Abstract
Pathogenic autosomal recessive mutations in the DJ-1 (Park7) or the PTEN-induced putative kinase 1 (Pink1 or PARK6) genes are associated with familial Parkinson’s disease (PD). It is not well known regarding the pathological mechanisms involving the DJ-1 and Pink1 mutations. Here we characterized DJ-1 and Pink1 knockout rats both through expression profiling and using quantitative autoradiography to measure the densities of the dopamine D1, D2, D3 receptors, vesicular monoamine transporter type-2 (VMAT2) and dopamine transporter (DAT) in the striatum of transgenic rats and wild type controls. Expression profiling with a commercially available array of 84 genes known to be involved in PD indicated that only the target gene was significantly downregulated in each transgenic rat model. D1 receptor, VMAT2, and DAT were measured using [3H]SCH23390, [3H]dihydrotetrabenazine, and [3H]WIN35428, respectively. No significant changes were observed in the density of DAT in either model. Although the densities of VMAT2 and D1 receptor were unchanged in Pink1 knockout, but both were increased in DJ-1 knockout rats. The densities of D2 and D3 receptors, determined by mathematical analysis of binding of radioligands [3H]WC-10 and [3H]raclopride, were significantly increased in both knockout models. These distinctive changes in the expression of dopamine presynaptic markers and receptors in the striatum may reflect different compensatory regulation of dopamine system in DJ-1 versus Pink1 knockout rat models of familial PD.
Keywords: DJ-1, Pink1, Parkinson’s disease, dopamine receptors, autoradiography
1. Introduction
Parkinson’s disease (PD) is the most common movement disorder, and is characterized by bradykinesia, rigidity, resting tremor and postural instability clinically. Significant loss of dopaminergic neurons in the pars compacta of the substantia nigra (SNpc) and formation of intraneuronal Lewy bodies (LBs) are the main neuropathological features in PD. Over 85% of PD cases are sporadic. Monogenic forms of this disease, resulting from pathogenic mutations in genes including DJ-1 (Park7) and PTEN-induced putative kinase 1 (Pink1 or PARK6), both of which are autosomal recessive, are associated with familial patients (less than 15% of all cases). Large exonic deletions or frame-shift truncations in DJ-1 and Pink1 are among the most frequent mutations found in these PD patients, suggesting a “loss of function” mechanism [1–3]. The mechanistic involvement of DJ-1 and Pink1 mutations in Parkinsonian etiology has not yet been elucidated.
DJ-1 and Pink1 knockout mice have been previously used to study the monogenic form of PD. These animals manifest no significant dopaminergic neuronal loss [4–8]. In the DJ-1 knockout mouse, although the evoked dopamine overflow is reduced [6], and dopamine release and reuptake are increased [5], the densities of dopamine transporter (DAT) [5, 6], vesicular monoamine transporter type-2 (VMAT2) [5] and dopamine D2 like receptor [6] remain unchanged. One study in the DJ-1 knockout mouse observed an increased DAT level in synaptosomes but not in cytoplasm [8]. In the Pink1 knockout mouse, dopamine release was decreased, and the density of striatal dopamine D1 and D2 like receptors was unchanged [7]. The lack of dopaminergic neuronal loss in these mouse models makes them less than ideal as PD models. Severe dopamine depletion in the brain, measured using positron emission tomography (PET) or single photon emission computed tomography (SPECT) is found in DJ-1 [9, 10] and Pink1 patients [11, 12]. One postmortem neuropathological study showed neuronal loss in the SNpc in familial PD patients with Pink1 mutations [11]. A DJ-1–nullizygous mouse was recently created which demonstrated progressive dopaminergic cell loss [13]. However, neither loss of striatal dopamine termini nor increase of striatal postsynaptic marker ΔFBJ was observed in this new model, indicating that both pre- and postsynaptic dopamine components are involved in compensatory regulation [13]. Unfortunately, the density of dopamine presynaptic markers and receptors was not determined in this study.
Because the rat has obvious advantages over the mouse for CNS studies, the Michael J Fox foundation provided funding to SAGE Labs to generate DJ-1 and Pink1 knockout rats as improved models of familial PD (See SAGE Labs website, http://www.sageresearchmodels.com/ and MJFF website, https://www.michaeljfox.org/). DJ-1 and Pink1 knockout rats demonstrate significant motor deficit and age-dependent neuronal loss in SNpc, consistent with human disease. In this study of post-mortem rat brains, we investigated the expression of an array of genes associated with PD and determined the absolute densities of dopamine D2 and D3 receptors using the D3 receptor-specific ligand [3H]WC-10 and the D2/D3 ligand [3H]raclopride [14]. We also measured the densities of dopamine D1 receptor, DAT and VMAT2 in the striatum of DJ-1 and Pink1 knockout rats by quantitative autoradiography. We observed a distinct regulation of dopamine presynaptic markers and receptors in DJ-1and PINK1 knockout rat model of PD.
2. Methods
DJ-1 and Pink1 knockout rats were created using zinc finger nuclease (ZFN) technology [15] and maintained at SAGE Labs, monitored by SAGE’s Institutional Animal Care and Use Committee (IACUC). The SAGE Labs IACUC closely oversees research and ensures that it is conducted in accordance with all provisions of the PHS Policy on Humane Care and Use of Laboratory Animals. Both transgenic rat lines are registered at the Rat Genome Database (RGD) under the following names: LE-Park7em1Sage−/− and LE-Pink1em1Sage−/−.
2.1 RNA isolation
Whole rat brains were collected from knockout animals and wild type littermates at 10 or 15 weeks of age for each experimental group, snap-frozen and stored at −80°C. Frozen tissues were homogenized by mortar and pestle in liquid nitrogen, and immediately used for RNA isolation. Total RNA isolation was performed by using the TRizol reagent (a monophasic solution of phenol and guanidine isothiocyanate) following the manufacturer’s instructions (Life Technologies, Carlsbad, CA). Isolated RNA was treated with RNAse-free DNase-I and purified on RNeasy columns (Qiagen, Germantown, MD).
2.2 PCR arrays
cDNA was prepared from 1 μg of total RNA using the RT2 First Strand cDNA synthesis kit (Qiagen) and used as templates for amplification with the Rat Parkinson’s Disease RT2 Profiler PCR Array (Qiagen), following the manufacturer’s protocols. In brief, 25 μl of the SYBR Green PCR cocktail was dispensed into each well of the 96-well PCR array using a multichannel pipettor. The SYBR Green PCR cocktail consisted of the 20 μl cDNA reaction, 1350 μl of 2× RT2 qPCR Master Mix and 1330 μl molecular grade water for a final volume of 2.7 ml. The recipe provides excess volume to allow multiple pipetting. Real-time PCR detection was performed with a CFX96 instrument (Bio-Rad, Hercules, CA) under the following PCR conditions: 95°C, 10 min; 40 cycles of 95°C, 15 sec, 60°C, 1 min.
2.3 Brain samples and tissue processing
Whole brains were carefully harvested from three DJ-1 knockout male rats at 8 months of age and three PINK1 knockout male rats at 6 months of age as well as three age-matched wild-type control male rats for each group and immediately snap-frozen in a dry ice/ethanol bath to preserve shape and then stored at −80°C until further processing.
Brains were sectioned at 20 μm on a cryostat and thaw-mounted onto Fisher Superfrost Plus slides, with 6 sets taken from the rostral through caudal striatum. Slides were stored at – 80°C until used for receptor autoradiography.
2.4 Radioligands and drug
[3H]WC-10 was custom synthesized by American Radiolabeled Chemicals (St Louis, Missouri, USA) by alkylation of the desmethyl precursor with [3H]methyl iodide. The specific activity of the radioligand was 80 Ci/mmol. The synthesis of [3H]WC-10 has been previously described [16]. Chemical reagents and the standard compounds used in this study were purchased from Sigma (St. Louis, MO) and Tocris (Ellisville, MO). [3H]Raclopride (76 Ci/mmol), [3H]SCH23390 (85 Ci/mmol) and [3H]WIN35428 (76 Ci/mmol) were purchased from Perkin Elmer Life Sciences (Boston, MA). [3H]Dihydrotetrabenazine ([3H]DTBZ) (20 Ci/mmol) was purchased from American Radiolabeled Chemicals (St Louis, Missouri, USA).
2.5 Quantitative autoradiography protocol
The quantitative autoradiography of the dopamine D1, D2, D3 receptors, vesicular monoamine transporter type-2 (VMAT2) and dopamine transporter (DAT) in the striatum of the transgenic rat brains was performed using Beta Imager 2000Z Digital Beta Imaging System (Biospace, France),
A total of 6 brain sections in the adjacent slide were chosen for each animal. Using known neuroanatomical markers, bilateral regions of interest were drawn freehand along the border of the entire striatum of serial brain sections from each individual rat brain to define the representative binding density for the striatum (Fig. 1, 2g). Quantitative analysis was performed with the program Beta-Vision Plus (BioSpace, France), and the absolute densities of dopamine D2 and D3 receptors using the D3-preferring radioligand [3H]WC-10 and the D2/D3 ligand, [3H]raclopride were calculated as previously described [14, 17].
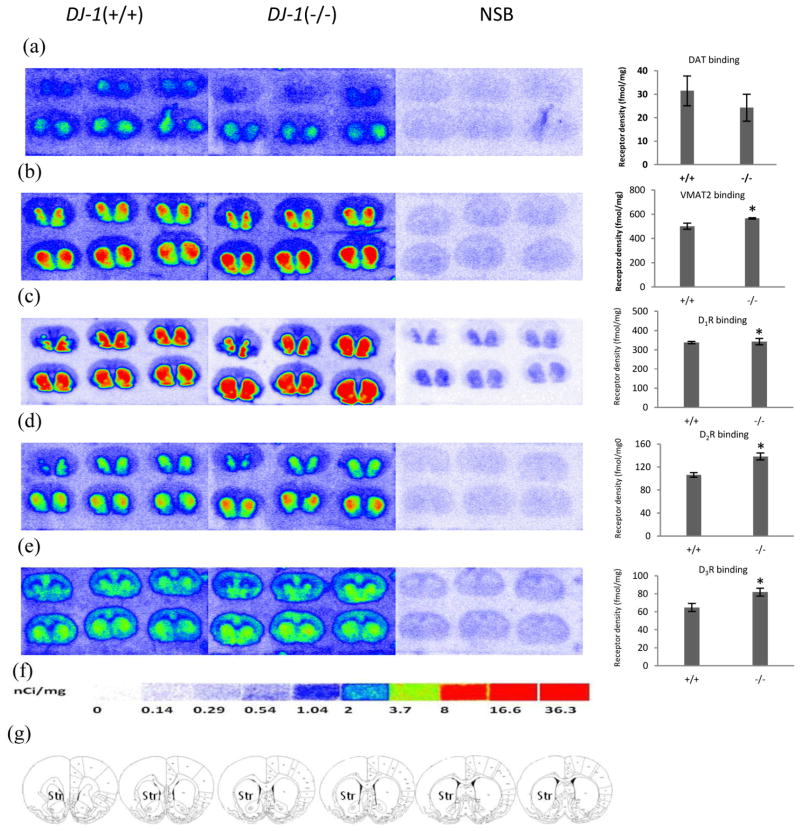
Figure 1. Quantitative autoradiographic analysis of DAT, DTBZ and dopamine receptors densities in the DJ-1 gene knockout rat models of PD.
Autoradiograms show binding of 8.5 nM [3H]WIN35428 (a), 23.5nM [3H]DTBZ (b), 3.8 nM [3H]SCH23390 (c), 4.4 nM [3H]raclopride (d) and 5.9 nM [3H]WC-10 (e) on multiple brain sections through the striatum in the DJ-1 gene knockout and wild-type littermate rat. Nonspecific binding was determined in the presence of 1 μM nomifensine (for [3H]WIN35428), 1 μM S(−)-tetrabenazine (for [3H]DTBZ), 1 μM (+) butaclamol (for [3H]SCH23390), 1 μM S(−)-eticlopride (for [3H]raclopride and [3H]WC-10). DAT binding did not significantly change in DJ-1 gene knockout rat (a). The densities of VMAT2, dopamine D1, D2 and D3 receptors were significantly increased in DJ-1 gene knockout rats (b, c, d, e). [3H]Microscale standards (ranging from 0 to 36.3 nCi/mg) were also counted (f). Schematic rat brain sections showing the rostral to caudal extent of the striatum, the region of interest in which DAT, VMAT2, D1, D2, and D3 receptors were quantified, across a total of six sections(g). NSB, nonspecific binding; Str, striatum. *p<0.05 for DJ-1 knockout rat vs. control rat.
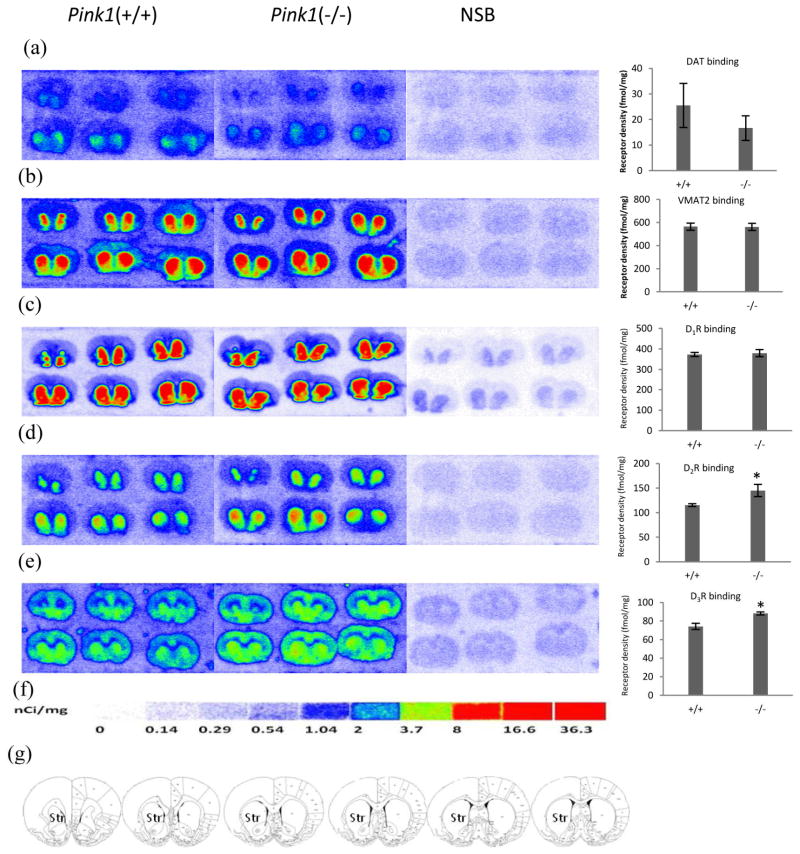
Figure 2. Quantitative autoradiographic analysis of DAT, DTBZ and dopamine receptors densities in the PINK1 gene knockout rat models of PD.
Autoradiograms show binding of 8.5 nM [3H]WIN35428 (a), 23.5 nM [3H]DTBZ (b), 3.8 nM [3H]SCH23390 (c), 4.4 nM [3H]raclopride(d) and 5.9 nM [3H]WC-10 (e) on multiple brain sections through the striatum in the PINK1 gene knockout and wild-type littermate rat. Nonspecific binding was determined in presence of 1 μM nomifensine (for [3H]WIN35428), 1 μM S(−)-tetrabenazine (for [3H]DTBZ),1 μM (+) butaclamol (for [3H]SCH23390), 1 μM S(−)-eticlopride (for [3H]raclopride and [3H]WC-10). DAT, VMAT2 and D1 receptor binding did not change in PINK1 gene knockout rat (a,b,c). The densities of dopamine D2 and D3 receptors were significant upregulated in PINK1 gene knockout rats (d, e). [3H]Microscale standards (ranging from 0 to 36.3 nCi/mg) were also counted (f). Schematic rat brain sections showing the rostral to caudal extent of the striatum, the region of interest in which DAT, VMAT2, D1, D2, and D3 receptors were quantified, across a total of six sections (g). NSB, nonspecific binding; Str, striatum. *p<0.05 for Pink1 knockout rat vs. control rat.
2.8 Statistical analysis
The apparent binding density for each receptor-bound radioligand was calculated using the specific activity of the radioligand expressed as fmol/mg tissue, as previously described [14]. The experimenter was blinded to all conditions during the analysis. Comparison of receptor densities between knockout rats and age-matched controls was analyzed by Student’s t-test.
3. Results
3.1 Confirmation of DJ-1 and Pink1 disruption
To confirm gene-specific knockout of DJ-1 and Pink1 in the transgenic rat models, total RNA from whole brain homogenates was analyzed for expression of PD-related genes using quantitative RT-PCR (see Methods). Compared to wild-type control rats, DJ-1 expression was 6- to 11-fold lower in DJ-1 knockout rats, and Pink1 expression was reduced 40-fold (Table1). Only a few minor changes in mRNA levels of other genes analyzed were noted, and the observed differences were seen mostly in the older animals, such as upregulation of Fjx1, NSF and synaptogyrin 3 in 15-week old DJ-1 knockout rats. However, the magnitude of these changes was small (2.3-, 2-, and 2.3-fold, respectively). DAT and D2 receptor expression did not change significantly based on mRNA levels. VMAT2 was slightly downregulated only in the 15-week DJ-1 knockout rat brains. Interestingly, the serotonin 2A receptor (Htr2a) was slightly upregulated in both knockout rat models at both time points tested.
Table 1.
Fold changes in mRNA expression levels in PD KO rat brains as assessed by PCR arrays
| Gene | GeneBank | Name | PARK7_KO 10 wks | PARK7_KO 15 wks | Pink1_KO 10 wks | Pink1_KO 15 wks |
|---|---|---|---|---|---|---|
| Park7 | nm_057143 | DJ-1 | −11.3 | −6.2 | ||
| Pink1 | nm_001106694 | PTEN induced putative kinase 1 | −41.4 | −42.7 | ||
| Fjx1 | nm_001108955 | four jointed box 1 | 2.3 | |||
| Htr2a | nm_017254 | serotonin 2A receptor | 2.5 | 2.6 | 2.2 | 3.0 |
| Nsf | nm_021748 | N-ethylmaleimide- sensitive factor | 2.0 | |||
| Slc18a2 | nm_013031 | vesicular monoamine transporter 2 (VMAT2) | −2.8 | |||
| Syngr3 | nm_001106985 | synaptogyrin 3 | 2.3 | |||
| Drd2 | nm_012547 | dopamine receptor D2 | −1.0 | 1.2 | −1.1 | 1.1 |
| Slc6a3 | nm_012694 | dopamine transporter (DAT) | −1.8 | 1.5 | 1.3 | −1.0 |
3.2 Quantitative analysis of dopamine D1, D2, D3 receptors, DAT and VMAT2 densities in the striatum of DJ-1 knockout rats
To determine the density of dopamine receptors and DAT and VMAT2, target-specific radioligand binding was measured using quantitative radiography. DJ-1 knockout rats were analyzed at eight months of age. Compared to wild type controls, the DJ-1 knockout rats exhibited no significant change in DAT density in the striatum (Fig. 1a) and a 14% increase in VMAT2 density; this was statistically significant (p = 0.05, t-test, Fig. 1b). Additionally, the densities of all three dopamine receptors were significantly increased in DJ-1 knockout rats in comparison with wild type controls: D1 receptor, 6% increase, p = 0.034, t-test (Fig. 1c), D2 receptor, 30% increase, p = 0.012, t-test (Fig. 1d), and D3 receptor, 26% increase, p = 0.047, t-test (Fig. 1e).
3.3 Quantitative analysis of dopamine D1, D2, D3 receptors, DAT and VMAT2 densities in the striatum of PINK1 knockout rats
In six months-old Pink1 knockout rats, the densities of DAT, VMAT2 and D1 receptor remained unchanged from those of wild type rats (Fig. 2a, b, c, respectively). However, the D2 receptor demonstrated a 26% increase in density in the Pink1 knockout rats over the wild type controls (P=0.029, t-test, Fig. 2d), while the D3 receptor showed a 19% increase over wild type controls (p=0.026, t-test, Fig. 2e).
4. Discussion
Gene expression profiling using a commercial array of 84 genes involved in PD demonstrated that disruption of DJ-1 and Pink1 genes in the transgenic rat models led to significant downregulation of the specific target gene expression. A few other genes involved in PD were slightly up- or downregulated at the mRNA level in the brains of DJ-1 and Pink1 knockout rats, but no global disturbance in PD-related gene expression patterns resulted from ZFN-targeted knockout of either the DJ-1 or Pink1 gene.
Most of the non-target mRNA expression changes found was observed in older DJ-1 knockout animals (15 weeks). The roles that these genes play in PD pathology are not yet clear. Four-jointed (Fj) protein regulates the development of the wing discs in drosophila, but its function in mammals is not known. It has been shown that loss of Fjx1, a mammalian ortholog of Fj, will increase number and branching of the neuronal dendrites [18]. N-Ethylmaleimide-sensitive factor (NSF) is involved in regulation of synaptic transmission [19]. A recent genetic study identified NSF as one of the candidate genes for study PD [20]. Synaptogyrin 3 is a synaptic vesicle protein, shown to interact with DAT and VMAT2, both functionally and physically [21]. Upregulation of 5HT2A receptor mRNA in both models is in agreement with the previous observations that loss of nigrostriatal cells causes an up-regulation of striatal 5-HT2AR mRNA [22]. All the receptors and transporters analyzed in this study, except for D1 receptor, were included in the PCR array. Yet, only VMAT2 showed slight decrease in the 15 week-old DJ-1 rats. All the other genes showed no change in expression on the mRNA level.
The density of dopamine presynaptic markers DAT and VMAT2 were measured using quantitative autoradiography in the striatum of Pink1 (at 6 months of age) and DJ-1 (at 8 months of age) knockout rats; significant neuronal loss was found in the SNpc. Surprisingly DAT density remained unchanged in both rat models, while VMAT 2 density was found to be slightly but significantly increased in DJ-1 knockout rats. Previously, DAT density in synaptosomes of DJ-1 knockout mice, which lack dopaminergic neuronal loss, has been reported to be either unchanged [5, 6] or increased [8]. A new DJ-1–nullizygous mouse model was recently created which shows significant dopaminergic cell loss as early as 2 months of age but without clear loss of striatal dopamine termini by TH staining; this has been attributed to sprouting of neurites within the nigrostriatal pathway [13]. This report is in line with our findings showing no significant reduction of dopamine pre-synaptic markers although there was a significant neuronal loss in SNpc in DJ-1 knockout rats. These observations seem to point to a compensatory mechanism in the presynaptic dopaminergic system of DJ-1-disrupted rodent brains. Consistent with these findings, SAGE has reported an increase in extracellular dopamine content without a corresponding change in its metabolites (DOPAC, HVA) (See SAGE Labs website, http://www.sageresearchmodels.com/ and MJFF website, https://www.michaeljfox.org/), indicating enhancement of dopamine release and reuptake in DJ-1 knock out rat, which can be mediated by DAT and VMAT2. Similarly, increased dopamine release and reuptake was also observed in DJ-1 knockout mice [5]. The changes in DAT and VMAT2 density in the Pink1 knockout rats resemble those of the DJ-1 knockout rats, implying that DJ-1 and Pink1 may function similarly in autosomal recessive PD. It is noteworthy that DAT binding has been reported to be decreased in familial PD patients with DJ-1 or Pink1 mutations [9, 11, 12]; this is in contrast to our current findings in the rat models. However, the changes in DAT and VMAT2 density in DJ-1 and Pink1 knockout rats may reflect a regulation of dopamine presynaptic markers in the preclinical or early stage of familial PD.
The density of dopamine D2 receptor was increased in the striatal regions of both DJ-1 and Pink1 knockout rats, which is in agreement with previous findings in neurotoxin induced PD models [23–27] and PD patients [28, 29]. In DJ-1–nullizygous mice recently created by Rousseaux et al [13], an increase in murine osteosarcoma viral oncogene homolog B (ΔFosB), a striatal postsynaptic marker was observed; this has also been reported in PD patients and neurotoxin-induced animal models of PD, indicating that there is a similar compensatory response in DJ-1 knockout mice. Our data suggests that the upregulation of striatal D2 receptors possibly be one of the postsynaptic compensatory responses upon neuronal loss in SNpc in DJ-1 and Pink1 knockout rats.
The increased dopamine D3 receptor density found in this study is in contrast to findings in 6-OHDA rats [30, 31] and MPTP treated monkey models of PD [32] as well as PD patients [33]. However increased striatal D3 receptor mRNA has been reported in monkey [34] and cat [35] MPTP-induced PD models, reflecting post-synaptic regulation. The D3 receptor was found to be increased in PD patients who responded well to L-dopa treatment [36]. It has been reported that BDNF from dopamine neurons is responsible for inducing normal expression of the dopamine D3 receptor both during development and in adulthood [37], however its regulation in PD may also depend on the time course of disease and the severity of neuronal loss in SNpc; dopaminergic therapy may also affect D3 receptor expression. Other factors including species differences, sample processing, selectivity of radioligands and method can also influence the published D3 receptor binding data. Nevertheless, the increase in D3 receptor density in the DJ-1 and Pink1 knockout rats in this study may reflect a compensatory upregulation upon neuronal loss in SNpc. Familial PD patients with DJ-1 or Pink1 mutations are characterized by early onset but good response to L-dopa treatment [9, 11]. Elevation of D3 receptors was positively correlated with L-dopa response in PD patients [36], although the relationship between this clinical observation and the regulation of D2 and D3 receptors needs to be further investigated.
In conclusion, this systematic measurement of dopamine presynaptic markers and receptors revealed alterations in dopaminergic regulation in DJ-1 and Pink1 knockout rats. To our knowledge, these are the first transgenic rat models of PD with dopaminergic neuron loss. Unchanged or elevated DAT and VMAT density may reflect compensatory regulation of dopamine presynaptic markers which can facilitate the turnover of extracellular dopamine; while the upregulation of dopamine D2 and D3 receptors suggests post-synaptic compensation for dopaminergic neuron loss caused by disruption of DJ-1 and Pink1 genes. These transgenic rat models of autosomal recessive PD can be potentially used for studying PD etiology as well as drug development.
Acknowledgments
This research was funded by NIH grants MH081281 and DA023957.
References
- 1.Bonifati V, et al. DJ-1( PARK7), a novel gene for autosomal recessive, early onset parkinsonism. Neurol Sci. 2003;24(3):159–60. doi: 10.1007/s10072-003-0108-0. [DOI] [PubMed] [Google Scholar]
- 2.Bonifati V, et al. Mutations in the DJ-1 gene associated with autosomal recessive early-onset parkinsonism. Science. 2003;299(5604):256–9. doi: 10.1126/science.1077209. [DOI] [PubMed] [Google Scholar]
- 3.Valente EM, et al. PINK1 mutations are associated with sporadic early-onset parkinsonism. Ann Neurol. 2004;56(3):336–41. doi: 10.1002/ana.20256. [DOI] [PubMed] [Google Scholar]
- 4.Chandran JS, et al. Progressive behavioral deficits in DJ-1-deficient mice are associated with normal nigrostriatal function. Neurobiol Dis. 2008;29(3):505–14. doi: 10.1016/j.nbd.2007.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen L, et al. Age-dependent motor deficits and dopaminergic dysfunction in DJ-1 null mice. J Biol Chem. 2005;280(22):21418–26. doi: 10.1074/jbc.M413955200. [DOI] [PubMed] [Google Scholar]
- 6.Goldberg MS, et al. Nigrostriatal dopaminergic deficits and hypokinesia caused by inactivation of the familial Parkinsonism-linked gene DJ-1. Neuron. 2005;45(4):489–96. doi: 10.1016/j.neuron.2005.01.041. [DOI] [PubMed] [Google Scholar]
- 7.Kitada T, et al. Impaired dopamine release and synaptic plasticity in the striatum of PINK1-deficient mice. Proc Natl Acad Sci U S A. 2007;104(27):11441–6. doi: 10.1073/pnas.0702717104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Manning-Bog AB, et al. Increased vulnerability of nigrostriatal terminals in DJ-1-deficient mice is mediated by the dopamine transporter. Neurobiol Dis. 2007;27(2):141–50. doi: 10.1016/j.nbd.2007.03.014. [DOI] [PubMed] [Google Scholar]
- 9.Dekker M, et al. Clinical features and neuroimaging of PARK7-linked parkinsonism. Mov Disord. 2003;18(7):751–7. doi: 10.1002/mds.10422. [DOI] [PubMed] [Google Scholar]
- 10.Dekker MC, et al. PET neuroimaging and mutations in the DJ-1 gene. J Neural Transm. 2004;111(12):1575–81. doi: 10.1007/s00702-004-0165-4. [DOI] [PubMed] [Google Scholar]
- 11.Samaranch L, et al. PINK1-linked parkinsonism is associated with Lewy body pathology. Brain. 2010;133(Pt 4):1128–42. doi: 10.1093/brain/awq051. [DOI] [PubMed] [Google Scholar]
- 12.Kessler KR, et al. Dopaminergic function in a family with the PARK6 form of autosomal recessive Parkinson’s syndrome. J Neural Transm. 2005;112(10):1345–53. doi: 10.1007/s00702-005-0281-9. [DOI] [PubMed] [Google Scholar]
- 13.Rousseaux MW, et al. Progressive dopaminergic cell loss with unilateral-to-bilateral progression in a genetic model of Parkinson disease. Proc Natl Acad Sci U S A. 2012;109(39):15918–23. doi: 10.1073/pnas.1205102109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xu J, et al. [3H]4-(dimethylamino)-N-(4-(4-(2-methoxyphenyl)piperazin-1-yl) butyl)benzamide: a selective radioligand for dopamine D(3) receptors. II. Quantitative analysis of dopamine D(3) and D(2) receptor density ratio in the caudate-putamen. Synapse. 2010;64(6):449–59. doi: 10.1002/syn.20748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cui X, et al. Targeted integration in rat and mouse embryos with zinc-finger nucleases. Nat Biotechnol. 2011;29(1):64–7. doi: 10.1038/nbt.1731. [DOI] [PubMed] [Google Scholar]
- 16.Xu J, et al. [(3)H]4-(Dimethylamino)-N-[4-(4-(2-methoxyphenyl)piperazin-1-yl)butyl]benzamide, a selective radioligand for dopamine D(3) receptors. I. In vitro characterization. Synapse. 2009;63(9):717–28. doi: 10.1002/syn.20652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sun J, et al. Dopamine D1, D2, D3 receptors, vesicular monoamine transporter type-2 (VMAT2) and dopamine transporter (DAT) densities in aged human brain. PLoS One. 2012;7(11):e49483. doi: 10.1371/journal.pone.0049483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Probst B, et al. The rodent Four-jointed ortholog Fjx1 regulates dendrite extension. Dev Biol. 2007;312(1):461–70. doi: 10.1016/j.ydbio.2007.09.054. [DOI] [PubMed] [Google Scholar]
- 19.Hong RM, et al. Association of N-ethylmaleimide-sensitive factor with synaptic vesicles. FEBS Lett. 1994;350(2–3):253–7. doi: 10.1016/0014-5793(94)00778-0. [DOI] [PubMed] [Google Scholar]
- 20.Karic A, Terzic R, Peterlin B. Identifying candidate genes for Parkinson’s disease by integrative genomics method. Biochem Med (Zagreb) 2011;21(2):174–81. doi: 10.11613/bm.2011.027. [DOI] [PubMed] [Google Scholar]
- 21.Egana LA, et al. Physical and functional interaction between the dopamine transporter and the synaptic vesicle protein synaptogyrin-3. J Neurosci. 2009;29(14):4592–604. doi: 10.1523/JNEUROSCI.4559-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang X, Andren PE, Svenningsson P. Changes on 5-HT2 receptor mRNAs in striatum and subthalamic nucleus in Parkinson’s disease model. Physiol Behav. 2007;92(1–2):29–33. doi: 10.1016/j.physbeh.2007.05.033. [DOI] [PubMed] [Google Scholar]
- 23.Joyce JN, et al. Hemiparkinsonism in a monkey after unilateral internal carotid artery infusion of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) is associated with regional ipsilateral changes in striatal dopamine D-2 receptor density. Brain Res. 1986;382(2):360–4. doi: 10.1016/0006-8993(86)91345-4. [DOI] [PubMed] [Google Scholar]
- 24.Graham WC, et al. Autoradiographic studies in animal models of hemi-parkinsonism reveal dopamine D2 but not D1 receptor supersensitivity. II. Unilateral intra-carotid infusion of MPTP in the monkey (Macaca fascicularis) Brain Res. 1990;514(1):103–10. doi: 10.1016/0006-8993(90)90440-m. [DOI] [PubMed] [Google Scholar]
- 25.Joyce JN. Differential response of striatal dopamine and muscarinic cholinergic receptor subtypes to the loss of dopamine. II. Effects of 6-hydroxydopamine or colchicine microinjections into the VTA or reserpine treatment. Exp Neurol. 1991;113(3):277–90. doi: 10.1016/0014-4886(91)90017-7. [DOI] [PubMed] [Google Scholar]
- 26.LaHoste GJ, Marshall JF. Chronic eticlopride and dopamine denervation induce equal nonadditive increases in striatal D2 receptor density: autoradiographic evidence against the dual mechanism hypothesis. Neuroscience. 1991;41(2–3):473–81. doi: 10.1016/0306-4522(91)90342-l. [DOI] [PubMed] [Google Scholar]
- 27.Gagnon C, et al. Effect of adding the D-1 agonist CY 208–243 to chronic bromocriptine treatment of MPTP-monkeys: regional changes of brain dopamine receptors. Prog Neuropsychopharmacol Biol Psychiatry. 1995;19(4):667–76. doi: 10.1016/0278-5846(95)00110-h. [DOI] [PubMed] [Google Scholar]
- 28.Rinne UK, et al. Positron emission tomography demonstrates dopamine D2 receptor supersensitivity in the striatum of patients with early Parkinson’s disease. Mov Disord. 1990;5(1):55–9. doi: 10.1002/mds.870050114. [DOI] [PubMed] [Google Scholar]
- 29.Sawle GV, et al. Asymmetrical pre-synaptic and post-synpatic changes in the striatal dopamine projection in dopa naive parkinsonism. Diagnostic implications of the D2 receptor status. Brain. 1993;116(Pt 4):853–67. doi: 10.1093/brain/116.4.853. [DOI] [PubMed] [Google Scholar]
- 30.Levesque D, et al. A paradoxical regulation of the dopamine D3 receptor expression suggests the involvement of an anterograde factor from dopamine neurons. Proc Natl Acad Sci U S A. 1995;92(5):1719–23. doi: 10.1073/pnas.92.5.1719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stanwood GD, et al. Quantitative autoradiographic mapping of rat brain dopamine D3 binding with [125I]7-OH-PIPAT: Evidence for the presence of D3 receptors on dopaminergic and nondopaminergic cell bodies and terminals. J Pharmacol Exp Ther. 2000;295(3):1223–31. [PubMed] [Google Scholar]
- 32.Quik M, et al. Expression of D(3) receptor messenger RNA and binding sites in monkey striatum and substantia nigra after nigrostriatal degeneration: effect of levodopa treatment. Neuroscience. 2000;98(2):263–73. doi: 10.1016/s0306-4522(00)00130-5. [DOI] [PubMed] [Google Scholar]
- 33.Boileau I, et al. Decreased binding of the D3 dopamine receptor-preferring ligand [11C]-(+)-PHNO in drug-naive Parkinson’s disease. Brain. 2009;132(Pt 5):1366–75. doi: 10.1093/brain/awn337. [DOI] [PubMed] [Google Scholar]
- 34.Todd RD, et al. Dynamic changes in striatal dopamine D2 and D3 receptor protein and mRNA in response to 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) denervation in baboons. J Neurosci. 1996;16(23):7776–82. doi: 10.1523/JNEUROSCI.16-23-07776.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wade TV, Rothblat DS, Schneider JS. Changes in striatal dopamine D3 receptor regulation during expression of and recovery from MPTP-induced parkinsonism. Brain Res. 2001;905(1–2):111–9. doi: 10.1016/s0006-8993(01)02513-6. [DOI] [PubMed] [Google Scholar]
- 36.Joyce JN, et al. Loss of response to levodopa in Parkinson’s disease and co-occurrence with dementia: role of D3 and not D2 receptors. Brain Res. 2002;955(1–2):138–52. doi: 10.1016/s0006-8993(02)03396-6. [DOI] [PubMed] [Google Scholar]
- 37.Guillin O, et al. BDNF controls dopamine D3 receptor expression and triggers behavioural sensitization. Nature. 2001;411(6833):86–9. doi: 10.1038/35075076. [DOI] [PubMed] [Google Scholar]