Abstract
Diffuse structural abnormalities in the prefrontal cortex have been reported in both schizophrenia patients and their nonpsychotic biological relatives. Additionally, working memory difficulties have long been documented in schizophrenia patients and have been associated with the genetic liability for the disorder. The present analysis investigated the relationship between prefrontal regional grey matter volumes and two facets of working memory in schizophrenia using a family study. Structural neuroimaging scans provided measurements of rostral middle, superior, and inferior prefrontal cortical grey matter volumes. Participants also completed a spatial working memory task that measured both short-term maintenance and manipulation of material in memory. Both schizophrenia patients and relatives had reduced superior and inferior frontal grey matter volumes. Schizophrenia patients demonstrated a spatial working memory deficit compared to both controls and relatives, with no greater impairment when required to manipulate material. Smaller prefrontal volumes in schizophrenia patients were associated with worse working memory performance. These relationships were absent in the nonpsychotic relatives and controls. Despite normative behavioural performance, nonpsychotic relatives demonstrated abnormalities in brain structure similar to those found in schizophrenia patients. Manipulation abilities were not more impaired than maintenance in schizophrenia patients. Consistent with other neuroimaging research, our results suggest that direct measures of the underlying biology may be more sensitive to the effects of the genetic liability for schizophrenia than behavioural measures.
Keywords: Schizophrenia, working memory, prefrontal cortex, family study, grey matter
1. INTRODUCTION
Prefrontal structural abnormalities and working memory difficulties have long been documented in schizophrenia patients and have also been found in their nonpsychotic family members (Cannon et al., 2002; Cannon et al., 1998; Snitz et al., 2006). The goal of the present study was to investigate prefrontal grey matter abnormalities and examine their relevance to different aspects of spatial working memory in schizophrenia patients and nonpsychotic first-degree biological relatives by using a cognitive neuroscience task that isolated working memory maintenance from manipulation processes (Cannon et al., 2005; Kim et al., 2004). Inclusion of both patients and family members allowed a better examination of genetic (familial) liability, as well as disease-related processes.
The prefrontal cortex has been shown to be consistently involved in working memory. Distinct components of the middle frontal region, the rostral and caudal areas, have been demarcated, with differing roles in working memory (Wager and Smith, 2003). The rostral area encompasses parts of Brodmann’s area 46, which is considered part of the dorsolateral prefrontal cortex, whereas the caudal area is considered part of the premotor region (Kikinis et al., 2010). In a meta-analysis, Brodmann’s area 46 was identified as being consistently activated during the manipulation of information held in working memory (Owen et al., 2005). Additionally, the superior frontal region had a role in continuous updating of content (Wager and Smith, 2003). Activations of the inferior frontal region were related to manipulation of information, primarily switching and inhibition (Wager and Smith, 2003). Furthermore, a meta-analysis of N-back working memory studies (which required remembering the stimulus that occurred “N” positions previously) demonstrated that the middle frontal region and inferior frontal region were consistently hypoactive, and the middle frontal and superior frontal region were consistently hyperactive in schizophrenia patients compared to controls (Glahn et al., 2005). In a meta-analysis of working memory studies in the relatives of schizophrenia patients, relatives showed hypofrontality in the right middle and inferior frontal regions and hyperfrontality in the right middle frontal region compared to controls, suggesting these abnormalities are related to the genetic liability for the disorder and cannot wholly be accounted for by disease process and medication (Goghari, 2010). Concurrent with prefrontal cortical activation abnormalities found to be associated with the genetic risk for schizophrenia (Goghari, 2010; Walton et al., 2013a; Walton et al., 2013b), prefrontal grey matter volume, including the sub-regions assessed in this paper, have shown associations with the genetic risk for the disorder (Bhojraj et al., 2011a; Bhojraj et al., 2011b; Chen et al., 2013; Rosso et al., 2010); however, this is not a wholly consistent finding (Goghari et al., 2007a, b), likely due to the heterogeneity in the samples studied and methods. Regardless, current literature supports investigating the relationship between prefrontal grey matter volumes and working memory abilities.
Working memory ability has been consistently demonstrated to be impaired in schizophrenia patients (Dickinson et al., 2007; Forbes et al., 2009) and in their family members (Snitz et al., 2006). One influential model of working memory by Baddeley (1992) includes a cognitive construct called the central executive, which controls attention and manipulates information, and secondary constructs called the phonological loop and visuospatial sketchpad, which store and rehearse information in short-term memory. Despite the acceptance of the varied processes termed working memory, the majority of schizophrenia studies have used tasks, such as the N-back and letter-number sequencing, which do not distinguish maintenance from manipulation processes. More recently, Cannon and colleagues have investigated maintenance and manipulation components using a task informed by findings from cognitive neuroscience approaches (Cannon et al., 2005; Glahn et al., 2002; Kim et al., 2004). Two studies have employed this task to investigate the maintenance and manipulation of spatial working memory content in schizophrenia (Cannon et al., 2005; Kim et al., 2004). The behavioural study demonstrated that schizophrenia patients were impaired in both aspects of spatial working memory, but were particularly impaired when manipulation of information was required (Kim et al., 2004). A second study evaluated the neural correlates of maintenance compared to manipulation, finding that when spatial manipulation of information was required, controls recruited the dorsolateral prefrontal cortex (BA 45 and 46) to a greater degree than schizophrenia patients (Cannon et al., 2005). Additionally, schizophrenia patients showed greater impairment in accuracy when manipulation of information held in working memory was required (Cannon et al., 2005). However, greater impairment when manipulation is required compared to maintenance is not a uniform finding in schizophrenia (Hill et al., 2010; Quee et al., 2011; Schlosser et al., 2008; Thakkar and Park, 2012). Thakkar and Park (2012) suggest that these divergent finding may be due to the differing demands on encoding and maintenance processes that are also present in the manipulation task.
To the best of our knowledge, maintenance and manipulation aspects of working memory have not been investigated using the Cannon spatial working memory tasks (Cannon et al., 2005; Kim et al., 2004) with a family study design. A better understanding of how genetic liability for schizophrenia affects different aspects of working memory could be an important advancement in mapping cognitive phenotypes onto genes predisposing the disorder. First, we examined whether prefrontal grey matter was reduced in schizophrenia patients and first-degree biological nonpsychotic relatives compared to controls. Second, we examined whether greater spatial manipulation than maintenance impairments would be replicated in an independent sample of schizophrenia patients and whether that pattern would also be found in relatives. Third, we investigated the relationship of spatial maintenance and manipulation working memory processes with prefrontal grey matter volume in schizophrenia patients, relatives, and healthy controls to determine whether behaviour and brain abnormalities were related. We hypothesized that schizophrenia patients and relatives would have less prefrontal volume compared to controls. We also predicted that schizophrenia patients and relatives would demonstrate impaired performance during the spatial working memory task, with greater impairment in the manipulation compared to maintenance condition. Last, we predicted that in schizophrenia patients, less grey matter in prefrontal areas would be related to worse spatial working memory task performance.
2. MATERIALS AND METHODS
2.1. Participants
Schizophrenia and schizoaffective probands were recruited from the Minneapolis VA Medical Center outpatient clinics and community support programs for the mentally ill. Research staff identified first-degree biological relatives by completing a pedigree with the proband. Controls were recruited through posting announcements in the community.
Twenty-four schizophrenia and schizoaffective patients (hereafter schizophrenia), 21 nonpsychotic relatives of schizophrenia patients, and 37 community control subjects participated in the structural MRI protocol and 30 schizophrenia patients, 25 nonpsychotic relatives, and 30 controls participated in the working memory task protocol. Seventeen schizophrenia patients, 15 relatives, and 18 controls participated in both the structural MRI and working memory task protocols. Schizophrenia patients and controls were excluded if English was their second language, for mental retardation, current alcohol abuse, current drug abuse/dependence, a current or past central nervous system condition, history of head injury with skull fracture or substantial loss of consciousness, a history of electroconvulsive therapy, and an age less than 18 or greater than 60. Controls were further excluded for a family history of psychosis or bipolar disorder. To maximize relative recruitment, relatives were excluded only if they were under the age of 18 and over the age of 60, had a lifetime diagnosis of a psychotic disorder, or unable to complete the protocol. However, no relative met criteria for an Axis II Cluster A disorder or current substance abuse/dependence, had IQ in the mental retardation range, or English as a second language. Three relatives had a history of a head injury and one relative had migraines. One control was on antipsychotic medications for his/her diagnoses of major depressive disorder (MDD), post-traumatic stress disorder (PTSD), and borderline personality disorder. The Minneapolis VA Medical Center and University of Minnesota Institution Review Boards approved the protocol.
2.2. Diagnosis and Assessment
The Structured Clinical Interview for DSM Disorders and the Psychosis Module of the Diagnostic Interview for Genetic Studies (Nurnberger et al., 1994) were completed with each participant. Axis II Cluster A traits were assessed with the Structured Interview for Schizotypy in relatives and controls (Kendler et al., 1989). A clinical psychologist reviewed all materials to determine DSM-IV-TR diagnoses. Schizophrenia patients’ current symptomatology was assessed using the Scale for the Assessment of Negative Symptoms (SANS; Andreasen, 1981) and the Scale for the Assessment of Positive Symptoms (SAPS; Andreasen, 1983). All participants had their psychiatric functioning assessed using the Brief Psychiatric Rating Scale (BPRS; Ventura et al., 1993). Handedness was determined by asking participants which hand they preferred overall.
Nonpsychotic relatives and controls were largely asymptomatic in terms of their current Axis 1 diagnoses, as also reflected in the their BPRS scores: 3 relatives had current Axis I diagnoses (1 individual with anxiety not otherwise specified (NOS), 1 individual with PTSD and MDD, and 1 individual with specific phobia) and 6 controls had current Axis I diagnoses (1 individual with depression NOS, 1 individual with PTSD, 1 individual with PTSD and dysthymia, and 3 individuals with specific phobia). The breakdown for a lifetime Axis I disorder diagnosis in the relatives was: 5 individuals with MDD; 2 individuals with PTSD; 1 individual with a specific phobia; 3 individuals with anxiety NOS; 8 individuals with alcohol abuse; 1 individual with substance (other than alcohol) dependence; 2 individuals with substance (other than alcohol) abuse; and 1 individual with an eating disorder NOS. The breakdown for a lifetime Axis I disorders in the controls was: 5 individuals with MDD; 1 individual with dysthymia; 2 individuals with depression NOS; 1 individual with bereavement; 1 individual with mood disorder due to substance use; 1 individual with obsessive-compulsive disorder; 3 individuals with PTSD; 3 individuals with a specific phobia; 10 individuals with alcohol abuse; 7 individuals with alcohol dependence; 3 individuals with substance (other than alcohol) abuse; 1 individual with substance (other than alcohol) dependence; 1 individual with bulimia nervosa; and 1 individual with attention deficit hyperactivity disorder. Some individuals had more than one disorder. The specific percentage of relatives and controls with a current and lifetime Axis I disorder for the different samples is presented in Table 1.
Table 1.
Participant characteristics.
| Structural MRI Sample | Working Memory Sample | Combined Sample | |||||||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| Schizophrenia | Relative | Control | Schizophrenia | Relative | Control | Schizophrenia | Relative | Control | |
| N | 24 | 21 | 37 | 30 | 25 | 30 | 17 | 15 | 18 |
| Age | 42.0 (10.0)a | 49.1 (6.8) | 41.6 (11.6)a | 42.1 (10.2)a | 49.7 (7.5) | 40.6 (12.6)a | 43.1 (10.1) | 48.8 (7.8) | 34.1 (10.0)ac |
| Gender (% female) | 25a | 62 | 46 | 23ab | 68 | 53 | 24a | 67 | 50 |
| Education (Years Completed) | 14.5 (2.5) | 14.6 (2.0) | 15.50 (1.9) | 14.5 (2.3)b | 15.0 (1.8) | 16.0 (1.7) | 14.7 (2.6) | 14.7 (2.2) | 15.9 (1.6) |
| Education (% HS completed) | 92 | 100 | 97 | 97 | 100 | 97 | 94 | 100 | 94 |
| Maternal Education (% HS completed) | 88 | 90 | 89 | 90 | 83 | 80 | 82 | 86 | 89 |
| Handedness (% right handed) | 96 | 84 | 86 | 82 | 91 | 96 | 94 | 85 | 94 |
| WAIS-III Vocabulary (Raw) | 45.2 (9.1)a | 53.8 (12.4) | 50.8 (9.7) | 48.0 (9.5) | 52.6 (10.2) | 52.9 (9.6) | 48.4 (7.1) | 54.9 (11.1) | 51.2 (10.2) |
| WAIS-III Block Design (Raw) | 35.8 (12.5) | 41.1 (11.0) | 41.0 (10.2) | 36.2 (12.1) | 41.9 (9.3) | 40.9 (10.9) | 32.6 (11.5)ab | 42.6 (9.9) | 42.7 (10.1) |
| BPRS Total | 45.7 (13.4) | 27.8 (7.3)c | 28.7 (4.3)c | 44.9 (14.3) | 31.5 (5.7)c | 27.3 (9.3)c | 44.7 (12.4) | 29.7 (3.9)c | 28.8 (5.6)c |
| SANS Total | 26.4 (15.6) | - | - | 25.6 (16.1) | - | - | 23.4 (15.3) | ||
| SAPS Total | 18.2 (16.7) | - | - | 17.4 (13.3) | - | - | 17.5 (15.7) | ||
| Axis I (% with any nonpsychotic current diagnosis) | - | 14 | 11 | - | 8 | 17 | - | 13 | 17 |
| Axis I (% with any nonpsychotic lifetime diagnosis) | - | 67 | 49 | - | 36 | 47 | - | 60 | 44 |
|
| |||||||||
| Medications | |||||||||
| Antipsychotic (Atypical, Typical; % on) | 92 (83, 17) | 0 | 3 (3, 0) | 87 (73, 17) | 8 (8, 0) | 3 (3, 0) | 88 (77, 18) | 0 | 6 (6, 0) |
| Antidepressants (% on) | 29 | 19 | 5 | 40 | 28 | 3 | 35 | 27 | 6 |
| Mood Stabilizer (% on) | 42 | 0 | 0 | 40 | 0 | 0 | 41 | 0 | 0 |
| Antianxiety (% on) | 21 | 0 | 0 | 23 | 0 | 0 | 24 | 0 | 0 |
| Antiparkinson (% on) | 25 | 0 | 0 | 20 | 4 | 0 | 29 | 0 | 0 |
| Other Psychiatric (% on) | 21 | 10 | 5 | 27 | 8 | 3 | 24 | 7 | 6 |
HS = high school; WAIS-III = Wechsler Adult Intelligence Scale, 3rd Edition; BPRS = Brief Psychiatric Rating Scale: 24 items, scores can range from 24–168; SANS = Scale for Assessment of Negative Symptoms: 20 items, scores can range from 0–100; SAPS = Scale for Assessment of Positive Symptoms: 30 items, scores can range from 0–150. The following notations were used for non-medication variables:
Less than relatives
Less than controls
Less than patients
Medication use was also recorded for participants. We compared groups on antidepressant and other psychiatric medication use as there were sufficient cases. We found overall group differences for both antidepressants (X2(2)=13.71, p=0.001) and other psychiatric medications (X2(2)=8.41, p=0.02). Specifically, schizophrenia patients were on more antidepressants (X2(1)=14.12, p<0.001) and other psychiatric medications (X2(1)=7.74, p=0.005) compared to controls, and relatives were on more antidepressants compared to controls (X2(1)=6.51, p=0.01). There were no differences between patients and relatives. The specific percentage of participants using medications for the different samples is presented in Table 1.
2.3. Structural Neuroimaging
Structural images were acquired using a standard MP-RAGE sequence (160 slices) on a 3T Siemens scanner at the Center for Magnetic Resonance Research, University of Minnesota. Images were collected with an 8-channel head coil system prior to a scanner upgrade after which images were collected with a 12-channel system. Parameters of the MP-RAGE sequence were (prior to scanner upgrade first, followed after scanner upgrade): TR=1600ms, 2300ms; TE=4.38, 2.98; Flip angle=15 degrees, 9 degrees; FOV=256mm, 256mm; thickness=1.0mm, 1.2mm. There was no difference in number of participants scanned in the three groups pre-post scanner upgrade (X2(2)=0.53, p=0.77). To assess the potential impact of scanner upgrade on the structural neuroimaging data, a MANOVA on the prefrontal brain regions was conducted with pre-post scanner as a factor in the model. There was an overall effect of scanner version on the structural MRI data (F(6, 71)=3.09, p=0.01), with greater grey matter volumes pre-scanner upgrade. Importantly, there was no interaction between scanner version and group (F(12, 144)=1.38, p=0.18). Furthermore, correlations assessed whether the association between prefrontal grey matter volume and working memory performance remained similar with and without scanner version as a covariate. The results demonstrated the significant and non-significant findings remained the same. Given these findings, it is unlikely the scanner version and head coil change would have affected the group differences.
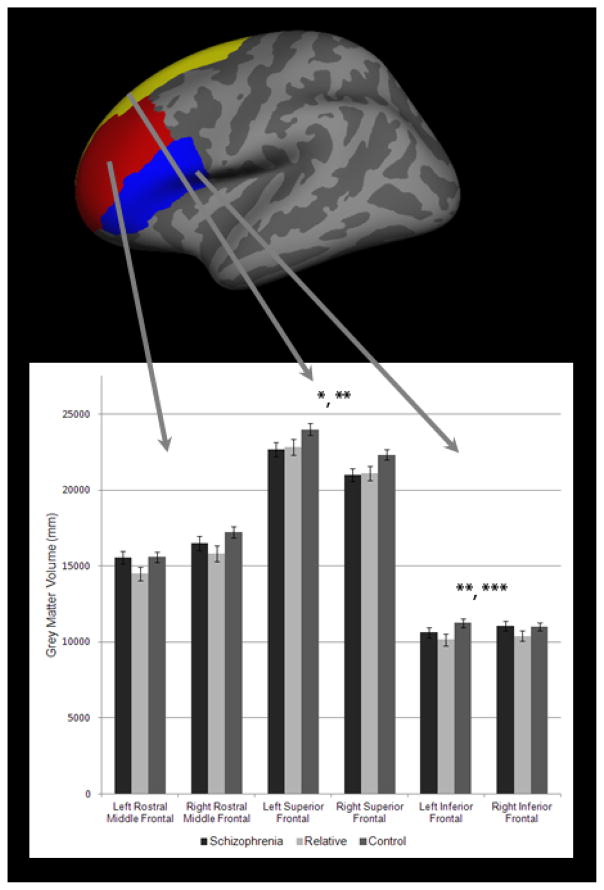
Volumetric segmentation, cortical reconstruction, and cortical and subcortical parcellations were performed with the Freesurfer image analysis suite, version 4.0.3 (http://surfer.nmr.mgh.harvard.edu/). Freesurfer processing included removal of non-brain tissue, automated Talairach transformation, watershed/surface deformation procedure, segmentation of the subcortical grey matter volumetric structures, intensity normalization, tessellation of the grey matter white matter boundary, and surface deformation (Dale et al., 1999; Fischl et al., 2002). Further data processing and analysis included surface inflation, registration to a spherical atlas, which utilized individual cortical folding patterns, and parcellation of the cerebral cortex (Fischl et al., 1999; Fischl et al., 2004). Freesurfer morphometric procedures demonstrated good test-retest reliability across scanner manufacturers and across field strengths (Han et al., 2006). This study focused on a priori defined rostral middle frontal, superior frontal, and inferior frontal regions (see Figure 1).
Figure 1.
Prefrontal grey matter regions depicted on an inflated Freesurfer brain and grey matter volumes in schizophrenia patients, nonpsychotic relatives, and controls
Bars represent means and brackets respresent standard errors.
*Patients less than controls bilaterally
**Relatives less than controls bilaterally
2.4. Spatial Working Memory Task
Participants completed a spatial working memory task with two conditions, maintenance (or “hold” as presented to participants), and maintenance and manipulation (hereafter referred to as manipulation or “flip” as presented to participants; see Figure 2) (Glahn et al., 2002). In the maintenance condition, participants were asked to remember the location of three filled circles and after a delay, respond whether a second set of three circles were a spatial match or not. In the manipulation condition, participants were asked to mentally mirror flip (i.e., mental rotation) the initial image consisting of three filled circles and after a delay respond if the second set of circles was a mirror flip or not. Cue and probe stimuli were each present for 1500ms, the inter-stimulus interval was 6000ms, and the inter-trial interval was 1500ms. The words “hold” and “flip” were present during the delay period. The maintenance and manipulation trials were presented in blocks of two, and the task alternated between trial types, with the task beginning with two maintenance trials. Before each condition, participants were informed whether the next set of trials was “hold” or “flip” trials. There were 20 maintenance and 20 manipulation trials.
Figure 2.
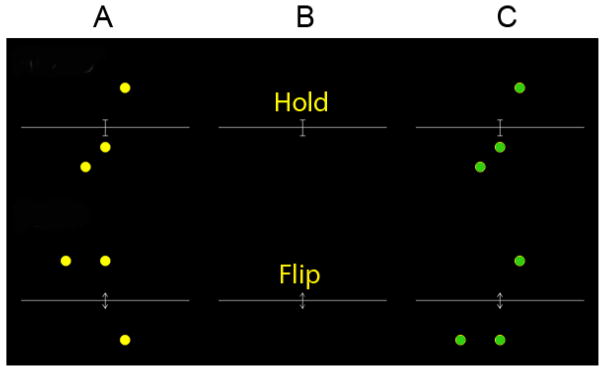
Maintenance and manipulation spatial working memory task
Example of a correct maintenance trial and a correct manipulation trial. The hold trial is the maintenance trial, which requires participants to maintain the information over a delay. The flip trial is the maintenance plus manipulation condition, which requires participants to mirror flip (i.e., mentally rotate) the initial image after a delay and respond if the second image was a mirror flip or not. A - represents the initial image made of three circles, B - represents the delay, and C -represents the probe to which the participants must respond. Cue and probe stimuli were each presented for 1500ms, the inter-stimulus interval was presented for 6000ms, and the inter-trial interval was presented for 1500ms.
2.5. Statistical Analyses
First, the normality of the data was tested using the one-sample Kolmogorov Smirnov analysis. All of the structural MRI data were normally distributed (p’s>0.22). The behavioural task data were largely normally distributed (p’s>0.08), other than for maintenance accuracy (p=0.008).
First, a 3 group (schizophrenia, relative, control) × 3 prefrontal region (rostral middle, superior, inferior) × 2 hemisphere (left, right) mixed model analysis of covariance (ANCOVA) was used to assess the effect of group and whether that effect varied by region and hemisphere. A 3 group × 2 hemisphere (left, right) ANCOVA was conducted for each region to follow-up on significant effects. Intracranial volume was included as a covariate in the structural analyses. A 3 group × 2 working memory condition (maintenance, manipulation) ANOVA was used to assess the effect of group and working memory condition on accuracy and reaction time data. For both the grey matter volume and working memory data, individual contrasts between groups were only conducted if there was an effect in the overall ANCOVA/ANOVA to control for multiple comparisons.
Given the group differences in age and gender, follow-up analyses of significant effects were conducted with age and gender as additional covariates. To examine the relationship between age with prefrontal grey matter volume and working memory dependent measures, linear regressions were conducted using group, age, and group by age interactions as predictors. To investigate the effects of gender on our variables, multivariate analysis of variance (MANOVA) and mixed-model ANOVAs were conducted. Greenhouse-Geisser correction is reported for ANCOVAs. Partial eta-squared effect sizes (η2) are also provided.
All correlations were assessed using the Pearson’s r. For the prefrontal regions that showed group differences, two-tail tests were conducted within group to examine the hypothesis that smaller grey matter volumes were associated with lower accuracy on the working memory task. Correlations were also used to assess the relationship between prefrontal grey matter and task performance, with symptoms (SAPS and SANS total scores) and WAIS-III sub-tests (vocabulary and block design). Steiger’s Z was used to determine whether correlations differed significantly within a group. Fisher’s Z-test was used for comparisons of correlations between groups.
3. RESULTS
3.1. Participants
Participant characteristics for the structural MRI and working memory samples are presented in Table 1. Statistics for the MRI sample and then the working memory sample are presented in the text. Characteristics of the individuals with both MRI and working memory data (i.e., combined sample) were similar and are presented in Table 1. The three groups differed for age in both samples (F(2, 79)=4.19, p=0.02; F(2, 82)=5.76, p=0.005), with relatives being older than patients (p=0.02; p=0.009) and controls (p=0.008; p=0.002). The three groups also differed in gender distribution (X2(2)=6.31, p=0.04; X2(2)=11.65, p=0.003), with the patient group having fewer females than the relative group (X2(1)=6.25, p=0.01; X2(1)=11.06, p=0.001) in both samples, and fewer females than in the control group (X2(1)=5.71, p=0.02) in the working memory sample. Groups did not differ for high school completion for participants (X2(2)=2.38, p=0.30; X2(2)=0.85, p=0.65), or for their mothers (X2(2)=0.07, p=0.97, X2(2)=1.18, p=0.55). There was no difference between groups for years of education in the structural MRI sample (F(2, 71)=1.92, p=0.15); however, there was a significant difference in the working memory sample (F(2, 78)=4.20, p=0.02) with post-hocs demonstrating schizophrenia patients having fewer years of education than controls (p=0.006). Groups did not differ for handedness distributions (X2(2)=1.74, p=0.42; X2(2)=2.75, p=0.25). In the structural MRI sample, groups differed on WAIS-III vocabulary sub-test score (F(2, 73)=3.58, p=0.03), with schizophrenia patients having lower scores than relatives (p=0.01) and marginally lower than controls (p=0.056); whereas, there was no significant difference in the working memory sample (F(2, 76)=2.09, p=0.13). No significant differences were found for block design in either sample (F(2, 73)=1.70, p=0.19; F(2, 76)=2.00, p=0.14). Last, groups differed on BPRS total score (F(2, 79)=34.42, p<0.001; F(2, 81)=21.97, p<0.001), with schizophrenia patients having higher symptomatology compared to controls (p’s<0.001) and relatives (p’s<0.001). Importantly relatives and controls did not differ on BPRS symptomology (p=0.70; p=0.15). Nor did relatives and controls differ for percentage of participants with a current Axis I diagnoses (X2=(1)=0.15, p=0.70; X2(1)=0.92, p=0.34). Furthermore, these groups did not differ for percentage of participants with a lifetime Axis I diagnoses (X2(1)=1.76, p=0.19; X2(1)=0.64, p=0.43). For relatives, superior frontal grey matter volume was reduced in individuals with a lifetime Axis I disorder compared to individuals without (F(1, 18)=4.29, p=0.05), when covarying for intracranial volume. No other measure showed a difference in either relatives or controls. Together, these findings suggest that differences between relatives and controls on the brain and working memory measures are likely not due to differences in symptoms or functioning.
3.2. Prefrontal Grey Matter Volume
3.2.1. Effects of Group
A 3 group (schizophrenia, relative, control) × 3 region (rostral middle frontal, superior frontal, and inferior frontal) × 2 hemisphere (left, right) ANCOVA was conducted to assess whether group differences varied by the prefrontal grey matter region and hemisphere. There was a significant main effect of group (F(2, 78)=3.68, p=0.03, partial η2=0.09) and intracranial volume covariate (F(1, 2)=80.83, p<0.001). There were no significant interactions between group and region (F(4, 155)=1.74, p=0.15, partial η2=0.04) or group and region and hemisphere (F(4, 138)=1.15, p=0.33, partial η2=0.03). To follow-up on the significant group effect, three 3 group × 2 hemisphere ANCOVAs on the rostral middle frontal, superior frontal, and inferior frontal grey matter volumes were conducted. To control for multiple comparisons, follow-up testing was conducted to determine whether specific groups differed only when a significant finding was demonstrated in this overall ANCOVA.
In the 3 group × 2 hemisphere ANCOVA of the rostral middle frontal region there was no significant effect of group (F(2, 78)=2.68, p=0.08, partial η2=0.06) or group by hemisphere interaction (F(2, 78)=1.14, p=0.33, η2=0.03); therefore, no follow-up testing was conducted for this region (see Figure 1).
In the 3 group × 2 hemisphere ANCOVA of superior frontal region, there was a significant main effect of group (F(2, 78)=4.01, p=0.02, partial η2=0.09) and a significant effect of the intracranial volume covariate (F(1, 78)=105.59, p<0.001). Follow-up ANCOVAs demonstrated both schizophrenia patients (F(1, 58)=5.36, p=0.02, partial η2=0.09) and nonpsychotic relatives (F(1, 55)=6.29, p=0.02, partial η2=0.10) had less bilateral superior frontal grey matter compared to controls. There were also significant effects of intracranial volume covariate (F’s=60.65–106.41, p’s<0.001). There was no significant difference between schizophrenia patients and nonpsychotic relatives in superior frontal grey matter volume (F(1, 42)=0.03, p=0.86, partial η2=0.001). No significant interactions between hemisphere and group in this region were found in any of ANCOVAs. When the additional covariates of age and gender were added to the ANCOVAs, the significant effect of group remained (F(1, 56)=4.59, p=0.04, partial η2=0.08) in the patients versus controls contrast and a trend towards a group effect (F(1, 53)=3.197, p=0.08, partial η2=0.06) remained in the relatives versus controls contrast.
In the 3 group × 2 hemisphere ANCOVA of the inferior frontal region, there was no main effect of group (F(2, 78)=2.11, p=0.13, partial η2=0.05); however, there was a significant group by hemisphere interaction (F(2, 78)=3.04, p=0.05, partial η2=0.07) and also an effect of the intracranial volume covariate (F(1, 78)=22.27, p<0.001). Follow-up ANCOVA of schizophrenia patients and controls demonstrated no main effect of group (F(1, 58)=0.49, p=0.49, partial η2=0.01); however, revealed a significant group by hemisphere (F(1, 58)=4.77, p=0.03, partial η2=0.08) interaction and effect of the intracranial volume covariate (F(1, 58)=16.58, p<0.001). Inspection of group means for inferior frontal volumes demonstrated that schizophrenia patients tended to have less volume in the left inferior frontal region compared to controls, whereas the groups were more comparable for the right inferior frontal region. The ANCOVA comparing nonpsychotic relatives and controls demonstrated a significant main effect of group (F(1, 55)=6.26, p=0.02, partial η2=0.10), with nonpsychotic relatives demonstrating less bilateral inferior prefrontal volume compared to controls. There was also a significant effect of the intracranial volume covariate (F(1, 55)=19.70, p<0.001); however, no interaction between group and hemisphere (F(1, 55)=2.61, p=0.11, partial η2=0.05) was found. In the ANCOVA comparing schizophrenia patients to relatives, no significant main effects of group (F(1, 42)=1.43, p=0.24, partial η2=0.03) or group by hemisphere interaction (F(1, 42)=0.47, p=0.499, partial η2=0.01) were found. When the additional covariates of age and gender were added into the model, there remained a significant group by hemisphere interaction (F(1, 56)=3.99, p=0.05, partial η2=0.07) in the patients versus controls contrast and there was a trend towards a group effect (F(1, 53)=3.52, p=0.07, partial η2=0.06) in the relatives versus controls contrast.
3.2.2. Effects of Age and Gender
Linear regressions demonstrated relationships between age and all the prefrontal grey matter regions (Beta’s=−0.42- −0.61, p’s<0.001–0.002), but no effects of group or interactions between group and age. A 3 group × 2 hemisphere × 2 gender MANCOVA with intracranial volume as a covariate was conducted to determine the effects of gender. The MANCOVA demonstrated there was no overall effect of gender across groups in the prefrontal regions (F(6,70)=0.92, p=0.49), nor a group by gender interaction (F(12, 142)=1.54, p=0.12).
3.3. Performance on the Spatial Working Memory Task
Effects of Group
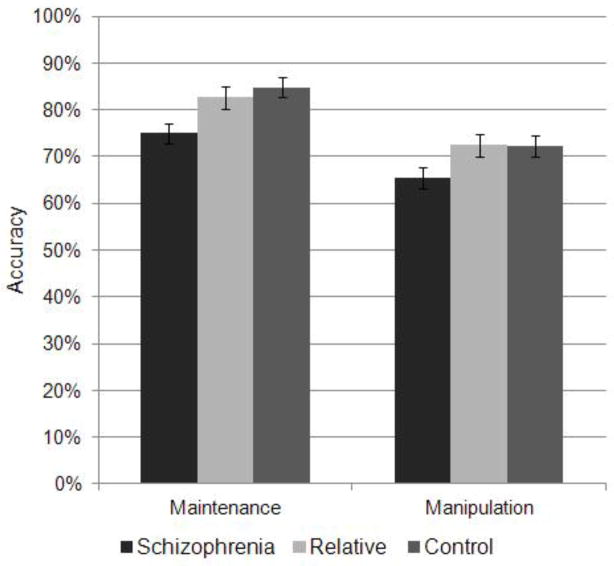
A 3 group × 2 working memory condition ANOVA evaluated whether there was a significant difference between the maintenance and manipulation conditions accuracy and whether that interacted with group. Least-square difference post-hocs were used to follow-up on the overall ANOVAs. The 3 group × 2 condition ANOVA demonstrated a significant main effect of working memory condition collapsed across group (F(1, 82)=48.29, p<0.001, partial η2=0.37), with the manipulation condition being more difficult than the maintenance condition. Additionally there was a significant main effect of group (F(2, 82)=6.55, p=0.002, partial η2=0.14) collapsing across conditions, with schizophrenia patients having reduced accuracy compared to controls (p=0.001) and nonpsychotic relatives (p=0.006; see Figure 3). There was no difference between relatives and controls (p=0.70). There was no significant interaction between working memory condition and group (F(2, 82)=0.40, p=0.67, partial η2=0.01) suggesting that the manipulation condition was not more impaired than the maintenance condition between the groups. When age and gender were included in the model as covariates, there was also a significant effect of group in patients versus controls contrast (F(1, 56)=10.86, p=0.002, partial η2=0.16) and patients versus relatives contrast (F(1, 51)=15.28, p<0.001, partial η2=0.23).
Figure 3.
Maintenance and manipulation spatial working memory task accuracy in schizophrenia patients, nonpsychotic relatives, and controls
Bars represent means and brackets are standard errors. Patients had reduced accuracy compared to controls and relatives across both maintenance and manipulation conditions. No differential effect of maintenance versus manipulation was found in patients.
Next, we specifically evaluated the two working memory conditions. There was a significant effect of group on maintenance accuracy (F(2, 82)=6.00, p=0.004, partial η2=0.13), with schizophrenia patients having reduced accuracy compared to controls (p=0.001) and nonpsychotic relatives (p=0.02). Nonpsychotic relatives did not differ from controls (p=0.47). There was a trend towards a significant effect of group on the manipulation condition (F(2, 82)=2.92, p=0.059, partial η2=0.07), with schizophrenia patients having reduced accuracy compared to controls (p=0.04) and nonpsychotic relatives (p=0.04). There was no significant difference between nonpsychotic relatives and controls (p=0.95). When age and gender were included in the models as covariates, significant effects of group for maintenance and manipulation conditions were found in the patients versus controls contrast (F(1, 56)=7.72, p=0.007, partial η2=0.12; F(1, 56)=6.27, p=0.02, partial η2=0.10 respectively) and for the patients versus relatives contrast (F(1, 51)=6.99, p=0.01, partial η2=0.12; F(1, 51)=12.92, p=0.001, partial η2=0.20 respectively).
A 3 group × 2 working memory condition ANOVA evaluated whether there was a significant difference between the maintenance and manipulation condition reaction times and whether that interacted with group. There was a main effect of working memory condition (F(1, 82)=77.14, partial η2=0.49), with the manipulation condition having longer reaction times than the maintenance condition. There was no significant main effect of group (F(2, 82)=0.92, p=0.40, partial η2=0.02) nor a group by hemisphere interaction (F(2, 82)=0.16, p=0.85, partial η2=0.004). Additionally, there were no significant effects of group on reaction times for either the maintenance (F(2, 82)=0.82, p=0.44, partial η2=0.02) or manipulation (F(2, 82)=0.82, p=0.45, partial η2=0.02) conditions.
3.3.4. Effects of Age and Gender
Linear regressions demonstrated relationships between age and working memory performance. For maintenance accuracy, there was an interaction between group (schizophrenia compared to controls) and age (Beta= −1.13, p=0.008) and for maintenance reaction times there was a relationship with age (Beta=0.40, p=0.005). Two 3 group × 2 gender MANOVAs were conducted to determine the effects of gender on accuracy and reaction times. For the accuracy data, across groups and working memory conditions there was no effect of gender (F(1, 79)=1.16, p=0.29) and no interaction between group and gender (F(2, 79)=0.72, p=0.49), gender and working memory condition (F(1, 79)=3.00, p=0.09), or interaction between group, gender, and working memory condition (F(2, 79)=0.67, p=0.52). For the reaction time data, there was no main effect of gender (F(1, 79)=0.75, p=0.39), group by gender interaction (F(2, 79)=0.79, p=0.46), or group by working memory interaction (F(1, 79)=1.85, p=0.18); however, there was a group by working memory condition by gender interaction (F(2, 79)=6.65, p=0.002), indicating that female schizophrenia patients had faster reaction times for the maintenance condition compared to the manipulation condition in contrast to males. This was different from the pattern compared to controls (F(1, 56)=10.03, p=0.002) and relatives (F(1, 51)=10.37, p=0.002).
3.4. Associations between Prefrontal Grey Matter Regions, Task Performance, Symptoms, and WAIS-III Sub-Tests
Superior frontal grey matter volumes were combined across hemispheres, given the lack of hemisphere specific effects. Inferior frontal grey matter volumes were analyzed separately for each hemisphere, given the group by hemisphere interaction in schizophrenia patients. These three volumes were correlated with accuracy for each group, in the two working memory conditions (see Table 2 and Figure 4). In schizophrenia patients, greater bilateral superior frontal volume was associated with greater accuracy on the manipulation condition (r=0.57, p=0.02) and marginally associated with accuracy in the maintenance condition (r=0.46, p=0.06). These correlations did not differ in significance (Steiger’s Z=0.46, p=0.65). The relationship between superior grey matter volume and accuracy did not exist in the other groups (r’s=−0.17- −0.20) and the correlations differed significantly between schizophrenia patients and both controls and relatives (Fisher’s Zs=2.15–2.18, p’s=0.03). For the inferior frontal grey matter volumes in schizophrenia patients, greater left hemisphere volume was associated with greater accuracy for the manipulation condition (r=0.56, p=0.02) and not for the maintenance condition (r=0.33, p=0.20). These correlations did not differ significantly (Steiger’s Z=0.99, p=0.32). The relationship between inferior grey matter volume and accuracy did not exist in the other groups (r’s=−0.04- −0.08). The correlations differed significantly between schizophrenia patients and controls (Fisher’s Z=1.93, p=0.05) and at a trend level with relatives (Fisher’s Z=1.71, p=0.09). For the right inferior frontal region there was no relationship between grey matter volume and manipulation or maintenance accuracy in any of the groups (r’s=−0.17–0.42).
Table 2.
Relationship between prefrontal grey matter volumes and spatial working memory task accuracy.
| Prefrontal Region | Task Condition | Schizophrenia | Relative | Control |
|---|---|---|---|---|
| Bilateral Superior | Maintenance | 0.46 (0.06) | 0.03 (0.92) | −0.21 (0.399) |
| Manipulation | 0.57 (0.02) | −0.20 (0.47) | −0.17 (0.50) | |
|
| ||||
| Left Inferior | Maintenance | 0.33 (0.20) | 0.26 (0.35) | −0.19 (0.46) |
| Manipulation | 0.56 (0.02) | −0.04 (0.898) | −0.08 (0.75) | |
|
| ||||
| Right Inferior | Maintenance | 0.11 (0.69) | 0.42 (0.12) | 0.004 (0.99) |
| Manipulation | 0.33 (0.19) | −0.17 (0.56) | −0.03 (0.89) | |
Values represent Pearson’s r (p value). Significant values are in bold.
Figure 4.
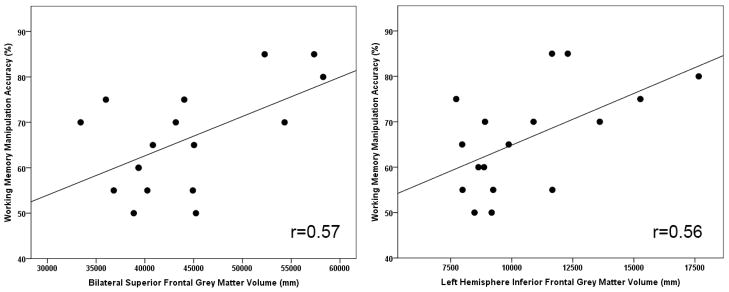
Relationship between spatial working memory manipulation accuracy and prefrontal grey matter volumes in schizophrenia patients
Positive and negative symptoms were not significantly correlated with prefrontal brain regions or behavioural task performance in schizophrenia patients. WAIS-III block design performance in schizophrenia patients was related to accuracy during the working memory manipulation task (r=0.44, p=0.03). There were no associations found between performance measures, cognitive functioning, and frontal grey matter volumes in the control and relative samples.
4. DISCUSSION
This study provides evidence that prefrontal lobe grey matter volumes are smaller in schizophrenia patients and nonpsychotic relatives compared to controls, suggesting that these abnormalities are related to the genetic liability for the disorder. Schizophrenia patients also demonstrated a generalized spatial working memory deficit that was not greater for manipulation than maintenance processes. In schizophrenia patients, grey matter deficits were predictive of impaired working memory performance. In contrast, relatives demonstrated intact spatial working memory behavioural performance. Consistent with numerous previous findings, schizophrenia patients demonstrated less grey matter prefrontal volume (Glahn et al., 2008; Shepherd et al., 2012) and impairments in working memory (Forbes et al., 2009; Lee and Park, 2005).
In this study, we found less grey matter volume in the bilateral superior and left inferior frontal prefrontal regions in schizophrenia patients compared to controls. The superior frontal region has been found to be reduced in schizophrenia (Ohtani et al., 2013; Xiao et al., 2013). Also, left hemisphere specific grey matter volume reductions in the inferior frontal region have been demonstrated previously in schizophrenia (Yoshihara et al., 2008) and have been related to poorer performance on a form of executive processing, semantic category switching (Ohtani et al., 2013). We also found nonpsychotic relatives of schizophrenia patients showed smaller prefrontal grey matter volumes than controls, reaching significance for the superior and inferior regions. Thus, aspects of reduced prefrontal gray matter volume may reflect genetic liability for schizophrenia (Bhojraj et al., 2011a; Cannon et al., 1998; Ho, 2007; Rosso et al., 2010); however, this is not a wholly consistent finding (Goghari et al., 2007a, b; Goldman et al., 2009).
Contrary to our hypothesis, we were unable to replicate the finding of a greater deficit during spatial manipulation compared to maintenance (Cannon et al., 2005; Kim et al., 2004). To assess potential reasons for this difference, we compared our study to that of Kim et al., (2004) as both studies were behavioural studies with very similar task presentation. Differences between the two studies included the age and gender distribution of the samples. Our patients and controls were in their early 40s whereas in the Kim et al. (2004) study, the participants were in their late 20s. Additionally, we had approximately a 25% female sample for patients and the Kim et al. (2004) study had 38% female sample for patients. In terms of spatial working memory performance, our controls had lower performance in both conditions, which was less pronounced for the maintenance condition (85% (SD=9) versus 88% (SD=10)) than for the manipulation condition (72% (SD=12) versus 77% (SD=11)). Another major difference, was that in our study, the patients had much lower maintenance accuracy (75% (SD=15) versus 81% (SD=15)) than in the Kim et al. (2004) study, but more similar manipulation accuracy (66% (SD=13) versus 64% (SD=16)). Hence, the differences between our findings could be due to differences in sample characteristics.
Nevertheless, the results of our study suggest that generalized cognitive dysfunction in schizophrenia may mask a specific cognitive deficit. Furthermore in contrast to the findings of greater manipulation related deficits, there has also been support for greater deficits in maintenance and not manipulation processes in schizophrenia (Hill et al., 2010; Quee et al., 2011; Schlosser et al., 2008; Thakkar and Park, 2012). Furthermore, Hill and colleagues (2010) found that deficits were less pronounced as manipulation load increased and Thakker and colleague (2012) found that manipulation abilities were superior in patients compared to controls for a mental rotation manipulation task. Our study supports these findings, as we found larger effect sizes for deficits in patients for the maintenance condition than the manipulation condition.
Alternatively, impairment in both manipulation and maintenance processes could reflect underlying neurobiological deficits in our patients. We found that the grey matter volume of the superior frontal cortex was associated with both manipulation and maintenance in schizophrenia. Additionally, neither superior nor inferior prefrontal grey matter volumes were more associated with manipulation than maintenance. In this study deficits were not observed for the rostral middle prefrontal cortex. The rostral middle frontal region (BA 46) is thought to be associated with manipulation of information (Kikinis et al., 2010; Wager and Smith, 2003). Cannon and colleagues (2005) found that a region including the rostral middle frontal area was the only region recruited to a greater extent in controls than patients during manipulation using this task. Perhaps the absence of rostral middle frontal abnormalities in our patient sample could explain a lack of greater spatial working memory deficits with manipulation.
Despite prefrontal structural abnormalities, relatives showed similar working memory performance to controls suggesting that reductions in grey matter volume were not severe enough to affect working memory function. Alternatively, nonpsychotic relatives may rely on compensatory brain mechanisms that preserve working memory performance.
Finally, the association of prefrontal grey matter volumes with working memory performance, and a lack of association with symptom ratings, is consistent with cognitive impairments in the disorder being generally more stable than symptomatology. Structural aspects of the brain are presumably more stable than functional elements; therefore, across participants with a range of symptomatology there might be a closer link between cognitive measures and brain structure.
Limitations of this study include a modest sample size; however, effect sizes were large enough to result in several significant findings. Power may not have been sufficient to detect performance deficits in patients’ relatives, which have been found in other work (Snitz et al., 2006); however, analysis of effect sizes suggest that the differences between relatives and controls for spatial working memory were negligible. Our samples were not matched for age and gender; however, we conducted analyses to investigate the relationship between these demographic variables and our dependent variables. Furthermore, our exclusion criteria for relatives were limited to facilitate recruitment and to enhance representativeness of first-degree relatives of schizophrenia patients.
In summary, schizophrenia patients demonstrated abnormalities in prefrontal grey matter that correlated with impaired working memory performance. Nonpsychotic relatives also demonstrated reduced prefrontal grey matter, but had intact working memory function. Family members may serve as a sample to investigate neural and behavioural compensatory mechanisms that lead to intact executive control to develop novel pharmacological and cognitive interventions.
Acknowledgments
ROLE OF FUNDING SOURCE
This work was supported by a Thesis Research Grant from the University of Minnesota; PGS Doctoral Award from the Natural Sciences and Engineering Research Council of Canada; University of Calgary Start-up, Seed, and Starter Grants; Canadian Institutes of Health Research Operating Grant and New Investigator Award to VMG; from the Clinical Science Merit Review Program Grant of the Department of Veterans Affairs to SRS; and from the National Institutes of Health (R24 MH069675 to SRS; R21 MH079262 to AWM; P41 RR008079 and P30 NS057091 to the Center for Magnetic Resonance Imaging at the University of Minnesota). The funding sources had no further role in study design; in the collection, analysis and interpretation of data; in the writing of the report; or in the decision to submit the paper for publication.
We gratefully acknowledge the Sponheim and MacDonald labs for their help with data collection. We thank Dr. Tyrone Cannon for providing the working memory task.
Footnotes
CONFLICT OF INTEREST
No authors report any potential conflicts of interest.
CONTRIBUTORS
Vina Goghari, Angus MacDonald, and Scott Sponheim designed the study and wrote the protocol. Vina Goghari managed the literature searches and analyses. Vina Goghari undertook the statistical analysis, and wrote the first draft of the manuscript. All authors contributed to and have approved the final manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Andreasen NC. Scale for the Assessment of Negative Symptoms (SANS) University of Iowa; Iowa: 1981. [PubMed] [Google Scholar]
- Andreasen NC. Scale for the Assessment of Positive Symptoms (SAPS) University of Iowa; Iowa: 1983. [Google Scholar]
- Baddeley A. Working Memory. Science. 1992;255 (5044):556–559. doi: 10.1126/science.1736359. [DOI] [PubMed] [Google Scholar]
- Bhojraj TS, Francis AN, Montrose DM, Keshavan MS. Grey matter and cognitive deficits in young relatives of schizophrenia patients. Neuroimage. 2011a;54:S287–S292. doi: 10.1016/j.neuroimage.2010.03.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhojraj TS, Sweeney JA, Prasad KM, Eack SM, Francis AN, Miewald JM, Montrose DM, Keshavan MS. Gray matter loss in young relatives at risk for schizophrenia: Relation with prodromal psychopathology. Neuroimage. 2011b;54:S272–S279. doi: 10.1016/j.neuroimage.2010.04.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannon TD, Glahn DC, Kim J, Van Erp TG, Karlsgodt K, Cohen MS, Nuechterlein KH, Bava S, Shirinyan D. Dorsolateral prefrontal cortex activity during maintenance and manipulation of information in working memory in patients with schizophrenia. Arch Gen Psychiatry. 2005;62 (10):1071–1080. doi: 10.1001/archpsyc.62.10.1071. [DOI] [PubMed] [Google Scholar]
- Cannon TD, Thompson PM, van Erp TG, Toga AW, Poutanen VP, Huttunen M, Lonnqvist J, Standerskjold-Nordenstam CG, Narr KL, Khaledy M, Zoumalan CI, Dail R, Kaprio J. Cortex mapping reveals regionally specific patterns of genetic and disease-specific gray-matter deficits in twins discordant for schizophrenia. Proc Natl Acad Sci U S A. 2002;99 (5):3228–3233. doi: 10.1073/pnas.052023499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannon TD, van Erp TG, Huttunen M, Lonnqvist J, Salonen O, Valanne L, Poutanen VP, Standertskjold-Nordenstam CG, Gur RE, Yan M. Regional gray matter, white matter, and cerebrospinal fluid distributions in schizophrenic patients, their siblings, and controls. Arch Gen Psychiatry. 1998;55 (12):1084–1091. doi: 10.1001/archpsyc.55.12.1084. [DOI] [PubMed] [Google Scholar]
- Chen JY, Calhoun VD, Pearlson GD, Perrone-Bizzozero N, Sui J, Turner JA, Bustillo JR, Ehrlich S, Sponheim SR, Canive JM, Ho BC, Liu JY. Guided exploration of genomic risk for gray matter abnormalities in schizophrenia using parallel independent component analysis with reference. Neuroimage. 2013;83:384–396. doi: 10.1016/j.neuroimage.2013.05.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale AM, Fischl B, Sereno MI. Cortical surface-based analysis. I. Segmentation and surface reconstruction. Neuroimage. 1999;9 (2):179–194. doi: 10.1006/nimg.1998.0395. [DOI] [PubMed] [Google Scholar]
- Dickinson D, Ramsey ME, Gold JM. Overlooking the obvious: a meta-analytic comparison of digit symbol coding tasks and other cognitive measures in schizophrenia. Arch Gen Psychiatry. 2007;64 (5):532–542. doi: 10.1001/archpsyc.64.5.532. [DOI] [PubMed] [Google Scholar]
- Fischl B, Salat DH, Busa E, Albert M, Dieterich M, Haselgrove C, van der Kouwe A, Killiany R, Kennedy D, Klaveness S, Montillo A, Makris N, Rosen B, Dale AM. Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron. 2002;33 (3):341–355. doi: 10.1016/s0896-6273(02)00569-x. [DOI] [PubMed] [Google Scholar]
- Fischl B, Sereno MI, Dale AM. Cortical surface-based analysis. II: Inflation, flattening, and a surface-based coordinate system. Neuroimage. 1999;9 (2):195–207. doi: 10.1006/nimg.1998.0396. [DOI] [PubMed] [Google Scholar]
- Fischl B, van der Kouwe A, Destrieux C, Halgren E, Segonne F, Salat DH, Busa E, Seidman LJ, Goldstein J, Kennedy D, Caviness V, Makris N, Rosen B, Dale AM. Automatically parcellating the human cerebral cortex. Cereb Cortex. 2004;14 (1):11–22. doi: 10.1093/cercor/bhg087. [DOI] [PubMed] [Google Scholar]
- Forbes NF, Carrick LA, McIntosh AM, Lawrie SM. Working memory in schizophrenia: a meta-analysis. Psychological Medicine. 2009;39 (6):889–905. doi: 10.1017/S0033291708004558. [DOI] [PubMed] [Google Scholar]
- Glahn DC, Kim J, Cohen MS, Poutanen VP, Therman S, Bava S, Van Erp TG, Manninen M, Huttunen M, Lonnqvist J, Standertskjold-Nordenstam CG, Cannon TD. Maintenance and manipulation in spatial working memory: dissociations in the prefrontal cortex. Neuroimage. 2002;17 (1):201–213. doi: 10.1006/nimg.2002.1161. [DOI] [PubMed] [Google Scholar]
- Glahn DC, Laird AR, Ellison-Wright I, Thelen SM, Robinson JL, Lancaster JL, Bullmore E, Fox PT. Meta-analysis of gray matter anomalies in schizophrenia: application of anatomic likelihood estimation and network analysis. Biol Psychiat. 2008;64 (9):774–781. doi: 10.1016/j.biopsych.2008.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glahn DC, Ragland JD, Abramoff A, Barrett J, Laird AR, Bearden CE, Velligan DI. Beyond hypofrontality: a quantitative meta-analysis of functional neuroimaging studies of working memory in schizophrenia. Human Brain Mapping. 2005;25 (1):60–69. doi: 10.1002/hbm.20138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goghari VM. Executive functioning brain abnormalities associated with the genetic liability for schizophrenia: An Activation Likelihood Estimation fMRI meta-analysis. Psychological Medicine. 2010;41:1239–1252. doi: 10.1017/S0033291710001972. [DOI] [PubMed] [Google Scholar]
- Goghari VM, Rehm K, Carter CS, MacDonald AW., 3rd Regionally specific cortical thinning and gray matter abnormalities in the healthy relatives of schizophrenia patients. Cereb Cortex. 2007a;17 (2):415–424. doi: 10.1093/cercor/bhj158. [DOI] [PubMed] [Google Scholar]
- Goghari VM, Rehm K, Carter CS, MacDonald AW., 3rd Sulcal thickness as a vulnerability indicator for schizophrenia. Br J Psychiatry. 2007b;191:229–233. doi: 10.1192/bjp.bp.106.034595. [DOI] [PubMed] [Google Scholar]
- Goldman AL, Pezawas L, Mattay VS, Fischl B, Verchinski BA, Chen Q, Weinberger DR, Meyer-Lindenberg A. Widespread reductions of cortical thickness in schizophrenia and spectrum disorders and evidence of heritability. Arch Gen Psychiatry. 2009;66 (5):467–477. doi: 10.1001/archgenpsychiatry.2009.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han X, Jovicich J, Salat D, van der Kouwe A, Quinn B, Czanner S, Busa E, Pacheco J, Albert M, Killiany R, Maguire P, Rosas D, Makris N, Dale A, Dickerson B, Fischl B. Reliability of MRI-derived measurements of human cerebral cortical thickness: the effects of field strength, scanner upgrade and manufacturer. Neuroimage. 2006;32 (1):180–194. doi: 10.1016/j.neuroimage.2006.02.051. [DOI] [PubMed] [Google Scholar]
- Hill SK, Griffin GB, Miura TK, Herbener ES, Sweeney JA. Salience of working-memory maintenance and manipulation deficits in schizophrenia. Psychol Med. 2010;40 (12):1979–1986. doi: 10.1017/S003329171000019X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho BC. MRI brain volume abnormalities in young, nonpsychotic relatives of schizophrenia probands are associated with subsequent prodromal symptoms. Schizophr Res. 2007;96 (1–3):1–13. doi: 10.1016/j.schres.2007.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendler KS, Lieberman JA, Walsh D. The Structured Interview for Schizotypy (SIS): a preliminary report. Schizophr Bull. 1989;15 (4):559–571. doi: 10.1093/schbul/15.4.559. [DOI] [PubMed] [Google Scholar]
- Kikinis Z, Fallon JH, Niznikiewicz M, Nestor P, Davidson C, Bobrow L, Pelavin PE, Fischl B, Yendiki A, McCarley RW, Kikinis R, Kubicki M, Shenton ME. Gray matter volume reduction in rostral middle frontal gyrus in patients with chronic schizophrenia. Schizophr Res. 2010;123 (2–3):153–159. doi: 10.1016/j.schres.2010.07.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Glahn DC, Nuechterlein KH, Cannon TD. Maintenance and manipulation of information in schizophrenia: further evidence for impairment in the central executive component of working memory. Schizophr Res. 2004;68 (2–3):173–187. doi: 10.1016/S0920-9964(03)00150-6. [DOI] [PubMed] [Google Scholar]
- Lee J, Park S. Working memory impairments in schizophrenia: a meta-analysis. Journal of Abnormal Psychology. 2005;114 (4):599–611. doi: 10.1037/0021-843X.114.4.599. [DOI] [PubMed] [Google Scholar]
- Nurnberger JI, Jr, Blehar MC, Kaufmann CA, York-Cooler C, Simpson SG, Harkavy-Friedman J, Severe JB, Malaspina D, Reich T. Diagnostic interview for genetic studies. Rationale, unique features, and training. NIMH Genetics Initiative. Arch Gen Psychiatry. 1994;51:849–859. doi: 10.1001/archpsyc.1994.03950110009002. discussion 863–864. [DOI] [PubMed] [Google Scholar]
- Ohtani T, Levitt JJ, Nestor PG, Kawashima T, Asami T, Shenton ME, Niznikiewicz M, McCarley RW. Prefrontal cortex volume deficit in schizophrenia: A new look using 3T MRI with manual parcellation. Schizophr Res. 2013 doi: 10.1016/j.schres.2013.10.026. [DOI] [PubMed] [Google Scholar]
- Owen AM, McMillan KM, Laird AR, Bullmore E. N-back working memory paradigm: a meta-analysis of normative functional neuroimaging studies. Hum Brain Mapp. 2005;25 (1):46–59. doi: 10.1002/hbm.20131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quee PJ, Eling PA, van der Heijden FM, Hildebrandt H. Working memory in schizophrenia: a systematic study of specific modalities and processes. Psychiatry Res. 2011;185 (1–2):54–59. doi: 10.1016/j.psychres.2009.08.012. [DOI] [PubMed] [Google Scholar]
- Rosso IM, Makris N, Thermenos HW, Hodge SM, Brown A, Kennedy D, Caviness VS, Faraone SV, Tsuang MT, Seidman LJ. Regional prefrontal cortex gray matter volumes in youth at familial risk for schizophrenia from the Harvard Adolescent High Risk Study. Schizophr Res. 2010;123 (1):15–21. doi: 10.1016/j.schres.2010.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlosser RGM, Koch K, Wagner G, Nenadic I, Roebel M, Schachtzabel C, Axer M, Schultz C, Reichenbach JR, Sauer H. Inefficient executive cognitive control in schizophrenia is preceded by altered functional activation during information encoding: An fMRI study. Neuropsychologia. 2008;46 (1):336–347. doi: 10.1016/j.neuropsychologia.2007.07.006. [DOI] [PubMed] [Google Scholar]
- Shepherd AM, Laurens KR, Matheson SL, Carr VJ, Green MJ. Systematic meta-review and quality assessment of the structural brain alterations in schizophrenia. Neurosci Biobehav Rev. 2012;36 (4):1342–1356. doi: 10.1016/j.neubiorev.2011.12.015. [DOI] [PubMed] [Google Scholar]
- Snitz BE, Macdonald AW, 3rd, Carter CS. Cognitive deficits in unaffected first-degree relatives of schizophrenia patients: a meta-analytic review of putative endophenotypes. Schizophrenia Bull. 2006;32 (1):179–194. doi: 10.1093/schbul/sbi048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thakkar KN, Park S. Impaired passive maintenance and spared manipulation of internal representations in patients with schizophrenia. Schizophr Bull. 2012;38 (4):787–795. doi: 10.1093/schbul/sbq159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ventura J, Lukoff D, Nuechterlein KH, Liberman RP, Green MF, Shaner A. Manual for the expanded Brief Psychiatric Rating Scale. International Journal of Methods in Psychiatric Research. 1993;3:221–224. [Google Scholar]
- Wager TD, Smith EE. Neuroimaging studies of working memory: a meta-analysis. Cogn Affect Behav Neurosci. 2003;3 (4):255–274. doi: 10.3758/cabn.3.4.255. [DOI] [PubMed] [Google Scholar]
- Walton E, Geisler D, Lee PH, Hass J, Turner JA, Liu J, Sponheim SR, White T, Wassink TH, Roessner V, Gollub RL, Calhoun VD, Ehrlich S. Prefrontal Inefficiency Is Associated With Polygenic Risk for Schizophrenia. Schizophr Bull. 2013a doi: 10.1093/schbul/sbt174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walton E, Turner J, Gollub RL, Manoach DS, Yendiki A, Ho BC, Sponheim SR, Calhoun VD, Ehrlich S. Cumulative Genetic Risk and Prefrontal Activity in Patients With Schizophrenia. Schizophrenia Bull. 2013b;39 (3):703–711. doi: 10.1093/schbul/sbr190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao Y, Zhang W, Lui S, Yao L, Gong Q. Similar and Different Gray Matter Deficits in Schizophrenia Patients and Their Unaffected Biological Relatives. Frontiers in psychiatry. 2013;4:150. doi: 10.3389/fpsyt.2013.00150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshihara Y, Sugihara G, Matsumoto H, Suckling J, Nishimura K, Toyoda T, Isoda H, Tsuchiya KJ, Takebayashi K, Suzuki K, Sakahara H, Nakamura K, Mori N, Takei N. Voxel-based structural magnetic resonance imaging (MRI) study of patients with early onset schizophrenia. Ann Gen Psychiatry. 2008;7:25. doi: 10.1186/1744-859X-7-25. [DOI] [PMC free article] [PubMed] [Google Scholar]