Abstract
Prenatal exposure to organophosphate pesticides (OPs) has been associated with adverse neurodevelopmental outcomes in childhood, including low IQ, Pervasive Developmental Disorder (PDD), attention problems and ADHD. Many of these disorders involve impairments in social functioning. Thus, we investigated the relationship between biomarkers of prenatal OP exposure and impaired reciprocal social behavior in childhood, as measured by the Social Responsiveness Scale (SRS). Using a multi-ethnic urban prospective cohort of mother-infant pairs in New York City recruited between 1998 and 2002 (n=404) we examined the relation between third trimester maternal urinary levels of dialkylphosphate (ΣDAP) OP metabolites and SRS scores among 136 children who returned for the 7–9 year visit. Overall, there was no association between OPs and SRS scores, although in multivariate adjusted models, associations were heterogeneous by race and by sex. Among blacks, each 10-fold increase in total diethylphosphates (ΣDEP) was associated with poorer social responsiveness (β = 5.1 points, 95% confidence interval (CI) 0.8, 9.4). There was no association amongst whites or Hispanics, or for total ΣDAP or total dimethylphosphate (ΣDMP) biomarker levels. Additionally, stratum-specific models supported a stronger negative association among boys for ΣDEPs (β = 3.5 points, 95% CI 0.2, 6.8), with no notable association among girls. Our results support an association of prenatal OP exposure with deficits in social functioning among blacks and among boys, although this may be in part reflective of differences in exposure patterns.
Keywords: Social Responsiveness Scale, organophosphate pesticides, environmental exposures, neurodevelopment
1. Introduction
Organophosphate pesticides (OPs) are acutely neurotoxic at high doses. The primary mechanism of action at these doses is inhibition of acetylcholinesterase, which leads to an accumulation of acetylcholine at the neuronal junction (Sultatos 1994). At low doses, OPs1 have several suspected mechanisms of action, including disruption of nuclear transcription factors (Dam et al. 2003), interference with neural cell development and neurotransmitter systems (Aldridge et al. 2005b), and altered synaptic formation (Qiao et al. 2003). Fetuses and babies are thought to be highly susceptible to OP exposure, due to the ready transmission of OPs through the placenta, and the immaturity of metabolic pathways required to process and excrete these compounds (Landrigan 1999; Whyatt et al. 2005). OPs were removed from the USA market for most residential uses in the early 2000’s, although exposure in the general population can still occur through dust reservoirs and ingestion from approved agricultural uses. (Stout II et al. 2009).
OP exposure has been negatively associated with a wide range of childhood cognitive and behavioral outcomes. Three independent birth cohort studies in the USA found prenatal OP exposure to be associated with lowered IQ in childhood (Bouchard et al. 2011; Engel et al. 2011; Eskenazi et al. 2007; Rauh et al. 2011), although the specific cognitive domains most strongly associated with exposure varied among populations. Two different cohort studies have found an association between prenatal OP exposure and Attention-Deficit Hyperactivity Disorder (ADHD)-like behaviors at 3 (Rauh et al. 2006) and 5 (Marks et al. 2010) years of age, although no association was found at 2 years (Eskenazi et al. 2007). Using a parent report instrument, the Child Behavior Checklist, these same studies also report associations with pervasive developmental disorder (PDD), an umbrella term which includes Autism Spectrum Disorders (ASDs) (Eskenazi et al. 2007; Rauh et al. 2006), although in both studies the number of putative cases was quite small.
The Social Responsiveness Scale (SRS) is a parent/caregiver survey designed to quantify impairments in reciprocal social behaviors (Constantino and Gruber 2005). While these impairments are useful in distinguishing autism spectrum disorder (ASD) from other child psychiatric conditions (Constantino and Gruber 2005), they are not necessarily specific to ASD, in that deficits in social reciprocity may also be found in conditions such as ADHD, language problems, maladaptive behavior, social anxiety, and mood disorders (Constantino et al. 2003; Hus et al. 2013; Pine et al. 2008; Reiersen et al. 2007). Therefore, higher scores on the SRS may highlight a neurobehavioral social impairment common to multiple neuropsychiatric conditions (Hus et al. 2013). Given that a number of recent studies have linked prenatal OP exposure with neuropsychiatric conditions that involve impaired social functioning (ADHD, PDD), we explored whether exposure to OPs in utero was associated with a continuous measure of impaired social responsiveness in childhood, and whether associations varied according to race/ethnicity or child sex.
2. METHODS
2.1 Cohort Enrollment and Follow-Up
The Mount Sinai Children’s Environmental Health Study is a prospective multiethnic cohort of primiparous women with singleton pregnancies who delivered at the Mount Sinai Hospital between May 1998 and July 2001 (Berkowitz et al. 2003; Berkowitz et al. 2004). Women were recruited at either the Mount Sinai Diagnostic and Treatment Center, which serves a predominantly minority East Harlem population, or at one of two private practices on the Upper East Side of Manhattan. Of the 479 mother-infant pairs that were successfully recruited, 75 were excluded for reasons described elsewhere (Engel et al. 2007), for a final cohort of 404 mother-infant pairs for whom birth data were available. During approximately the third trimester of pregnancy, maternal urinary specimens were obtained and questionnaires were administered to participants to obtain information on sociodemographic characteristics, medical history, and lifestyle factors. Delivery characteristics and birth outcomes were obtained from a perinatal database. Women were invited to return with their child for follow-up visits at ages 1, 2, 4, 6 and 7–9 years. At the 7–9 year evaluation, mothers completed the SRS (n=136).
2.3 Reciprocal Social Behavior Outcomes
The SRS is a 65-item caregiver rating scale of social behaviors characteristic of autism spectrum and related disorders for children ages 4 to 18 (Constantino and Gruber 2005). Each item rates the frequency of a behavior on a 4 point Likert scale, with higher scores indicating more symptoms of impairment (Constantino and Gruber 2005). Raw scores are sex-standardized to T-scores with a mean of 50 and a standard deviation of 10, and are calculated separately for boys and girls. The SRS has good test-retest temporal stability, parent-parent and parent-teacher interrater agreement and discriminate and concurrent validity (Constantino and Gruber 2005). T-scores over 60 are described as possible indicators of mild/moderate impairment, and T-scores over 75 are possible indicators of severe impairment.
2.2 Pesticide metabolite measurements
Maternal urine samples were collected between 25 and 40 weeks’ gestation (mean of 31.2 weeks, sd of 3.7) and were analyzed by the Centers for Disease Control and Prevention (Atlanta, Georgia) for six dialkylphosphate (DAP, in nm/L) metabolites in two batches using lyophilization, derivatization to form chloropropyl phosphate esters and analysis using gas chromatography-tandem mass spectrometry. Isotope dilution quantification was performed with 10% quality control samples included in each run (Bravo et al. 2004).
Metabolite levels that were missing due to analytic interference were imputed using regression analysis to predict the missing metabolite from the other non-missing metabolites measured for that woman (n=6/136 (4.4%) of DEP metabolites, n=0 of DMP metabolites), as has been previously described (Engel et al. 2007). Prior to imputation, samples below the limit of detection were assigned a value of the . Diethyl- (DEP) and dimethylphosphate (DMP) metabolites were then summed on a molar basis (as nm/L) to respectively obtain total diethylphosphates (ΣDEP) and total dimethylphosphates (ΣDMP)(Barr et al. 2011). These were together summed to obtain total dialkylphosphates (ΣDAPs). Overall, approximately 97%, 89%, and 90% of the cohort had detectable levels of DEP and DMP metabolites, respectively.
2.4 Statistical Analyses
All analyses were performed in SAS version 9.3 (SAS Institute, Inc, Cary, NC). ΣDAPs, ΣDEPs, and ΣDMPs were transformed using log base 10 to approximate a normal distribution. Very dilute urine samples containing less than 10 mg/dL of creatinine (n=1) were excluded from statistical analyses, consistent with methods previously described (Engel et al. 2007; Eskenazi et al. 2004). OP metabolite biomarker levels (nm/L) were examined continuously on a log10 basis and using creatinine-corrected tertiles (nm/gC).
Multivariate linear regression was used to analyze relationships between log10 ΣDAPs and the dependent variable, continuous SRS total scores. Potential covariates for the model were selected after examining a directed acyclic graph (DAG) for confounding. Variables that the DAG identified as likely mediators or colliders were not considered for inclusion in the model (Rothman 2008). After constructing our DAG, and due to our small sample size, we used a backward elimination method to obtain the most parsimonious model (Weng et al. 2009). We eliminated covariates that did not change the estimate of the main effect by more than 20% unless they improved the precision of the model. Analyzing the DAG yielded a potential covariate list that included maternal age, maternal education (dichotomous for high school or less), race/ethnicity (disjoint class variable for White, Black, Hispanic), marital status (dichotomous for married/living with partner or single), child sex, any smoking during pregnancy, breastfeeding, housing status (categorical indicators for public housing, private rentals, and ownership), child age in months at the time of testing, and urine creatinine. After backwards selection, final included variables were maternal education, race/ethnicity, marital status, child age, housing status, and natural log transformed creatinine. Five mothers had missing data on either education or marital status and were excluded from analyses, while two mothers had missing ΣDEP data and were excluded from models examining ΣDEPs and ΣDAPs. Previous studies have suggested that organophosphate pesticide associations may be differential by sex (Marks et al. 2010; Rauh et al. 2012), and previous studies in this population have suggested that effects may also be differential by race (Engel et al. 2011). Thus, additional models testing effect measure modification by child sex and race/ethnicity were also explored (interaction α < 0.20), and effects were estimated for each race and sex strata regardless of interaction p-values due to this a priori hypothesis. Although an alpha of 0.20 increases the type I error rate, we accepted this rate in exchange for investigating potentially meaningful associations by strata. We could not simultaneously test interactions by race and sex due to small cell size. Finally, we examined F-tests in ANOVA analyses and trend tests of tertiles in multivariate linear regression analyses of the creatinine-adjusted log10 total metabolites by sex and by race to assess linearity of the dose-response curve, in addition to analyzing restricted cubic splines of the dose-response function in exploratory analyses (data not shown).
3. Results
3.1 Demographics and Characteristics
Mothers were primarily young (59% <22 years), unmarried (57%) minorities (85% non-white) who had achieved a high school education or less (63%). Approximately 18% reported smoking at any time during pregnancy (Table 1). OP metabolite concentrations are summarized in Table 2. The geometric mean (GM) level of ΣDAPs was 76.9 nm/L (GSD 4.6), while the GM for ΣDEPs was 17.4 nm/L (GSD 4.63), and the GM for ΣDEPs was 41.7 (GSD 6.06). The average SRS total t score was 52.9 (SD 10.6), with 30 children scoring above 60 (the cut-off for mild/moderate impairment), and 6 children scoring above 75 (the cut-off for severe impairment) (Table 2).
Table 1.
Characteristics of Study Population (n = 136)a
| Characteristic | Category | N | % |
|---|---|---|---|
| Maternal Education | High School or less | 82 | 63% |
| Some college or higher | 49 | 37% | |
| Missing | 5 | ||
|
| |||
| Marital Status | Married or Living with Baby’s Father | 58 | 43% |
| Not Married or living with partner | 76 | 57% | |
| Missing | 2 | ||
|
| |||
| Race/Ethnicity | Hispanic | 71 | 52% |
| Black | 45 | 33% | |
| White | 20 | 15% | |
| Missing | 0 | ||
|
| |||
| Sex | Male | 68 | 50% |
| Female | 68 | 50% | |
| Missing | 0 | ||
|
| |||
| Any Smoking During Pregnancy | No | 112 | 82% |
| Yes | 24 | 18% | |
| Missing | 0 | ||
|
| |||
| Mothers Age | < 22 years | 80 | 59% |
| 22–34 years | 45 | 33% | |
| 35 years and older | 11 | 8% | |
Excludes subjects with Creatinine ≤10 mg/dL
Table 2.
Descriptive Statistics of Dialkylphosphate Metabolite Biomarker levels and Social Responsiveness Scale (SRS) Total Score (n = 136)
| N | Geometric Mean (GSD) | Min | Max | IQR | 5/95 percentile | ||
|---|---|---|---|---|---|---|---|
| ΣDAP nm/L | All | 134 | 76.9 (4.59) | 0.50 | 4990 | 6.09 | 8.45/7112 |
| Boys | 68 | 72.5 (3.27) | 0.50 | 1240 | 6.45 | 8.63/475 | |
| Girls | 66 | 81.7 (6.16) | 0.50 | 4990 | 7.78 | 0.49/956 | |
| Blacks | 44 | 90.5 (6.14) | 0.50 | 4990 | 6.91 | 7.48/1240 | |
| Whites & Hispanics | 90 | 71.0 (3.92) | 0.50 | 814 | 5.52 | 8.48/475 | |
|
| |||||||
| ΣDEP nm/L | All | 134 | 17.4 (4.63) | 0.50 | 345 | 5.56 | 0.49/118 |
| Boys | 68 | 14.5 (4.78) | 0.50 | 208 | 5.16 | 0.49/104 | |
| Girls | 66 | 21.0 (4.43) | 0.50 | 345 | 7.06 | 0.49/120 | |
| Blacks | 44 | 15.7 (4.90) | 0.50 | 345 | 5.90 | 0.49/135 | |
| Whites & Hispanics | 90 | 18.3 (4.53) | 0.50 | 223 | 6.14 | 0.49/113 | |
|
| |||||||
| ΣDMP nm/L | All | 135 | 41.7 (6.06) | 0.50 | 4900 | 12.92 | 0.49/755 |
| Boys | 68 | 38.1 (4.83) | 0.50 | 1240 | 9.49 | 3.96/444 | |
| Girls | 67 | 45.7 (7.50) | 0.50 | 4900 | 16.04 | 0.49/908 | |
| Blacks | 45 | 49.8 (8.50) | 0.50 | 4900 | 14.09 | 0.49/1240 | |
| Whites & Hispanics | 90 | 38.2 (5.02) | 0.50 | 788 | 9.46 | 3.96/415 | |
|
| |||||||
| Creatinine mg/dL | All | 136 | 69.3 (2.19) | 12.7 | 342 | 3.31 | 16.7/246 |
| Boys | 68 | 69.0 (2.29) | 12.7 | 342 | 3.26 | 16.2/241 | |
| Girls | 68 | 69.6 (2.10) | 13.7 | 271 | 3.02 | 20.9/246 | |
| Blacks | 45 | 75.6 (2.38) | 12.7 | 342 | 4.07 | 14.5/271 | |
| Whites & Hispanics | 91 | 66.4 (2.09) | 14.4 | 270 | 3.08 | 18.0/194 | |
|
| |||||||
| SRS Total Score1 | All | 136 | 52.9 (10.6) | 38.0 | 84.0 | 15.0 | 39.0/74.0 |
| Boys | 68 | 52.4 (10.4) | 38.0 | 84.0 | 16.5 | 38.0/72.0 | |
| Girls | 68 | 53.4 (10.8) | 38.0 | 82.0 | 14.5 | 41.0/75.0 | |
| Blacks | 45 | 53.9 (11.9) | 38.0 | 82.0 | 14.0 | 40.0/76.0 | |
| Whites & Hispanics | 91 | 52.4 (9.90) | 38.0 | 84.0 | 15.0 | 39.0/72.0 | |
Arithmetic means and standard deviations, not geometric means, are reported for SRS total scores
3.2 Organophosphate Pesticide Metabolites and SRS Scores
Overall, there were no associations between prenatal ΣDAPs, ΣDEPs, or ΣDMPs and total SRS t-scores (Table 3). However, associations between prenatal total diethylphosphate metabolite levels and SRS total score were heterogeneous by race and by sex (ΣDEP p-interaction = 0.06 for race and ΣDEP p-interaction = 0.12 for sex) in multivariate adjusted models. Stratum-specific estimates indicated stronger associations between ΣDEP and SRS total scores among boys than girls, and stronger associations among blacks than whites and Hispanics. Because the stratum specific effects for whites and Hispanics were similar (data not shown), we combined these strata to improve our overall power.
Table 3.
Dialkylphosphate Metabolite Associations with Total Social Responsiveness Scale Score, by Race and by Sex
| Log10 ΣDAP Unadjustedc β (95% CI) | Log10 ΣDAP Adjusted β (95% CI) | Log10 ΣDEP Unadjustedc β (95% CI) | Log10 ΣDEP Adjusted β (95% CI) | Log10 ΣDMP Unadjusted β (95% CI) | Log10 ΣDMP Adjusted β (95% CI) | |
|---|---|---|---|---|---|---|
| All n=129 | −0.9 (−3.6, 1.7) | 0.3 (−2.3, 2.9) | 1.1 (−1.6, 3.8) | 1.8 (−0.7, 4.3) | −1.0 (−3.3, 1.3) | −0.2 (−2.4, 2.0) |
| Blacka n=42 | −0.6 (−4.5, 3.4) | 1.8 (−2.2, 5.8) | 2.3 (−2.2, 6.8) | 5.1 (0.8, 9.4) | −0.9 (−4.2, 2.4) | 0.4 (−2.8, 3.7) |
| Whites and Hispanics n=87 | −1.3 (−5.0, 2.3) | −1.0 (−4.3, 2.3) | 0.5 (−2.7, 3.8) | 0.2 (−2.8, 3.2) | −1.2 (−4.3, 1.9) | −1.1 (−3.9, 1.7) |
| P interaction | 0.78 | 0.29 | 0.54 | 0.06 | 0.89 | 0.48 |
| Boysb (n = 66) | 0.7 (−4.0, 5.5) | 1.6 (−3.0, 6.1) | 2.4 (−1.2, 6.1) | 3.5 (0.2, 6.8) | −0.2 (−3.9, 3.5) | 0.7 (−2.8, 4.2) |
| Girls(n = 63) | −1.7 (−4.9, 1.5) | −0.4 (−3.6, 2.8) | −0.5 (−4.4, 3.4) | −0.4 (−4.1, 3.3) | −1.6 (−4.5, 1.3) | −0.8 (−3.7, 2.0) |
| P interaction | 0.41 | 0.50 | 0.28 | 0.12 | 0.56 | 0.52 |
Race interaction models adjusted for education, marital status, sex, housing status, child age, and creatinine
Sex interaction models adjusted for education, marital status, creatinine, housing status, child age, and three category race variable (black, Hispanic, white)
“Unadjusted” models adjusted for loge creatinine
Bolded estimates indicate p<0.05 for stratum-specific effects and p < 0.20 for interaction p-values
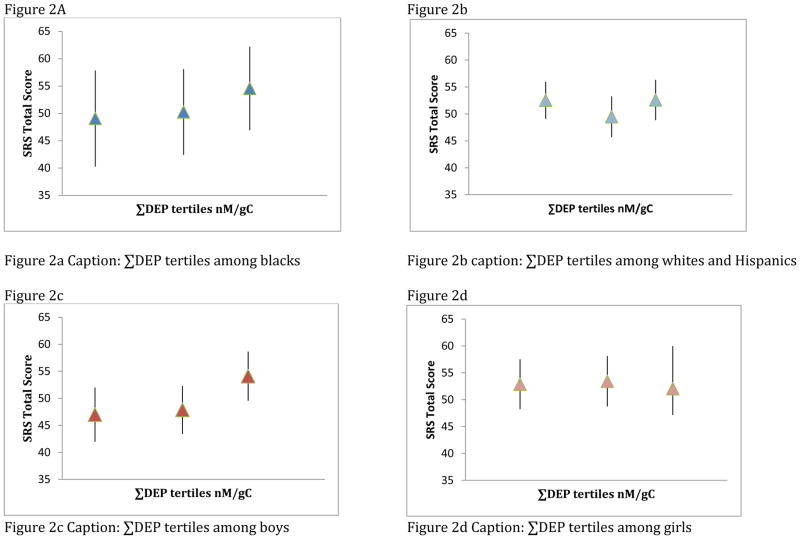
In multivariate adjusted models including a race interaction term, increasing ΣDEP biomarker levels were associated with poorer scores on the SRS among blacks (β = 5.1 points, 95% CI 0.8, 9.4), with no effect among whites or Hispanics (β =0.2 points, 95% CI −2.8, 3.2). Likewise, associations were stronger among boys (β = 3.5 points, 95% CI 0.2, 6.8), than girls (β = −0.4 points, 95% CI −4.1, 3.3) (Table 3 and Figure 1a). In both cases, due to our small sample size, confidence intervals were wide; however the overall ΣDEP estimate also supported the suggestion of poorer SRS scores with increasing metabolite levels (β =1.8 points, 95% –0.7, 4.3). ΣDEP tertiles (nm/gC) suggested a dose-dependent effect on total SRS scores among blacks and among boys, although the trend test and F-test were only significant for boys (p for trend boys =0.01 and F-test for boys=0.03; p for trend blacks= 0.21, F-test for blacks=0.43. Additionally, tertiles 1 and 2 for males were not different from each other (p=0.76), although both were different from tertile 3 (tertile 1 vs tertile 3 p=0.02, tertile 2 vs tertile 3 p=0.03), which may be suggestive of non-linearity among boys (Figure 2).
Figure 1. Overall and Stratum-Specific Associations of Log10 Dialkylphosphate Metabolite levels and Total SRS Score.
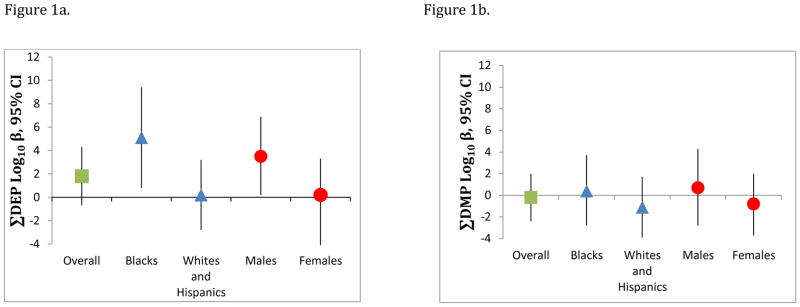
Figure 1a shows the association between increasing log10 ΣDEPs and total SRS t-scores according to race and sex. Stronger adverse associations with SRS score were found for blacks and boys, with no associations among whites/Hispanics and girls (Race p-interaction = 0.06; Sex p-interaction= 0.12). Figure 1b shows null associations between log10 ΣDMPs and SRS total t-scores overall, within strata of Race/ethnicity and sex, and no interaction (Race p-interaction =0.48, Sex-interaction =0.52 ). Models adjusted for log creatinine, mother’s marital status, mother’s education, housing status, child age, race, and sex.
Figure 2. Associations of ΣDEP tertiles with predicted SRS Total Scores within strata of Race/Ethnicity and Sex.
Tertiles suggest a weak dose-response relationship among Blacks (p-trend = 0.12) and no association among whites/Hispanics (p-trend=0.71). Race-specific tertiles as a group did not predict total SRS t-scores (Type 3 F-test p for Tertile: Blacks = 0.43; Whites/Hispanics = 0.36). In sex-specific analysis, tertiles suggest an elevated association in the third tertile among boys (p-trend= 0.01) and no relationship among girls (p-trend =0.79). Sex-specific tertiles as a group predicted SRS total scores among boys, but not among girls (Type 3 F-test p for Tertile: Boys =0.03; Girls = 0.90).
Neither overall, nor within strata of race/ethnicity or child sex did we see any notable associations of prenatal total ΣDAP or ΣDMP with total SRS score (Table 3 and Figure 1b). In general, effects hovered around the null (Figure 1b).
We tested response bias with Satterthwaite pooled t-tests to determine whether mothers with SRS follow-up data were different from mothers who did not return for the SRS follow-up visit, with respect to maternal education, prenatal smoking, alcohol consumption during pregnancy, marital status, and mother’s age at birth. There was no evidence of response bias except for marital status, in that mothers who were married or living with the baby’s father at follow-up were significantly less likely to return for a follow-up visit than unmarried mothers.
4. Discussion
4.1 Findings
This study adds to the growing body of literature investigating the neurobehavioral consequences of prenatal OP exposure. Although we found little evidence of any overall association of dialkylphosphate metabolite concentrations with social-reciprocal behavioral deficits, there was some suggestion of an association between increasing prenatal ΣDEP metabolite concentrations and adverse SRS scores among blacks and among boys. One of DEPs parent compounds is chlorpyrifos, which has previously been implicated in studies of the association between prenatal exposure to pesticides and child neurodevelopment (Rauh et al. 2011; Rauh et al. 2006), and was widely used in New York City before the year 2000 (Thier et al. 1998).
4.2 Previous Research
Unfortunately, there are few cohort studies of prenatal exposure to organophosphate pesticides and social behavior in childhood, and thus the literature is in large part attributable to a limited number of relatively small cohorts that have been studied intensively over a period of years, principally in the Salinas valley of California (Eskenazi et al. 2003), or in urban (Berkowitz et al. 2003; Perera et al. 2002) and semi-urban (Rauch et al. 2012) environments. The nature and extent of exposure in these populations is likely to vary substantially, given differences in parent compound applications, source, and time period. Nonetheless, some troubling patterns have begun to emerge. In a primarily Hispanic agricultural cohort, the CHAMACOS study, Eskenazi et al (2007) reported a relationship between prenatal exposure to total ΣDAPs and ΣDMPs and parent-report based PDD in 2 year olds (measured by the Child Behavior Checklist), with no association found for ΣDEPs (Eskenazi et al. 2007). Notably, reported geometric means in the agricultural cohort for ΣDMPs were approximately twice the geometric mean in our cohort, and these differential levels of exposure may account for some of the differences in findings. It is also possible that the associations of ΣDAPs in the CHAMACOS cohort may be driven by the dimethylphosphate metabolites, since there was no association for ΣDEPs and the amount of ΣDMPs was considerably greater than the amount of ΣDEPs. In the same cohort, Marks et al reported a relationship between prenatal exposure to total ΣDAPs and ΣDEPs and a derived variable for ADHD based on several instruments used to evaluate behavior (Marks et al. 2010). They further reported sex-specific effects for several of the outcome variables, including a sex-specific effect in males for the derived composite ADHD indicator. Rauh et al. (2006) reported an association between prenatal exposure to chlorpyrifos (which devolves into DEPs) and PDD in 2–3 year olds in an inner city cohort of blacks and Dominicans in New York City. They also reported associations between prenatal exposure to chlorpyrifos and attention problems and ADHD problems, as measured by the CBCL.
While our findings generally do not support evidence of an association with total ΣDAPs or ΣDMPs in any strata, they do provide evidence of an association with ΣDEP metabolites and social reciprocal deficits among blacks and among boys in our urban, inner-city population. The differences in associations between ΣDEPs, ΣDMPs, and ΣDAPs and behavior across studies may in part reflect the non-specificity of these biomarkers of exposure, as they arise from multiple different parent compounds of varying toxicity. ΣDEPs in our population, for instance, may reflect a different mixture of parent compounds than ΣDEPs in the CHAMACOS cohort. The difference in associations we observed according to race/ethnicity may reflect varying sources of exposure, different genotype frequencies for genes that govern metabolism and detoxification of organophosphate pesticides (Thyagarajan et al. 2008), and/or unmeasured social confounding. While the mean levels of ΣDEPs for whites and blacks were within one standard deviation of each other (Table 2), blacks had more variability in measured levels of ΣDEPs. The white population in this study is likely to have been primarily exposed to OPs through diet, which includes a mixture of parent compound and preformed metabolite exposure, the latter of which would not be toxic. Exposure through the diet is further complicated by co-exposures to potentially beneficial nutrients, such as folate consumption during the preconceptual and early pregnancy period, which has been found to be associated with lower risk of ASD (Schmidt et al. 2011), improved social competence scores, and fewer symptoms of inattention (Julvez et al. 2009). These co-exposures may compete with the toxic effects of exposure to parent OP compounds. Alternatively, a high proportion of the black and Hispanic populations studied here lived in low-income housing (Engel et al. 2011) and may have been exposed to indoor pest treatments, although we controlled for housing in our models. The lack of an effect in Hispanics may be due to differing genotypes, or to protective exposures from different dietary patterns. There is suggestive evidence that paraoxonase 1 (PON1) phenotype may modify associations of OPs with neurobehavior (Eskenazi et al. 2010). Unfortunately, our sample size is too small to reliably test interactions with PON1 genotype while also assessing interactions with race and/or sex, although genetic susceptibility may be an important source of variability. Unmeasured confounding by social factors may also play a role; although we considered Medicaid status, housing type, maternal education, and marital status in our models, there may still be residual confounding by other social factors that are associated with race, prenatal pesticide exposure, and social responsiveness.
Our study found stronger associations of ΣDEP with social deficits among boys than girls. Boys are almost five times more likely than girls to be diagnosed with autism (Baio 2012), and are more than twice as likely as girls to be diagnosed with ADHD (Visser 2010). This could be indicative of greater environmental sensitivities in boys, or it could be due to a diagnostic or reporting bias if social deficits have a higher degree of recognition in boys. This finding could also be spurious. However, previous studies have found sex-specific effects of organophosphate pesticides on attention in boys (Marks et al. 2010), and animal studies suggest there may be greater neurological effects in males in response to chlorpyrifos (Slotkin and Seidler 2005), or other sex-specific effects of organophosphate pesticides (Ricceri et al. 2006; Slotkin et al. 2008); thus our results seem plausible and are in line with the evolving literature.
Prenatal exposure to the organophosphate pesticide chlorpyrifos has also been found to result in structural changes in the brain (Rauh et al. 2012). In a structural MRI study of 5–11 year olds exposed prenatally to chlorpyrifos, higher levels of chlorpyrifos were associated with bilateral enlargement of temporal lobes, unilateral enlargement in the right hemisphere of the frontal lobe, and enlargement in the cuneus and precuneus (both of which are in the occipital lobe) (Rauh et al. 2012). Structural brain differences have also been found in children with autism and ADHD. Autistic 2–4 year old children have been found to have enlarged frontal and temporal lobes, and enlarged amygdala and hippocampi (Carper and Courchesne 2005; Courchesne et al. 2007; Sparks et al. 2002). Children with ADHD also have unilateral enlargement in the right frontal hemisphere, specifically the prefrontal cortex (Krain and Castellanos 2006). Animal models of in utero exposure to organophosphates provide further evidence that they play a role in depression and anxiety, which are also associated with higher SRS scores (Pine et al. 2008). Mouse and rat models support the hypothesis that in utero exposure to low-levels of chlorpyrifos alters serotoninergic functioning in the absence of cholinergic effects. Serotonin is a critical neurotransmitter in the regulation of anxiety and depression, and OP exposure also increases behaviors associated with anxiety and depression (Aldridge et al. 2005a; Venerosi et al. 2010). Animal models have also shown that adult exposure to low-levels of chlorpyrifos impairs attention and increases impulsivity in rats (Middlemore-Risher et al. 2010).
While it may be tempting to equate deficits in social responsiveness with ASD, there is no evidence actually associating prenatal exposure to organophosphate pesticides with development of ASD. Although previous studies have linked OP exposure to CBCL scores that are indicative of PDD, an umbrella diagnosis that includes ASD, these studies likely suffered from a relatively high degree of outcome misclassification. Population-based studies suggest the frequency of ASD in the general population to be approximately 1/88 children (Baio 2012). Rauh (2006) reported that 4.7% of the NYC cohort met the cut-off for PDD, which yielded 11 cases and an OR estimate of 5.39 with a 95% confidence interval of 1.2, 24.11. This large confidence interval suggests sparse data. Additionally, Eskenazi (2007) reported that 14.4% of the CHAMACOS cohort met criteria for clinically significant PDD, which is much higher than the expected ~1% prevalence of autism in the general population, indicating a likelihood that, as with the SRS, the CBCL’s clinically significant cutoff may have a low positive predictive value. However, even with misclassification in the precise clinical diagnosis, these studies and ours are suggestive of a relationship between prenatal organophosphate pesticide exposure and social impairments, which are common across multiple neuropsychiatric conditions, including ADHD, autism, depression, and mood disorders.
4.3 Study Limitations
Finally, there are several limitations to our study that should be considered while interpreting our results. Although the sample size at birth (n=404) is comparable in size to other longitudinal birth cohorts in the United States, the substantial loss-to-follow-up has resulted in a relatively small sample at ages 7 to 9 for this study. In our study, loss-to-follow-up appeared to only be associated with marital status, which was adjusted for in this analysis. Even so, it is possible that other unmeasured covariates are associated with loss-to-follow-up, which could result in residual uncontrolled confounding. In addition, the small sample size available for analysis results in reduced power for statistical associations and interaction analyses. It is possible that the heterogeneity in associations we observed according to race are attributable in part to residual confounding by social factors that we cannot account for with our existing covariates. Additionally, we lack postnatal exposure measurements in the children that would allow us to consider whether exposure during other periods of development might impact social functioning. Although one study that examined associations between prenatal organophosphate pesticide exposure and IQ in childhood found that postnatal exposure was not an important predictor of the outcome when prenatal exposure was accounted for (Bouchard et al. 2011), it is still possible that postnatal exposure to organophosphate pesticides accounts for some of the variability in childhood neurobehavioral development. Nonetheless we would not expect postnatal exposure to bias any prenatal exposure associations given that it would temporally follow prenatal exposure. Still, future studies should consider whether later exposure windows are relevant to child social functioning and neurobehavioral development.
5. Conclusions
We report an association between prenatal biomarkers of ΣDEPs and poorer scores on the Social Responsiveness Scale among blacks and possibly among boys in New York City. Race-specific effects may be due to varying sources of exposure or other susceptibility factors, while sex-specific effects may be due to enhanced environmental sensitivity of boys. These results indicate a possible relationship between prenatal organophosphate pesticide use and social impairment in childhood, which is a common component of multiple prevalent neuropsychiatric conditions of childhood.
HIGHLIGHTS.
We examined associations of prenatal OP biomarkers and childhood social reciprocity
Associations varied within strata of race/ethnicity, and within strata of sex
Increasing exposure was adversely associated with social responsiveness among blacks and among boys
No associations were found among whites or Hispanics, or among girls
Acknowledgments
Funding: This work was supported by National Institute for Environmental Health Sciences/U.S. Environmental Protection Agency Children’s Center grants ES09584 and R827039, the New York Community Trust, and the Agency for Toxic Substances and Disease Registry/Centers for Disease Control and Prevention (CDC)/Association of Teachers of Preventive Medicine. The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the CDC. M. Furlong supported by the University of North Carolina Graduate School’s Merit Fellowship.
We gratefully acknowledge the tremendous contributions of G. Berkowitz in developing the New York Children’s Environmental Health cohort. We also thank the participants, as well as the efforts of Kelly Nichols, Karen Ireland and Martha Lievano.
Footnotes
Abbreviations: Organophosphate Pesticide (OP); Social Responsiveness Scale (SRS); Autism Spectrum Disorders (ASD); Attention-Deficit-Hyperactivity Disorder (ADHD); Child Behavior Checklist (CBCL), Magnetic Resonance Imaging (MRI), Pervasive Developmental Disorder (PDD); ΣDialkylphosphates (DAPs); ΣDiethylphosphate (DEPs); ΣDimethylphosphates (DMPs)
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aldridge JE, Levin ED, Seidler FJ, Slotkin TA. Developmental exposure of rats to chlorpyrifos leads to behavioral alterations in adulthood, involving serotonergic mechanisms and resembling animal models of depression. Environmental health perspectives. 2005a;113:527. doi: 10.1289/ehp.7867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aldridge JE, Meyer A, Seidler FJ, Slotkin TA. Alterations in central nervous system serotonergic and dopaminergic synaptic activity in adulthood after prenatal or neonatal chlorpyrifos exposure. Environmental health perspectives. 2005b;113:1027. doi: 10.1289/ehp.7968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baio J. Morbidity and Mortality Weekly Report. Atlanta, Georgia: Centers for Disease Control and Prevention; 2012. Prevalence of autism spectrum disorders - autism and developmental disabilities monitoring network, 14 sites, United States, 2008. [PubMed] [Google Scholar]
- Barr DB, Wong L-Y, Bravo R, Weerasekera G, Odetokun M, Restrepo P, Kim D-G, Fernandez C, Perez J, Gallegos M. Urinary concentrations of dialkylphosphate metabolites of organophosphorus pesticides: National Health and Nutrition Examination Survey 1999–2004. International journal of environmental research and public health. 2011;8:3063–3098. doi: 10.3390/ijerph8083063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berkowitz GS, Obel J, Deych E, Lapinski R, Godbold J, Liu Z, Landrigan PJ, Wolff MS. Exposure to indoor pesticides during pregnancy in a multiethnic, urban cohort. Environmental Health Perspectives. 2003;111:79. doi: 10.1289/ehp.5619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berkowitz GS, Wetmur JG, Birman-Deych E, Obel J, Lapinski RH, Godbold JH, Holzman IR, Wolff MS. In utero pesticide exposure, maternal paraoxonase activity, and head circumference. Environmental health perspectives. 2004;112:388. doi: 10.1289/ehp.6414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouchard MF, Chevrier J, Harley KG, Kogut K, Vedar M, Calderon N, Trujillo C, Johnson C, Bradman A, Barr DB. Prenatal exposure to organophosphate pesticides and IQ in 7-year-old children. Environmental health perspectives. 2011;119:1189. doi: 10.1289/ehp.1003185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bravo R, Caltabiano LM, Weerasekera G, Whitehead RD, Fernandez C, Needham LL, Bradman A, Barr DB. Measurement of dialkyl phosphate metabolites of organophosphorus pesticides in human urine using lyophilization with gas chromatography-tandem mass spectrometry and isotope dilution quantification. Journal of Exposure Science and Environmental Epidemiology. 2004;14:249–259. doi: 10.1038/sj.jea.7500322. [DOI] [PubMed] [Google Scholar]
- Carper RA, Courchesne E. Localized enlargement of the frontal cortex in early autism. Biological psychiatry. 2005;57:126–133. doi: 10.1016/j.biopsych.2004.11.005. [DOI] [PubMed] [Google Scholar]
- Constantino JN, Gruber CP. Social Responsiveness Scale. Los Angeles, CA: 2005. [Google Scholar]
- Constantino JN, Hudziak JJ, Todd RD. Deficits in reciprocal social behavior in male twins: evidence for a genetically independent domain of psychopathology. Journal of the American Academy of Child & Adolescent Psychiatry. 2003;42:458–467. doi: 10.1097/01.CHI.0000046811.95464.21. [DOI] [PubMed] [Google Scholar]
- Courchesne E, Pierce K, Schumann CM, Redcay E, Buckwalter JA, Kennedy DP, Morgan J. Mapping early brain development in autism. Neuron. 2007;56:399–413. doi: 10.1016/j.neuron.2007.10.016. [DOI] [PubMed] [Google Scholar]
- Dam K, Seidler FJ, Slotkin TA. Transcriptional biomarkers distinguish between vulnerable periods for developmental neurotoxicity of chlorpyrifos: implications for toxicogenomics. Brain research bulletin. 2003;59:261–265. doi: 10.1016/s0361-9230(02)00874-2. [DOI] [PubMed] [Google Scholar]
- Engel SM, Berkowitz GS, Barr DB, Teitelbaum SL, Siskind J, Meisel SJ, Wetmur JG, Wolff MS. Prenatal organophosphate metabolite and organochlorine levels and performance on the Brazelton Neonatal Behavioral Assessment Scale in a multiethnic pregnancy cohort. American journal of epidemiology. 2007;165:1397–1404. doi: 10.1093/aje/kwm029. [DOI] [PubMed] [Google Scholar]
- Engel SM, Wetmur J, Chen J, Zhu C, Barr DB, Canfield RL, Wolff MS. Prenatal exposure to organophosphates, paraoxonase 1, and cognitive development in childhood. Environmental health perspectives. 2011;119:1182. doi: 10.1289/ehp.1003183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eskenazi B, Bradman A, Gladstone EA, Jaramillo S, Birch K, Holland N. CHAMACOS, a longitudinal birth cohort study: lessons from the fields. Journal of Children’s Health. 2003;1:3–27. [Google Scholar]
- Eskenazi B, Harley K, Bradman A, Weltzien E, Jewell NP, Barr DB, Furlong CE, Holland NT. Association of in utero organophosphate pesticide exposure and fetal growth and length of gestation in an agricultural population. Environmental health perspectives. 2004;112:1116. doi: 10.1289/ehp.6789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eskenazi B, Huen K, Marks A, Harley KG, Bradman A, Barr DB, Holland N. PON1 and neurodevelopment in children from the CHAMACOS study exposed to organophosphate pesticides in utero. Environmental health perspectives. 2010;118:1775. doi: 10.1289/ehp.1002234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eskenazi B, Marks AR, Bradman A, Harley K, Barr DB, Johnson C, Morga N, Jewell NP. Organophosphate pesticide exposure and neurodevelopment in young Mexican-American children. Environmental health perspectives. 2007;115:792. doi: 10.1289/ehp.9828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hus V, Bishop S, Gotham K, Huerta M, Lord C. Factors influencing scores on the Social Responsiveness Scale. Journal of Child Psychology and Psychiatry. 2013;54:216–224. doi: 10.1111/j.1469-7610.2012.02589.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Julvez J, Fortuny J, Mendez M, Torrent M, Ribas-Fitó N, Sunyer J. Maternal use of folic acid supplements during pregnancy and four-year-old neurodevelopment in a population-based birth cohort. Paediatric and perinatal epidemiology. 2009;23:199–206. doi: 10.1111/j.1365-3016.2009.01032.x. [DOI] [PubMed] [Google Scholar]
- Krain AL, Castellanos FX. Brain development and ADHD. Clinical psychology review. 2006;26:433–444. doi: 10.1016/j.cpr.2006.01.005. [DOI] [PubMed] [Google Scholar]
- Landrigan PJ. Risk assessment for children and other sensitive populations. Annals of the New York Academy of Sciences. 1999;895:1–9. doi: 10.1111/j.1749-6632.1999.tb08073.x. [DOI] [PubMed] [Google Scholar]
- Marks AR, Harley K, Bradman A, Kogut K, Barr DB, Johnson C, Calderon N, Eskenazi B. Organophosphate pesticide exposure and attention in young Mexican-American children: the CHAMACOS study. Environmental health perspectives. 2010;118:1768. doi: 10.1289/ehp.1002056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Middlemore-Risher M, Buccafusco J, Terry A., Jr Repeated exposures to low-level chlorpyrifos results in impairments in sustained attention and increased impulsivity in rats. Neurotoxicology and teratology. 2010;32:415–424. doi: 10.1016/j.ntt.2010.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perera FP, Illman SM, Kinney PL, Whyatt RM, Kelvin EA, Shepard P, Evans D, Fullilove M, Ford J, Miller RL. The challenge of preventing environmentally related disease in young children: community-based research in New York City. Environmental Health Perspectives. 2002;110:197. doi: 10.1289/ehp.02110197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pine DS, Guyer AE, Goldwin M, Towbin KA, Leibenluft E. Autism spectrum disorder scale scores in pediatric mood and anxiety disorders. Journal of the American Academy of Child & Adolescent Psychiatry. 2008;47:652–661. doi: 10.1097/CHI.0b013e31816bffa5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiao D, Seidler FJ, Tate CA, Cousins MM, Slotkin TA. Fetal chlorpyrifos exposure: adverse effects on brain cell development and cholinergic biomarkers emerge postnatally and continue into adolescence and adulthood. Environmental health perspectives. 2003;111:536. doi: 10.1289/ehp.5828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauch SA, Braun JM, Barr DB, Calafat AM, Khoury J, Montesano MA, Yolton K, Lanphear BP. Associations of prenatal exposure to organophosphate pesticide metabolites with gestational age and birth weight. Environmental health perspectives. 2012;120:1055. doi: 10.1289/ehp.1104615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauh V, Arunajadai S, Horton M, Perera F, Hoepner L, Barr DB, Whyatt R. Seven-year neurodevelopmental scores and prenatal exposure to chlorpyrifos, a common agricultural pesticide. Environmental health perspectives. 2011;119:1196. doi: 10.1289/ehp.1003160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauh VA, Garfinkel R, Perera FP, Andrews HF, Hoepner L, Barr DB, Whitehead R, Tang D, Whyatt RW. Impact of prenatal chlorpyrifos exposure on neurodevelopment in the first 3 years of life among inner-city children. Pediatrics. 2006;118:e1845–e1859. doi: 10.1542/peds.2006-0338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauh VA, Perera FP, Horton MK, Whyatt RM, Bansal R, Hao X, Liu J, Barr DB, Slotkin TA, Peterson BS. Brain anomalies in children exposed prenatally to a common organophosphate pesticide. Proceedings of the National Academy of Sciences. 2012;109:7871–7876. doi: 10.1073/pnas.1203396109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiersen AM, Constantino JN, Volk HE, Todd RD. Autistic traits in a population-based ADHD twin sample. Journal of Child Psychology and Psychiatry. 2007;48:464–472. doi: 10.1111/j.1469-7610.2006.01720.x. [DOI] [PubMed] [Google Scholar]
- Ricceri L, Venerosi A, Capone F, Cometa MF, Lorenzini P, Fortuna S, Calamandrei G. Developmental neurotoxicity of organophosphorous pesticides: fetal and neonatal exposure to chlorpyrifos alters sex-specific behaviors at adulthood in mice. Toxicological sciences. 2006;93:105–113. doi: 10.1093/toxsci/kfl032. [DOI] [PubMed] [Google Scholar]
- Rothman KGS, Lash T. Modern Epidemiology. Philadelphia, PA: Lippincott Williams & Wilkins; 2008. [Google Scholar]
- Schmidt RJ, Hansen RL, Hartiala J, Allayee H, Schmidt LC, Tancredi DJ, Tassone F, Hertz-Picciotto I. Prenatal vitamins, one-carbon metabolism gene variants, and risk for autism. Epidemiology (Cambridge, Mass) 2011;22:476. doi: 10.1097/EDE.0b013e31821d0e30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slotkin TA, Bodwell BE, Ryde IT, Levin ED, Seidler FJ. Exposure of neonatal rats to parathion elicits sex-selective impairment of acetylcholine systems in brain regions during adolescence and adulthood. Environmental health perspectives. 2008;116:1308. doi: 10.1289/ehp.11451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slotkin TA, Seidler FJ. The alterations in CNS serotonergic mechanisms caused by neonatal chlorpyrifos exposure are permanent. Developmental brain research. 2005;158:115–119. doi: 10.1016/j.devbrainres.2005.06.008. [DOI] [PubMed] [Google Scholar]
- Sparks B, Friedman S, Shaw D, Aylward E, Echelard D, Artru A, Maravilla K, Giedd J, Munson J, Dawson G. Brain structural abnormalities in young children with autism spectrum disorder. Neurology. 2002;59:184–192. doi: 10.1212/wnl.59.2.184. [DOI] [PubMed] [Google Scholar]
- Stout DM, II, Bradham KD, Egeghy PP, Jones PA, Croghan CW, Ashley PA, Pinzer E, Friedman W, Brinkman MC, Nishioka MG. American Healthy Homes Survey: a national study of residential pesticides measured from floor wipes. Environmental science & technology. 2009;43:4294–4300. doi: 10.1021/es8030243. [DOI] [PubMed] [Google Scholar]
- Sultatos LG. Mammalian toxicology of organophosphorus pesticides. Journal of Toxicology and Environmental Health, Part A Current Issues. 1994;43:271–289. doi: 10.1080/15287399409531921. [DOI] [PubMed] [Google Scholar]
- Thier AL, Enck J, Klossner C, Advocates E, Albany N. Plagued by Pesticides: An Analysis of New York State and New York City’s 1997 Pesticide Use and Sales Data. 1998 [Google Scholar]
- Thyagarajan B, Jacobs DR, Carr JJ, Alozie O, Steffes MW, Kailash P, Hayes JH, Gross MD. Factors associated with paraoxonase genotypes and activity in a diverse, young, healthy population: the Coronary Artery Risk Development in Young Adults (CARDIA) study. Clinical chemistry. 2008;54:738–746. doi: 10.1373/clinchem.2007.099044. [DOI] [PubMed] [Google Scholar]
- Venerosi A, Ricceri L, Rungi A, Sanghez V, Calamandrei G. Gestational exposure to the organophosphate chlorpyrifos alters social–emotional behaviour and impairs responsiveness to the serotonin transporter inhibitor fluvoxamine in mice. Psychopharmacology. 2010;208:99–107. doi: 10.1007/s00213-009-1713-2. [DOI] [PubMed] [Google Scholar]
- Visser SBRH, Danielson ML, Perou R. Morbidity and Mortality Weekly Report (MMWR) Atlanta, GA: 2010. Increasing Prevalence of Parent-Reported Attention-Deficit/Hyperactivity Disorder among Children -- United States, 2003 and 2007. [PubMed] [Google Scholar]
- Weng H-Y, Hsueh Y-H, Messam LLM, Hertz-Picciotto I. Methods of covariate selection: directed acyclic graphs and the change-in-estimate procedure. American journal of epidemiology. 2009;169:1182–1190. doi: 10.1093/aje/kwp035. [DOI] [PubMed] [Google Scholar]
- Whyatt R, Camann D, Perera F, Rauh V, Tang D, Kinney P, Garfinkel R, Andrews H, Hoepner L, Barr D. Biomarkers in assessing residential insecticide exposures during pregnancy and effects on fetal growth. Toxicology and applied pharmacology. 2005;206:246–254. doi: 10.1016/j.taap.2004.11.027. [DOI] [PubMed] [Google Scholar]