Abstract
Multimodal CT/MRI has dramatically changed the approach to ischemic stroke management, as noninvasive CT/MRI images detail brain tissue or parenchyma, angiography or vessel status, and collateral perfusion or blood flow in regions of the brain vulnerable to ischemic injury. Such snapshots of the dynamic process of cerebral ischemia may be used to gauge reversibility and therapeutic opportunities. Treatment of acute stroke may be rapidly tailored to clinical scenarios based on imaging correlation of ischemia, vessel status, and perfusion. Serial or repeated imaging from the initial presentation to later stages of the hospital course may illustrate infarct growth, persisting occlusion, reocclusion, recanalization, reperfusion, and hemorrhagic transformation. From acute stroke to rehabilitation phases and subsequent prevention, multimodal CT/MRI has emerged as a key tool to track the process of stroke and the impact of our therapeutic interventions.
Keywords: stroke, acute ischemia, imaging, thrombolysis, collateral circulation, hemodynamics
Introduction
Advanced imaging technologies, including multi-modal CT/MRI, have dramatically changed the approach to ischemic stroke management during the last decade. Multimodal CT/MRI provides noninvasive images of brain tissue or parenchyma, angiography or vessels, and perfusion or blood flow in regions of the brain vulnerable to ischemic injury. Such comprehensive information on potential arterial occlusions, downstream perfusion, and evolving injury are routinely utilized in clinical practice. Research efforts have extended the utility of these tools to identify biomarkers of cerebral ischemia, although clinical trials have yet to simplify the vast resulting imaging data into streamlined protocols.1 Overwhelming emphasis has been placed on the use of baseline or initial imaging predictors of clinical stroke outcomes, with relatively minimal consideration of intervening treatments and other influential factors. The discrepancy between current clinical use and research applications of multimodal CT/MRI has not, however, deterred rapid adoption of this technology by clinicians to routinely evaluate stroke patients and guide stepwise decision making throughout the hospital course into the chronic, outpatient setting.
Imaging in clinical context
Stroke imaging has developed into a specialized approach that integrates advanced multimodal CT/MRI techniques with pertinent clinical information at the bedside. Images of brain vessels, per-fusion, and potential injury must be interpreted in light of clinical history and examination to guide subsequent decision making. This facet is in sharp contrast to the traditional notion that blinded readings or interpretations are optimal. In fact, imaging is an extension of the clinical evaluation, providing subtle clues to critical pathophysiology rather than findings that are obvious from the physical or neurological examination. For instance, imaging of a large infarction, obvious by clinical examination, may not be as important as subtle indicators of the pattern of parenchymal injury that informs clinicians about important stroke mechanisms or sub-types. Imaging has therefore emerged as an overall approach to patient management, rather than a mere diagnostic test. Most commonly, a clinical differential diagnosis including stroke is entertained, and multimodal CT/MRI may help to confirm or rule out ischemia. Often, a specific question or area of the brain is the focus of imaging efforts, such as the possibility of a left middle cerebral artery (MCA) occlusion that explains acute symptoms of right hemiparesis and aphasia (Fig. 1). If thrombotic occlusion of this vessel is suspect, then specific sequences such as gradient-recalled echo (GRE) acquisitions may be useful to depict an associated blooming artifact of the thrombosed arterial segment.2 Although a standard CT/MRI protocol is commonly used at most institutions, the specific components or sequences may be tailored to the clinical question.3 Even further modification of scan parameters may help in answering the specific clinical question. In recent years, postprocessing of noninvasive CT/MR angiography (CTA/MRA) and per-fusion (CTP/PWI) imaging may be performed at the scanner or later to provide detailed information regarding vascular status. Once images are available for review, interpretation in light of clinical details is critical. Noncontrast CT scans in acute stroke are often designated as unremarkable despite the presence of early ischemic findings that denote dynamic pathophysiology. The description of these findings and potential interpretation by verbal or written report are critical, and the need for serial imaging should be emphasized when appropriate. Serial or repeated imaging from the initial presentation to later stages of the hospital course may illustrate infarct growth, persisting occlusion, reocclusion, recanalization, reperfusion, and hemorrhagic transformation.4
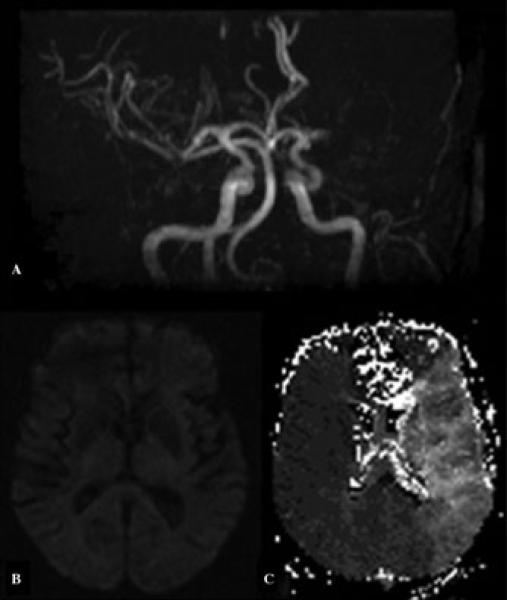
Figure 1.
Multimodal MRI, including (A) time-of-flight MR angiography, (B) diffusion-weighted imaging, and (C) perfusion-weighted imaging, in acute stroke due to left middle cerebral artery occlusion.
Vascular occlusion and collaterals
During the hyperacute phase of stroke, as the patient arrives in the emergency department, rapid imaging with CT/MRI is the first priority in order to confirm a suspicion of ischemia and to rule out hemorrhage or nonvascular disorders (Fig. 2). Clinicians often employ imaging to simply rule out stroke in patients that present with neurological emergencies. It should be remembered that vascular correlates exist in almost all neurological disorders. From a stroke standpoint, it is important to consider neurovascular structures at many levels, from large arteries and veins with compensatory collaterals, to the micro-circulation, including the blood–brain barrier, per-fusion of the penumbra, or areas at risk of evolving infarction and hemorrhagic transformation.
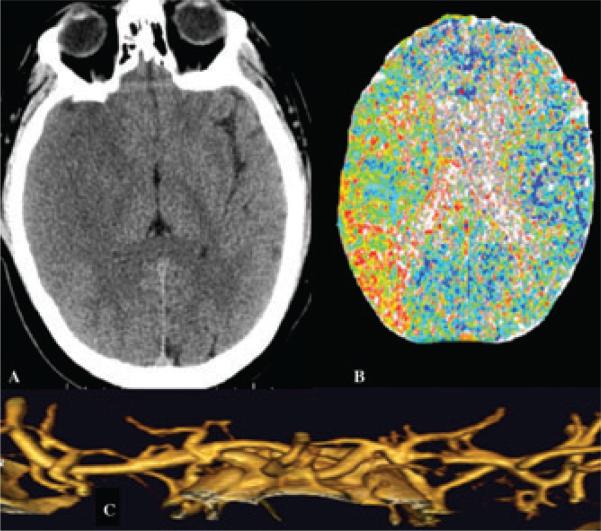
Figure 2.
Multimodal CT, including (A) noncontrast CT, (B) CT perfusion, and (C) CT angiography, in acute stroke due to right middle cerebral artery distribution ischemia after partial reperfusion.
Knowledge of common arterial and venous anatomy and pathophysiology or function remains a prerequisite for ideal application of multimodal CT/MRI in stroke. Almost every imaging modality, including the multimodal components of parenchymal images, CTA/MRA and CTP/PWI, provide important details about acute ischemic stroke. Vessel imaging may reveal an arterial occlusion or stenosis, and in some cases, the morphology or imaging of the vessel wall may indicate dissection as an important mechanism. In other cases, the pattern of ischemic lesions in the brain may demarcate the border between vascular territories where perfusion is minimal and emboli are least likely to wash out.5 Noninvasive CTA/MRA may provide distinct information about vessel status with respect to structural anatomy or morphology and flow or physiology. CTA emphasizes anatomical features of the vessel lumen and adjacent wall, where atherosclerotic plaque or dissection may be visualized. The nature of the CTA study, with relatively prolonged scan acquisitions, tends to obliterate any temporal information about flow in the vessel. Conversely, time-of-flight (TOF) MRA accentuates the relative amount of flow in a vessel, with a paucity of information about anatomical details. Contrast-enhanced MRA techniques may provide better characterization of the luminal anatomy or degree of stenosis. Finally, phase-contrast MRA can provide unique data on not just the amount but also on the direction of blood flow in large vessels. This approach, however, is impractical in acute stroke due to the longer time required to acquire the scans.
These vascular imaging studies are often used to illustrate occlusion or blockage of a feeding artery to the brain in the setting of acute ischemic stroke; yet the potential to illustrate collateral flow patterns may be equally important. Collaterals deliver compensatory blood flow to downstream regions of the brain when an artery is blocked.6 The amount and nature of collateral perfusion may therefore be important in determining the likelihood of tissue being preserved from an evolving infarction or a worsening stroke. CTA collateral grading schemes may provide gross distinctions of the extent of such protective features, yet CTP may provide further information, as the source images may demonstrate the actual vessel extent of collaterals with simultaneous measurement of downstream perfusion.7 This use of CTP source images may be superior to CTA for detection of collaterals because of the temporal information provided by CTP. On MRI, the fluid-attenuated inversion recovery (FLAIR) sequences may show vascular hyperintensities due to slow retrograde flow in the leptomeningeal vessels feeding the cortex (Fig. 3).8 Such subtle findings may be an important clue to the presence of a proximal arterial occlusion in the diagnosis of acute ischemic stroke. Conversely, a noncontrast CT scan may demonstrate increased densities along cortical sulci, a finding that indicates an increased blood pool in the cortical vessels due either to compensatory vasodilation in the presence of a proximal occlusion or to hyperemic reperfusion of an infarct.
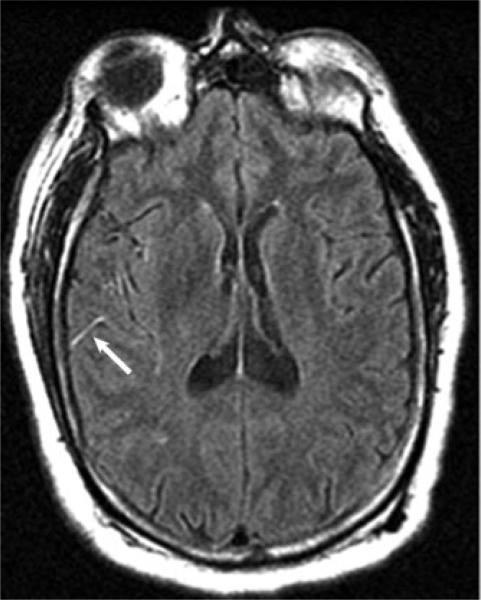
Figure 3.
FLAIR vascular hyperintensity (arrow) of slow right posterior cerebral artery collateral flow into the adjacent territory of an occluded middle cerebral artery during acute stroke.
Ischemic injury
Imaging diagnosis of acute ischemic stroke complements clinical examination by providing detailed information about the extent of evolving injury in the core and the potential therapeutic target within the surrounding penumbra or areas at risk. Both multimodal CT and MRI can be used to confirm a diagnosis of stroke by documenting ischemic changes on noncontrast CT or diffusion-weighted imaging (DWI) sequences, while also demonstrating whether an arterial occlusion is present on noninvasive angiography, with estimation in the degree or extent of reduced blood flow with CTP or PWI. The use of such techniques is not mandatory or universal, as the imaging approach to diagnosis of acute stroke often varies based on institutional practices and the clinical scenario, including such details as time from onset of symptoms. In general, any component of the multimodal CT/MRI study that aids diagnosis should be used to guide specific decision making. While multimodal CT protocols are fairly standard, MRI protocols for acute ischemic stroke often vary. Resolution varies with both CT and MRI, depending on scan acquisition parameters. Detailed resolution may enhance delineation of subtle features on MRI or even CT, yet such increased resolution may introduce excessive noise to signal on perfusion studies. Increased coverage has recently become available with CT, which now permits whole brain coverage. Use of a routine protocol at any institution may accelerate diagnosis and better inform clinical decisions. Most commonly, the multimodal CT/MRI provides a snapshot of the dynamic process of cerebral ischemia, with hints available from the perfusion imaging to gauge reversibility. The physiological clock of tissue status available from imaging provides far more information than the previous standard of time from symptom onset. Research in acute stroke imaging has failed, however, to define unequivocal definitions that can be universally applied across all cases, as the time course or evolution of injury may be different in each individual. Serial imaging, including repeated studies, may depict this aspect of cerebral ischemia, but serial imaging is clearly impractical during acute stroke in which urgent treatment must be initiated. During the earliest stages of ischemia—within the first few hours after symptom onset—detailed imaging with multimodal CT/MRI may not be needed, as reversibility with prompt treatment and delays incurred by additional diagnostic studies may make multimodal CT/MRI superfluous. Multimodal CT/MRI has also recently demonstrated interesting early findings associated with hemorrhagic stroke, such as the CTA spot sign that may predict hematoma expansion and perihematomal changes on CT/MRI of edema.9
Diagnosis of ischemia may be confirmed and systematically evaluated with use of the Alberta Stroke Program Early CT Score (ASPECTS) scheme on either CT or MRI.10 This grading system divides supratentorial regions into cortical and subcortical to provide a summary score for extent of ischemic changes. This system complements casual observation of other early ischemic changes, such as subtle hypodensity at the cortical surface, insular ribbon sign, or lenticular obscuration. Today, MRI may be used instead of CT because the ability to detect hemorrhage and simultaneously depict ischemia is superior compared with CT. As the first sequence acquired during MRI, DWI may depict acute ischemia; however, this should not be immediately equated with an infarct, as areas of restricted diffusion correlate with various levels of metabolic derangement on PET.11 Although some DWI lesions may be reversible, most hyperacute DWI lesions will likely evolve into mature infarctions, particularly if reperfusion is delayed or ineffective. DWI provides further useful information, including patterns that may provide information regarding causes or mechanisms of stroke;12 for example, embolic patterns and borderzone ischemia may be noted. When exact time of onset is unclear, the presence of DWI findings without FLAIR changes may be informative about approximate duration or severity of ischemia.13 This DWI-FLAIR mismatch may be used to guide revascularization decisions; ongoing trials will confirm the utility of this approach in coming years. Even when large vessel findings may not be evident, DWI and GRE sequences may characterize the extent of small vessel or microvascular disease.14
Hypoperfusion
Perfusion imaging with either CTP or PWI has become a routine tool in assessment of stroke patients. Although evidence lags with respect to the literature on perfusion imaging as an indispensable tool for acute stroke evaluation, many centers utilize per-fusion imaging with either CT or MRI to triage patients for revascularization. Indirect markers of salvageable tissue constructed from mismatch combinations between clinical findings and CT, DWI, and vessel occlusion on MRA, and other variants, all suggest the presence of hypoperfusion in the affected territory. Perfusion imaging, however, yields an intuitive and direct imaging approach to ischemic stroke, as hypoperfusion is the principal pathophysiology that leads to brain injury. Hypoperfusion on acute stroke imaging studies may be characterized by the degree of delay in time parameters for blood flow transit and the dispersion of the nutrient blood pool measured as cerebral blood volume (CBV) within a region. The mathematics for CT or MRI perfusion techniques that utilize bolus tracking of intravascular contrast is not specific to imaging modality. The relationship between contrast concentration and signal intensity is dissimilar between CT and MRI; yet once concentration curves can be generated during postprocessing, the calculation of parameters such as cerebral blood flow (CBF), mean transit time, and cerebral blood volume are identical. Arterial spin-labeled (ASL) per-fusion imaging uses endogenous labeling of blood flow in the proximal arteries to measure downstream perfusion without the need for exogenous contrast agents.15 This technique, therefore, holds specific advantage over standard perfusion imaging, as the ASL studies can be easily repeated to measure changes in blood flow. Until recently, ASL was used to focus on CBF during earlier arterial phases, rather than the more familiar parameters of transit time and CBV. Multidelay acquisitions with ASL that allow for scanning during later stages of perfusion through the capillary bed and venous system may soon expand the capabilities of ASL in acute stroke.
All perfusion imaging techniques may be limited by poor contrast opacification or timing errors due to decreased cardiac output, curtailed acquisitions that fail to capture the influential venous stages of perfusion, patient motion, and permeability changes in the blood-brain barrier (BBB). Perhaps the most significant limitation in perfusion imaging with either CT or MRI is the variability of results produced by different postprocessing software packages.
Imaging the treatment of acute ischemic stroke
Treatment of acute stroke may be rapidly tailored based on imaging correlation of ischemia, vessel status, and perfusion with clinical features. Revascularization during the initial 4.5 h after symptom onset may be implemented with intravenous thrombolysis, yet imaging may be critical to identifying those unlikely to respond to tissue plasminogen activator (t-PA) or to selecting appropriate candidates for other vascular interventions during later times. Endovascular treatments such as thrombectomy may be indicated by the presence of early vessel signs of occlusion in the absence of prohibitive changes in downstream per-fusion or established ischemia. Unequivocal benefit of such interventions remains to be proven. A mismatch pattern, as defined by the DEFUSE trialists, may segregate optimal candidates for later thrombolysis, without incurring additional harm, by avoiding treatment in those with malignant mismatch patterns.16,17 These patterns were originally establishedfor MRI mismatch betweenspecific DWI and PWI patterns; however, other thresholded parameters and approaches with CT are now under investigation. Early vessel signs, including the hyperdense MCA sign on a CT or a blooming artifact on GRE MRI, may indicate red cell–rich thrombotic occlusions rather than fibrin-laden blockages.2 In the coming years, imaging strategies that reliably distinguish cases based on collateral status may also prove useful.18 Meanwhile, practical approaches include consideration of clinical CT and clinical DWI mismatches in which the severity of the neurological deficit is compared with the extent and location of the lesion on imaging.19,20 Severe deficits with small lesions strongly suggest a large area of hypoperfusion that affects potentially reversible tissues.
Monitoring the response to various acute stroke therapies may be facilitated with the use of multi-modal CT/MRI. As previously noted, the dynamic nature of cerebral ischemia is best characterized by serial imaging. Clinical response is often difficult to predict and may also not be obviously linked to specific mechanisms. For instance, resolution of symptoms or rapid improvement may not be due to arterial recanalization, as patients may improve with head down-positioning, due to improved residual flow or collateral perfusion despite persisting proximal arterial occlusion. Conversely, there are numerous causes of early neurological deterioration that may be disclosed with imaging. Serial imaging may characterize the changes on each component of multimodal CT/MRI. For example, infarct growth or hemorrhage may be measured with serial or repeated parenchymal imaging. Similarly, recanalization may be chronicled with serial noninvasive angiography techniques, and reperfusion may be illustrated with repeat CTP or PWI. Recanalization and reperfusion are related yet distinct components of revascularization that may lead to clinical improvement in acute stroke.21 Recanalization, or opening of the proximal arterial occlusion, may be an important technical outcome of a drug or device, whereas recanalization without more extensive reperfusion may not lead to clinical improvement. Reperfusion, however, is more closely linked with clinical outcomes. Serial perfusion imaging may demonstrate reperfusion, yet the same techniques must be applied to definitively assess the extent and location of reperfusion. The most complex aspect of stroke imaging may relate to the vast heterogeneity of voxel or tissue fate within a region of the brain.22 For example, a given patient may have areas that have already reperfused at baseline and yet other areas that remain hypoperfused. In order to accurately document recanalization or reperfusion, one would want to use serial or repeated imaging with the same technique at each time point.
Hyperperfusion and hemorrhagic transformation
Finally, hyperperfusion may manifest on perfusion imaging, culminating in hemorrhagic transformation.23 Such hyperperfusion may be most obvious with ASL techniques (Fig. 4). Standard imaging time points for monitoring stroke evolution and response to therapy include baseline, several to 24 h after revascularization, and tissue fate at day 7. Hyper-perfusion may develop at any time point, but the hemorrhagic risk likely increases in the presence of permeability changes in the blood–brain barrier (BBB). Permeability or leakage of contrast may be measured from CTP or PWI source images, thereby avoiding the use of longer, dedicated acquisitions when time is of the essence.24 After thrombolysis or other revascularization therapies, patients may be evaluated with repeated imaging during their intensive care unit (ICU) course. Although unexplored to date, monitoring of infarct patterns, hemorrhage, and hemodynamics with serial imaging may be important for optimizing patient outcomes in the ICU. On rare occasions, recurrent strokes may ensue. Use of advanced imaging approaches for early diagnosis and treatment of acute ischemic stroke also facilitates implementation of early secondary prevention algorithms. Often, the mechanism or underlying subtype of ischemic stroke is easily ascertained from use of multimodal CT/MRI, thereby permitting prompt use of prevention strategies. Prognostication is possible and allows for planning of ideal rehabilitation approaches.
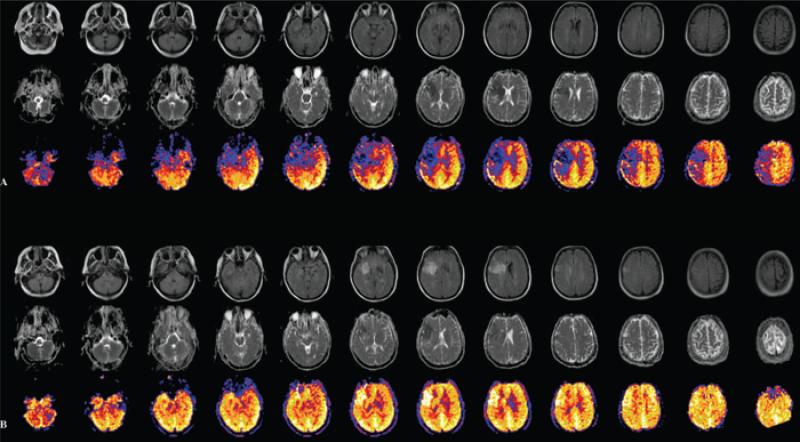
Figure 4.
Serial MRI at (A) baseline and (B) 3 hours after revascularization of right middle cerebral artery distribution stroke revealing FLAIR evolution of ischemia (top rows) with apparent diffusion coefficient evidence of ischemic injury (middle rows) and arterial spin-labeled perfusion evidence (bottom rows) of hypoperfusion changing to hyperperfusion after treatment.
Conclusions
In summary, imaging with multimodal CT/MRI has become an extension of the clinical exam, confirming diagnosis of stroke and specific pathophysiology and facilitating treatment decisions and monitoring of dynamic changes during the first few days after stroke onset. From acute stroke to rehabilitation and subsequent prevention, multimodal CT/MRI has emerged as a key tool to track the process of stroke and the impact of therapeutic interventions.
Acknowledgments
The authors acknowledge funding from NIHNINDS K23 NS054084, P50 NS044378, and K24 NS072272.
Footnotes
Conflicts of interest
The authors declare no conflicts of interest.
References
- 1.Wintermark M, Albers GW, Alexandrov AV, et al. Acute stroke imaging research roadmap. Stroke. 2008;39:1621–1628. doi: 10.1161/STROKEAHA.107.512319. [DOI] [PubMed] [Google Scholar]
- 2.Liebeskind DS, Sanossian N, Yong WH, et al. CT and MRI early vessel signs reflect clot composition in acute stroke. Stroke. 2011;42:1237–1243. doi: 10.1161/STROKEAHA.110.605576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liebeskind DS, Kidwell CS. Advanced MR imaging of acute stroke: the University of California at Los Angeles endovascular therapy experience. Neuroimaging Clin. N. Am. 2005;15:455–466. xiii. doi: 10.1016/j.nic.2005.06.002. [DOI] [PubMed] [Google Scholar]
- 4.Scalzo F, Hao Q, Alger JR, et al. Regional prediction of tissue fate in acute ischemic stroke. Ann. Biomed. Eng. 2012 May 17; doi: 10.1007/s10439-012-0591-7. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Caplan LR, Hennerici M. Impaired clearance of emboli (washout) is an important link between hypoper-fusion, embolism, and ischemic stroke. Arch. Neurol. 1998;55:1475–1482. doi: 10.1001/archneur.55.11.1475. [DOI] [PubMed] [Google Scholar]
- 6.Liebeskind DS. Collateral circulation. Stroke. 2003;34:2279–2284. doi: 10.1161/01.STR.0000086465.41263.06. [DOI] [PubMed] [Google Scholar]
- 7.Miteff F, Levi CR, Bateman GA, et al. The independent predictive utility of computed tomography angiographic collateral status in acute ischaemic stroke. Brain. 2009;132:2231–2238. doi: 10.1093/brain/awp155. [DOI] [PubMed] [Google Scholar]
- 8.Sanossian N, Saver JL, Alger JR, et al. Angiography reveals that fluid-attenuated inversion recovery vascular hyperintensities are due to slow flow, not thrombus. AJNR Am. J. Neuroradiol. 2009;30:564–568. doi: 10.3174/ajnr.A1388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Demchuk AM, Dowlatshahi D, Rodriguez-Luna D, et al. Prediction of haematoma growth and outcome in patients with intracerebral haemorrhage using the CT-angiography spot sign (PREDICT): a prospective observational study. Lancet Neurol. 2012;11:307–314. doi: 10.1016/S1474-4422(12)70038-8. [DOI] [PubMed] [Google Scholar]
- 10.Barber PA, Demchuk AM, Zhang J, Buchan AM. Validity and reliability of a quantitative computed tomography score in predicting outcome of hyperacute stroke before thrombolytic therapy. ASPECTS Study Group: Alberta Stroke Programme Early CT Score. Lancet. 2000;355:1670–1674. doi: 10.1016/s0140-6736(00)02237-6. [DOI] [PubMed] [Google Scholar]
- 11.Donswijk ML, Jones PS, Guadagno JV, et al. T2*-weighted MRI versus oxygen extraction fraction PET in acute stroke. Cerebrovasc. Dis. 2009;28:306–313. doi: 10.1159/000229017. [DOI] [PubMed] [Google Scholar]
- 12.Kang DW, Chalela JA, Ezzeddine MA, Warach S. Association of ischemic lesion patterns on early diffusion-weighted imaging with TOAST stroke subtypes. Arch. Neurol. 2003;60:1730–1734. doi: 10.1001/archneur.60.12.1730. [DOI] [PubMed] [Google Scholar]
- 13.Thomalla G, Cheng B, Ebinger M, et al. DWI-FLAIR mismatch for the identification of patients with acute ischaemic stroke within 4.5 h of symptom onset (PRE-FLAIR): a multicentre observational study. Lancet Neurol. 2011;10:978–986. doi: 10.1016/S1474-4422(11)70192-2. [DOI] [PubMed] [Google Scholar]
- 14.Wardlaw JM, Lewis SC, Keir SL, et al. Cerebral microbleeds are associated with lacunar stroke defined clinically and radiologically, independently of white matter lesions. Stroke. 2006;37:2633–2636. doi: 10.1161/01.STR.0000240513.00579.bf. [DOI] [PubMed] [Google Scholar]
- 15.Zaharchuk G. Arterial spin label imaging of acute ischemic stroke and transient ischemic attack. Neuroimaging Clin. N. Am. 2011;21:285–301. doi: 10.1016/j.nic.2011.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mlynash M, Lansberg MG, De Silva DA, et al. Refining the definition of the malignant profile: insights from the DEFUSE-EPITHET pooled data set. Stroke. 2011;42:1270–1275. doi: 10.1161/STROKEAHA.110.601609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Albers GW, Thijs VN, Wechsler L, et al. Magnetic resonance imaging profiles predict clinical response to early reperfusion: the diffusion and perfusion imaging evaluation for understanding stroke evolution (DEFUSE) study. Ann. Neurol. 2006;60:508–517. doi: 10.1002/ana.20976. [DOI] [PubMed] [Google Scholar]
- 18.Liebeskind DS. Imaging the future of stroke: I. Ischemia. Ann. Neurol. 2009;66:574–590. doi: 10.1002/ana.21787. [DOI] [PubMed] [Google Scholar]
- 19.Choi JY, Pary JK, Alexandrov AV, et al. Does clinical-CT “mismatch” predict early response to treatment with recombinant tissue plasminogen activator? Cerebrovasc. Dis. 2006;22:384–388. doi: 10.1159/000094856. [DOI] [PubMed] [Google Scholar]
- 20.Davalos A, Blanco M, Pedraza S, et al. The clinical-DWI mismatch: a new diagnostic approach to the brain tissue at risk of infarction. Neurology. 2004;62:2187–2192. doi: 10.1212/01.wnl.0000130570.41127.ea. [DOI] [PubMed] [Google Scholar]
- 21.Liebeskind DS. Recanalization and reperfusion in acute ischemic stroke. F1000 Med. Rep. 2010;2 doi: 10.3410/M2-71. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.del Zoppo GJ, Sharp FR, Heiss WD, Albers GW. Heterogeneity in the penumbra. J. Cereb. Blood Flow Metab. 2011;31:1836–1851. doi: 10.1038/jcbfm.2011.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kidwell CS, Saver JL, Mattiello J, et al. Diffusion-perfusion MRI characterization of post-recanalization hyperperfusion in humans. Neurology. 2001;57:2015–2021. doi: 10.1212/wnl.57.11.2015. [DOI] [PubMed] [Google Scholar]
- 24.Bang OY, Buck BH, Saver JL, et al. Prediction of hemorrhagic transformation after recanalization therapy using T2*-permeability magnetic resonance imaging. Ann. Neurol. 2007;62:170–176. doi: 10.1002/ana.21174. [DOI] [PubMed] [Google Scholar]