connor, MA, Ms. Xue-Yan Zhang, MA for editorial assistance, and Mr. B. Douglas Ward, MS, for supervision in statistical analysis. We also would like to thank the reviewer and our editor of Behavioural Brain Research for their helpful comments and suggestions. This study was supported by Chinese Ministry of Science and Technology grants: 2003CB51540 (ZY), and National Institute of Health grants on Drug Abuse: DA10214 (SJL).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure
All authors have made substantial intellectual and physical contributions to this paper in one or more of the following areas: design or conceptualization of the study, data acquisition, data analysis and interpretation, drafting and revising the manuscript. All authors have given final approval of this manuscript. None of the authors of this paper have reported any possible conflict of interest, financial or otherwise, related directly or indirectly to this work.
References
- 1.Koob GF, Volkow ND. Neurocircuitry of Addiction. Neuropsychopharmacology Reviews. 2010;35:217–238. doi: 10.1038/npp.2009.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sutherland MT, McHugh MJ, Pariyadath V, Stein EA. Resting state functional connectivity in addiction: Lessons learned and a road ahead. Neuroimage. 2012;62:2281–2295. doi: 10.1016/j.neuroimage.2012.01.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dalley JW, Everitt BJ, Robbins TW. Impulsivity, compulsivity, and top-down cognitive control. Neuron. 2011;69:680–694. doi: 10.1016/j.neuron.2011.01.020. [DOI] [PubMed] [Google Scholar]
- 4.Volkow ND, Li T-K. Science and Society: Drug addiction: the neurobiology of behaviour gone awry. Nat Rev Neurosci. 2004;5:963–970. doi: 10.1038/nrn1539. [DOI] [PubMed] [Google Scholar]
- 5.Yang Z, Xie J, Shao Y-C, Xie C-M, Fu L-P, Li D-J, et al. Dynamic neural responses to cue-reactivity paradigms in heroin-dependent users: an fMRI study. Hum Brain Mapp. 2009;30:766–775. doi: 10.1002/hbm.20542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bechara A. Decision making, impulse control and loss of willpower to resist drugs: a neurocognitive perspective. Nat Neurosci. 2005;8:1458–1463. doi: 10.1038/nn1584. [DOI] [PubMed] [Google Scholar]
- 7.Noël X, Van Der Linden M, Bechara A. The Neurocognitive Mechanisms of Decision-making, Impulse Control, and Loss of Willpower to Resist Drugs. Psychiatry (Edgmont) 2006;3:30–41. [PMC free article] [PubMed] [Google Scholar]
- 8.Hyman SE. Addiction: A Disease of Learning and Memory. Focus. 2007;5:220–228. [Google Scholar]
- 9.Robbins TW, Ersche KD, Everitt BJ. Drug Addiction and the Memory Systems of the Brain. Ann N Y Acad Sci. 2008;1141:1–21. doi: 10.1196/annals.1441.020. [DOI] [PubMed] [Google Scholar]
- 10.Volkow ND, Wang G-J, Fowler JS, Tomasi D. Addiction circuitry in the human brain. Annu Rev Pharmacol Toxicol. 2012;52:321–336. doi: 10.1146/annurev-pharmtox-010611-134625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Torregrossa MM, Corlett PR, Taylor JR. Aberrant learning and memory in addiction. Neurobiol Learn Mem. 2011;96:609–623. doi: 10.1016/j.nlm.2011.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Drummond DC. Theories of drug craving, ancient and modern. J Clin Psychol. 2001;96:33–46. doi: 10.1046/j.1360-0443.2001.961333.x. [DOI] [PubMed] [Google Scholar]
- 13.Grant S, London ED, Newlin DB, Villemagne VL, Liu X, Contoreggi C, et al. Activation of memory circuits during cue-elicited cocaine craving. Proc Natl Acad Sci USA. 1996;93:12040–12045. doi: 10.1073/pnas.93.21.12040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schultz W, Dayan P, Montague PR. A Neural Substrate of Prediction and Reward. Science. 1997;275:1593–1599. doi: 10.1126/science.275.5306.1593. [DOI] [PubMed] [Google Scholar]
- 15.Volkow N, Li T-K. The neuroscience of addiction. Nat Neurosci. 2005;8:1429–1430. doi: 10.1038/nn1105-1429. [DOI] [PubMed] [Google Scholar]
- 16.Everitt BJ, Robbins TW. Neural systems of reinforcement for drug addiction: from actions to habits to compulsion. Nat Neurosci. 2005;8:1481–1489. doi: 10.1038/nn1579. [DOI] [PubMed] [Google Scholar]
- 17.Xie C, Shao Y, Ma L, Zhai T, Ye E, Fu L, et al. Imbalanced functional link between valuation networks in abstinent heroin-dependent subjects. Mol Psychiatry. 2014;19:10–12. doi: 10.1038/mp.2012.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ma N, Liu Y, Li N, Wang C-X, Zhang H, Jiang X-F, et al. Addiction related alteration in resting-state brain connectivity. Neuroimage. 2010;49:738–744. doi: 10.1016/j.neuroimage.2009.08.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang Y, Tian J, Yuan K, Liu P, Zhuo L, Qin W, et al. Distinct resting-state brain activities in heroin-dependent individuals. Brain Res. 2011;1402:46–53. doi: 10.1016/j.brainres.2011.05.054. [DOI] [PubMed] [Google Scholar]
- 20.Upadhyay J, Maleki N, Potter J, Elman I, Rudrauf D, Knudsen J, et al. Alterations in brain structure and functional connectivity in prescription opioid-dependent patients. Brain. 2010;133:2098–2114. doi: 10.1093/brain/awq138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Squire LR. Declarative and nondeclarative memory: multiple brain systems supporting learning and memory. Journal of Cognitive Neuroscience. 1992;4:232–243. doi: 10.1162/jocn.1992.4.3.232. [DOI] [PubMed] [Google Scholar]
- 22.Delgado MR, Dickerson KC. Reward-related learning via multiple memory systems. Biol Psychiatry. 2012;72:134–141. doi: 10.1016/j.biopsych.2012.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Müller CP. Episodic memories and their relevance for psychoactive drug use and addiction. Front Behav Neurosci. 2013;7:1–13. doi: 10.3389/fnbeh.2013.00034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Squire LR. Memory systems of the brain: A brief history and current perspective. Neurobiol Learn Mem. 2004;82:171–177. doi: 10.1016/j.nlm.2004.06.005. [DOI] [PubMed] [Google Scholar]
- 25.Milner B, Squire LR, Kandel ER. Cognitive neuroscience and the study of memory. Neuron. 1998;20:445–468. doi: 10.1016/s0896-6273(00)80987-3. [DOI] [PubMed] [Google Scholar]
- 26.Bast T. Toward an integrative perspective on hippocampal function: from the rapid encoding of experience to adaptive behavior. Reviews in the Neurosciences. 2007;18:253–281. doi: 10.1515/revneuro.2007.18.3-4.253. [DOI] [PubMed] [Google Scholar]
- 27.Shohamy D, Adcock RA. Dopamine and adaptive memory. Trends Cogn Sci (Regul Ed) 2010;14:464–472. doi: 10.1016/j.tics.2010.08.002. [DOI] [PubMed] [Google Scholar]
- 28.Hyman SE. Addiction: a disease of learning and memory. Am J Psychiatry. 2005;162:1414–1422. doi: 10.1176/appi.ajp.162.8.1414. [DOI] [PubMed] [Google Scholar]
- 29.Wimmer GE, Shohamy D. Preference by association: how memory mechanisms in the hippocampus bias decisions. Science. 2012;338:270–273. doi: 10.1126/science.1223252. [DOI] [PubMed] [Google Scholar]
- 30.Fu L-P, Bi G-h, Zou Z-t, Wang Y, Ye E-m, Ma L, et al. Impaired response inhibition function in abstinent heroin dependents: an fMRI study. Neurosci Lett. 2008;438:322–326. doi: 10.1016/j.neulet.2008.04.033. [DOI] [PubMed] [Google Scholar]
- 31.Xie C, Li S-J, Shao Y, Fu L, Goveas J, Ye E, et al. Identification of hyperactive intrinsic amygdala network connectivity associated with impulsivity in abstinent heroin addicts. Beh Brain Res. 2011;216:639–646. doi: 10.1016/j.bbr.2010.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Patton JH, Stanford MS, Barratt ES. Factor structure of the Barratt impulsiveness scale. J Clin Psychol. 1995;51:768–774. doi: 10.1002/1097-4679(199511)51:6<768::aid-jclp2270510607>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 33.Fransson P. Spontaneous low-frequency BOLD signal fluctuations: An fMRI investigation of the resting-state default mode of brain function hypothesis. Hum Brain Mapp. 2005;26:15–29. doi: 10.1002/hbm.20113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fox MD, Raichle ME. Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging. Nat Rev Neurosci. 2007;8:700–711. doi: 10.1038/nrn2201. [DOI] [PubMed] [Google Scholar]
- 35.Good CD, Johnsrude IS, Ashburner J, Henson RN, Friston KJ, Frackowiak RS. A voxel-based morphometric study of ageing in 465 normal adult human brains. Neuroimage. 2001;14:21–36. doi: 10.1006/nimg.2001.0786. [DOI] [PubMed] [Google Scholar]
- 36.Bari A, Robbins TW. Inhibition and impulsivity: behavioral and neural basis of response control. Progress in neurobiology. 2013;108:44–79. doi: 10.1016/j.pneurobio.2013.06.005. [DOI] [PubMed] [Google Scholar]
- 37.O'Doherty J, Dayan P, Schultz J, Deichmann R, Friston K, Dolan RJ. Dissociable Roles of Ventral and Dorsal Striatum in Instrumental Conditioning. Science. 2004;304:452–454. doi: 10.1126/science.1094285. [DOI] [PubMed] [Google Scholar]
- 38.Hare TA, O'Doherty J, Camerer CF, Schultz W, Rangel A. Dissociating the role of the orbitofrontal cortex and the striatum in the computation of goal values and prediction errors. J Neurosci. 2008;28:5623–5630. doi: 10.1523/JNEUROSCI.1309-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yin HH, Knowlton BJ. The role of the basal ganglia in habit formation. Nat Rev Neurosci. 2006;7:464–476. doi: 10.1038/nrn1919. [DOI] [PubMed] [Google Scholar]
- 40.McClure SM, Laibson DI, Loewenstein G, Cohen JD. Separate neural systems value immediate and delayed monetary rewards. Science. 2004;306:503–507. doi: 10.1126/science.1100907. [DOI] [PubMed] [Google Scholar]
- 41.McClure SM, Ericson KM, Laibson DI, Loewenstein G, Cohen JD. Time discounting for primary rewards. J Neurosci. 2007;27:5796–5804. doi: 10.1523/JNEUROSCI.4246-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hare TA, Camerer CF, Knoepfle DT, Rangel A. Value computations in ventral medial prefrontal cortex during charitable decision making incorporate input from regions involved in social cognition. J Neurosci. 2010;30:583–590. doi: 10.1523/JNEUROSCI.4089-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Montague PR, Berns GS. Neural economics and the biological substrates of valuation. Neuron. 2002;36:265–284. doi: 10.1016/s0896-6273(02)00974-1. [DOI] [PubMed] [Google Scholar]
- 44.Sanfey AG, Loewenstein G, McClure SM, Cohen JD. Neuroeconomics: cross-currents in research on decision-making. Trends Cogn Sci (Regul Ed) 2006;10:108–116. doi: 10.1016/j.tics.2006.01.009. [DOI] [PubMed] [Google Scholar]
- 45.Monterosso J, Piray P. Neuroeconomics and the Study of Addiction. Biol Psychiatry. 2012;75:107–112. doi: 10.1016/j.biopsych.2012.03.012. [DOI] [PubMed] [Google Scholar]
- 46.Hare TA, Camerer CF, Rangel A. Self-control in decision-making involves modulation of the vmPFC valuation system. Science. 2009;324:646–648. doi: 10.1126/science.1168450. [DOI] [PubMed] [Google Scholar]
- 47.Mansouri FA, Tanaka K, Buckley MJ. Conflict-induced behavioural adjustment: a clue to the executive functions of the prefrontal cortex. Nat Rev Neurosci. 2009;10:141–152. doi: 10.1038/nrn2538. [DOI] [PubMed] [Google Scholar]
- 48.Modirrousta M, Fellows LK. Dorsal medial prefrontal cortex plays a necessary role in rapid error prediction in humans. J Neurosci. 2008;28:14000–14005. doi: 10.1523/JNEUROSCI.4450-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Polli FE. Rostral and dorsal anterior cingulate cortex make dissociable contributions during antisaccade error commission. Proceedings of the National Academy of Sciences. 2005;102:15700–15705. doi: 10.1073/pnas.0503657102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Forman SD, Dougherty GG, Casey BJ, Siegle GJ, Braver TS, Barch DM, et al. Opiate addicts lack error-dependent activation of rostral anterior cingulate. Biol Psychiatry. 2004;55:531–537. doi: 10.1016/j.biopsych.2003.09.011. [DOI] [PubMed] [Google Scholar]
- 51.Lee TMC, Zhou W-H, Luo X-J, Yuen KSL, Ruan X-Z, Weng X-C. Neural activity associated with cognitive regulation in heroin users: A fMRI study. Neurosci Lett. 2005;382:211–216. doi: 10.1016/j.neulet.2005.03.053. [DOI] [PubMed] [Google Scholar]
- 52.Schmidt A, Borgwardt S, Gerber H, Schmid O, Wiesbeck GA, Riecher-Rössler A, et al. Altered prefrontal connectivity after acute heroin administration during cognitive control. Int J Neuropsychopharmacol. 2014:1–11. doi: 10.1017/S1461145714000297. [DOI] [PubMed] [Google Scholar]
- 53.Hong LE, Gu H, Yang Y, Ross TJ, Salmeron BJ, Buchholz B, et al. Association of nicotine addiction and nicotine's actions with separate cingulate cortex functional circuits. Arch Gen Psychiatry. 2009;66:431–441. doi: 10.1001/archgenpsychiatry.2009.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gu H, Salmeron BJ, Ross TJ, Geng X, Zhan W, Stein EA, et al. Mesocorticolimbic circuits are impaired in chronic cocaine users as demonstrated by resting-state functional connectivity. Neuroimage. 2010;53:593–601. doi: 10.1016/j.neuroimage.2010.06.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Montague PR. Neuroeconomics: a view from neuroscience. Funct Neurol. 2007;22:219–234. [PubMed] [Google Scholar]
- 56.Kable JW, Glimcher PW. The neural correlates of subjective value during intertemporal choice. Nat Neurosci. 2007;10:1625–1633. doi: 10.1038/nn2007. [DOI] [PMC free article] [PubMed] [Google Scholar]