Abstract
Several cycloaddition catalysts and reagents were surveyed for their effectiveness toward cyclizing alkynenitriles with cyanamides. Catalytic amounts of FeI2, iPrPDAI and Zn were found to effectively catalyze the [2+2+2] cycloaddition of a variety of cyanamides and alkynenitriles to afford bicyclic 2-aminopyrimidines.
Since the 1948 report by Reppe of acetylene cyclotrimerization in the presence of a transition metal, the field of [2+2+2] cycloaddition has grown immensely.1 Today, a myriad of carbocycles can be created from various combinations of unsaturated hydrocarbons.2 The next development in this field was the incorporation of heteroatoms into cyclic products. Heterocycles (such as pyridones,3 piperidines,4 pyridines5 etc.) of great complexity can now be synthesized from numerous coupling partners.6 These systems supply a highly efficient method to form multiple bonds in a single step and are of paramount importance in organic synthesis.
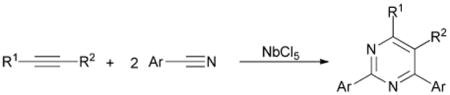
The domain of [2+2+2] cycloaddition is poised to enter a new step in its evolution. Cyclic compounds with multiple heteroatoms constitute another vast collection of compounds attracting considerable interest. Such motifs are abound in pharmaceuticals, natural products, and other biologically active compounds as well as in polymers and supramolecules.7 Despite such importance, the use of [2+2+2] cycloaddition for the construction of these compounds has received little attention. Such a reaction requires the incorporation of two heteroatom coupling partners to one hydrocarbon coupling partner. However, the latter substrates are typically more reactive in cycloaddition and lead to products that exclude the less reactive heteroatom coupling partner.8 For example, the combination of alkynes and excess nitriles in the presence of a variety of [2+2+2] cycloaddition catalysts affords either pyridines or substituted benzenes, or a mixture of thereof.9 Even when employing nitrile as a solvent, incorporation of multiple nitrogen atoms is typically not observed.10 Despite this significant obstacle, limited examples demonstrate that strategies to access cyclic compounds with multiple heteroatoms are possible. The first example by Hoberg involved the cycloaddition of an alkyne and two isocyanates in the presence of stoichiometric Ni(0) producing a pyrimidine dione.11 Our lab revisited this reactivity and successfully developed the first catalytic [2+2+2] route to pyrimidine diones.12 This work was later followed up by Kondo and co-workers.13 A more recent example by Obora utilizes a niobium Lewis acid to afford pyrimidines from an alkyne and two nitriles (eqn (1)).14 While this method is regioselec-tive, it requires an excess of NbCl5 that must be added in six portions over the course of the reaction. Additionally, yields are moderate and this system is limited to aromatic nitriles. Nevertheless, based on the reactivity of Nb complexes, pyrimidine formation likely involves a nucleophilic attack of the nitrile nitrogen onto a Nb coordinated alkyne complex rather than a Nb catalyzed cycloaddition reaction. Such a scarcity of examples in this area led us to seek a new metal-catalyzed [2+2+2] cycloaddition method for the creation of cyclic compounds with two heteroatoms.
 |
(1) |
 |
(2) |
 |
(3) |
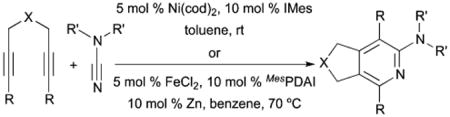
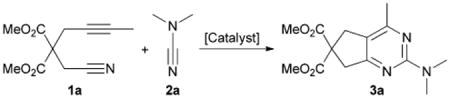
Our group demonstrated that cyanamides perform exceptionally well in Ni- and Fe-catalyzed cycloadditions with diynes (eqn (2)).15,16 Additionally, we showed that utilizing alkynenitriles aid in the incorporation of nitrogen into cycloaddition products when using an iron catalyst.17 We report herein a novel Fe-catalyzed cycloaddition reaction between alkynenitriles and cyanamides to provide 2-aminopyrimidines (eqn (3)). The abundant biological activity of 2-aminopyrimidines make such a reaction desirable because complex substitution patterns can be obtained from simple starting materials.18,19
We began by surveying the field of [2+2+2] transition metal catalysts under their previously developed conditions (Table 1).20 Rhodium, cobalt, nickel, and ruthenium catalysts (entries 1–7) were ineffective while iridium (entry 8) only afforded a trace of 3a as detected by GCMS. Additionally, niobium, gold, silver and Cu reagents provided no products (entries 9–13). FeI2/dppp was ineffective (entries 14 and 15). However, our previous Fe(OAc)2–p-OMe,iPrPDAI system17,21 provided a trace of 2-aminopyrimidine (entry 16). Curiously, our FeCl2/MesPDAI catalyst (entry 17), under the conditions used to produce 2-aminopyridines,16 afforded 2-aminopyridine 3a (Fig. 1) in 16% NMR yield. This result was auspicious considering the low cost, high terrestrial abundance and relatively low toxicity of iron as compared to other metal catalysts.
Table 1. Catalyst/reagent screening.
| Entry | Substrates (equiv.) | Conditionsa | Yield 3ab (%) |
|---|---|---|---|
| 1 | 1a (1), 2a (2) | 5 mol% Rh(cod)2 BF4/BINAP | n.d.c |
| 2 | 1a (1), 2a (5) | 15 mol% CoCp(CO)2 | n.d. |
| 3 | 1a (1), 2a (3) | 10 mol% CoCl2, 10 mol% dppe 20 mol% Zn | n.d. |
| 4 | 1a (1), 2a (1.5) | 10 mol% Ni(cod)2, 20 mol% SIPr | n.d. |
| 5 | 1a (1), 2a (1.5) | 10 mol% Ni(cod)2, 20 mol% IMes | n.d. |
| 6 | 1a (1), 2a (1.5) | 10 mol% Ni(cod)2, 10 mol% Xantphos | n.d. |
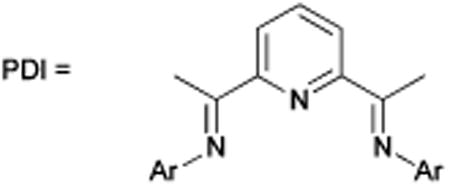
| 7 | 1a (1), 2a (1.5) | 2 mol% Cp*RuCl(cod) | n.d. |
| 8 | 1a (1), 2a (3) | 2 mol% [Ir(cod)Cl]2, 4 mol% DPPF | Trace |
| 9 | 1a (1), 2a (3) | 1.2 equiv. NbCl5 | n.d. |
| 10 | 1a (1), 2a (3) | 5 mol% Ph3PAuOPOF2 | n.d. |
| 11 | 1a (1), 2a (3) | 5 mol% AuCl3, 1.1 equiv. MsOH | n.d. |
| 12 | 1a (1), 2a (3) | 5 mol% AuPEt3Cl, 5 mol% AgSbF6 | n.d. |
| 13 | 1a (1), 2a (3) | 10 mol% AgOTf, 10 mol% CuBr | n.d. |
| 14 | 1a (1), 2a (3) | 10 mol% FeI2, 20 mol% dppp 20 mol% Zn | n.d. |
| 15 | 1a (1), 2a (3) | 10 mol% FeI2, 20 mol% dppp 20 mol% Zn, 20 mol% ZnI2 | n.d. |
| 16 | 1a (1), 2a (2) | 20 mol% Fe(OAc)2, 40 mol% Zn, 26 mol% p-OMe,iPrPDAIm | Trace |
| 17 | 1a (1), 2a (2) | 10 mol% FeCl2, 20 mol% MesPDAI 20 mol% Zn | 16d |
Fig. 1.
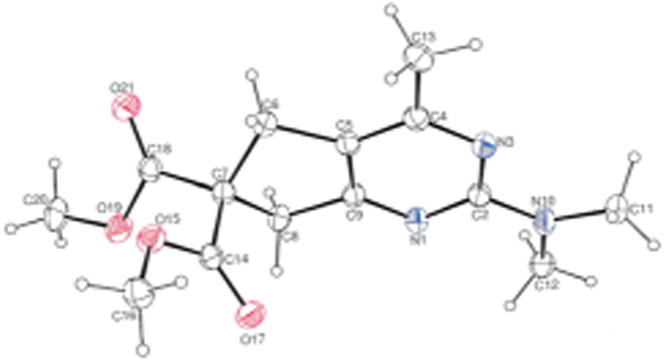
Ortep of 3a.
Expensive and highly toxic benzene was replaced with toluene at the outset of optimization.22 We initially identified Fe(OAc)2 as the optimum iron source; however, subsequent optimization with Fe(OAc)2 was unproductive. We then turned to FeI2, which allowed for significant improvements. Decreasing concentration, increasing cyanamide loading to 3 equiv. and ensuing ligand optimization led to sound improvements in yields. Phosphine, amine, carbene and bisimine ligands were ineffective while PDI ligands could only afford traces of product.23 Reducing temperature to 40 °C and increasing Zn dust loading to 30 mol% allowed for catalyst loading to be reduced to 5 mol%. At lower concentrations, the reduction of iron pre-catalyst by Zn is perhaps more difficult leading to the necessity for higher loading. Our final conditions utilize a 1: 3 ratio of alkynenitrile with 5 mol% FeI2, 10 mol% iPrPDAI, 30 mol% Zn dust in toluene at 40 °C (eqn (4)).
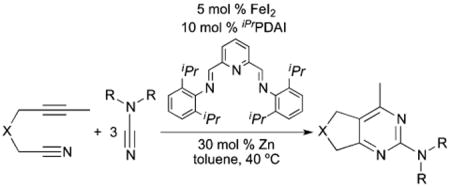 |
(4) |
Our final optimized conditions were applied to various combinations of substrates (Table 2). The model substrates 1a and 2a afforded 3a in 90% yield in 12 hours (entry 1); however, other substrates required extended reaction times. Cyclic cyanamides were incorporated providing 3b, 3c, and 3d in 40, 82, and 35% yields, respectively (entry 2–4). Me/ Ph-cyanamide 2e provided 3e in 40% yield over 3 days (entry 5). Under our standard conditions, 1b was unreactive. However, in the presence of 30 mol% ZnI2, 3f was obtained in 27% yield (entry 6). While ZnI2 is known to aid in cobalt catalysis by stabilizing Co(I) species,24 its effect in iron-catalyzed reactions remains unclear.8,25 Applying ZnI2 to all reactions involving 1a resulted in increased reaction times and decreased yields. This conflicting result has been observed in a previous study.25 Finally, the challenging terminal alkynenitrile 1c failed to react under these conditions (entry 7). Efforts to replace the cyanamide substrate with an unactivated nitrile such as PhCN were ineffective as were 3-component cyclizations of 4-CH3PhCCH with either 2 equivalents of free nitriles (i.e., PhCN) or cyanamides (i.e., 2a). Although yields are modest in many cases, this work demonstrates that bicyclic 2-aminopyrimidines with complex substitution patterns can be prepared through Fe-catalyzed cycloaddition chemistry. In some cases however, yields are high indicating that this system could be synthetically useful if optimization is done on a case-by-case basis.
Table 2. Substrate scope.
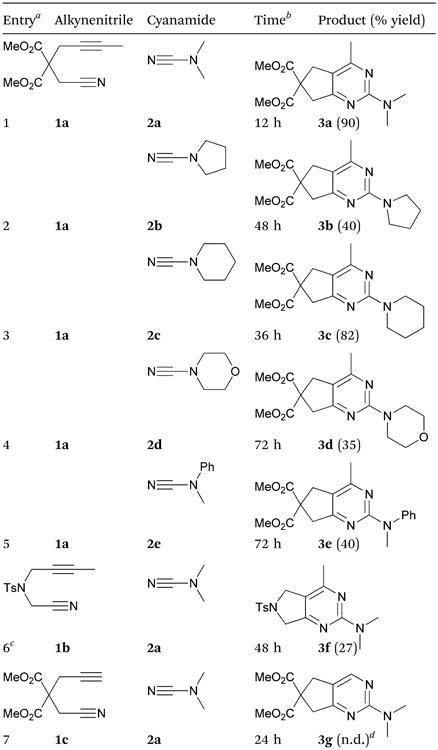
|
Conditions: 5 mol% FeI2, 10 mol% iPrPDAI, 30 mol% Zn dust, toluene, 40 °C.
Reaction progress monitored by GC.
Conditions: 5 mol% FeI2, 10 mol% iPrPDAI, 30 mol% Zn dust, 30 mol% ZnI2, toluene, 40 °C.
n.d. = not detected.
We have disclosed the first catalytic [2+2+2] cycloaddition to produce aromatic diazaheterocycles. What is especially remarkable is that iron, which has traditionally been an inefficient cycloaddition catalyst for nitrile incorporation, can now incorporate multiple nitriles into aromatic products. Furthermore, traditionally more efficient catalysts were ineffective toward this strategy of 2-aminopyrimidine synthesis. We are actively studying the properties of iron/PDAI catalysts in the hope that a better understanding of these systems will lead to improved yields and expanded substrate scopes.
Supplementary Material
Footnotes
Electronic supplementary information (ESI) available. CCDC 944058. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c3cc44422h
Notes and references
- 1.Reppe W, Schichting O, Klager K, Toepel T. Justus Liebigs Ann Chem. 1948;560:1–92. [Google Scholar]
- 2.(a) Kotha S, Brahmachary E, Lahiri K. Eur J Org Chem. 2005:4741. [Google Scholar]; (b) Chopade PR, Louie J. Adv Synth Catal. 2006;348:2307. [Google Scholar]; (c) Evans PA, Baum EW, Fazal AN, Lai KW, Robinson JE, Sawyer JR. ARKIVOC. 2006:338. [Google Scholar]; (d) Galan BR, Rovis T. Angew Chem, Int Ed. 2009;48:2830. doi: 10.1002/anie.200804651. [DOI] [PMC free article] [PubMed] [Google Scholar]; (e) Rajca A, Rajca S. Angew Chem, Int Ed. 2009;49:672. doi: 10.1002/anie.200905421. [DOI] [PubMed] [Google Scholar]; (f) Tanaka K. Chem – Asian J. 2009;4:508. doi: 10.1002/asia.200800378. [DOI] [PubMed] [Google Scholar]
- 3.(a) Duong HA, Cross JJ, Louie J. J Am Chem Soc. 2004;126:11438. doi: 10.1021/ja046477i. [DOI] [PubMed] [Google Scholar]; (b) Oberg KM, Lee EE, Rovis T. Tetrahedron. 2009;65:5056. doi: 10.1016/j.tet.2009.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.(a) Ho KYT, Aïssa C. Chem – Eur J. 2012;18:3486. doi: 10.1002/chem.201200167. [DOI] [PubMed] [Google Scholar]; (b) Kumar P, Louie J. Org Lett. 2012;14:2026. doi: 10.1021/ol300534j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.(a) McCormick MM, Duong HA, Zuo G, Louie J. J Am Chem Soc. 2005;127:5030. doi: 10.1021/ja0508931. [DOI] [PubMed] [Google Scholar]; (b) Hyster TK, Rovis T. Chem Commun. 2011;47:11846. doi: 10.1039/c1cc15248c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.(a) Varela JA, Saá C. Chem Rev. 2003;103:3787. doi: 10.1021/cr030677f. [DOI] [PubMed] [Google Scholar]; (b) Heller B, Hapke M. Chem Soc Rev. 2007;36:1085. doi: 10.1039/b607877j. [DOI] [PubMed] [Google Scholar]; (c) Perreault S, Rovis T. Chem Soc Rev. 2009;38:3149. doi: 10.1039/b816702h. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Tanaka K. Heterocycles. 2012;85:1017. [Google Scholar]; (e) Wang C, Wan B. Chin Sci Bull. 2012;57:2338. [Google Scholar]
- 7.(a) Gulevich AV, Dudnik AS, Chernyak N, Gevorgyan V. Chem Rev. 2013;113:3084. doi: 10.1021/cr300333u. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Hanan GS, Volkmer D, Schubert US, Lehn JM, Baum G, Genske D. Angew Chem, Int Ed Engl. 1997;36:1842. [Google Scholar]
- 8.Liu Y, Yan X, Yang N, Xi C. Catal Commun. 2011;12:489. [Google Scholar]
- 9.(a) Pillai SM, Ohnishi r, Ichikawa M. J Chem Soc, Chem Commun. 1990:246. [Google Scholar]; (b) Costa M, Dias FS, Chiusoli GP, Gazzola GL. J Organomet Chem. 1995:47. [Google Scholar]; (c) Knoch F, Kremer F, Schmidt U, Zenneck U. Organometallics. 1996;15:2713. [Google Scholar]; (d) Wang C, Li X, Wu F, Wan B. Angew Chem, Int Ed. 2011;50:7162. doi: 10.1002/anie.201102001. [DOI] [PubMed] [Google Scholar]
- 10.(a) Hilt G, Vogler T, Hess W, Galbiati F. Chem Commun. 2005:1417. doi: 10.1039/b417832g. [DOI] [PubMed] [Google Scholar]; (b) Hilt G, Hess W, Vogler T, Hengst C. J Organomet Chem. 2005:5170. [Google Scholar]; (c) Hsieh JC, Cheng CH. Chem Commun. 2008:2992. doi: 10.1039/b801870g. [DOI] [PubMed] [Google Scholar]
- 11.Hoberg H, Oster BW. J Organomet Chem. 1983:359. [Google Scholar]
- 12.Duong HA, Louie J. Tetrahedron. 2006;62:7552. [Google Scholar]
- 13.Kondo T, Nomura M, Ura Y, Wada K, Mitsudo T. Tetrahedron Lett. 2006;47:7107. [Google Scholar]
- 14.Satoh Y, Yasuda K, Obora Y. Organometallics. 2012;31:5235. [Google Scholar]
- 15.(a) Stolley RM, Maczka MT, Louie J. Eur J Org Chem. 2011:3815. doi: 10.1002/ejoc.201100428. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Kumar P, Prescher S, Louie J. Angew Chem, Int Ed. 2011;50:10694. doi: 10.1002/anie.201104475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lane TK, D'Souza BR, Louie J. J Org Chem. 2012;77:7555. doi: 10.1021/jo3012418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.D'Souza BR, Lane TK, Louie J. Org Lett. 2011;13:2936. doi: 10.1021/ol2009939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Reviews: Koroleva EV, Gusak KN, Ignatovich ZV. Russ Chem Rev. 2010;79:655.Hill MD, Movassaghi M. Chem-Eur J. 2008;14:6836. doi: 10.1002/chem.200800014.von Angerer S. Sci Synth. 2004:379. section 16.2.Christopherson RI, Lyons SD, Wilson PK. Acc Chem Res. 2002;35:961. doi: 10.1021/ar0000509.
- 19.(a) Anand N, Singh P, Sharma A, Tiwari S, Singh V, Singh DK, Srivastava KK, Singh BN, Tripathi RP. Bioorg Med Chem. 2012;20:5150. doi: 10.1016/j.bmc.2012.07.009. [DOI] [PubMed] [Google Scholar]; (b) Han YT, Choi GI, Son D, Kim NJ, Yun H, Lee S, Chang DJ, Hong HS, Kim H, Ha HJ, Kim YH, Park HJ, Lee J, Suh YG. J Med Chem. 2012;55:9120. doi: 10.1021/jm300172z. [DOI] [PubMed] [Google Scholar]; (c) Giridhar R, Tamboli RS, Ramajayam R, Prajapati DG, Yadav MR. Eur J Med Chem. 2012;50:428. doi: 10.1016/j.ejmech.2012.01.035. [DOI] [PubMed] [Google Scholar]; (d) Lebar MD, Hahn KN, Mutka T, Maignan P, McClintock JB, Amsler CD, van Olphen A, Kyle DE, Baker BJ. Bioorg Med Chem. 2011;19:5756. doi: 10.1016/j.bmc.2011.08.033. [DOI] [PubMed] [Google Scholar]; (e) Perl NR, Ide ND, Prajapati S, Perfect HH, Duron SG, Gin YD. J Am Chem Soc. 2010;132:1802. doi: 10.1021/ja910831k. [DOI] [PMC free article] [PubMed] [Google Scholar]; (f) Lin YL, Huang RL. J Nat Prod. 1997;60:982. doi: 10.1021/np970159y. [DOI] [PubMed] [Google Scholar]
- 20.(a) Rh (Table 1, entry 1): Tanaka K, Suzuki N, Nishida G. Eur J Org Chem. 2006:3917.(b) For CpCo(CO)2 (entry 2) Boñaga LVR, Zhang HC, Maryanoff BE. Chem Commun. 2004:2394. doi: 10.1039/b410012c.(c) CoCl2 (entry 3): Sugiyama YK, Okamoto S. Synthesis. 2011:2247.(d) Ni (entries 4–6): see ref. 15; (e) Ru (entry 7): Yamamoto Y, Kinpara K, Saigoku T, Takagishi H, Okuda S, Nishiyama H, Itoh K. J Am Chem Soc. 2005;127:605. doi: 10.1021/ja045694g.(f) Ir (entry 8): Onodera G, Shimizu Y, Kimura J, Kobayashi J, Evihara Y, Kondo K, Sakata K, Takeuchi R. J Am Chem Soc. 2012;134:10515. doi: 10.1021/ja3028394.(g) NbCl5 (entry 9): see ref. 14; (h) Ph3AuOPOF2 (entry 10): Kim SM, Park JH, Chung YK. Chem Commun. 2011;47:6719. doi: 10.1039/c1cc11127b.(i) AuCl3 (entry 11): Xiao Y, Zhang L. Org Lett. 2012;14:4662. doi: 10.1021/ol302102h.(j) AuPEt3Cl/AgSbF6 (entry 12): Fernandez-Garcia JM, Fernandez-Rodriguez MA, Aguilar E. Org Lett. 2011;13:5172. doi: 10.1021/ol202046y.(k) AgOTf/CuBr (entry 13): Li S, Luo Y, Wu J. Org Lett. 2011;13:4312. doi: 10.1021/ol201653j.(l) FeI2/dppp (entry 14) see ref. 9d; (m) Fe(OAc)2/p-OMe,iPrPDAI (entry 16) see ref. 17; (n) FeCl2/MesPDAI (entry 17) see ref. 16.
- 21.PDAI = bis(aldimino) pyridine naming system was introduced by Chirik: Russell SK, Milsmann C, Lobkovsky E, Weyhermüller T, Chirik PJ. Inorg Chem. 2011;50:3159. doi: 10.1021/ic102186q.
- 22.See ESI† for details.
-
23.PDI = bis(imino) pyridine naming system was introduced by Chirik: Bart SC, Lobkovsky E, Chirik PJ. J Am Chem Soc. 2004;126:13794. doi: 10.1021/ja046753t.
- 24.Seka S, Buriez O, Périchon J. Chem–Eur J. 2003;9:3597. doi: 10.1002/chem.200204604. [DOI] [PubMed] [Google Scholar]
- 25.Wang C, Wang D, Xu F, Pan B, Wan B. J Org Chem. 2013;78:3065. doi: 10.1021/jo400057t. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


