Abstract
Activation of the alternative pathway of the complement system has been implicated in the pathogenesis of age-related macular degeneration. Membrane attack complex (MAC) has been identified mainly on the Bruch’s membrane and drusen underlying the retinal pigment epithelium (RPE). Membrane cofactor protein (CD46) preferentially regulates the alternative pathway of complement. The aim of this study was to evaluate the potential of increasing CD46 expression on RPE cells using an adenovirus as a gene therapy approach to reduce alternative pathway-mediated damage to RPE cells. We generated a recombinant adenovirus vector expressing human CD46 (hCD46) and delivered the vector to murine hepatocytes and RPE cells in vitro. After incubation in human serum in conditions in which the classical pathway of complement was blocked, we measured alternative pathway-mediated damage of these cells by quantifying lysis and MAC formation. Adenovirus expressing hCD46 was delivered to the subretinal space of adult mice, and 1 week later, ocular flat mounts were challenged with human serum and the levels of complement-mediated damage was quantified. Adenovirus-mediated delivery of hCD46 localizes to the basal and lateral surfaces of RPE cells where it offers protection from alternative pathway-mediated damage, but not classical, allowing the classical pathway to function unhindered.
Keywords: adenovirus, age-related macular degeneration, CD46
INTRODUCTION
Gene transfer approaches to ocular tissues using adenovirus, as the gene delivery vector have been found to be safe in a number of clinical trials.1,2 Recombinant adenovirus constructs have a significant tropism for retinal pigment epithelial (RPE) cells3 and can persist in this tissue in rodents for at least 1 year (latest time point examined)4 or potentially for the ‘lifetime’ of the animal.5 In addition, helper-dependent adenovirus vectors can accommodate transgene expression cassettes up to 36 Kb,6,7 allowing for the expression of multiple transgenes as well as the inclusion of native gene-regulatory elements.
Several diseases have been linked to the activation of the complement cascade, a component of the innate immune system, including atypical hemolytic uremic syndrome (aHUS), arthritis, multiple sclerosis, lupus and age-related macular degeneration (AMD). AMD is the leading cause of blindness amongst the elderly in the developed world.8,9 Currently, the only Food and Drug Administration-approved treatment for AMD targets the less prevalent yet highly debilitating ‘wet’ form of the disease.10 Although this treatment (ranibizumab/lucentis) is highly effective, it does not affect the 90% of AMD patients that suffer from the ‘dry’ form of the disease. A window of opportunity exists for treating ‘dry’ AMD before its progression to an advanced stage of ‘dry’ or the more severe ‘wet’ form. Early signs of disease can be detected by the formation of extracellular deposits generally referred to as ‘drusen’ between the RPE and Bruch’s membrane, abnormalities in pigmentation, or mottling.11
Although there is considerable debate on the precise mechanism underlying drusen formation, mostly composed of proteins and lipids, there is agreement that they represent a site of chronic inflammation mediated by the complement cascade.12 The complement cascade is a component of the innate immune system that can be triggered by pathogens (classical pathway) as well as constitutively or spontaneously (alternative pathway). In addition to normal ‘tickover’, activation of the alternative pathway is triggered by C3 hydrolysis, leading to a cascade of cleavage events that result in the production of anaphylatoxins, opsonins and ultimately the formation of a lytic pore on the cell surface termed as membrane attack complex (MAC). MAC has been observed mainly on the Bruch’s membrane and drusen underlying the RPE in AMD patients.13 Importantly, proteins of the complement pathway have been identified in drusen,11 and prediction models strongly indicate a higher incidence of AMD in patients with polymorphisms in genes encoding alternative pathway components, such as complement factor H, complement factor B and complement factor 3.14
Because activation of the alternative pathway has been implicated in AMD, we wished to identify a complement regulator that will specifically block the alternative arm of the complement system and use adenovirus to locally deliver the regulator to the back of the eye. Membrane cofactor protein (CD46) is a cofactor for factor I which has previously been identified as a key regulator of the alternative pathway.15 Mutations in CD46 have been identified as a major risk factor for developing diseases associated with chronic alternative pathway activity, such as aHUS.16 Although the importance of CD46 expression has been defined for aHUS, its role in AMD has not been fully evaluated. Analysis of the expression pattern for CD46 in normal human eyes has revealed that it is expressed on the basal and lateral surfaces of RPE cells.17 As the basal surface of RPE cells are exposed to drusen, this places CD46 in a prime location to dampen alternative pathway activity. In a state of chronic inflammation, such as AMD, the levels of CD46 may not be sufficient to protect the RPE cells from complement-mediated attack. Therefore, increasing the expression of CD46 in RPE cells may be one way to restore the balance between alternative complement activation and regulation in AMD patients.
Both CD46 and factor H negatively regulate the alternative pathway by acting as co-factors for C3b cleavage. Polymorphisms in the soluble regulator factor H have been implicated as a major risk factor for developing AMD. Using adenovirus to efficiently deliver CD46, a membrane-bound complement regulator, to RPE cells may provide localized protection from complement attack by cleaving C3b. This may be a way to compensate for factor H in patients with polymorphisms. To our knowledge, this is the first time adenovirus has been used to test the efficacy of CD46 for protecting against a complement-mediated disease. Although we have focused on AMD, our results may suggest the need to extend testing into other disease models, such as aHUS. Currently, kidney transplantation has been the most successful treatment for aHUS patients. Adenoviral delivery of CD46 may be a viable option that bypasses the limited availability of kidney donors for aHUS patients.
Previously, we have described the development of a novel murine model of complement activation to test the capacity for adenoviral delivery of the human complement regulator CD59 to protect against human MAC deposition.18 This humanized murine model provides a convenient approach to evaluate the role of CD46 in the murine retina. In this study, we further adapted this model such that complement activation occurs primarily through the alternative pathway and used this model to evaluate the role of human CD46 in protecting murine RPE cells from alternative pathway-mediated complement attack. To our knowledge, this is the first time adenoviral delivery of CD46 has been evaluated for its role in protecting RPE cells from alternative complement pathway activity. Our results highlight the need for further investigation of adenoviral delivery of CD46 and its potential use as a therapeutic for AMD.
RESULTS
Expression of human CD46 from adenovirus in human embryonic retinoblasts and mouse hepatocytes
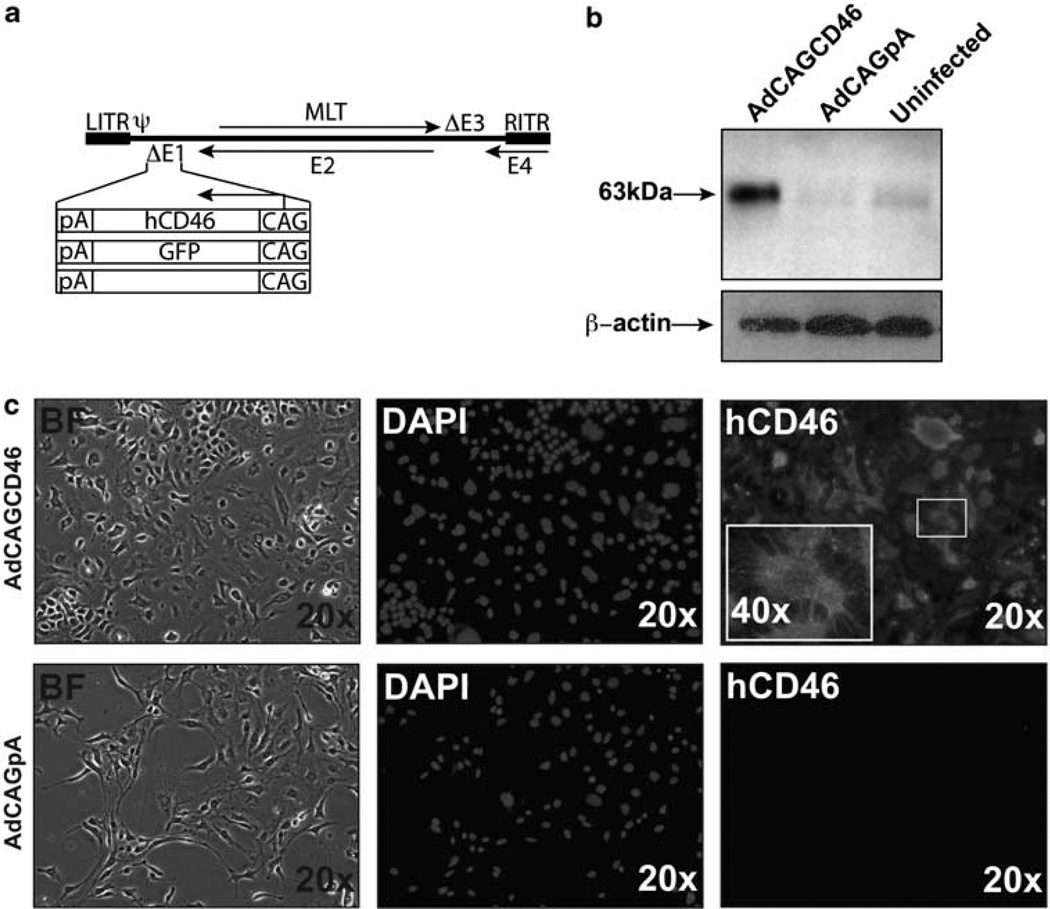
Human CD46 is a transmembrane protein ranging in size from 48 to 68 kDa.19 There are four isoforms that all contain four conserved short consensus sequences, an O-glycosylated serine-/threonine-/proline-rich area, a hydrophobic transmembrane portion and an intracellular domain. To evaluate the ability of human CD46 (hCD46) to protect tissues against human complement-mediated insult, we first cloned hCD46 into an adenoviral vector under the control of a chicken β-actin promoter (CAG) to generate AdCAGCD46. A control adenovirus that does not have a transgene (AdCAGpA) and an adenovirus expressing green florescent protein (GFP) (AdCAGGFP) were also generated, the GFP-expressing virus for the purpose of labeling the site of transduction following an in vivo injection (Figure 1a). Expression of hCD46 was confirmed by western blot of lysate collected from AdCAGCD46-infected human embryonic retinoblasts. We observed a band at 63kDa corresponding to the predicted molecular weight of the BC isoform of hCD46 (ref 19; Figure 1b). A faint band in the AdCAGpA and uninfected lanes was also detected. This is not surprising, as it has been shown that the majority of human cells express CD46 (ref. 20). The ability of adenovirus-expressed hCD46 to localize to the cell membrane was confirmed by immuno-cytochemistry of non-permeabilized mouse hepatocytes (Hepa 1c1c7) infected with either AdCAGCD46 or AdCAGpA. The AdCAGCD46-infected hepatocytes show robust expression on the cell membrane, whereas no hCD46 is detected on AdCAGpA-infected cells (Figure 1c).
Figure 1.
AdCAGCD46 is expressed in human embryonic retinoblast cells and on the membrane of mouse Hepa 1c1c7 cells. (a) Adenoviral constructs: expression cassettes for human CD46, GFP and without transgene were inserted into the E1-deleted region of Ad5. (b) Western blot for hCD46 showing expression in human embryonic retinoblast cells following infection with AdCAGCD46. (c) Mouse Hepa1c1c7 cells infected with AdCAGCD46 and stained with mouse anti-human CD46 (MEM258) showing incorporation of CD46 into the cell membrane. BF, bright field; CAG, chicken β-actin promoter; pA, rabbit globin poly A signal; LITR, left inverted terminal repeat; RITR, right inverted terminal repeat; ψ, packaging signal; MLT, major late transcript; E1–E4, early regions 1–4; DAPI, 4’,6-diamidino-2-phenylindole.
AdCAGCD46 protects mouse hepatocyte (Hepa1c1c7) cells from lysis mediated by the alternative, but not classical, complement pathway
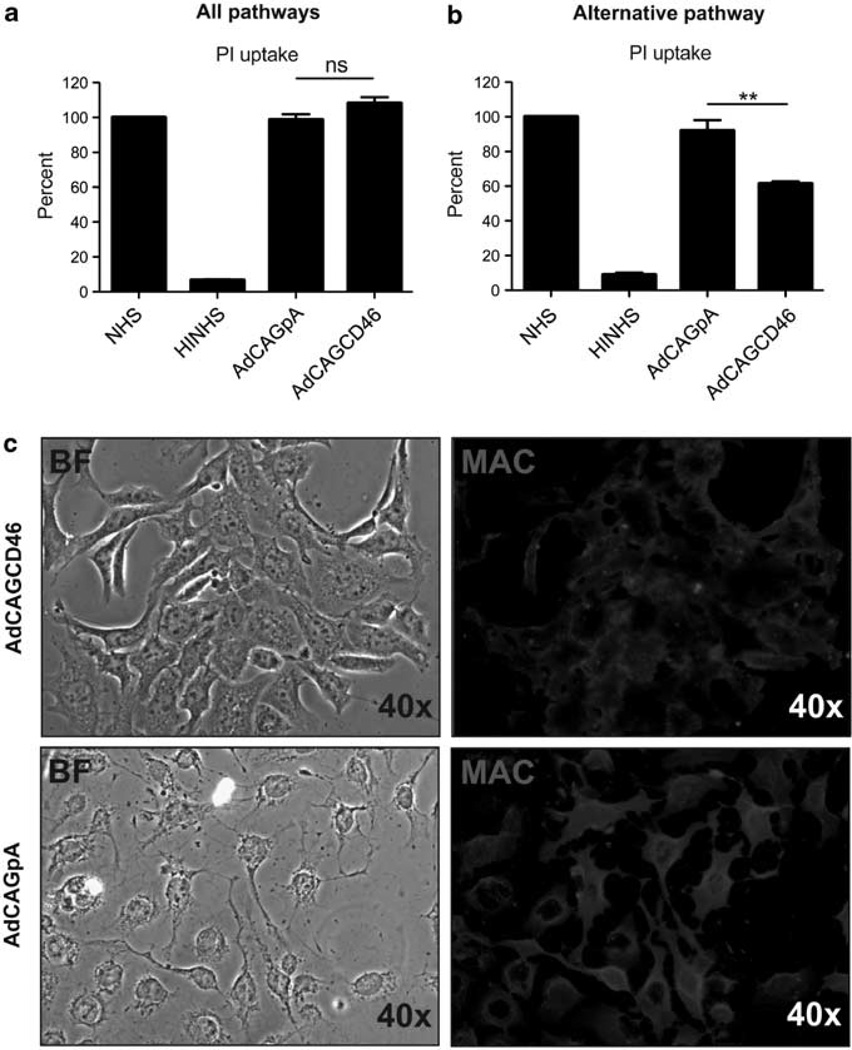
It has previously been shown that hCD46 has a higher affinity for binding C3b relative to C4b, resulting in increased inhibition of convertase formation of the alternative pathway15 Therefore, we evaluated the ability of AdCAGCD46 to protect cells from complement-mediated cell lysis concomitantly from the classical and alternative complement pathways, or specifically the alternative pathway. Hepa1c1c7 cells pre-incubated with either AdCAGCD46 or AdCAGpA for 3 days were treated with either 25 µg ml−1 emmprin antibody followed by 10% normal human serum (NHS), or 7mm magnesium, 10mm EGTA (MgEGTA) treated NHS (for inhibition of the classical pathway) and cell lysis quantified by fluorescence-activated cell sorter (FACS) analysis of propidium iodide (PI) uptake. Complement activation was performed on cells in suspension eliminating the possibility for dead cells to escape FACS analysis by coming off the plate. Forward and side scatter did not reveal any difference between groups indicating no difference in cell toxicity (data not shown). When the classical pathway of the complement system was active, we observed no significant protection from lysis in AdCAGCD46-infected cells relative to those infected with AdCAGpA (Figure 2a). However, when the classical pathway was inactivated, such that cell lysis occurred primarily by the alternative pathway, we observed a 39 ± 0.88% (P=0.008) reduction in the amount of PI uptake by AdCAGCD46 relative to AdCAGpA (Figure 2b). The difference in PI uptake between AdCAGCD46- and AdCAGpA-infected cells should correlate with the difference in the amount of MAC deposited on the membranes of these cells. To test this hypothesis, we infected Hepa1c1c7 cells with either AdCAGCD46 or AdCAGpA for 3 days, and then activated the alternative pathway by incubating the cells with 25 µg ml−1 emmprin followed by 10% NHS and MgEGTA. We determined the amount of human MAC deposition using a monoclonal antibody against the C5b9 complex. Cells infected with AdCAGCD46 have less MAC deposited on their surface (Figure 2c) than cells infected with AdCAGpA. In addition, the morphology of the cells remains largely unaffected by MAC deposition relative to those cells infected with AdCAGpA. Together, these data suggest that AdCAGCD46 confers protection from MAC-mediated cell lysis occurring mainly through the alternative pathway.
Figure 2.
AdCAGCD46 protects mouse Hepa1c1c7 cells from alternative pathway-mediated, but not classical pathway-mediated, MAC deposition. (a) FACS analysis of PI uptake showing that when all pathways of the complement system are activated hCD46 has no significant effect on MAC deposition on mouse Hepa1c1c7 cells. (b) FACS analysis of PI uptake showing that when the alternative arm of the complement system alone is active, hCD46 protects mouse Hepa1c1c7 cells from MAC deposition by 39 ± 0.88% (**P=0.008). (c) Following alternative pathway activation, hCD46 expressing Hepa1c1c7 cells appear healthier and have less MAC deposited on the membrane. (Representative of three separate experiments performed in duplicate each time.) NHS, normal human serum; HINHS, heat-inactivated normal human serum; ns, not significant; PI, propidium iodide; BF, bright field; MAC, membrane attack complex.
AdCAGCD46 protects mouse primary RPE cells from alternative pathway-mediated MAC deposition
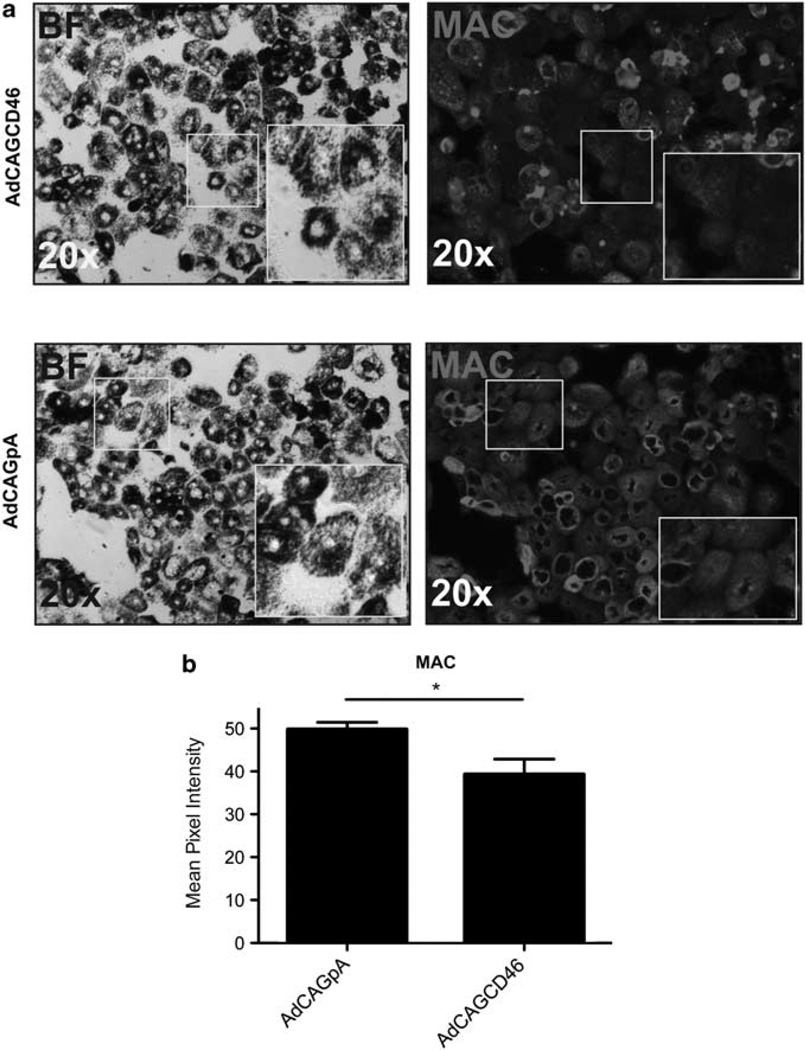
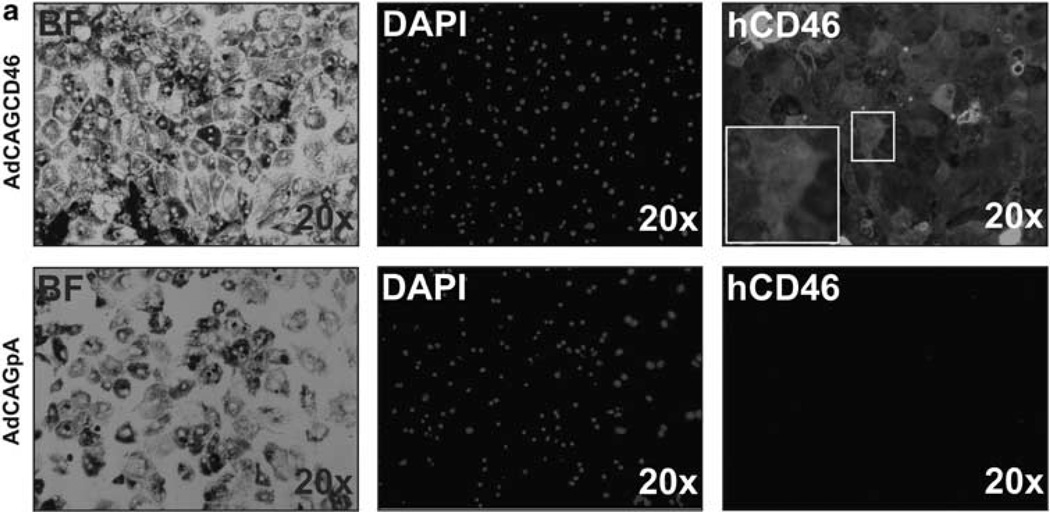
As MAC has been observed on RPE cells in AMD patients and the alternative pathway has been implicated to have a pivotal role in this disease,11,14 we wished to determine whether AdCAGCD46-infected mouse primary RPE cells are protected from human MAC deposition-mediated primarily by the alternative complement pathway. We have previously shown that RPE cells are more resistant to MAC deposition than Hepa1c1c7 cells,18 requiring higher concentrations of antibody and serum. RPE cells were pre-incubated with 50 µg ml−1 emmprin antibody followed by MgEGTA and 50% NHS. MAC was detected by immunohistochemistry. Quantification of intensity of MAC staining revealed a reduction of 21 ±1.9% (P=0.04) MAC deposition on RPE cells infected with AdCAGCD46 relative to AdCAGpA (Figure 3a and b). To ensure that the reduction in MAC is not a consequence of RPE cell loss from serum exposure, we counted the cells after the assay. The same number of cells are remaining on the AdCAGCD46 treated slide as the AdCAGpA treated (data not shown). As the reduction in MAC was less than that of hepatocytes, we considered the possibility that this might be because of the lower infection rate of RPE cells and/or reduced expression of hCD46. To test this, we confirmed the expression of hCD46 from AdCAGCD46 in mouse primary RPE cells by immunohistochemistry. AdCAGCD46-infected cells showed almost 100% transduction and showed strong expression of hCD46 on the cell membrane, whereas AdCAGpA-infected cells had no detectable expression (Figure 4a). It may be more likely then that the reduced inhibition of MAC deposition on RPE cells may be because of the increased amount of antibody (and, therefore, increased activation of Classical pathway) required to activate complement on this cell type.
Figure 3.
AdCAGCD46 protects mouse primary RPE cells from alternative pathway mediated MAC deposition. (a) Staining intensity of MAC is not uniform on cells infected with AdCAGCD46 and has areas of reduced MAC deposition. AdCAGpA-infected cells display uniform MAC deposition. (b) Quantification of MAC pixel intensity shows an overall reduction in staining intensity of 21±1.9% (*P=0.04) on AdCAGCD46-infected RPE cells compared with AdCAGpA. (Representative of three separate experiments performed in duplicate each time.)
Figure 4.
hCD46 is expressed in mouse primary RPE cells following infection with AdCAGCD46. (a) Mouse primary RPE cells efficiently express hCD46 on their membranes following infection with AdCAGCD46. No hCD46 is detectable in cells infected with AdCAGpA. BF, bright field; DAPI, 4′,6-diamidino-2-phenylindole.
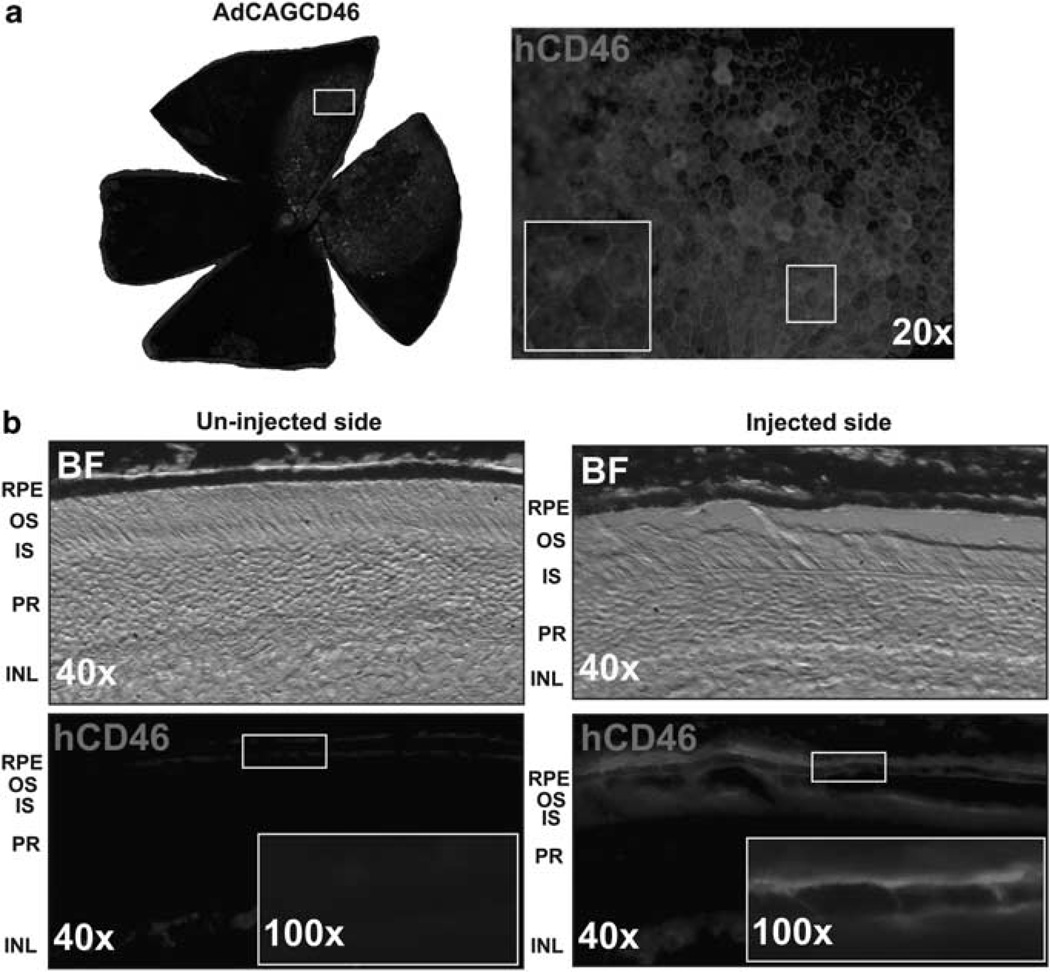
hCD46 is expressed on the basal and lateral surface of mouse RPE cells following a sub-retinal injection of AdCAGCD46
In the human eye, CD46 is expressed on both the basal and lateral surfaces of RPE cells.17 To determine whether hCD46 can be expressed in a similar pattern in mouse RPE cells in vivo, we injected AdCAGCD46 into the sub-retinal space of adult mice. After 8 days, the eyes were harvested and examined for CD46 expression. Flat mounts of the eyecups revealed robust expression in RPE cells with increased intensity of expression observed at the intercellular junctions (Figure 5a). To determine if expression is also on the basal and lateral surfaces of these cells, cross-sections were taken through the injection site and compared with an un-injected region of the eyecup. The injected region demonstrates expression mainly on the basal and lateral surface of the RPE cells, consistent with that seen in human eyes (Figure 5b).
Figure 5.
hCD46 is expressed on mouse RPE cells following a sub-retinal injection of AdCAGCD46. (a) Flat mount of a mouse eyecup with the RPE cells exposed reveals a patch of CD46 expression at 8 days after a sub-retinal injection of AdCAGCD46. (b) Cross-sections through the injection site show that hCD46 expression is strongest on the basal and lateral surface of the RPE cells and that there is no detectable hCD46 expression in the un-injected region of the same eye. BF, bright field; RPE, retinal pigment epithelium; OS, outer segments; IS, inner segments; PR, photoreceptors; INL, inner nuclear layer.
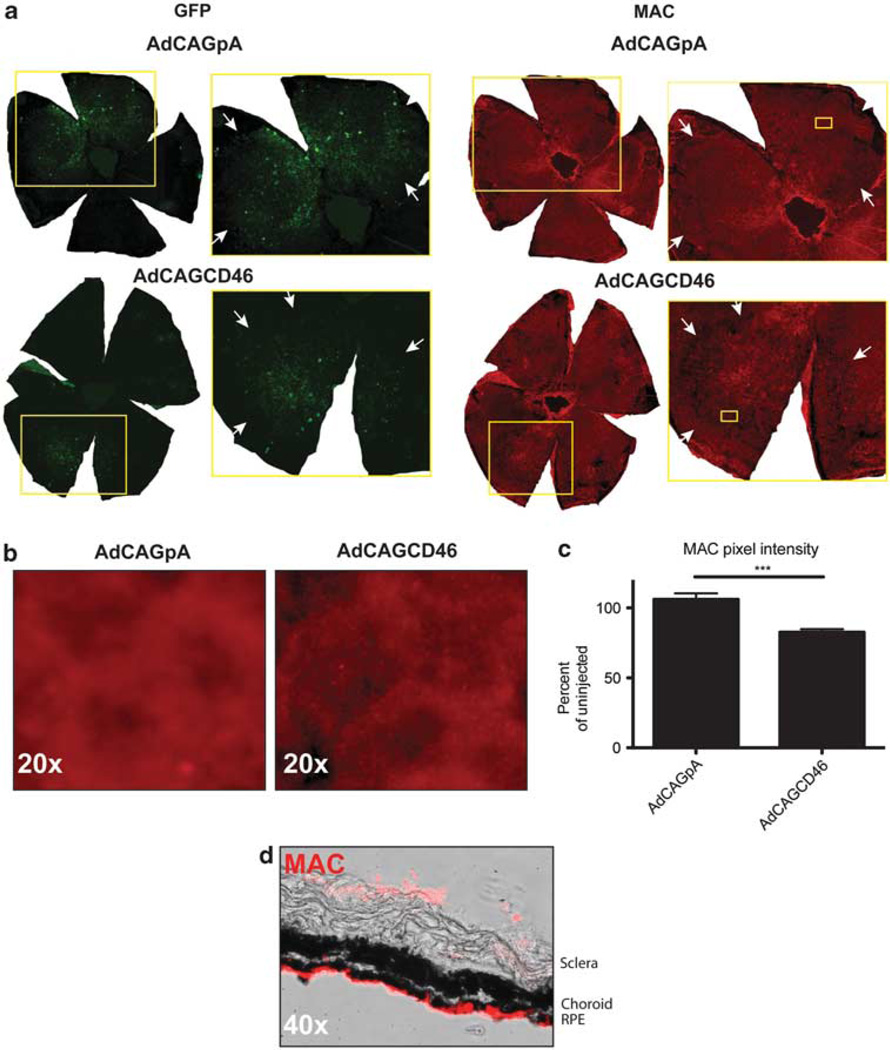
AdCAGCD46 protects mouse RPE cells from alternative pathway-mediated MAC deposition following a sub-retinal injection
As we were able to recapitulate the expression pattern of hCD46 in mouse RPE cells, we then considered whether hCD46 expressed from an adenovirus on mouse RPE cells in vivo could offer protection from human MAC deposited via the alternative pathway. Adult mice were injected into the sub-retinal space with either AdCAGCD46 or AdCAGpA. To identify the injection site, each virus was mixed with an adenovirus expressing GFP. After 8 days, the eyes were enucleated, and the lens, cornea and retina were removed to expose the RPE cells. The eyecup was treated with 140 µg ml−1 emmprin followed by 50% NHS and MgEGTA. MAC formation was detected using a monoclonal antibody to the C5b9 complex. Before the treatment of serum, GFP expression was robust. After serum treatment, GFP expression was patchy due to damage of RPE cells by the serum. Within the region of AdCAGCD46 expression, there was a significant reduction in the amount of MAC staining relative to AdCAGpA-injected eyes (Figure 6a and b). We determined a 24 ± 4.5% (P=0.0001) reduction in MAC deposition in AdCAGCD46-injected eyes relative to AdCAGpA (Figure 6c). To determine whether MAC is deposited equally on the apical and basal surface of the RPE cells, we performed the assay on un-injected eyes and then prepared cross-sections through the RPE. We found MAC to be deposited almost exclusively on the apical surface (Figure 6d).
Figure 6.
Sub-retinal delivery of AdCAGCD46 reduces the amount of alternative pathway-mediated MAC deposition on RPE cells. (a) Immunohistochemistry for MAC complex on eyecups reveals a visible decrease in MAC pixel intensity for AdCAGCD46 relative to AdCAGpA. Arrows in the inset demarcate the periphery of the injected area. (b) Higher magnification of the boxed region of MAC staining shown in a. (c) Quantification of MAC pixel intensity showing a 24±4.5% (***P=0.0001) reduction in MAC staining in AdCAGCD46-transduced eyecups relative to AdCAGpA-injected. (Representative images for AdCAGpA n=7 and AdCAGCD46 n=9.) (c) Cross-sections of an un-injected eye exposed to serum then stained for MAC indicate most of the MAC is deposited on the apical surface of RPE cells.
DISCUSSION
Recent evidence suggests that increased activity of the complement system has a significant role in the pathogenesis of AMD. Such evidence includes the presence of fragments of the complement cascade closely associated with drusen11 or presence of MAC on the surface of RPE and choroidal blood vessels.13 Genetic studies have identified polymorphisms in complement genes of both alternative and classical pathways, including factor H, factor B, C2 and C3.14 The most significant risk of developing AMD, however, is associated with polymorphisms in the alternative pathway regulator factor H.21 As the complement cascade is an important first line of defense for the immune system, we considered regulators that would allow it to function locally in ocular tissues and conservatively by dampening only the portion of the complement cascade that has been implicated as being overactive in this disease, that is, the alternative pathway.
We have previously shown localized protection of mouse RPE cells against human MAC deposition in an ex vivo humanized mouse model of complement deposition.18 In that study, human CD59 expressed from an adenovirus specifically on RPE cells, efficiently blocked MAC deposition occurring as a result of all complement pathways. With evidence accumulating implicating the alternative pathway in AMD, we chose to evaluate the potential of a complement regulator known as membrane cofactor protein (CD46) that has previously been shown to preferentially dampen the alternative pathway.15 In our study, CD46, despite robust expression on the membrane of mouse hepatocytes transduced with a CD46-expressing adenovirus, displayed no inhibition of complement-mediated cell lysis conferred in the presence of all pathways, but indicated 39 ± 0.88% protection against lysis when examined specifically in the context of the alternative pathway.
One reason for this preference may be that CD46 has a higher binding affinity for the alternative pathway component C3b relative to the classical component C4b. However, another reason may be that it takes longer for CD46 to cleave C3b and C4b than it does for a regulator such as CD59 to disrupt the MAC complex. This could be due to CD46 being a co-factor for cleavage, requiring binding of the serine protease factor I to cleave C3b and C4b. Therefore, limiting the process of MAC deposition to the alternative pathway may allow enough time for CD46 to bind C3b and recruit factor I before the convertase is formed. Furthermore, it has been shown that after the convertase is formed, CD46 can no longer bind to either C3b or C4b.22,23 This suggests that once convertase has assembled on the cell membrane, the rate of C3 cleavage may become so rapid that the amount of C3b becomes overwhelming for CD46. It is interesting to speculate whether this may be the case on RPE cells in human AMD.
CD46 in human retinal tissue is localized to the basal and lateral surface of RPE cells.17 This places CD46 close to the site of complement activation in AMD patients, in which MAC deposition has been observed in RPE cells, drusen and choroidal endothelial cells. Of particular interest is the recent observation that CD46 expression is reduced on the RPE of AMD patients.24 Polymorphisms in CD46 have been linked to the kidney disease aHUS,25 another disease resulting from overactivation in the alternative arm of complement.16 Renal allographs, which provide wild-type expression of CD46, have been a viable therapy for patients suffering from aHUS due to CD46 polymorphisms.26,27 Our results show that CD46 expressed from an adenovirus in vivo in mice recapitulates the pattern of CD46 expression observed in human RPE, and that expression is sufficient to provide 24 ± 4.5% protection against an acute insult from human complement attack mediated by the alternative pathway. Figure 5 illustrates that CD46 is most abundantly localized to the basal surface of mouse RPE cells after injection of AdCAGCD46, with some expression also on the apical and lateral surfaces. This pattern of expression recapitulates that observed in human RPE.17 One of the limitations of our assay is that only the apical side of the RPE is exposed directly to emmprin antibody and human serum such that MAC deposition occurs almost exclusively on the apical surface. Therefore, while we noticed significant protection against MAC deposition, we were not able to confirm the potential of the ability of CD46 being able to block MAC deposition also on the basal surface due to the limitations inherent in our assay.
The strong linkage observed between polymorphisms in factor H and AMD prompt consideration of CD46 as a viable therapeutic option, as both function at the same step of the complement cascade. Previous studies have shown that CD46 can compensate for the loss or reduction in factor H activity in human serum,15 a situation analogous to that proposed for AMD patients with factor H polymorphisms.
In summary, we have shown that CD46 delivered to murine retina in vivo localizes to the basal and lateral membrane of RPE cells and that this expression is sufficient to confer protection against human complement attack generated by the alternative pathway. Considering the evidence for the role of the alternative pathway in AMD, as well as the specificity of CD46 as a regulator of the alternative pathway, a precision that would allow the rest of the complement system to remain active against invading pathogens, the use of CD46 as a treatment for AMD prompts further investigation.
MATERIALS AND METHODS
Cell lines and primary RPE cell culture
HEPA 1c1c7 and 293 cell lines were obtained from American Type Culture Collection (Manassas, VA, USA). Cell culture reagents were purchased from Invitrogen Life Technologies (Carlsbad, CA, USA). Cells (293 and human embryonic retinoblasts28) were maintained in Dulbecco’s modified Eagle’s medium/10% fetal calf serum (FBS). Hepa1c1c7 cells were maintained in α-minimum essential medium (MEM)/10% FBS.
Primary mouse RPE cells were obtained from 6- to 10-week-old C57Bl/6 mice. Eyes were enucleated, and the lens, cornea and retina were removed to reveal the RPE cell layer. Eyecups were incubated in 200 µl of 0.25% trypsin/ EDTA for 1 h at 37 °C. RPE cells were pulled off in sheets and resuspended in 20 µl αMEM/10% FBS. The suspension was placed in the center of an eight-well poly-d-lysine-coated chamber slide (Becton Dickinson, Franklin Lakes, NJ, USA) for 10min to allow cells to adhere to the plate, and an additional 130 µl αMEM/10% FBS was added to each well. Cells were kept in a humidified incubator at 37 °C with 5% CO2 for 3 days before use.
Adenovirus construction
E1/E3-deleted adenovirus serotype 5 was used to express human CD46 (hCD46) or no transgene. hCD46, American Type Culture Collection (MGC-26544), was excised from pBluescriptR using EcoRI and SspI, and inserted into pCAGEN using EcoRI and EcoRV (gift from C Cepko, Harvard Medical School, Boston, MA, USA) between a cytomegalovirus enhancer/CAG and a rabbit globin polyadenylation termination sequence. The sequence containing CAG, hCD46 and polyadenylation was excised from pCAGEN using SpeI and HindIII, and was inserted into pShuttle29 using XbaI and HindIII. The pShuttle was recombined with Adeasy-1 then linearized and transfected into 293 cells. Virus production has been previously described.30 Following initial transfection virus was amplified in 911 cells. Viral purification was carried out using the adenopure purification kit (Puresyn Inc., Malvern, PA, USA) and viral titer determined at an optical density of 260 using a spectrophotometer, and then plaque purified as previously described.7 The hCD46- and polyadenylation-, previously published18, expressing viruses are subsequently referred to as AdCAGCD46 and AdCAGpA, respectively.
Western blotting
Cells (911) were infected with either AdCAGCD46 or AdCAGpA at a multiplicity of infection of 1000. After 24 h, cells were collected by trypsin treatment and centrifugation, cells lysed in lysis buffer (SDS/triton) containing protease inhibitors (leupeptin, aprotinin and phenylmethanesulfonylfluoride), and lysate run on a 12.5% Tris HCL precast gel (Biorad, Hercules, CA, USA). Following transfer, the nylon membrane was probed with a mouse anti-human CD46 antibody (MEM258, Serotec, Raleigh, NC, USA) at a dilution of 1:1000. A horseradish peroxidase-conjugated secondary antibody was used followed by detection with luminol (Pierce chemiluminescent substrate kit, Pierce, Rockford, IL, USA).
Complement assay on Hepa 1c1c7 cells
Hepa1c1c7 cells were infected (multiplicity of infection 1000) for 3 days with AdCAGCD46 or AdCAGpA in αMEM/2% FBS. For FACS analysis, the cells were collected by trypsinization (0.25%/EDTA), resuspended in 1 × phosphate-buffered saline (PBS) containing 0.5% FBS, centrifuged at 1200r.p.m. per 4 °C, and 5×105 cells resuspended in 500µl ice-cold Gelatin Veronal Buffer containing Ca2 and Mg2 (GVB2+; Complement Technology, Tyler, TX, USA). Complement was then activated on the suspension of 5× 105 cells and all cells were used for FACS so that forward and side scatter could be assessed for cell viability. To activate all complement pathways, 25µgml−1 rat anti-mouse emmprin (MCA2283, Serotec) was added for 30min at 4°C followed by 10% NHS (Sigma, St Louis, MO, USA), 37 °C for 1h with constant rotary motion. Cells were treated similarly with 10% heat-inactivated NHS (HINHS; 56 °C for 1 h) as control. Cell lysis was determined by the PI exclusion method. PI was added to each sample (1 µl PI into 500 µl GVB2+) and 2.5 × 104 cells were counted by FACS (Becton Dickinson) for PI uptake (CellQuest Pro software, Becton Dickinson).
To preferentially activate the alternative pathway, cells were collected as described above and resuspended in GVB2+. A total of 5×105 cells were pre-incubated with 25µgml−1 rat anti-mouse emmprin antibody and incubated for 30min at 4 °C. Either 10% NHS or 10% HINHS containing MgEGTA 7 mm magnesium, 10 mm EGTA was added and incubated at 37 °C for 1 h.
For MAC staining of Hepa1c1c7, cells were plated into an eight-well chamber slide (Becton Dickinson) and infected with either AdCAGCD46 or AdCAGpA for 3 days (multiplicity of infection 1000). Each well was incubated with 25 µg ml−1 emmprin for 30 min at 4°C followed by either 10% NHS or 10% HINHS in GVB2+ at 37 °C for 5min with MgEGTA to de-activate the classical pathway. Cells were washed twice with cold 1× PBS, fixed for 15min in 10% neutral-buffered formalin and stored in 1× PBS. Cell lysis was determined using the PI exclusion method as described above.
Complement assay on primary mouse RPE cells
Cells were infected with either AdCAGCD46 or AdCAGpA at an multiplicity of infection of 1000 for 3 days in αMEM/10% FBS. The media was removed and 50 µg ml−1 rat anti-mouse emmprin was added and incubated at room temperature for 1 h. Immediately following emmprin treatment, 50% NHS or 50% HINHS containing MgEGTA was added and cells incubated at 37 °C for 1 h. Cells were washed three times in cold 1 × PBS, fixed in 10% neutral-buffered formalin for 15min and stored in 1× PBS at 4°C.
Complement assay on mouse eyecups
At 8 days after subretinal injection of adenovirus, mice were killed using CO2, and the lens, cornea and were retina removed. The eyecup was incubated in GVB2+ containing 140 µg ml−1 rat anti-mouse emmprin for 1h at 4 °C. Either 50% NHS or 50% HINHS was added directly to the GVB2+/emmprin and incubated for 4min at 37 °C. MgEGTA was added to each sample and incubated for an additional 56min at 37 °C. Samples were washed three times in cold 1× PBS and fixed overnight in 4% paraformaldehyde at 4 °C.
Immunohistochemistry
To detect membrane expression of CD46 on hepa1c1c7 and primary RPE cell cultures, cells were incubated in mouse anti-human CD46 (clone MEM258, Serotec; 1:50) in 3% normal goat serum (NGS; Jackson Immunoresearch, West Grove, PA, USA) at 4 °C for 3 h before fixation. Cells were fixed in 10% neutral-buffered formalin overnight at 4 °C. Secondary detection was performed with Cy3 goat anti-mouse (37.5 ng ml−1 ) in 3% NGS for 1 h. To detect CD46 in fixed 14-µm frozen sections and mouse eyecups, samples were pre-treated with 6% NGS and incubated in mouse anti-human CD46 (clone E4.3, BD Pharmingen, San Jose, CA, USA; 1:100) containing 0.5% triton overnight at 4 °C. Secondary detection was performed with Cy3 goat anti-mouse (37.5 ng ml−1) in 0.5% triton for 1 h. To detect the MAC complex, samples were incubated with mouse anti-human C5b-9 (clone aE11, Abcam, Cambridge, MA, USA; 1:100) containing 6% NGS and 0.05% triton for 2.5 h. Secondary detection was carried out with Cy3 goat anti-mouse (37.5 ng ml−1 ) containing 3% NGS and 0.05% triton for 1.5 h.
Sub-retinal injections
The use of animals in this study was in accordance with the ARVO (Association for Research in Vision and Ophthalmology) statement for the use of animals in ophthalmic and vision research. C57Bl/6 mice were purchased from Jackson laboratories, bred and maintained in a 12-h light–dark cycle and cared for in accordance with federal, state, and local regulations. Mice (6–10 week old) were anesthetized by intraperitoneal injection of xylazine (10 mg ml−1)/ketamine (1 mg ml−1). Subretinal injections were carried out as previously described30 using the transcleral–transchoroidal approach with a 32-gauge needle attached to a 5 µl glass syringe (Hamilton, Reno, NV, USA). Either 1 µl of an empty vector control mixture containing nine parts AdCAGpA (total 5× 107 particles) and one part AdCAGGFP (total 1×106 particles) or CD46 vector containing nine parts AdCAGCD6 (total 5× 107 particles) and one part AdCAGGFP (total 1×106 particles) were injected.
Statistical analysis
Experiments were carried out in duplicate at least three times. Error bars represent standard deviation from the mean. Where appropriate, significance was calculated using Student’s t-test.
ACKNOWLEDGEMENTS
This study was supported by grants to RK-S from The Ellison Foundation, The Virginia B Smith Trust and grants to the Department of Ophthalmology at Tufts University from the Lions Eye Foundation and Research to Prevent Blindness.
Footnotes
CONFLICT OF INTEREST
The authors declare no conflict of interest.
References
- 1.Chevez-Barrios P, Chintagumpala M, Mieler W, Paysse E, Boniuk M, Kozinetz C, et al. Response of retinoblastoma with vitreous tumor seeding to adenovirus-mediated delivery of thymidine kinase followed by ganciclovir. J Clin Oncol. 2005;23:7927–7935. doi: 10.1200/JCO.2004.00.1883. [DOI] [PubMed] [Google Scholar]
- 2.Campochiaro PA, Nguyen QD, Shah SM, Klein ML, Holz E, Frank RN, et al. Adenoviral vector-delivered pigment epithelium-derived factor for neovascular age-related macular degeneration: results of a phase I clinical trial. Hum Gene Theropy. 2006;17:167–176. doi: 10.1089/hum.2006.17.167. [DOI] [PubMed] [Google Scholar]
- 3.Sweigard JH, Cashman SM, Kumar-Singh R. Adenovirus vectors targeting distinct cell types in the retina. Invest Ophthalmol Vis Sci. 2010;51:2219–2228. doi: 10.1167/iovs.09-4367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lamartina S, Cimino M, Roscilli G, Dammassa E, Lazzaro D, Rota R, et al. Helper-dependent adenovirus for the gene therapy of proliferative retinopathies: stable gene transfer, regulated gene expression and therapeutic efficacy. J Gene Med. 2007;9:862–874. doi: 10.1002/jgm.1083. [DOI] [PubMed] [Google Scholar]
- 5.Kim IH, Józkowicz A, Piedra PA, Oka K, Chan L. Lifetime correction of genetic deficiency in mice with a single injection of helper-dependent adenoviral vector. Proc Natl Acad Sci USA. 2001;98:13282–13287. doi: 10.1073/pnas.241506298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Parks RJ, Chen L, Anton M, Sankar U, Rudnicki MA, Graham FL. A helper-dependent adenovirus vector system: removal of helper virus by Cre-mediated excision of the viral packaging signal. Proc Natl Acad Sci USA. 1996;93:13565–13570. doi: 10.1073/pnas.93.24.13565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kumar-Singh R, Yamashita CK, Tran K, Farber DB. Construction of encapsidated (gutted) adenovirus minichromosomes and their application to rescue of photoreceptor degeneration. Methods Enzymol. 2000;316:724–743. doi: 10.1016/s0076-6879(00)16759-x. [DOI] [PubMed] [Google Scholar]
- 8.Klein R, Klein BE, Knudtson MD, Meuer SM, Swift M, Gangnon RE. Fifteen-year cumulative incidence of age-related macular degeneration: the Beaver Dam Eye Study. Ophthalmology. 2007;114:253–262. doi: 10.1016/j.ophtha.2006.10.040. [DOI] [PubMed] [Google Scholar]
- 9.van Leeuwen R, Klaver CC, Vingerling JR, Hofman A, de Jong PT. Epidemiology of age-related maculopathy: a review. Eur J Epidemiol. 2003;18:845–854. doi: 10.1023/a:1025643303914. [DOI] [PubMed] [Google Scholar]
- 10.Ozkiris A. Anti-VEGF agents for age-related macular degeneration. Expert Opin Ther Pat. 2010;20:103–118. doi: 10.1517/13543770902762885. [DOI] [PubMed] [Google Scholar]
- 11.Hageman GS, Luthert PJ, Victor Chong NH, Johnson LV, Anderson DH, Mullins RF. An integrated hypothesis that considers drusen as biomarkers of immune-mediated processes at the RPE-Bruch’s membrane interface in aging and age-related macular degeneration. Prog Retin Eye Res. 2001;20:705–732. doi: 10.1016/s1350-9462(01)00010-6. [DOI] [PubMed] [Google Scholar]
- 12.Johnson LV, Leitner WP, Staples MK, Anderson DH. Complement activation and inflammatory processes in Drusen formation and age related macular degeneration. Exp Eye Res. 2001;73:887–896. doi: 10.1006/exer.2001.1094. [DOI] [PubMed] [Google Scholar]
- 13.Anderson DH, Mullins RF, Hageman GS, Johnson LV. A role for local inflammation in the formation of drusen in the aging eye. Am J Ophthalmol. 2002;134:411–431. doi: 10.1016/s0002-9394(02)01624-0. [DOI] [PubMed] [Google Scholar]
- 14.Reynolds R, Hartnett ME, Atkinson JP, Giclas PC, Rosner B, Seddon JM. Plasma complement components and activation fragments: associations with age-related macular degeneration genotypes and phenotypes. Invest Ophthalmol Vis Sci. 2009;50:5818–5827. doi: 10.1167/iovs.09-3928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Barilla-LaBarca ML, Liszewski MK, Lambris JD, Hourcade D, Atkinson JP. Role of membrane cofactor protein (CD46) in regulation of C4b and C3b deposited on cells. J Immunol. 2002;168:6298–6304. doi: 10.4049/jimmunol.168.12.6298. [DOI] [PubMed] [Google Scholar]
- 16.Kavanagh D, Richards A, Atkinson J. Complement regulatory genes and hemolytic uremic syndromes. Annu Rev Med. 2008;59:293–309. doi: 10.1146/annurev.med.59.060106.185110. [DOI] [PubMed] [Google Scholar]
- 17.Vogt SD, Barnum SR, Curcio CA, Read RW. Distribution of complement anaphylatoxin receptors and membrane-bound regulators in normal human retina. Exp Eye Res. 2006;83:834–840. doi: 10.1016/j.exer.2006.04.002. [DOI] [PubMed] [Google Scholar]
- 18.Ramo K, Cashman SM, Kumar-Singh R. Evaluation of adenovirus-delivered human CD59 as a potential therapy for AMD in a model of human membrane attack complex formation on murine RPE. Invest Ophthalmol Vis Sci. 2008;49:4126–4136. doi: 10.1167/iovs.08-2025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Post TW, Liszewski MK, Adams EM, Tedja I, Miller EA, Atkinson JP. Membrane cofactor protein of the complement system: alternative splicing of serine/threonine/proline-rich exons and cytoplasmic tails produces multiple isoforms that correlate with protein phenotype. J Exp Med. 1991;174:93–102. doi: 10.1084/jem.174.1.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McNearney T, Ballard L, Seya T, Atkinson JP. Membrane cofactor protein of complement is present on human fibroblast, epithelial, and endothelial cells. J Clin Invest. 1989;84:538–545. doi: 10.1172/JCI114196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Edwards AO, Ritter IIIR, Abel KJ, Manning A, Panhuysen C, Farrer LA. Complement factor H polymorphism and age-related macular degeneration. Science. 2005;308:421–424. doi: 10.1126/science.1110189. [DOI] [PubMed] [Google Scholar]
- 22.Gao LJ, Guo SY, Cai YQ, Gu PQ, Su YJ, Gong H, et al. Cooperation of decay-accelerating factor and membrane cofactor protein in regulating survival of human cervical cancer cells. BMC Cancer. 2009;9:384. doi: 10.1186/1471-2407-9-384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brodbeck WG, Mold C, Atkinson JP, Medof ME. Cooperation between decay-accelerating factor and membrane cofactor protein in protecting cells from autologous complement attack. J Immunol. 2000;165:3999–4006. doi: 10.4049/jimmunol.165.7.3999. [DOI] [PubMed] [Google Scholar]
- 24.Vogt SD, Curcio CA, Wang L, Li CM, McGwin G, Jr, Medeiros NE, et al. Altered retinal pigment epithelium morphology is associated with decreased expression of complement regulatory protein CD46 and ion transporter MCT3 in geographic atrophy of age-related maculopathy. Invest Ophthalmol Vis Sci. 2009;50:4180. E-Abstract. [Google Scholar]
- 25.Richards A, Kemp EJ, Liszewski MK, Goodship JA, Lampe AK, Decorte R, et al. Mutations in human complement regulator, membrane cofactor protein (CD46), predispose to development of familial hemolytic uremic syndrome. Proc Natl Acad Sci USA. 2003;100:12966–12971. doi: 10.1073/pnas.2135497100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Caprioli J, Noris M, Brioschi S, Pianetti G, Castelletti F, Bettinaglio P, et al. Genetics of HUS: the impact of MCP, CFH, and IF mutations on clinical presentation, response to treatment, and outcome. Blood. 2006;108:1267–1279. doi: 10.1182/blood-2005-10-007252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kavanagh D, Goodship TH. Membrane cofactor protein and factor I: mutations and transplantation. Semin Thromb Hemost. 2006;32:155–159. doi: 10.1055/s-2006-939771. [DOI] [PubMed] [Google Scholar]
- 28.Fallaux FJ, Kranenburg O, Cramer SJ, Houweling A, Van Ormondt H, Hoeben RC, et al. Characterization of 911: a new helper cell line for the titration and propagation of early region 1-deleted adenoviral vectors. Hum Gene Theropy. 1996;7:215–222. doi: 10.1089/hum.1996.7.2-215. [DOI] [PubMed] [Google Scholar]
- 29.He TC, Zhou S, da Costa LT, Yu J, Kinzler KW, Vogelstein B. A simplified system for generating recombinant adenoviruses. Proc Natl Acad Sci USA. 1998;95:2509–2514. doi: 10.1073/pnas.95.5.2509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cashman SM, Morris DJ, Kumar-Singh R. Adenovirus type 5 with adenovirus type 37 fiber uses sialic acid as a cellular receptor. Virology. 2004;324:129–139. doi: 10.1016/j.virol.2004.04.001. [DOI] [PubMed] [Google Scholar]