Abstract
Objective
The objective of this study was to determine whether maternal plasma concentrations of soluble α-klotho are different between women with microbial invasion of the intra-amniotic cavity (MIAC) and those without MIAC among preterm labor and intact membranes (PTL) or preterm prelabor rupture of membranes (pPROM).
Methods
A cross-sectional study was conducted to include women in the following groups:1) PTL with MIAC (n=14); 2) PTL without MIAC (n=79); 3) pPROM with MIAC (n=30); and 4) pPROM without MIAC (n=33). MIAC was defined as a positive amniotic fluid culture for microorganisms (aerobic/anaerobic bacteria or genital mycoplasmas). Amniotic fluid samples were obtained within 48 hours from maternal blood collection. Plasma concentration of soluble α-klotho was determined by ELISA.
Results
1) The median plasma concentration (pg/mL) of soluble α-klotho was significantly lower in patients with MIAC than in those without MIAC (787.0 vs. 1117.8; p <0.001); 2) Among patients with PTL, those with MIAC had a lower median plasma concentration (pg/mL) of soluble α-klotho than those without MIAC (787.0 vs. 1138.9; p=0.007); 3) Among patients with pPROM, those with MIAC had a lower median plasma concentration (pg/mL) of soluble α-klotho than those without MIAC (766.4 vs. 1001.6; p=0.045); 4) There was no significant difference in the median plasma concentration of soluble α-klotho between PPROM without MIAC and PTL without MIAC (1001.6 pg/mL vs. 1138.9 pg/mL, respectively; p=0.5); 5) After adjustment for potential confounders (maternal age, tobacco use, gestational age at venipuncture), soluble α-klotho remained significantly associated with MIAC (p= 0.02); and 6) Among patients without MIAC, smoking was significantly associated with a lower median plasma concentration soluble α-klotho than in non-smokers (794.2 pg/mL vs. 1382.0 pg/mL, respectively; p<0.001); however, this difference was not observed in patients with MIAC.
Conclusions
Intra-amniotic infection occurring at preterm gestations (regardless of membrane status) was associated with a decrease in maternal plasma concentrations of soluble α-klotho. Moreover, among patients without infection, the plasma concentration of soluble α-klotho was lower in smokers.
Keywords: Intrauterine infection, prematurity, inflammation, preterm labor, preterm prelabor rupture of membranes
Introduction
Preterm birth is the leading cause of neonatal morbidity and mortality worldwide[52, 81, 154], with 40-45% following spontaneous preterm labor (PTL) and 25-30% following preterm prelabor rupture of membranes (pPROM)[172]. Preterm parturition is a syndrome with multiple etiologies[154], including uterine overdistension[122, 129, 130, 144, 179], uterine ischemia[7, 30, 56, 90, 91, 108, 181, 183], abnormal allogenic recognition[87, 89, 102, 103, 133, 200], allergic-like reaction[20, 21, 43, 44, 159, 160], cervical disease[3, 6, 15, 16, 36, 38, 53, 64-66, 79, 80, 85, 138, 139, 174, 189, 194], endocrine disorders[17, 35, 67, 70, 86, 119-121, 145, 148, 182, 188, 195, 211], intrauterine inflammation and microbial invasion of the amniotic cavity (MIAC)[4, 9, 12, 27-29, 31, 40, 41, 47, 48, 52, 54, 55, 57-61, 64, 69, 71, 89, 91, 100, 111-118, 131, 134, 140, 149-158, 161-166, 168-171, 173, 177, 193, 204, 208]. The presence of MIAC is associated with increased risk of adverse perinatal outcomes, such as early preterm birth[164, 165, 171, 177, 202, 207], neonatal sepsis[104, 167], bronchopulmonary dysplasia[48, 206], neonatal white matter brain lesions[203, 210], and cerebral palsy[13, 14, 176, 205, 209]. Although MIAC is considered to be confined to the amniotic cavity and not a systemic condition, studies with flow cytometry have shown phenotypic and metabolic changes in leukocytes[45, 46] as well as changes in maternal cytokines concentration [22, 24, 34, 41, 62, 63, 68, 125, 126, 141, 143, 198]. These observations suggest that biomarkers for MIAC may be found in the maternal circulation.
Klotho is an aging suppressor gene [96] that has gained interest due to the potential anti-inflammatory properties of its secreted protein, soluble α-klotho [105, 107]. The importance of soluble α-klotho in vitamin D metabolism has recently been investigated [135, 180]; however, its role in the maternal circulation in the presence of pregnancy complications, such as MIAC, has not been reported.
Decreased concentrations of plasma soluble α-klotho have been found in patients with cardiovascular disease [39, 123, 175], diabetes [37], and early stages of chronic kidney disease[1, 142, 178]. However, little information is available in the context of infection. Since soluble α-klotho reduces tumor necrosis factor – alpha (TNF-α) induced expression of adhesion molecules and NF-κβ activation in human umbilical vein endothelial cells [107] this protein may play a role in host defense and in the control of the inflammatory response [105, 107, 124, 137, 212, 213].
The aim of this study was to determine whether the plasma concentration of soluble α-klotho during pregnancy is associated with MIAC in patients presenting with PTL or pPROM.
Materials and Methods
Study Design
A cross-sectional study of women with singleton gestations between 20 to 34 weeks was conducted to include women in the following groups: 1) PTL with MIAC; 2) PTL without MIAC; 3) pPROM with MIAC; and 4) pPROM without MIAC.
Eligible patients were approached at the Detroit Medical Center/Hutzel Women's Hospital in Detroit, Michigan. Patients with 1 or more of the following conditions were excluded: 1) multifetal gestation; 2) hypertensive disorder of pregnancy; 3) renal disease; 4) fetuses with chromosomal and/or congenital anomalies; and 6) medically indicated preterm delivery.
Amniocentesis was performed at the clinician's discretion to rule out MIAC. Amniotic fluid samples were analyzed for white blood cell count (WBC), glucose cultured for aerobic/anaerobic bacteria or genital Mycoplasmas. The results of amniotic fluid testing were used in clinical management.
All patients provided informed consent prior to the collection of plasma and amniotic fluid samples. The collection and utilization of samples were approved by the Institutional Review Board of the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD/NIH/DHHS).
Definition and study procedures
Gestational age was determined by the last menstrual period or earliest available ultrasound if dating by ultrasound was not consistent with the last menstrual period.
Spontaneous PTL was defined as the presence of regular uterine contractions (frequency of at least 4 contractions in 20 minutes), cervical dilation of ≥ 2cm, effacement of ≥ 80%, or documented cervical change. PPROM was defined as spontaneous amniorrhexis before the onset of spontaneous labor, confirmed by vaginal pooling, ferning, positive nitrazine and/or amnio dye test. Maternal blood samples were collected at the time of diagnosis of PTL or pPROM.
Amniocentesis was performed within 48 hours from the maternal venipuncture. MIAC was defined as a positive amniotic fluid culture for microorganisms (aerobic/anaerobic bacteria or genital Mycoplasmas).
Sample Collection
Maternal blood samples were obtained by venipuncture and collected in tubes containing EDTA. Amniotic fluid samples were obtained by transabdominal amniocentesis, transferred to the laboratory in a sterile capped syringe, and cultured for aerobic/anaerobic bacteria and genital Mycoplasmas. The samples were centrifuged at 1300G for 10 minutes at 4°C and stored at −70°C. Laboratory personnel were blinded to the clinical diagnosis.
Human plasma soluble α-klotho ELISA
Maternal plasma concentrations of soluble α-klotho were determined by sensitive and specific immunoassays (Immuno-Biological Laboratories America Inc., Minneapolis, MN) utilizing a sandwich enzyme based technique. These immunoassays had been validated for plasma determination of the analytes. The inter- and intra-assay coefficients of variation were 4.6% and 3.9%, respectively. The sensitivity of the assays was 48 pg/ml.
Statistical Analysis
The Kolmogorov-Smirnov and Shapiro-Wilk tests were used to test for normality of arithmetic data distributions. The Kruskal-Wallis test was used for comparison of continuous variables among three or more groups. The Chi-square test for Fisher's exact was used to examine differences in proportions. Bivariate comparisons of continuous variables were evaluated using the Mann-Whitney U test or Fisher exact test. General linear models were constructed to further examine the relationship between log transformed soluble α-klotho and study group, controlling for potentially confounding factors, which included gestational age at maternal venipuncture, maternal race, nulliparity, smoking status, and duration of sample storage. A two-sided p-value of <0.05 was considered statistically significant. The statistical packages employed were SPSS version 19 (IBM Corp, Armonk, NY) and SAS version 9.3 (Cary, NC).
Results
During the study period, 156 patients met the inclusion criteria and the study groups were: 1) PTL with MIAC (n=14); 2) PTL without MIAC (n=79); 3) pPROM with MIAC (n=30); and 4) pPROM without MIAC (n=33). There were 44 patients with MIAC (28.2%) and 110 (70.5%) patients delivered prior to 34 weeks of gestation. The demographic and clinical characteristics of the study population are summarized in Table 1. As expected, the median gestational age at maternal venipuncture and at delivery, the duration of sample storage, and median soluble α-klotho plasma concentrations differed between groups. There were also differences between the groups in age, nulliparity, and smoking status.
Table 1.
Demographic and Clinical Characteristics of Study Population
| Clinical Characteristics | Preterm Labor, without MIAC (n=79) | Preterm Labor, with MIAC (n=14) | pPROM, without MIAc (n=33) | pPROM, with MIAC (n=30) | p-value |
|---|---|---|---|---|---|
| Maternal age (years) | 22.0 (19.0-26.0) | 23.5 (21.0-27.0) | 26.0 (21.0-31.0) | 24.5 (22.0-33.0) | 0.005 |
| Black Race | 67 (85.9%) | 11 (84.6%) | 28 (84.6%) | 28 (93.3%) | 0.9 |
| Nulliparity | 31 (39.2%) | 7 (50%) | 9 (27.3%) | 8 (26.7%) | 0.3 |
| Smoking | 20 (25.6%) | 6 (42.9%) | 15 (45.5%) | 18 (62.1%) | 0.005 |
| Gestational Age at venipuncture (weeks) | 30.6 (26.9-32.6) | 24.1 (23.3-25.0) | 30.7 (29.0-32.0) | 30.4 (27-32.4) | <0.001 |
| Gestational Age at delivery (weeks) | 33.7 (28.4-37) | 24.6 (23.4-25.9) | 31.7 (30.1-33.4) | 31.2 (27.1-32.6) | <0.001 |
| 1960 | 679 | 1730 | 1412 | ||
| Birthweight (grams) | (1110-2740) | (525-1060) | (1420-2100) | (980-1880) | <0.001 |
| Duration of sample storage (years) | 10.1 (9.7-10.8) | 10.3 (9.8-11.0) | 10.5 (10.0-11.0) | 10.0 (9.6-10.6) | 0.2 |
| 1138.9 | 787 | 1001.6 | 766.4 | ||
| alpha-klotho (pg/mL) | (791.7-1688.5) | (551.9-1055.9) | (661.3-1820.7) | (447.2-1177.6) | 0.001 |
Values are expressed as number (percentage) or median (interquartile range). A p value <0.05 was considered statistically significant. Missing data are: Race, n=2; Nulliparity, n=3; Smoking, n=3, Birth weight, n=2
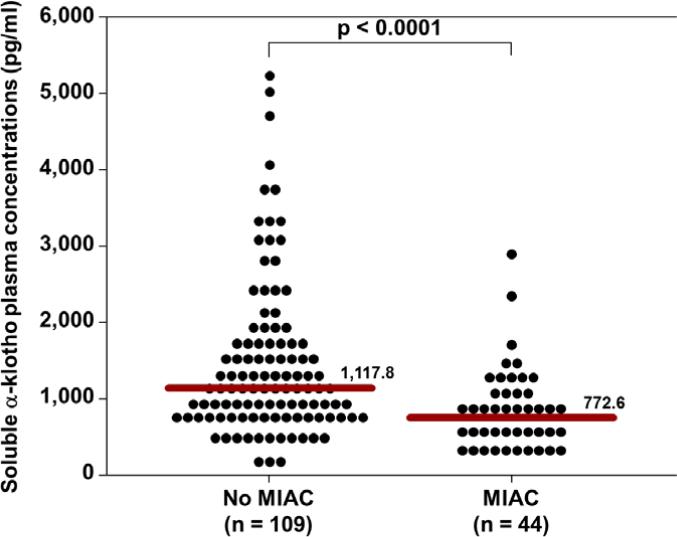
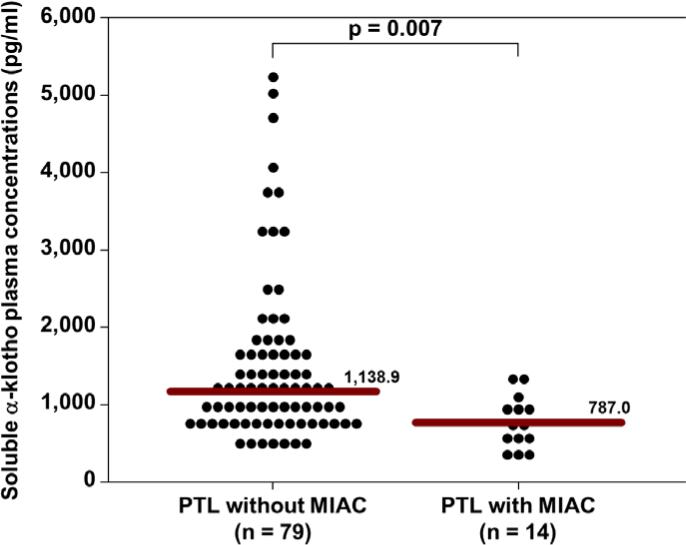
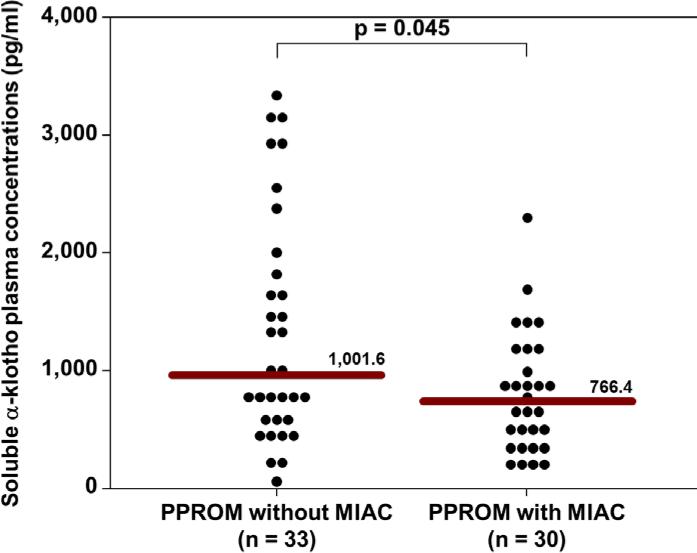
The median soluble α-klotho plasma concentration (pg/mL) was significantly lower in patients with MIAC than in those without MIAC (772.6 vs 1117.8; p<0.0001, Figure 1). The median soluble α-klotho plasma concentration (pg/mL) was also significantly lower in patients presenting in PTL with MIAC than in those without MIAC (787.0 vs. 1138.9; p=0.007, Figure 2). Similarly, the median soluble α-klotho plasma concentration (pg/mL) was significantly lower in pPROM patients with MIAC than in those without MIAC (766.4 vs. 1001.6; p=0.045, Figure 3). There was no significant difference in the median plasma concentration (pg/mL) of soluble α-klotho between patients with PTL (without MIAC) and those with pPROM (without MIAC) (1138.9 vs. 1001.6; p=0.5).
Figure 1.
The median plasma soluble α-klotho concentration was significantly lower in patients with MIAC than in those without MIAC (772.6 pg/mL vs. 117.8 pg/mL; P<0.0001).
Figure 2.
The median plasma soluble α-klotho concentration was significantly lower in patients presenting in preterm labor with MIAC than in those without MIAC (787.0 pg/mL vs. 1138.9 pg/mL; P=0.007).
Figure 3.
The median plasma soluble α-klotho concentration was significantly lower in patients presenting in preterm prelabor rupture of membranes with MIAC than in those without MIAC (766.4 pg/mL vs. 1001.6 pg/mL; P=0.045).
Differences in the mean log soluble α-klotho concentrations between MIAC and negative MIAC groups remained significant after adjustment for maternal age, gestational age at venipuncture, and smoking status (p= 0.02). This final model also revealed that maternal smoking was independently associated with the log soluble α-klotho concentration (p=0.0007). The mean log soluble α-klotho concentration was 23% lower among patients with MIAC compared to those without MIAC, and was 30.6% lower among smokers than in nonsmokers, when holding other factors constant.
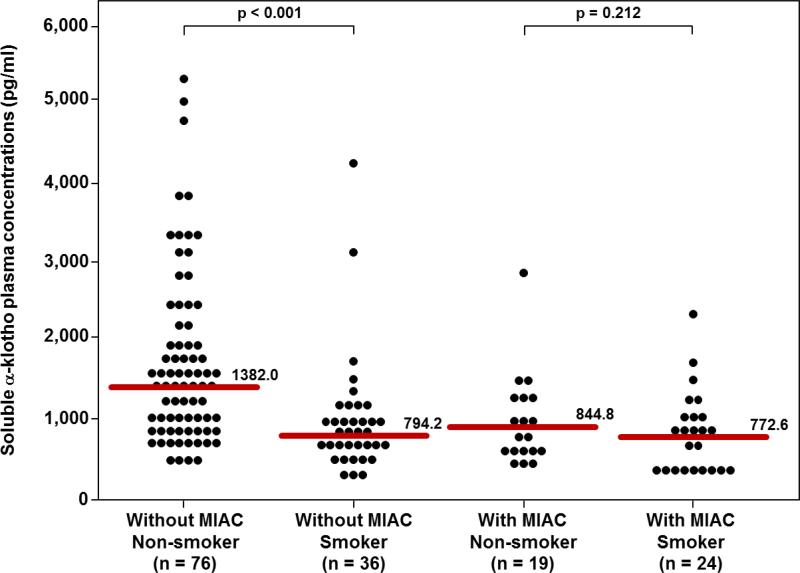
The relationship between smoking status and soluble α-klotho concentration appeared to differ as a function of MIAC (figure 4). Among patients without MIAC, the median plasma concentration (pg/mL) of soluble α-klotho was significantly lower in smokers than in non-smokers (794.2 vs. 1382.0; p<0.0001). The difference in the median plasma concentration (pg/mL) of soluble α-klotho concentration between smoker and non-smokers was not observed in patients with MIAC (772.6 vs. 844.8; p=0.2). Moreover, model fit as reflected by an increase in adjusted r2 improved when including the effect modification term improved when including the effect-modification term of smoking status. This model revealed that in the setting of MIAC, smoking status was not associated with a difference in mean log soluble α-klotho concentration (p=0.8). In contrast, among patients without MIAC, the mean log soluble α-klotho concentration was significantly lower among smokers than non-smokers (Bonferroni p<0.001).
Figure 4.
Among patients without MIAC, the median plasma soluble α-klotho concentration was significantly lower in smokers than in non-smokers (794.2 pg/mL vs. 1382.0 pg/mL, P<0.0001). In contrast, no significant difference in the median plasma α-klotho concentration was observed between smokers and non-smokers among patients with MIAC.
Comments
Principal findings of this study
The principal findings of this study are: 1.) the median plasma concentration of soluble α-klotho was significantly lower in patients with MIAC than those without MIAC and this was consistent in both the PTL and pPROM groups when evaluated separately; 2) the relationship between soluble α-klotho concentration and MIAC remained significant after adjustment for potential confounders; and 3) among patients without MIAC, the plasma concentration of soluble α-klotho was significantly lower in pregnant patients who reported smoking than in those who did not.
Our results suggest that soluble α-klotho concentration is associated with MIAC. Two plausible explanations are: 1) the host response to the MIAC includes a decrease in soluble α-klotho concentration; 2) a lower concentration of klotho may confer a susceptibility to infection.
Biology of klotho
Transgenic mice that have a functional loss of the klotho gene exhibit a phenotype that closely resembles patients with premature-aging syndromes, such as short lifespan, osteoporosis, age-related skin changes, ectopic calcifications, atherosclerosis and infertility[94, 96, 109, 110]. In contrast, overexpression of the klotho gene in mice extends the life span and increases resistance to oxidative stress[11, 98, 196, 201]. The human klotho gene produces two transcripts through alternative RNA splicing to form two types of klotho: a membrane bound and a secreted protein (soluble α-klotho)[110]. Klotho is primarily expressed in the distal convoluted tubule of the kidney, parathyroid, and choroid plexus in the brain, but expression also occurs in other tissues, including the placenta[96].
Membrane bound klotho forms a complex with fibroblast growth factor (FGF) receptors and functions as a necessary co-receptor for FGF23[95, 97, 128, 146]. Membrane bound klotho also plays an integral part in the regulation of phosphate, calcium and vitamin D homeostasis in human and mice[2, 5, 8, 84, 136, 190]. Lack of expression in either klotho or FGF23 result in phosphate retention and a premature-aging syndrome in the mouse[94, 101, 128, 146, 147]. The extracellular domain of the klotho protein is cleaved on the surface by membrane-anchored protease, such as A Disintegrin and Metalloproteinase Domain-containing protein 10 (ADAM10), ADAM17, and Beta-secretase 1(BACE1). This leads to the release of the secreted form of klotho (soluble α-klotho) into blood, urine, and cerebrospinal fluid[18, 32, 83, 191]. Soluble α-klotho is thought to play an important role in protecting cells and tissues from oxidative stress[51, 196, 201, 214].
Soluble α-klotho regulates the activity of several ion channels and ion transporters on cell surfaces, such as calcium channel TRPV5 (transient receptor potential cation channel, subfamily V, member 5), potassium channel ROMK1 (potassium inwardly-rectifying channel, subfamily J, member 1), and npt2a (Type 2a Na-phosphate cotransporter)[25, 26, 77, 78, 99, 106]. Soluble α-klotho can also suppress activation of the insulin/insulin-like growth factor-1 (IGF-1) receptor and canonical Wnt signaling induced by Wnt3[32, 72, 75, 94, 127, 186, 199].
Klotho and vitamin D metabolism
Expression of klotho is found predominantly (although not exclusively) in the kidney, parathyroid, and choroid plexus [96]. Prior studies have focused on the roles of Klotho in chronic renal disorders[37, 74-76, 142, 178, 184, 214], disorders of calcium metabolism[2, 5, 8, 73, 82, 84, 190], hypertension,[33, 123, 132, 175, 197] and aging[49, 93, 96, 109, 187, 191]. Kuro-o et al demonstrated that klotho is also expressed in the placenta; however, of pregnancy complications has not been investigated [96]. Ohata et al proposed that klotho may play an important role in mineral metabolism in the neonate. The investigators measured circulating α-klotho concentrations in the umbilical cord plasma of the healthy neonates, plasma of the neonate 4 days after delivery, maternal plasma, and that of healthy non-pregnant controls [135]. The concentration of soluble α-klotho in umbilical venous blood was significantly higher than in neonatal plasma 4 days after delivery. A negative correlation was also found between FGF23 and soluble α-klotho concentrations in the cord blood samples, which was consistent with the results of previous studies in healthy children and adult volunteers [135]. Multiple regression analysis revealed that soluble α-klotho concentration was independently associated with phosphate; thus, the authors concluded that klotho in the umbilical cord blood might be a useful biomarker for calcium and phosphate metabolism in the fetus. Recently, Siahanidou et al, demonstrated that plasma concentration of soluble α-klotho was significantly lower in preterm than in full-term infants, and that it was correlated with body weight, body length, 1,25-dihydroxy-vitamin D concentration, malondialdehyde concentration, and gestational age at delivery[180]. Therefore, soluble α-klotho may play a role in vitamin D metabolism and oxidative stress in preterm neonates.
An anti-inflammatory role of klotho
Several investigators have described the protective role of soluble α-klotho from oxidative stress [11, 23, 51, 185, 196, 201, 214]. Wang et al demonstrated that klotho suppresses Nox2 expression and significantly decreases superoxide production in rat aortic smooth muscle cells, thus attenuating apoptosis[196]. Similarly, Zuo et al demonstrated that a disruption in klotho expression led to an increase in superoxide production, causing aging-related kidney damage[214]. Therefore, klotho plays an important protective role in the regulation of oxidative stress. The mechanism by which soluble α-klotho protects cells and tissues from oxidative stress is unknown; however, it may act through multiple ion channels and group factors, such as insulin, insulin-like growth factor (IGF-I) and the Wnt signaling pathway, which is integral in cell to cell communication (i.e. cell proliferation and differentiation)[32, 72, 75, 78, 94, 127, 192, 199].
Klotho has been investigated as a protective protein against “harmful” inflammation[49, 105, 107]. Liu et al reported that intracellular klotho interacts with the retinoic-acid-inducible gene-I (RIG-I) resulting in the inhibition of RIG-I induced expression of IL-5 and IL-8 in vitro studies of human umbilical vein endothelial cells (HUVECs) and mice[105]. Maekawa et al demonstrated a similar protective effect of klotho [107]. The administration of klotho protein administration attenuated tumor necrosis factor-α (TNF-α) induced expression of intracellular adhesion molecule-1 (ICAM-1), vascular cell adhesion molecule-1 (VCAM-1), NF-κB activation and IκB phosphylation. These findings suggest that klotho may have a role in the modulation of endothelial response to the inflammatory mediators.
Our findings that plasma concentrations of α-klotho were lower in MIAC than in those without MIAC are consistent with 2 previous studies [124, 137]. Ohyama et al, reported that the administration of lipopolysaccharide to mice resulted in a significantly decreased the expression of klotho[137]. The administration of the proinflammatory cytokines such as TNF-like weak inducer of apoptosis (TWEAK) and TNF-α, resulted in decreased expression of klotho in the kidney in mice[124]. These results suggest that klotho is downregulated in response to microbial products or proinflammatory cytokines, resulting in accelerated aging of organs seen in chronic renal disease.
The presence of intraamniotic inflammation/infection is known to be significantly associated with increased risks of adverse perinatal outcomes, such as early preterm birth and neonatal morbidity, in patients with PTL or pPROM [13, 14, 48, 104, 164, 167, 171, 176, 177, 202, 203, 205-207, 209, 210]. Elevated maternal plasma concentrations of inflammatory cytokines in preterm gestations complicated by PTL or pPROM are associated with an increased risk of intra-amniotic infection [22, 24, 34, 41, 62, 63, 68, 125, 126, 141, 143, 198]. The findings herein demonstrate that MIAC is associated with decreased maternal plasma concentrations of soluble α-klotho.
Decreased plasma concentration of α-klotho in smokers
This is the first report to find a relationship between exposure to tobacco and decreased plasma concentration of soluble α-klotho. No studies have been published on the effect of smoking on soluble α-klotho concentrations in non-pregnant subjects. Smoking is known to be associated with aging and this has been attributed to chronic inflammation and oxidative stress which tend to be more severe in women[19]. Indeed, the term “inflammaging” has been coiled to stress the relationship between chronic inflammation and aging[42, 50]. Moreover, smoking is associated with reduction in telomere length, a putative molecular marker of aging[10]. Klotho has anti-aging properties and protect against oxidative stress. The findings that plasma concentration of α-klotho is lower in smokers suggest that the aging effect of smoking may be mediated by a decrease in the anti-aging factor klotho. A relative deficiency in α-klotho will make subjects more susceptible to oxidative stress. Further studies are required to test this hypothesis.
Strengths and limitations
This is the first study to evaluate changes of soluble α-klotho in the context of infection. Replication of this finding is desirable for both pregnant and non-pregnant subjects. Limitations are those inherent to an observational cross-sectional study in which a temporal relationship cannot be established.
Conclusion
Patients who present with preterm labor or preterm prelabor rupture of membranes with MIAC have a lower median plasma concentration of soluble α-klotho than in those without MIAC at the time of presentation. These results suggest that klotho plays a protective role during pregnancy. However, further studies are required to evaluate whether patients who subsequently developed MIAC began the pregnancy with lower concentrations of soluble α-klotho, making them susceptible to infection, or vice versa. Prospective studies appear warranted to evaluate soluble α-klotho as a non-invasive biomarker for the prediction of infection and inflammation in pregnancies with adverse outcomes.
Acknowledgement
This research was supported, in part, by the Perinatology Research Branch, Division of Intramural Research, Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health, Department of Health and Human Services (NICHD/NIH); and, in part, with Federal funds from NICHD, NIH under Contract No. HHSN275201300006C.
Footnotes
Presented at the 33rd annual meeting of the Society for Maternal-Fetal Medicine, February 11-16, 2013, San Francisco, CA
References
- 1.Akimoto T, Shiizaki K, Sugase T, Watanabe Y, Yoshizawa H, Otani N, et al. The relationship between the soluble Klotho protein and the residual renal function among peritoneal dialysis patients. Clin Exp Nephrol. 2012;16:442–7. doi: 10.1007/s10157-011-0582-2. [DOI] [PubMed] [Google Scholar]
- 2.Alexander RT, Woudenberg-Vrenken TE, Buurman J, Dijkman H, Van Der Eerden BC, Van Leeuwen JP, et al. Klotho prevents renal calcium loss. J Am Soc Nephrol. 2009;20:2371–9. doi: 10.1681/ASN.2008121273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Andersen HF, Nugent CE, Wanty SD, Hayashi RH. Prediction of risk for preterm delivery by ultrasonographic measurement of cervical length. Am J Obstet Gynecol. 1990;163:859–67. doi: 10.1016/0002-9378(90)91084-p. [DOI] [PubMed] [Google Scholar]
- 4.Andrews WW, Hauth JC, Goldenberg RL, Gomez R, Romero R, Cassell GH. Amniotic fluid interleukin-6: correlation with upper genital tract microbial colonization and gestational age in women delivered after spontaneous labor versus indicated delivery. Am J Obstet Gynecol. 1995;173:606–12. doi: 10.1016/0002-9378(95)90290-2. [DOI] [PubMed] [Google Scholar]
- 5.Anour R, Andrukhova O, Ritter E, Zeitz U, Erben RG. Klotho lacks a vitamin D independent physiological role in glucose homeostasis, bone turnover, and steady-state PTH secretion in vivo. PLoS One. 2012;7:e31376. doi: 10.1371/journal.pone.0031376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Arabin B, Halbesma JR, Vork F, Hubener M, Van Eyck J. Is treatment with vaginal pessaries an option in patients with a sonographically detected short cervix? J Perinat Med. 2003;31:122–33. doi: 10.1515/JPM.2003.017. [DOI] [PubMed] [Google Scholar]
- 7.Arias F, Rodriquez L, Rayne SC, Kraus FT. Maternal placental vasculopathy and infection: two distinct subgroups among patients with preterm labor and preterm ruptured membranes. Am J Obstet Gynecol. 1993;168:585–91. doi: 10.1016/0002-9378(93)90499-9. [DOI] [PubMed] [Google Scholar]
- 8.Asai O, Nakatani K, Tanaka T, Sakan H, Imura A, Yoshimoto S, et al. Decreased renal alpha-Klotho expression in early diabetic nephropathy in humans and mice and its possible role in urinary calcium excretion. Kidney Int. 2012;81:539–47. doi: 10.1038/ki.2011.423. [DOI] [PubMed] [Google Scholar]
- 9.Athayde N, Romero R, Maymon E, Gomez R, Pacora P, Yoon BH, et al. Interleukin 16 in pregnancy, parturition, rupture of fetal membranes, and microbial invasion of the amniotic cavity. Am J Obstet Gynecol. 2000;182:135–41. doi: 10.1016/s0002-9378(00)70502-3. [DOI] [PubMed] [Google Scholar]
- 10.Babizhayev MA, Yegorov YE. Smoking and health: association between telomere length and factors impacting on human disease, quality of life and life span in a large population-based cohort under the effect of smoking duration. Fundam Clin Pharmacol. 2011;25:425–42. doi: 10.1111/j.1472-8206.2010.00866.x. [DOI] [PubMed] [Google Scholar]
- 11.Balasubramanian P, Longo VD. Linking Klotho, Nrf2, MAP kinases and aging. Aging (Albany NY) 2010;2:632–3. doi: 10.18632/aging.100219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bashiri A, Horowitz S, Huleihel M, Hackmon R, Dukler D, Mazor M. Elevated concentrations of interleukin-6 in intra-amniotic infection with Ureaplasma urealyticum in asymptomatic women during genetic amniocentesis. Acta Obstet Gynecol Scand. 1999;78:379–82. [PubMed] [Google Scholar]
- 13.Berger A, Witt A, Haiden N, Kaider A, Klebermasz K, Fuiko R, et al. Intrauterine infection with Ureaplasma species is associated with adverse neuromotor outcome at 1 and 2 years adjusted age in preterm infants. J Perinat Med. 2009;37:72–8. doi: 10.1515/JPM.2009.016. [DOI] [PubMed] [Google Scholar]
- 14.Berger A, Witt A, Haiden N, Kretzer V, Heinze G, Kohlhauser C. Microbial invasion of the amniotic cavity at birth is associated with adverse short-term outcome of preterm infants. J Perinat Med. 2003;31:115–21. doi: 10.1515/JPM.2003.016. [DOI] [PubMed] [Google Scholar]
- 15.Berghella V, Roman A, Daskalakis C, Ness A, Baxter JK. Gestational age at cervical length measurement and incidence of preterm birth. Obstet Gynecol. 2007;110:311–7. doi: 10.1097/01.AOG.0000270112.05025.1d. [DOI] [PubMed] [Google Scholar]
- 16.Bevis KS, Biggio JR. Cervical conization and the risk of preterm delivery. Am J Obstet Gynecol. 2011;205:19–27. doi: 10.1016/j.ajog.2011.01.003. [DOI] [PubMed] [Google Scholar]
- 17.Blanks AM, Vatish M, Allen MJ, Ladds G, De Wit NC, Slater DM, et al. Paracrine oxytocin and estradiol demonstrate a spatial increase in human intrauterine tissues with labor. J Clin Endocrinol Metab. 2003;88:3392–400. doi: 10.1210/jc.2002-021212. [DOI] [PubMed] [Google Scholar]
- 18.Bloch L, Sineshchekova O, Reichenbach D, Reiss K, Saftig P, Kuro-O M, et al. Klotho is a substrate for alpha-, beta-and gamma-secretase. FEBS Lett. 2009;583:3221–4. doi: 10.1016/j.febslet.2009.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bridges AB, Scott NA, Parry GJ, Belch JJ. Age, sex, cigarette smoking and indices of free radical activity in healthy humans. Eur J Med. 1993;2:205–8. [PubMed] [Google Scholar]
- 20.Bytautiene E, Romero R, Vedernikov YP, El-Zeky F, Saade GR, Garfield RE. Induction of premature labor and delivery by allergic reaction and prevention by histamine H1 receptor antagonist. Am J Obstet Gynecol. 2004;191:1356–61. doi: 10.1016/j.ajog.2004.06.092. [DOI] [PubMed] [Google Scholar]
- 21.Bytautiene E, Vedernikov YP, Saade GR, Romero R, Garfield RE. Endogenous mast cell degranulation modulates cervical contractility in the guinea pig. Am J Obstet Gynecol. 2002;186:438–45. doi: 10.1067/mob.2002.120488. [DOI] [PubMed] [Google Scholar]
- 22.Canzoneri BJ, Grotegut CA, Swamy GK, Brancazio LR, Sinclair T, Heine PR, et al. Maternal serum interleukin-6 levels predict impending funisitis in preterm premature rupture of membranes after completion of antibiotics. J Matern Fetal Neonatal Med. 2012;25:1329–32. doi: 10.3109/14767058.2011.632794. [DOI] [PubMed] [Google Scholar]
- 23.Carracedo J, Buendia P, Merino A, Madueno JA, Peralbo E, Ortiz A, et al. Klotho modulates the stress response in human senescent endothelial cells. Mech Ageing Dev. 2012;133:647–54. doi: 10.1016/j.mad.2012.09.002. [DOI] [PubMed] [Google Scholar]
- 24.Carroll SG, Papaioannou S, Davies ET, Nicolaides KH. Maternal assessment in the prediction of intrauterine infection in preterm prelabor amniorrhexis. Fetal Diagn Ther. 1995;10:290–6. doi: 10.1159/000264246. [DOI] [PubMed] [Google Scholar]
- 25.Cha SK, Hu MC, Kurosu H, Kuro-O M, Moe O, Huang CL. Regulation of renal outer medullary potassium channel and renal K(+) excretion by Klotho. Mol Pharmacol. 2009;76:38–46. doi: 10.1124/mol.109.055780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cha SK, Ortega B, Kurosu H, Rosenblatt KP, Kuro OM, Huang CL. Removal of sialic acid involving Klotho causes cell-surface retention of TRPV5 channel via binding to galectin-1. Proc Natl Acad Sci U S A. 2008;105:9805–10. doi: 10.1073/pnas.0803223105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chaiworapongsa T, Hong JS, Hull WM, Romero R, Whitsett JA. Amniotic fluid concentration of surfactant proteins in intra-amniotic infection. J Matern Fetal Neonatal Med. 2008;21:663–70. doi: 10.1080/14767050802215664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chaiworapongsa T, Romero R, Espinoza J, Kim YM, Edwin S, Bujold E, et al. Macrophage migration inhibitory factor in patients with preterm parturition and microbial invasion of the amniotic cavity. J Matern Fetal Neonatal Med. 2005;18:405–16. doi: 10.1080/14767050500361703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chaiworapongsa T, Romero R, Kim JC, Kim YM, Blackwell SC, Yoon BH, et al. Evidence for fetal involvement in the pathologic process of clinical chorioamnionitis. Am J Obstet Gynecol. 2002;186:1178–82. doi: 10.1067/mob.2002.124042. [DOI] [PubMed] [Google Scholar]
- 30.Chaiworapongsa T, Romero R, Tarca A, Kusanovic JP, Mittal P, Kim SK, et al. A subset of patients destined to develop spontaneous preterm labor has an abnormal angiogenic/anti-angiogenic profile in maternal plasma: evidence in support of pathophysiologic heterogeneity of preterm labor derived from a longitudinal study. J Matern Fetal Neonatal Med. 2009;22:1122–39. doi: 10.3109/14767050902994838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chaiworapongsa T, Romero R, Tolosa JE, Yoshimatsu J, Espinoza J, Kim YM, et al. Elevated monocyte chemotactic protein-1 in amniotic fluid is a risk factor for pregnancy loss. J Matern Fetal Neonatal Med. 2002;12:159–64. doi: 10.1080/jmf.12.3.159.164. [DOI] [PubMed] [Google Scholar]
- 32.Chen CD, Podvin S, Gillespie E, Leeman SE, Abraham CR. Insulin stimulates the cleavage and release of the extracellular domain of Klotho by ADAM10 and ADAM17. Proc Natl Acad Sci U S A. 2007;104:19796–801. doi: 10.1073/pnas.0709805104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cheng X, Zhou Q, Lin S, Wu R. Fosinopril and valsartan intervention in gene expression of Klotho, MMP-9, TIMP-1, and PAI-1 in the kidney of spontaneously hypertensive rats. Zhong Nan Da Xue Xue Bao Yi Xue Ban. 2010;35:1048–56. doi: 10.3969/j.issn.1672-7347.2010.10.004. [DOI] [PubMed] [Google Scholar]
- 34.Cobo T, Tsiartas P, Kacerovsky M, Holst RM, Hougaard DM, Skogstrand K, et al. Maternal inflammatory response to microbial invasion of the amniotic cavity: analyses of multiple proteins in the maternal serum. Acta Obstet Gynecol Scand. 2012;92:61–8. doi: 10.1111/aogs.12028. [DOI] [PubMed] [Google Scholar]
- 35.Condon JC, Hardy DB, Kovaric K, Mendelson CR. Up-regulation of the progesterone receptor (PR)-C isoform in laboring myometrium by activation of nuclear factor-kappaB may contribute to the onset of labor through inhibition of PR function. Mol Endocrinol. 2006;20:764–75. doi: 10.1210/me.2005-0242. [DOI] [PubMed] [Google Scholar]
- 36.Crane JM, Hutchens D. Use of transvaginal ultrasonography to predict preterm birth in women with a history of preterm birth. Ultrasound Obstet Gynecol. 2008;32:640–5. doi: 10.1002/uog.6143. [DOI] [PubMed] [Google Scholar]
- 37.Devaraj S, Syed B, Chien A, Jialal I. Validation of an immunoassay for soluble Klotho protein: decreased levels in diabetes and increased levels in chronic kidney disease. Am J Clin Pathol. 2012;137:479–85. doi: 10.1309/AJCPGPMAF7SFRBO4. [DOI] [PubMed] [Google Scholar]
- 38.Dijkstra K, Funai EF, O'neill L, Rebarber A, Paidas MJ, Young BK. Change in cervical length after cerclage as a predictor of preterm delivery. Obstet Gynecol. 2000;96:346–50. doi: 10.1016/s0029-7844(00)00924-8. [DOI] [PubMed] [Google Scholar]
- 39.Donate-Correa J, Mora-Fernandez C, Martinez-Sanz R, Muros-De-Fuentes M, Perez H, Meneses-Perez B, et al. Expression of FGF23/KLOTHO system in human vascular tissue. Int J Cardiol. 2011 doi: 10.1016/j.ijcard.2011.08.850. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 40.Fidel PL, Jr., Romero R, Cutright J, Wolf N, Gomez R, Araneda H, et al. Treatment with the interleukin-I receptor antagonist and soluble tumor necrosis factor receptor Fc fusion protein does not prevent endotoxin-induced preterm parturition in mice. J Soc Gynecol Investig. 1997;4:22–6. doi: 10.1177/107155769700400104. [DOI] [PubMed] [Google Scholar]
- 41.Fidel PL, Jr., Romero R, Wolf N, Cutright J, Ramirez M, Araneda H, et al. Systemic and local cytokine profiles in endotoxin-induced preterm parturition in mice. Am J Obstet Gynecol. 1994;170:1467–75. doi: 10.1016/s0002-9378(94)70180-6. [DOI] [PubMed] [Google Scholar]
- 42.Franceschi C, Capri M, Monti D, Giunta S, Olivieri F, Sevini F, et al. Inflammaging and anti-inflammaging: a systemic perspective on aging and longevity emerged from studies in humans. Mech Ageing Dev. 2007;128:92–105. doi: 10.1016/j.mad.2006.11.016. [DOI] [PubMed] [Google Scholar]
- 43.Garfield RE, Bytautiene E, Vedernikov YP, Marshall JS, Romero R. Modulation of rat uterine contractility by mast cells and their mediators. Am J Obstet Gynecol. 2000;183:118–25. doi: 10.1067/mob.2000.105741. [DOI] [PubMed] [Google Scholar]
- 44.Garfield RE, Irani AM, Schwartz LB, Bytautiene E, Romero R. Structural and functional comparison of mast cells in the pregnant versus nonpregnant human uterus. Am J Obstet Gynecol. 2006;194:261–7. doi: 10.1016/j.ajog.2005.05.011. [DOI] [PubMed] [Google Scholar]
- 45.Gervasi MT, Chaiworapongsa T, Naccasha N, Blackwell S, Yoon BH, Maymon E, et al. Phenotypic and metabolic characteristics of maternal monocytes and granulocytes in preterm labor with intact membranes. Am J Obstet Gynecol. 2001;185:1124–9. doi: 10.1067/mob.2001.117681. [DOI] [PubMed] [Google Scholar]
- 46.Gervasi MT, Chaiworapongsa T, Naccasha N, Pacora P, Berman S, Maymon E, et al. Maternal intravascular inflammation in preterm premature rupture of membranes. J Matern Fetal Neonatal Med. 2002;11:171–5. doi: 10.1080/jmf.11.3.171.175. [DOI] [PubMed] [Google Scholar]
- 47.Gervasi MT, Romero R, Bracalente G, Erez O, Dong Z, Hassan SS, et al. Midtrimester amniotic fluid concentrations of interleukin-6 and interferon-gamma-inducible protein-10: evidence for heterogeneity of intraamniotic inflammation and associations with spontaneous early (<32 weeks) and late (>32 weeks) preterm delivery. J Perinat Med. 2012;40:329–43. doi: 10.1515/jpm-2012-0034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ghezzi F, Gomez R, Romero R, Yoon BH, Edwin SS, David C, et al. Elevated interleukin-8 concentrations in amniotic fluid of mothers whose neonates subsequently develop bronchopulmonary dysplasia. Eur J Obstet Gynecol Reprod Biol. 1998;78:5–10. doi: 10.1016/s0301-2115(97)00236-4. [DOI] [PubMed] [Google Scholar]
- 49.Giunta B, Fernandez F, Nikolic WV, Obregon D, Rrapo E, Town T, et al. Inflammaging as a prodrome to Alzheimer's disease. J Neuroinflammation. 2008;5:51. doi: 10.1186/1742-2094-5-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Giunta S. Is inflammaging an auto[innate]immunity subclinical syndrome? Immun Ageing. 2006;3:12. doi: 10.1186/1742-4933-3-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gold PW, Licinio J, Pavlatou MG. Pathological parainflammation and endoplasmic reticulum stress in depression: potential translational targets through the CNS insulin, klotho and PPAR-gamma systems. Mol Psychiatry. 2012;18:154–65. doi: 10.1038/mp.2012.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Goldenberg RL, Culhane JF, Iams JD, Romero R. Epidemiology and causes of preterm birth. Lancet. 2008;371:75–84. doi: 10.1016/S0140-6736(08)60074-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Goldenberg RL, Iams JD, Das A, Mercer BM, Meis PJ, Moawad AH, et al. The Preterm Prediction Study: sequential cervical length and fetal fibronectin testing for the prediction of spontaneous preterm birth. National Institute of Child Health and Human Development Maternal-Fetal Medicine Units Network. Am J Obstet Gynecol. 2000;182:636–43. doi: 10.1067/mob.2000.104212. [DOI] [PubMed] [Google Scholar]
- 54.Gomez R, Ghezzi F, Romero R, Munoz H, Tolosa JE, Rojas I. Premature labor and intra-amniotic infection. Clinical aspects and role of the cytokines in diagnosis and pathophysiology. Clin Perinatol. 1995;22:281–342. [PubMed] [Google Scholar]
- 55.Gomez R, Romero R, Edwin SS, David C. Pathogenesis of preterm labor and preterm premature rupture of membranes associated with intraamniotic infection. Infect Dis Clin North Am. 1997;11:135–76. doi: 10.1016/s0891-5520(05)70347-0. [DOI] [PubMed] [Google Scholar]
- 56.Gomez R, Romero R, Nien JK, Medina L, Carstens M, Kim YM, et al. Idiopathic vaginal bleeding during pregnancy as the only clinical manifestation of intrauterine infection. J Matern Fetal Neonatal Med. 2005;18:31–7. doi: 10.1080/14767050500217863. [DOI] [PubMed] [Google Scholar]
- 57.Goncalves LF, Chaiworapongsa T, Romero R. Intrauterine infection and prematurity. Ment Retard Dev Disabil Res Rev. 2002;8:3–13. doi: 10.1002/mrdd.10008. [DOI] [PubMed] [Google Scholar]
- 58.Gotsch F, Romero R, Chaiworapongsa T, Erez O, Vaisbuch E, Espinoza J, et al. Evidence of the involvement of caspase-1 under physiologic and pathologic cellular stress during human pregnancy: a link between the inflammasome and parturition. J Matern Fetal Neonatal Med. 2008;21:605–16. doi: 10.1080/14767050802212109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gotsch F, Romero R, Erez O, Vaisbuch E, Kusanovic JP, Mazaki-Tovi S, et al. The preterm parturition syndrome and its implications for understanding the biology, risk assessment, diagnosis, treatment and prevention of preterm birth. J Matern Fetal Neonatal Med. 2009;22(Suppl 2):5–23. doi: 10.1080/14767050902860690. [DOI] [PubMed] [Google Scholar]
- 60.Gravett MG, Hitti J, Hess DL, Eschenbach DA. Intrauterine infection and preterm delivery: evidence for activation of the fetal hypothalamic-pituitary-adrenal axis. Am J Obstet Gynecol. 2000;182:1404–13. doi: 10.1067/mob.2000.106180. [DOI] [PubMed] [Google Scholar]
- 61.Gray DJ, Robinson HB, Malone J, Thomson RB., Jr Adverse outcome in pregnancy following amniotic fluid isolation of Ureaplasma urealyticum. Prenat Diagn. 1992;12:111–7. doi: 10.1002/pd.1970120206. [DOI] [PubMed] [Google Scholar]
- 62.Gulati S, Agrawal S, Raghunandan C, Bhattacharya J, Saili A, Agarwal S, et al. Maternal serum interleukin-6 and its association with clinicopathological infectious morbidity in preterm premature rupture of membranes: a prospective cohort study. J Matern Fetal Neonatal Med. 2012;25:1428–32. doi: 10.3109/14767058.2011.638952. [DOI] [PubMed] [Google Scholar]
- 63.Gulati S, Bhatnagar S, Raghunandan C, Bhattacharjee J. Interleukin-6 as a predictor of subclinical chorioamnionitis in preterm premature rupture of membranes. Am J Reprod Immunol. 2012;67:235–40. doi: 10.1111/j.1600-0897.2011.01084.x. [DOI] [PubMed] [Google Scholar]
- 64.Hassan S, Romero R, Hendler I, Gomez R, Khalek N, Espinoza J, et al. A sonographic short cervix as the only clinical manifestation of intra-amniotic infection. J Perinat Med. 2006;34:13–9. doi: 10.1515/JPM.2006.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hassan SS, Romero R, Berry SM, Dang K, Blackwell SC, Treadwell MC, et al. Patients with an ultrasonographic cervical length < or =15 mm have nearly a 50% risk of early spontaneous preterm delivery. Am J Obstet Gynecol. 2000;182:1458–67. doi: 10.1067/mob.2000.106851. [DOI] [PubMed] [Google Scholar]
- 66.Hassan SS, Romero R, Tarca AL, Nhan-Chang CL, Mittal P, Vaisbuch E, et al. The molecular basis for sonographic cervical shortening at term: identification of differentially expressed genes and the epithelial-mesenchymal transition as a function of cervical length. Am J Obstet Gynecol. 2010;203:472, e1–72, e14. doi: 10.1016/j.ajog.2010.06.076. [DOI] [PubMed] [Google Scholar]
- 67.Hassan SS, Romero R, Vidyadhari D, Fusey S, Baxter JK, Khandelwal M, et al. Vaginal progesterone reduces the rate of preterm birth in women with a sonographic short cervix: a multicenter, randomized, double-blind, placebo-controlled trial. Ultrasound Obstet Gynecol. 2011;38:18–31. doi: 10.1002/uog.9017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hatzidaki E, Gourgiotis D, Manoura A, Korakaki E, Bossios A, Galanakis E, et al. Interleukin-6 in preterm premature rupture of membranes as an indicator of neonatal outcome. Acta Obstet Gynecol Scand. 2005;84:632–8. doi: 10.1111/j.0001-6349.2005.00747.x. [DOI] [PubMed] [Google Scholar]
- 69.Helmig BR, Romero R, Espinoza J, Chaiworapongsa T, Bujold E, Gomez R, et al. Neutrophil elastase and secretory leukocyte protease inhibitor in prelabor rupture of membranes, parturition and intra-amniotic infection. J Matern Fetal Neonatal Med. 2002;12:237–46. doi: 10.1080/jmf.12.4.237.246. [DOI] [PubMed] [Google Scholar]
- 70.Henderson D, Wilson T. Reduced binding of progesterone receptor to its nuclear response element after human labor onset. Am J Obstet Gynecol. 2001;185:579–85. doi: 10.1067/mob.2001.116753. [DOI] [PubMed] [Google Scholar]
- 71.Hillier SL, Krohn MA, Kiviat NB, Watts DH, Eschenbach DA. Microbiologic causes and neonatal outcomes associated with chorioamnion infection. Am J Obstet Gynecol. 1991;165:955–61. doi: 10.1016/0002-9378(91)90447-y. [DOI] [PubMed] [Google Scholar]
- 72.Hiyama A, Arai F, Sakai D, Yokoyama K, Mochida J. The effects of oxygen tension and antiaging factor Klotho on Wnt signaling in nucleus pulposus cells. Arthritis Res Ther. 2012;14:R105. doi: 10.1186/ar3830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Holecki M, Chudek J, Wiecek A, Titz-Bober M, Dulawa J. The serum level of fibroblast growth factor-23 and calcium-phosphate homeostasis in obese perimenopausal women. Int J Endocrinol. 2011;2011:707126. doi: 10.1155/2011/707126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hu MC, Kuro-O M, Moe OW. Klotho and kidney disease. J Nephrol. 2010;23(Suppl 16):S136–44. [PMC free article] [PubMed] [Google Scholar]
- 75.Hu MC, Kuro-O M, Moe OW. Secreted klotho and chronic kidney disease. Adv Exp Med Biol. 2012;728:126–57. doi: 10.1007/978-1-4614-0887-1_9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hu MC, Shi M, Zhang J, Quinones H, Kuro-O M, Moe OW. Klotho deficiency is an early biomarker of renal ischemia-reperfusion injury and its replacement is protective. Kidney Int. 2010;78:1240–51. doi: 10.1038/ki.2010.328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Huang CL. Regulation of ion channels by secreted Klotho. Adv Exp Med Biol. 2012;728:100–6. doi: 10.1007/978-1-4614-0887-1_7. [DOI] [PubMed] [Google Scholar]
- 78.Huang CL. Regulation of ion channels by secreted Klotho: mechanisms and implications. Kidney Int. 2010;77:855–60. doi: 10.1038/ki.2010.73. [DOI] [PubMed] [Google Scholar]
- 79.Iams JD, Goldenberg RL, Meis PJ, Mercer BM, Moawad A, Das A, et al. The length of the cervix and the risk of spontaneous premature delivery. National Institute of Child Health and Human Development Maternal Fetal Medicine Unit Network. N Engl J Med. 1996;334:567–72. doi: 10.1056/NEJM199602293340904. [DOI] [PubMed] [Google Scholar]
- 80.Iams JD, Johnson FF, Sonek J, Sachs L, Gebauer C, Samuels P. Cervical competence as a continuum: a study of ultrasonographic cervical length and obstetric performance. Am J Obstet Gynecol. 1995;172:1097–103. doi: 10.1016/0002-9378(95)91469-2. discussion 104-6. [DOI] [PubMed] [Google Scholar]
- 81.Iams JD, Romero R, Culhane JF, Goldenberg RL. Primary, secondary, and tertiary interventions to reduce the morbidity and mortality of preterm birth. Lancet. 2008;371:164–75. doi: 10.1016/S0140-6736(08)60108-7. [DOI] [PubMed] [Google Scholar]
- 82.Ichikawa S, Imel EA, Kreiter ML, Yu X, Mackenzie DS, Sorenson AH, et al. A homozygous missense mutation in human KLOTHO causes severe tumoral calcinosis. J Musculoskelet Neuronal Interact. 2007;7:318–9. [PubMed] [Google Scholar]
- 83.Imura A, Iwano A, Tohyama O, Tsuji Y, Nozaki K, Hashimoto N, et al. Secreted Klotho protein in sera and CSF: implication for post-translational cleavage in release of Klotho protein from cell membrane. FEBS Lett. 2004;565:143–7. doi: 10.1016/j.febslet.2004.03.090. [DOI] [PubMed] [Google Scholar]
- 84.Imura A, Tsuji Y, Murata M, Maeda R, Kubota K, Iwano A, et al. alpha-Klotho as a regulator of calcium homeostasis. Science. 2007;316:1615–8. doi: 10.1126/science.1135901. [DOI] [PubMed] [Google Scholar]
- 85.Jakobsson M, Gissler M, Paavonen J, Tapper AM. Loop electrosurgical excision procedure and the risk for preterm birth. Obstet Gynecol. 2009;114:504–10. doi: 10.1097/AOG.0b013e3181b052de. [DOI] [PubMed] [Google Scholar]
- 86.Karalis K, Goodwin G, Majzoub JA. Cortisol blockade of progesterone: a possible molecular mechanism involved in the initiation of human labor. Nat Med. 1996;2:556–60. doi: 10.1038/nm0596-556. [DOI] [PubMed] [Google Scholar]
- 87.Kim CJ, Romero R, Kusanovic JP, Yoo W, Dong Z, Topping V, et al. The frequency, clinical significance, and pathological features of chronic chorioamnionitis: a lesion associated with spontaneous preterm birth. Mod Pathol. 2010;23:1000–11. doi: 10.1038/modpathol.2010.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kim CJ, Yoon BH, Park SS, Kim MH, Chi JG. Acute funisitis of preterm but not term placentas is associated with severe fetal inflammatory response. Hum Pathol. 2001;32:623–9. doi: 10.1053/hupa.2001.24992. [DOI] [PubMed] [Google Scholar]
- 89.Kim SY, Romero R, Tarca AL, Bhatti G, Kim CJ, Lee J, et al. Methylome of fetal and maternal monocytes and macrophages at the feto-maternal interface. Am J Reprod Immunol. 2012;68:8–27. doi: 10.1111/j.1600-0897.2012.01108.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kim YM, Bujold E, Chaiworapongsa T, Gomez R, Yoon BH, Thaler HT, et al. Failure of physiologic transformation of the spiral arteries in patients with preterm labor and intact membranes. Am J Obstet Gynecol. 2003;189:1063–9. doi: 10.1067/s0002-9378(03)00838-x. [DOI] [PubMed] [Google Scholar]
- 91.Kim YM, Chaiworapongsa T, Gomez R, Bujold E, Yoon BH, Rotmensch S, et al. Failure of physiologic transformation of the spiral arteries in the placental bed in preterm premature rupture of membranes. Am J Obstet Gynecol. 2002;187:1137–42. doi: 10.1067/mob.2002.127720. [DOI] [PubMed] [Google Scholar]
- 92.Kim YM, Romero R, Chaiworapongsa T, Kim GJ, Kim MR, Kuivaniemi H, et al. Toll-like receptor-2 and -4 in the chorioamniotic membranes in spontaneous labor at term and in preterm parturition that are associated with chorioamnionitis. Am J Obstet Gynecol. 2004;191:1346–55. doi: 10.1016/j.ajog.2004.07.009. [DOI] [PubMed] [Google Scholar]
- 93.King GD, Chen C, Huang MM, Zeldich E, Brazee PL, Schuman ER, et al. Identification of novel small molecules that elevate Klotho expression. Biochem J. 2012;441:453–61. doi: 10.1042/BJ20101909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Kuro-O M. Klotho and aging. Biochim Biophys Acta. 2009;1790:1049–58. doi: 10.1016/j.bbagen.2009.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Kuro-O M. Overview of the FGF23-Klotho axis. Pediatr Nephrol. 2010;25:583–90. doi: 10.1007/s00467-009-1260-4. [DOI] [PubMed] [Google Scholar]
- 96.Kuro-O M, Matsumura Y, Aizawa H, Kawaguchi H, Suga T, Utsugi T, et al. Mutation of the mouse klotho gene leads to a syndrome resembling ageing. Nature. 1997;390:45–51. doi: 10.1038/36285. [DOI] [PubMed] [Google Scholar]
- 97.Kurosu H, Ogawa Y, Miyoshi M, Yamamoto M, Nandi A, Rosenblatt KP, et al. Regulation of fibroblast growth factor-23 signaling by klotho. J Biol Chem. 2006;281:6120–3. doi: 10.1074/jbc.C500457200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kurosu H, Yamamoto M, Clark JD, Pastor JV, Nandi A, Gurnani P, et al. Suppression of aging in mice by the hormone Klotho. Science. 2005;309:1829–33. doi: 10.1126/science.1112766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Kusaba T, Okigaki M, Matui A, Murakami M, Ishikawa K, Kimura T, et al. Klotho is associated with VEGF receptor-2 and the transient receptor potential canonical-1 Ca2+ channel to maintain endothelial integrity. Proc Natl Acad Sci U S A. 2010;107:19308–13. doi: 10.1073/pnas.1008544107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Kusanovic JP, Romero R, Mazaki-Tovi S, Chaiworapongsa T, Mittal P, Gotsch F, et al. Resistin in amniotic fluid and its association with intra-amniotic infection and inflammation. J Matern Fetal Neonatal Med. 2008;21:902–16. doi: 10.1080/14767050802320357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Larsson T, Marsell R, Schipani E, Ohlsson C, Ljunggren O, Tenenhouse HS, et al. Transgenic mice expressing fibroblast growth factor 23 under the control of the alpha1(I) collagen promoter exhibit growth retardation, osteomalacia, and disturbed phosphate homeostasis. Endocrinology. 2004;145:3087–94. doi: 10.1210/en.2003-1768. [DOI] [PubMed] [Google Scholar]
- 102.Lee J, Romero R, Xu Y, Kim JS, Park JY, Kusanovic JP, et al. Maternal HLA panel-reactive antibodies in early gestation positively correlate with chronic chorioamnionitis: evidence in support of the chronic nature of maternal anti-fetal rejection. Am J Reprod Immunol. 2011;66:510–26. doi: 10.1111/j.1600-0897.2011.01066.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Lee J, Romero R, Xu Y, Kim JS, Topping V, Yoo W, et al. A signature of maternal anti-fetal rejection in spontaneous preterm birth: chronic chorioamnionitis, anti-human leukocyte antigen antibodies, and C4d. PLoS One. 2011;6:e16806. doi: 10.1371/journal.pone.0016806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Lee SE, Romero R, Jung H, Park CW, Park JS, Yoon BH. The intensity of the fetal inflammatory response in intraamniotic inflammation with and without microbial invasion of the amniotic cavity. Am J Obstet Gynecol. 2007;197:294, e1–6. doi: 10.1016/j.ajog.2007.07.006. [DOI] [PubMed] [Google Scholar]
- 105.Liu F, Wu S, Ren H, Gu J. Klotho suppresses RIG-I-mediated senescence-associated inflammation. Nat Cell Biol. 2011;13:254–62. doi: 10.1038/ncb2167. [DOI] [PubMed] [Google Scholar]
- 106.Lu P, Boros S, Chang Q, Bindels RJ, Hoenderop JG. The beta-glucuronidase klotho exclusively activates the epithelial Ca2+ channels TRPV5 and TRPV6. Nephrol Dial Transplant. 2008;23:3397–402. doi: 10.1093/ndt/gfn291. [DOI] [PubMed] [Google Scholar]
- 107.Maekawa Y, Ishikawa K, Yasuda O, Oguro R, Hanasaki H, Kida I, et al. Klotho suppresses TNF-alpha-induced expression of adhesion molecules in the endothelium and attenuates NF-kappaB activation. Endocrine. 2009;35:341–6. doi: 10.1007/s12020-009-9181-3. [DOI] [PubMed] [Google Scholar]
- 108.Major CA, De Veciana M, Lewis DF, Morgan MA. Preterm premature rupture of membranes and abruptio placentae: is there an association between these pregnancy complications? Am J Obstet Gynecol. 1995;172:672–6. doi: 10.1016/0002-9378(95)90591-x. [DOI] [PubMed] [Google Scholar]
- 109.Manya H, Akasaka-Manya K, Endo T. Klotho protein deficiency and aging. Geriatr Gerontol Int. 2010;10(Suppl 1):S80–7. doi: 10.1111/j.1447-0594.2010.00596.x. [DOI] [PubMed] [Google Scholar]
- 110.Matsumura Y, Aizawa H, Shiraki-Iida T, Nagai R, Kuro-O M, Nabeshima Y. Identification of the human klotho gene and its two transcripts encoding membrane and secreted klotho protein. Biochem Biophys Res Commun. 1998;242:626–30. doi: 10.1006/bbrc.1997.8019. [DOI] [PubMed] [Google Scholar]
- 111.Maymon E, Ghezzi F, Edwin SS, Mazor M, Yoon BH, Gomez R, et al. The tumor necrosis factor alpha and its soluble receptor profile in term and preterm parturition. Am J Obstet Gynecol. 1999;181:1142–8. doi: 10.1016/s0002-9378(99)70097-9. [DOI] [PubMed] [Google Scholar]
- 112.Maymon E, Romero R, Chaiworapongsa T, Berman S, Conoscenti G, Gomez R, et al. Amniotic fluid matrix metalloproteinase-8 in preterm labor with intact membranes. Am J Obstet Gynecol. 2001;185:1149–55. doi: 10.1067/mob.2001.118165. [DOI] [PubMed] [Google Scholar]
- 113.Maymon E, Romero R, Chaiworapongsa T, Kim JC, Berman S, Gomez R, et al. Value of amniotic fluid neutrophil collagenase concentrations in preterm premature rupture of membranes. Am J Obstet Gynecol. 2001;185:1143–8. doi: 10.1067/mob.2001.118166. [DOI] [PubMed] [Google Scholar]
- 114.Maymon E, Romero R, Pacora P, Gervasi MT, Bianco K, Ghezzi F, et al. Evidence for the participation of interstitial collagenase (matrix metalloproteinase 1) in preterm premature rupture of membranes. Am J Obstet Gynecol. 2000;183:914–20. doi: 10.1067/mob.2000.108879. [DOI] [PubMed] [Google Scholar]
- 115.Maymon E, Romero R, Pacora P, Gervasi MT, Edwin SS, Gomez R, et al. Matrilysin (matrix metalloproteinase 7) in parturition, premature rupture of membranes, and intrauterine infection. Am J Obstet Gynecol. 2000;182:1545–53. doi: 10.1067/mob.2000.107652. [DOI] [PubMed] [Google Scholar]
- 116.Maymon E, Romero R, Pacora P, Gomez R, Athayde N, Edwin S, et al. Human neutrophil collagenase (matrix metalloproteinase 8) in parturition, premature rupture of the membranes, and intrauterine infection. Am J Obstet Gynecol. 2000;183:94–9. doi: 10.1067/mob.2000.105344. [DOI] [PubMed] [Google Scholar]
- 117.Maymon E, Romero R, Pacora P, Gomez R, Mazor M, Edwin S, et al. A role for the 72 kDa gelatinase (MMP-2) and its inhibitor (TIMP-2) in human parturition, premature rupture of membranes and intraamniotic infection. J Perinat Med. 2001;29:308–16. doi: 10.1515/JPM.2001.044. [DOI] [PubMed] [Google Scholar]
- 118.Mazor M, Hershkovitz R, Ghezzi F, Maymon E, Horowitz S, Leiberman JR. Intraamniotic infection in patients with preterm labor and twin pregnancies. Acta Obstet Gynecol Scand. 1996;75:624–7. doi: 10.3109/00016349609054686. [DOI] [PubMed] [Google Scholar]
- 119.Mesiano S, Chan EC, Fitter JT, Kwek K, Yeo G, Smith R. Progesterone withdrawal and estrogen activation in human parturition are coordinated by progesterone receptor A expression in the myometrium. J Clin Endocrinol Metab. 2002;87:2924–30. doi: 10.1210/jcem.87.6.8609. [DOI] [PubMed] [Google Scholar]
- 120.Mesiano S, Wang Y, Norwitz ER. Progesterone receptors in the human pregnancy uterus: do they hold the key to birth timing? Reprod Sci. 2011;18:6–19. doi: 10.1177/1933719110382922. [DOI] [PubMed] [Google Scholar]
- 121.Mesiano S, Welsh TN. Steroid hormone control of myometrial contractility and parturition. Semin Cell Dev Biol. 2007;18:321–31. doi: 10.1016/j.semcdb.2007.05.003. [DOI] [PubMed] [Google Scholar]
- 122.Millar LK, Stollberg J, Debuque L, Bryant-Greenwood G. Fetal membrane distention: determination of the intrauterine surface area and distention of the fetal membranes preterm and at term. Am J Obstet Gynecol. 2000;182:128–34. doi: 10.1016/s0002-9378(00)70501-1. [DOI] [PubMed] [Google Scholar]
- 123.Moe SM. Klotho: A Master Regulator of Cardiovascular Disease? Circulation. 2012;125:2181–3. doi: 10.1161/CIRCULATIONAHA.112.104828. [DOI] [PubMed] [Google Scholar]
- 124.Moreno JA, Izquierdo MC, Sanchez-Nino MD, Suarez-Alvarez B, Lopez-Larrea C, Jakubowski A, et al. The inflammatory cytokines TWEAK and TNFalpha reduce renal klotho expression through NFkappaB. J Am Soc Nephrol. 2011;22:1315–25. doi: 10.1681/ASN.2010101073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Murtha AP, Greig PC, Jimmerson CE, Roitman-Johnson B, Allen J, Herbert WN. Maternal serum interleukin-6 concentrations in patients with preterm premature rupture of membranes and evidence of infection. Am J Obstet Gynecol. 1996;175:966–9. doi: 10.1016/s0002-9378(96)80033-0. [DOI] [PubMed] [Google Scholar]
- 126.Murtha AP, Sinclair T, Hauser ER, Swamy GK, Herbert WN, Heine RP. Maternal serum cytokines in preterm premature rupture of membranes. Obstet Gynecol. 2007;109:121–7. doi: 10.1097/01.AOG.0000250474.35369.12. [DOI] [PubMed] [Google Scholar]
- 127.Nagasu H, Satoh M, Kuwabara A, Yorimitsu D, Kidokoro K, Nishi Y, et al. Overexpression of klotho protein modulates uninephrectomy-induced compensatory renal hypertrophy by suppressing IGF-I signals. Biochem Biophys Res Commun. 2011;407:39–43. doi: 10.1016/j.bbrc.2011.02.089. [DOI] [PubMed] [Google Scholar]
- 128.Nakatani T, Sarraj B, Ohnishi M, Densmore MJ, Taguchi T, Goetz R, et al. In vivo genetic evidence for klotho-dependent, fibroblast growth factor 23 (Fgf23) -mediated regulation of systemic phosphate homeostasis. FASEB J. 2009;23:433–41. doi: 10.1096/fj.08-114397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Nemeth E, Millar LK, Bryant-Greenwood G. Fetal membrane distention: II. Differentially expressed genes regulated by acute distention in vitro. Am J Obstet Gynecol. 2000;182:60–7. doi: 10.1016/s0002-9378(00)70491-1. [DOI] [PubMed] [Google Scholar]
- 130.Nemeth E, Tashima LS, Yu Z, Bryant-Greenwood GD. Fetal membrane distention: I. Differentially expressed genes regulated by acute distention in amniotic epithelial (WISH) cells. Am J Obstet Gynecol. 2000;182:50–9. doi: 10.1016/s0002-9378(00)70490-x. [DOI] [PubMed] [Google Scholar]
- 131.Nhan-Chang CL, Romero R, Kusanovic JP, Gotsch F, Edwin SS, Erez O, et al. A role for CXCL13 (BCA-1) in pregnancy and intra-amniotic infection/inflammation. J Matern Fetal Neonatal Med. 2008;21:763–75. doi: 10.1080/14767050802244946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Nzietchueng R, El Shamieh S, Benachour H, Labat C, Herbeth B, Ndiaye NC, et al. Klotho KL-VS genotype is involved in blood pressure regulation. Clin Chim Acta. 2011;412:1773–7. doi: 10.1016/j.cca.2011.05.032. [DOI] [PubMed] [Google Scholar]
- 133.Ogge G, Romero R, Lee DC, Gotsch F, Than NG, Lee J, et al. Chronic chorioamnionitis displays distinct alterations of the amniotic fluid proteome. J Pathol. 2011;223:553–65. doi: 10.1002/path.2825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Oh KJ, Lee SE, Jung H, Kim G, Romero R, Yoon BH. Detection of ureaplasmas by the polymerase chain reaction in the amniotic fluid of patients with cervical insufficiency. J Perinat Med. 2010;38:261–8. doi: 10.1515/JPM.2010.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Ohata Y, Arahori H, Namba N, Kitaoka T, Hirai H, Wada K, et al. Circulating levels of soluble alpha-Klotho are markedly elevated in human umbilical cord blood. J Clin Endocrinol Metab. 2011;96:E943–7. doi: 10.1210/jc.2010-2357. [DOI] [PubMed] [Google Scholar]
- 136.Ohnishi M, Nakatani T, Lanske B, Razzaque MS. Reversal of mineral ion homeostasis and soft-tissue calcification of klotho knockout mice by deletion of vitamin D 1alpha-hydroxylase. Kidney Int. 2009;75:1166–72. doi: 10.1038/ki.2009.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Ohyama Y, Kurabayashi M, Masuda H, Nakamura T, Aihara Y, Kaname T, et al. Molecular cloning of rat klotho cDNA: markedly decreased expression of klotho by acute inflammatory stress. Biochem Biophys Res Commun. 1998;251:920–5. doi: 10.1006/bbrc.1998.9576. [DOI] [PubMed] [Google Scholar]
- 138.Owen J, Yost N, Berghella V, Macpherson C, Swain M, Dildy GA, 3rd, et al. Can shortened midtrimester cervical length predict very early spontaneous preterm birth? Am J Obstet Gynecol. 2004;191:298–303. doi: 10.1016/j.ajog.2003.11.025. [DOI] [PubMed] [Google Scholar]
- 139.Owen J, Yost N, Berghella V, Thom E, Swain M, Dildy GA, 3rd, et al. Mid-trimester endovaginal sonography in women at high risk for spontaneous preterm birth. JAMA. 2001;286:1340–8. doi: 10.1001/jama.286.11.1340. [DOI] [PubMed] [Google Scholar]
- 140.Park KH, Chaiworapongsa T, Kim YM, Espinoza J, Yoshimatsu J, Edwin S, et al. Matrix metalloproteinase 3 in parturition, premature rupture of the membranes, and microbial invasion of the amniotic cavity. J Perinat Med. 2003;31:12–22. doi: 10.1515/JPM.2003.002. [DOI] [PubMed] [Google Scholar]
- 141.Park KH, Kim SN, Oh KJ, Lee SY, Jeong EH, Ryu A. Noninvasive prediction of intra-amniotic infection and/or inflammation in preterm premature rupture of membranes. Reprod Sci. 2012;19:658–65. doi: 10.1177/1933719111432869. [DOI] [PubMed] [Google Scholar]
- 142.Pavik I, Jaeger P, Ebner L, Poster D, Krauer F, Kistler AD, et al. Soluble Klotho and autosomal dominant polycystic kidney disease. Clin J Am Soc Nephrol. 2012;7:248–57. doi: 10.2215/CJN.09020911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Perrone G, Anceschi MM, Capri O, Galoppi P, Pizzulo S, Buccheri M, et al. Maternal C-reactive protein at hospital admission is a simple predictor of funisitis in preterm premature rupture of membranes. Gynecol Obstet Invest. 2012;74:95–9. doi: 10.1159/000337717. [DOI] [PubMed] [Google Scholar]
- 144.Phelan JP, Park YW, Ahn MO, Rutherford SE. Polyhydramnios and perinatal outcome. J Perinatol. 1990;10:347–50. [PubMed] [Google Scholar]
- 145.Pieber D, Allport VC, Hills F, Johnson M, Bennett PR. Interactions between progesterone receptor isoforms in myometrial cells in human labour. Mol Hum Reprod. 2001;7:875–9. doi: 10.1093/molehr/7.9.875. [DOI] [PubMed] [Google Scholar]
- 146.Razzaque MS. FGF23, klotho and vitamin D interactions: What have we learned from in vivo mouse genetics studies? Adv Exp Med Biol. 2012;728:84–91. doi: 10.1007/978-1-4614-0887-1_5. [DOI] [PubMed] [Google Scholar]
- 147.Razzaque MS, Sitara D, Taguchi T, St-Arnaud R, Lanske B. Premature aging-like phenotype in fibroblast growth factor 23 null mice is a vitamin D-mediated process. FASEB J. 2006;20:720–2. doi: 10.1096/fj.05-5432fje. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Rezapour M, Backstrom T, Lindblom B, Ulmsten U. Sex steroid receptors and human parturition. Obstet Gynecol. 1997;89:918–24. doi: 10.1016/s0029-7844(97)00116-6. [DOI] [PubMed] [Google Scholar]
- 149.Romero R, Avila C, Brekus CA, Morotti R. The role of systemic and intrauterine infection in preterm parturition. Ann N Y Acad Sci. 1991;622:355–75. doi: 10.1111/j.1749-6632.1991.tb37880.x. [DOI] [PubMed] [Google Scholar]
- 150.Romero R, Avila C, Santhanam U, Sehgal PB. Amniotic fluid interleukin 6 in preterm labor. Association with infection. J Clin Invest. 1990;85:1392–400. doi: 10.1172/JCI114583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Romero R, Brody DT, Oyarzun E, Mazor M, Wu YK, Hobbins JC, et al. Infection and labor. III. Interleukin-1: a signal for the onset of parturition. Am J Obstet Gynecol. 1989;160:1117–23. doi: 10.1016/0002-9378(89)90172-5. [DOI] [PubMed] [Google Scholar]
- 152.Romero R, Emamian M, Quintero R, Wan M, Hobbins JC, Mitchell MD. Amniotic fluid prostaglandin levels and intra-amniotic infections. Lancet. 1986;1:1380. doi: 10.1016/s0140-6736(86)91685-5. [DOI] [PubMed] [Google Scholar]
- 153.Romero R, Espinoza J, Chaiworapongsa T, Kalache K. Infection and prematurity and the role of preventive strategies. Semin Neonatol. 2002;7:259–74. doi: 10.1016/s1084-2756(02)90121-1. [DOI] [PubMed] [Google Scholar]
- 154.Romero R, Espinoza J, Kusanovic JP, Gotsch F, Hassan S, Erez O, et al. The preterm parturition syndrome. BJOG. 2006;113(Suppl 3):17–42. doi: 10.1111/j.1471-0528.2006.01120.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Romero R, Espinoza J, Rogers WT, Moser A, Nien JK, Kusanovic JP, et al. Proteomic analysis of amniotic fluid to identify women with preterm labor and intra-amniotic inflammation/infection: the use of a novel computational method to analyze mass spectrometric profiling. J Matern Fetal Neonatal Med. 2008;21:367–88. doi: 10.1080/14767050802045848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Romero R, Gomez R, Galasso M, Munoz H, Acosta L, Yoon BH, et al. Macrophage inflammatory protein-1 alpha in term and preterm parturition: effect of microbial invasion of the amniotic cavity. Am J Reprod Immunol. 1994;32:108–13. doi: 10.1111/j.1600-0897.1994.tb01101.x. [DOI] [PubMed] [Google Scholar]
- 157.Romero R, Gomez R, Ghezzi F, Yoon BH, Mazor M, Edwin SS, et al. A fetal systemic inflammatory response is followed by the spontaneous onset of preterm parturition. Am J Obstet Gynecol. 1998;179:186–93. doi: 10.1016/s0002-9378(98)70271-6. [DOI] [PubMed] [Google Scholar]
- 158.Romero R, Kadar N, Hobbins JC, Duff GW. Infection and labor: the detection of endotoxin in amniotic fluid. Am J Obstet Gynecol. 1987;157:815–9. doi: 10.1016/s0002-9378(87)80061-3. [DOI] [PubMed] [Google Scholar]
- 159.Romero R, Kusanovic JP, Gomez R, Lamont R, Bytautiene E, Garfield RE, et al. The clinical significance of eosinophils in the amniotic fluid in preterm labor. J Matern Fetal Neonatal Med. 2010;23:320–9. doi: 10.3109/14767050903168465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Romero R, Kusanovic JP, Munoz H, Gomez R, Lamont RF, Yeo L. Allergy-induced preterm labor after the ingestion of shellfish. J Matern Fetal Neonatal Med. 2010;23:351–9. doi: 10.3109/14767050903177193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Romero R, Mazor M, Morrotti R, Avila C, Oyarzun E, Insunza A, et al. Infection and labor. VII. Microbial invasion of the amniotic cavity in spontaneous rupture of membranes at term. Am J Obstet Gynecol. 1992;166:129–33. doi: 10.1016/0002-9378(92)91845-2. [DOI] [PubMed] [Google Scholar]
- 162.Romero R, Mazor M, Wu YK, Sirtori M, Oyarzun E, Mitchell MD, et al. Infection in the pathogenesis of preterm labor. Semin Perinatol. 1988;12:262–79. [PubMed] [Google Scholar]
- 163.Romero R, Nores J, Mazor M, Sepulveda W, Oyarzun E, Parra M, et al. Microbial invasion of the amniotic cavity during term labor. Prevalence and clinical significance. J Reprod Med. 1993;38:543–8. [PubMed] [Google Scholar]
- 164.Romero R, Quintero R, Oyarzun E, Wu YK, Sabo V, Mazor M, et al. Intraamniotic infection and the onset of labor in preterm premature rupture of the membranes. Am J Obstet Gynecol. 1988;159:661–6. doi: 10.1016/s0002-9378(88)80030-9. [DOI] [PubMed] [Google Scholar]
- 165.Romero R, Roslansky P, Oyarzun E, Wan M, Emamian M, Novitsky TJ, et al. Labor and infection. II. Bacterial endotoxin in amniotic fluid and its relationship to the onset of preterm labor. Am J Obstet Gynecol. 1988;158:1044–9. doi: 10.1016/0002-9378(88)90216-5. [DOI] [PubMed] [Google Scholar]
- 166.Romero R, Salafia CM, Athanassiadis AP, Hanaoka S, Mazor M, Sepulveda W, et al. The relationship between acute inflammatory lesions of the preterm placenta and amniotic fluid microbiology. Am J Obstet Gynecol. 1992;166:1382–8. doi: 10.1016/0002-9378(92)91609-e. [DOI] [PubMed] [Google Scholar]
- 167.Romero R, Savasan ZA, Chaiworapongsa T, Berry SM, Kusanovic JP, Hassan SS, et al. Hematologic profile of the fetus with systemic inflammatory response syndrome. J Perinat Med. 2011;40:19–32. doi: 10.1515/JPM.2011.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Romero R, Schaudinn C, Kusanovic JP, Gorur A, Gotsch F, Webster P, et al. Detection of a microbial biofilm in intraamniotic infection. Am J Obstet Gynecol. 2008;198:135, e1–5. doi: 10.1016/j.ajog.2007.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Romero R, Shamma F, Avila C, Jimenez C, Callahan R, Nores J, et al. Infection and labor. VI. Prevalence, microbiology, and clinical significance of intraamniotic infection in twin gestations with preterm labor. Am J Obstet Gynecol. 1990;163:757–61. doi: 10.1016/0002-9378(90)91063-i. [DOI] [PubMed] [Google Scholar]
- 170.Romero R, Sibai B, Caritis S, Paul R, Depp R, Rosen M, et al. Antibiotic treatment of preterm labor with intact membranes: a multicenter, randomized, double-blinded, placebo-controlled trial. Am J Obstet Gynecol. 1993;169:764–74. doi: 10.1016/0002-9378(93)90003-2. [DOI] [PubMed] [Google Scholar]
- 171.Romero R, Sirtori M, Oyarzun E, Avila C, Mazor M, Callahan R, et al. Infection and labor. V. Prevalence, microbiology, and clinical significance of intraamniotic infection in women with preterm labor and intact membranes. Am J Obstet Gynecol. 1989;161:817–24. doi: 10.1016/0002-9378(89)90409-2. [DOI] [PubMed] [Google Scholar]
- 172.Romero R, Yeo L, Miranda J, Hassan SS, Conde-Agudelo A, Chaiworapongsa T. A blueprint for the prevention of preterm birth: vaginal progesterone in women with a short cervix. J Perinat Med. 2013;41:27–44. doi: 10.1515/jpm-2012-0272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Romero R, Yoon BH, Kenney JS, Gomez R, Allison AC, Sehgal PB. Amniotic fluid interleukin-6 determinations are of diagnostic and prognostic value in preterm labor. Am J Reprod Immunol. 1993;30:167–83. doi: 10.1111/j.1600-0897.1993.tb00618.x. [DOI] [PubMed] [Google Scholar]
- 174.Sadler L, Saftlas A, Wang W, Exeter M, Whittaker J, Mccowan L. Treatment for cervical intraepithelial neoplasia and risk of preterm delivery. JAMA. 2004;291:2100–6. doi: 10.1001/jama.291.17.2100. [DOI] [PubMed] [Google Scholar]
- 175.Semba RD, Cappola AR, Sun K, Bandinelli S, Dalal M, Crasto C, et al. Plasma klotho and cardiovascular disease in adults. J Am Geriatr Soc. 2011;59:1596–601. doi: 10.1111/j.1532-5415.2011.03558.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Shen Y, Yu HM, Yuan TM, Gu WZ, Wu YD. Intrauterine infection induced oligodendrocyte injury and inducible nitric oxide synthase expression in the developing rat brain. J Perinat Med. 2007;35:203–9. doi: 10.1515/JPM.2007.058. [DOI] [PubMed] [Google Scholar]
- 177.Shim SS, Romero R, Hong JS, Park CW, Jun JK, Kim BI, et al. Clinical significance of intra-amniotic inflammation in patients with preterm premature rupture of membranes. Am J Obstet Gynecol. 2004;191:1339–45. doi: 10.1016/j.ajog.2004.06.085. [DOI] [PubMed] [Google Scholar]
- 178.Shimamura Y, Hamada K, Inoue K, Ogata K, Ishihara M, Kagawa T, et al. Serum levels of soluble secreted alpha-Klotho are decreased in the early stages of chronic kidney disease, making it a probable novel biomarker for early diagnosis. Clin Exp Nephrol. 2012;16:722–9. doi: 10.1007/s10157-012-0621-7. [DOI] [PubMed] [Google Scholar]
- 179.Shynlova O, Williams SJ, Draper H, White BG, Macphee DJ, Lye SJ. Uterine stretch regulates temporal and spatial expression of fibronectin protein and its alpha 5 integrin receptor in myometrium of unilaterally pregnant rats. Biol Reprod. 2007;77:880–8. doi: 10.1095/biolreprod.107.062356. [DOI] [PubMed] [Google Scholar]
- 180.Siahanidou T, Garatzioti M, Lazaropoulou C, Kourlaba G, Papassotiriou I, Kino T, et al. Plasma soluble alpha-klotho protein levels in premature and term neonates: correlations with growth and metabolic parameters. Eur J Endocrinol. 2012;167:433–40. doi: 10.1530/EJE-12-0476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Signore CC, Sood AK, Richards DS. Second-trimester vaginal bleeding: correlation of ultrasonographic findings with perinatal outcome. Am J Obstet Gynecol. 1998;178:336–40. doi: 10.1016/s0002-9378(98)80022-7. [DOI] [PubMed] [Google Scholar]
- 182.Smith R. Alterations in the hypothalamic pituitary adrenal axis during pregnancy and the placental clock that determines the length of parturition. J Reprod Immunol. 1998;39:215–20. doi: 10.1016/s0165-0378(98)00023-0. [DOI] [PubMed] [Google Scholar]
- 183.Strigini FA, Lencioni G, De Luca G, Lombardo M, Bianchi F, Genazzani AR. Uterine artery velocimetry and spontaneous preterm delivery. Obstet Gynecol. 1995;85:374–7. doi: 10.1016/0029-7844(94)00420-I. [DOI] [PubMed] [Google Scholar]
- 184.Sugiura H, Yoshida T, Shiohira S, Kohei J, Mitobe M, Kurosu H, et al. Reduced Klotho Expression Level in Kidney Aggravates Renal Interstitial Fibrosis. Am J Physiol Renal Physiol. 2012;302:F1252–64. doi: 10.1152/ajprenal.00294.2011. [DOI] [PubMed] [Google Scholar]
- 185.Sun CY, Chang SC, Wu MS. Suppression of Klotho expression by protein-bound uremic toxins is associated with increased DNA methyltransferase expression and DNA hypermethylation. Kidney Int. 2012;81:640–50. doi: 10.1038/ki.2011.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Sze L, Bernays RL, Zwimpfer C, Wiesli P, Brandle M, Schmid C. Excessively high soluble Klotho in patients with acromegaly. J Intern Med. 2012;272:93–7. doi: 10.1111/j.1365-2796.2012.02542.x. [DOI] [PubMed] [Google Scholar]
- 187.Takahasi Y, Kuro-O M, Ishikawa F. Aging mechanisms. Proc Natl Acad Sci U S A. 2000;97:12407–08. doi: 10.1073/pnas.210382097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Tan H, Yi L, Rote NS, Hurd WW, Mesiano S. Progesterone receptor-A and -B have opposite effects on proinflammatory gene expression in human myometrial cells: implications for progesterone actions in human pregnancy and parturition. J Clin Endocrinol Metab. 2012;97:E719–30. doi: 10.1210/jc.2011-3251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.To MS, Skentou C, Liao AW, Cacho A, Nicolaides KH. Cervical length and funneling at 23 weeks of gestation in the prediction of spontaneous early preterm delivery. Ultrasound Obstet Gynecol. 2001;18:200–3. doi: 10.1046/j.1469-0705.2001.00437.x. [DOI] [PubMed] [Google Scholar]
- 190.Tsujikawa H, Kurotaki Y, Fujimori T, Fukuda K, Nabeshima Y. Klotho, a gene related to a syndrome resembling human premature aging, functions in a negative regulatory circuit of vitamin D endocrine system. Mol Endocrinol. 2003;17:2393–403. doi: 10.1210/me.2003-0048. [DOI] [PubMed] [Google Scholar]
- 191.Turan K, Ata P. Effects of intra- and extracellular factors on anti-aging klotho gene expression. Genet Mol Res. 2011;10:2009–23. doi: 10.4238/vol10-3gmr1261. [DOI] [PubMed] [Google Scholar]
- 192.Utsugi T, Ohno T, Ohyama Y, Uchiyama T, Saito Y, Matsumura Y, et al. Decreased insulin production and increased insulin sensitivity in the klotho mutant mouse, a novel animal model for human aging. Metabolism. 2000;49:1118–23. doi: 10.1053/meta.2000.8606. [DOI] [PubMed] [Google Scholar]
- 193.Vaisbuch E, Romero R, Erez O, Kusanovic JP, Mazaki-Tovi S, Gotsch F, et al. Clinical significance of early (< 20 weeks) vs. late (20-24 weeks) detection of sonographic short cervix in asymptomatic women in the midtrimester. Ultrasound Obstet Gynecol. 2010;36:471–81. doi: 10.1002/uog.7673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Vaisbuch E, Romero R, Mazaki-Tovi S, Erez O, Kusanovic JP, Mittal P, et al. The risk of impending preterm delivery in asymptomatic patients with a nonmeasurable cervical length in the second trimester. Am J Obstet Gynecol. 2010;203:446, e1–9. doi: 10.1016/j.ajog.2010.05.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Wagner GP, Tong Y, Emera D, Romero R. An evolutionary test of the isoform switching hypothesis of functional progesterone withdrawal for parturition: humans have a weaker repressive effect of PR-A than mice. J Perinat Med. 2012;40:345–51. doi: 10.1515/jpm-2011-0256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Wang Y, Kuro OM, Sun Z. Klotho gene delivery suppresses Nox2 expression and attenuates oxidative stress in rat aortic smooth muscle cells via the cAMP-PKA pathway. Aging Cell. 2012 doi: 10.1111/j.1474-9726.2012.00796.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Wang Y, Sun Z. Klotho gene delivery prevents the progression of spontaneous hypertension and renal damage. Hypertension. 2009;54:810–7. doi: 10.1161/HYPERTENSIONAHA.109.134320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Watts DH, Krohn MA, Hillier SL, Wener MH, Kiviat NB, Eschenbach DA. Characteristics of women in preterm labor associated with elevated C-reactive protein levels. Obstet Gynecol. 1993;82:509–14. [PubMed] [Google Scholar]
- 199.Wolf I, Levanon-Cohen S, Bose S, Ligumsky H, Sredni B, Kanety H, et al. Klotho: a tumor suppressor and a modulator of the IGF-1 and FGF pathways in human breast cancer. Oncogene. 2008;27:7094–105. doi: 10.1038/onc.2008.292. [DOI] [PubMed] [Google Scholar]
- 200.Xu Y, Tarquini F, Romero R, Kim CJ, Tarca AL, Bhatti G, et al. Peripheral CD300a+CD8+ T lymphocytes with a distinct cytotoxic molecular signature increase in pregnant women with chronic chorioamnionitis. Am J Reprod Immunol. 2012;67:184–97. doi: 10.1111/j.1600-0897.2011.01088.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Yamamoto M, Clark JD, Pastor JV, Gurnani P, Nandi A, Kurosu H, et al. Regulation of oxidative stress by the anti-aging hormone klotho. J Biol Chem. 2005;280:38029–34. doi: 10.1074/jbc.M509039200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Yoon BH, Chang JW, Romero R. Isolation of Ureaplasma urealyticum from the amniotic cavity and adverse outcome in preterm labor. Obstet Gynecol. 1998;92:77–82. doi: 10.1016/s0029-7844(98)00122-7. [DOI] [PubMed] [Google Scholar]
- 203.Yoon BH, Jun JK, Romero R, Park KH, Gomez R, Choi JH, et al. Amniotic fluid inflammatory cytokines (interleukin-6., interleukin-1beta, and tumor necrosis factor-alpha), neonatal brain white matter lesions, and cerebral palsy. Am J Obstet Gynecol. 1997;177:19–26. doi: 10.1016/s0002-9378(97)70432-0. [DOI] [PubMed] [Google Scholar]
- 204.Yoon BH, Oh SY, Romero R, Shim SS, Han SY, Park JS, et al. An elevated amniotic fluid matrix metalloproteinase-8 level at the time of mid-trimester genetic amniocentesis is a risk factor for spontaneous preterm delivery. Am J Obstet Gynecol. 2001;185:1162–7. doi: 10.1067/mob.2001.117678. [DOI] [PubMed] [Google Scholar]
- 205.Yoon BH, Park CW, Chaiworapongsa T. Intrauterine infection and the development of cerebral palsy. BJOG. 2003;110(Suppl 20):124–7. doi: 10.1016/s1470-0328(03)00063-6. [DOI] [PubMed] [Google Scholar]
- 206.Yoon BH, Romero R, Jun JK, Park KH, Park JD, Ghezzi F, et al. Amniotic fluid cytokines (interleukin-6, tumor necrosis factor-alpha, interleukin-1 beta, and interleukin-8) and the risk for the development of bronchopulmonary dysplasia. Am J Obstet Gynecol. 1997;177:825–30. doi: 10.1016/s0002-9378(97)70276-x. [DOI] [PubMed] [Google Scholar]
- 207.Yoon BH, Romero R, Moon JB, Shim SS, Kim M, Kim G, et al. Clinical significance of intra-amniotic inflammation in patients with preterm labor and intact membranes. Am J Obstet Gynecol. 2001;185:1130–6. doi: 10.1067/mob.2001.117680. [DOI] [PubMed] [Google Scholar]
- 208.Yoon BH, Romero R, Park JS, Chang JW, Kim YA, Kim JC, et al. Microbial invasion of the amniotic cavity with Ureaplasma urealyticum is associated with a robust host response in fetal, amniotic, and maternal compartments. Am J Obstet Gynecol. 1998;179:1254–60. doi: 10.1016/s0002-9378(98)70142-5. [DOI] [PubMed] [Google Scholar]
- 209.Yoon BH, Romero R, Park JS, Kim CJ, Kim SH, Choi JH, et al. Fetal exposure to an intra-amniotic inflammation and the development of cerebral palsy at the age of three years. Am J Obstet Gynecol. 2000;182:675–81. doi: 10.1067/mob.2000.104207. [DOI] [PubMed] [Google Scholar]
- 210.Yoon BH, Romero R, Yang SH, Jun JK, Kim IO, Choi JH, et al. Interleukin-6 concentrations in umbilical cord plasma are elevated in neonates with white matter lesions associated with periventricular leukomalacia. Am J Obstet Gynecol. 1996;174:1433–40. doi: 10.1016/s0002-9378(96)70585-9. [DOI] [PubMed] [Google Scholar]
- 211.Zakar T, Hertelendy F. Progesterone withdrawal: key to parturition. Am J Obstet Gynecol. 2007;196:289–96. doi: 10.1016/j.ajog.2006.09.005. [DOI] [PubMed] [Google Scholar]
- 212.Zhang H, Li Y, Fan Y, Wu J, Zhao B, Guan Y, et al. Klotho is a target gene of PPAR-gamma. Kidney Int. 2008;74:732–9. doi: 10.1038/ki.2008.244. [DOI] [PubMed] [Google Scholar]
- 213.Zhang R, Zheng F. PPAR-gamma and aging: one link through klotho? Kidney Int. 2008;74:702–4. doi: 10.1038/ki.2008.382. [DOI] [PubMed] [Google Scholar]
- 214.Zuo Z, Lei H, Wang X, Wang Y, Sonntag W, Sun Z. Aging-related kidney damage is associated with a decrease in klotho expression and an increase in superoxide production. Age (Dordr) 2011;33:261–74. doi: 10.1007/s11357-010-9176-2. [DOI] [PMC free article] [PubMed] [Google Scholar]