Abstract
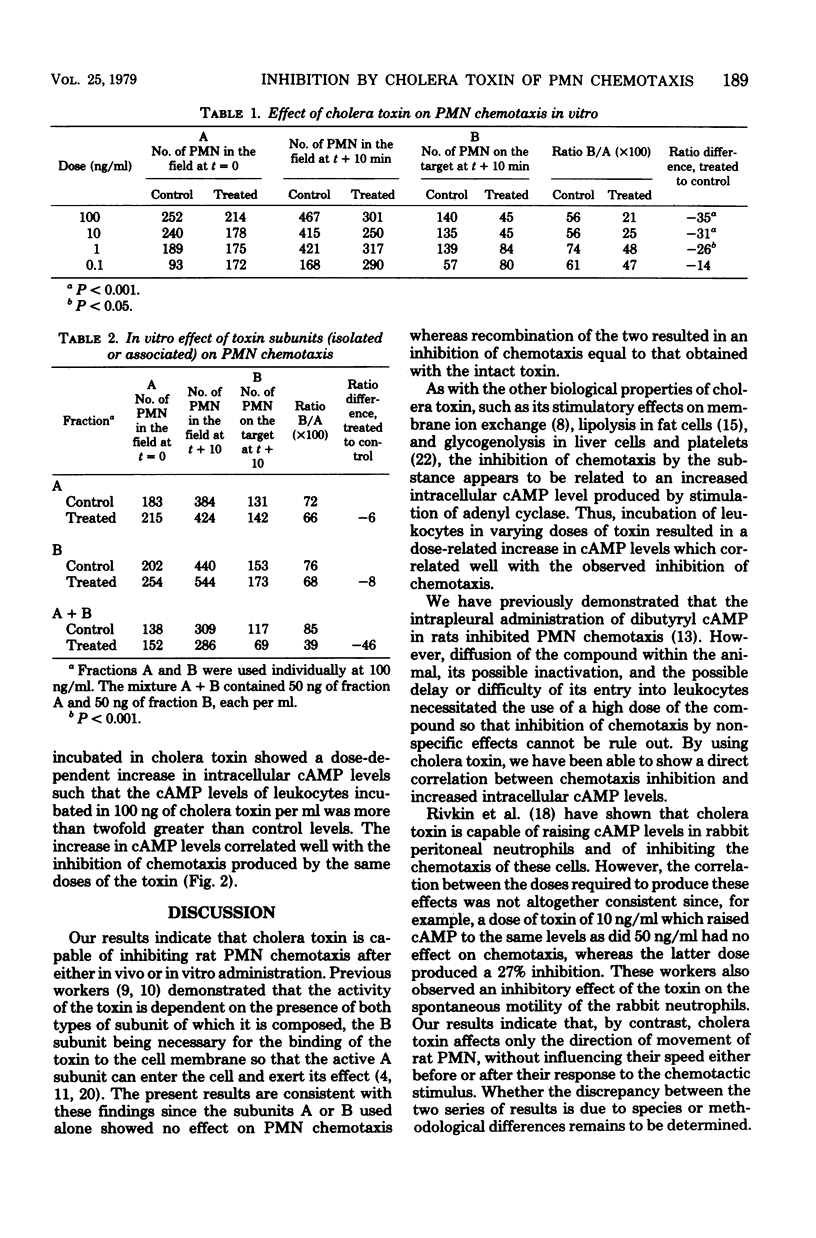
The effect of cholera toxin on the chemotaxis of rat polymorphonuclear leukocytes (PMN) was studied using a technique in which the movement of the cells towards a laser-lysed erythrocyte is followed under a phase-contrast microscrope. In vitro studies indicated that the intact toxin was capable of inhibiting PMN chemotaxis in a dose-dependent manner at doses ranging from 1 to 100 ng/ml. Subunits A and B of the toxin were without inhibitory activity when used alone, but after recombination their ability to inhibit chemotaxis was similar to that of the intact toxin, suggesting that the toxin is acting intracellularly. Cholera toxin has been reported to act in other systems via stimulation of adenyl cyclase with consequent elevation of intracellular cyclic adenosine 5'-monophosphate (cAMP) levels. It appears that this mechanism may also account for its ability to inhibit chemotaxis since there was a correlation, at all doses tested, between inhibition of chemotaxis and increased intracellular cAMP levels. Cholera toxin was also found to be active in vivo in that, after intrapleural injection of the toxin, the chemotaxis of cells subsequently recovered from the pleural cavity was markedly reduced. These results support previous findings which suggest that modification of leukocyte cAMP levels can influence the chemotactic responsiveness of these cells.
Full text
PDF

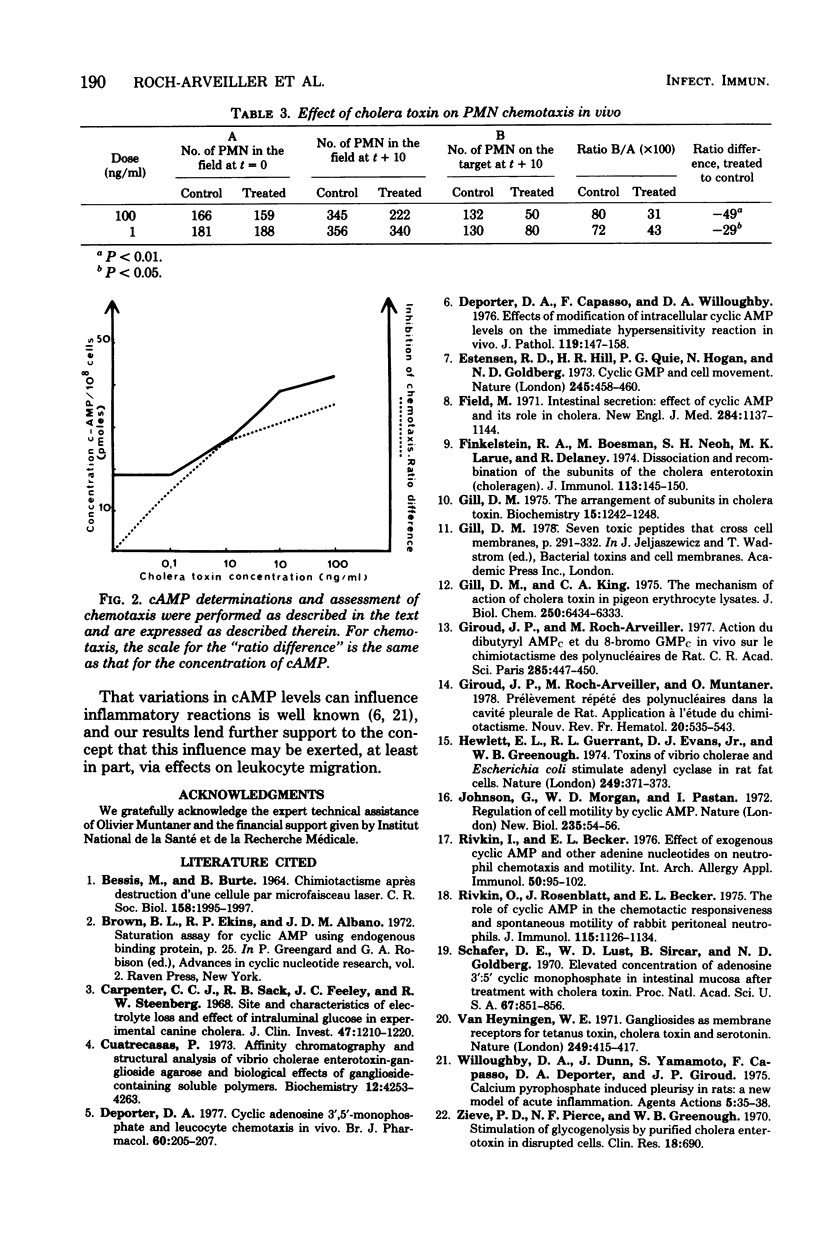
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BESSIS M., BURTE B. CHIMIOTACTISME APR'ES DESTRUCTION D'UNE CELLULE PAR MICROFAISCEAUX LASER. C R Seances Soc Biol Fil. 1964;158:1995–1997. [PubMed] [Google Scholar]
- Carpenter C. C., Sack R. B., Feeley J. C., Steenberg R. W. Site and characteristics of electrolyte loss and effect of intraluminal glucose in experimental canine cholera. J Clin Invest. 1968 May;47(5):1210–1220. doi: 10.1172/JCI105810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuatrecasas P., Parikh I., Hollenberg M. D. Affinity chromatography and structural analysis of Vibrio cholerae enterotoxin-ganglioside agarose and the biological effects of ganglioside-containing soluble polymers. Biochemistry. 1973 Oct 9;12(21):4253–4264. doi: 10.1021/bi00745a033. [DOI] [PubMed] [Google Scholar]
- Deporter D. A., Capasso F., Willoughby D. A. Effects of modification of intracellularcyclic AMP levels on the imediate hypersensitivity reaction in vivo. J Pathol. 1976 Jul;119(3):147–158. doi: 10.1002/path.1711190304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deporter D. A. Cyclic adenosine 3',5'-monophosphate and leucocyte chemotaxis in vivo. Br J Pharmacol. 1977 Jun;60(2):205–207. doi: 10.1111/j.1476-5381.1977.tb07742.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estensen R. D., Hill H. R., Quie P. G., Gogan N., Goldberg N. D. Cyclic GMP and cell movement. Nature. 1973 Oct 26;245(5426):458–460. doi: 10.1038/245458a0. [DOI] [PubMed] [Google Scholar]
- Field M: Intestinal secretion: effect of cyclic AMP and its role in cholera. N Engl J Med. 1971 May 20;284(20):1137–1144. doi: 10.1056/NEJM197105202842008. [DOI] [PubMed] [Google Scholar]
- Finkelstein R. A., Boesman M., Neoh S. H., LaRue M. K., Delaney R. Dissociation and recombination of the subunits of the cholera enterotoxin (choleragen). J Immunol. 1974 Jul;113(1):145–150. [PubMed] [Google Scholar]
- Gill D. M. The arrangement of subunits in cholera toxin. Biochemistry. 1976 Mar 23;15(6):1242–1248. doi: 10.1021/bi00651a011. [DOI] [PubMed] [Google Scholar]
- Giroud J. P., Roch-Arveiller M. Action du dibutyryl AMPC et du 8-bromo GMPC in vivo sur le chimiotactisme des polynucléaires de rat. C R Acad Sci Hebd Seances Acad Sci D. 1977 Sep 12;285(4):447–450. [PubMed] [Google Scholar]
- Giroud J. P., Roch-Arveiller M., Muntaner O. Prélèvement répété des polynucléaires dans la cavité pleurale du rat application a l'étude du chimiotactisme. Nouv Rev Fr Hematol. 1979 Jan 30;20(4):535–543. [PubMed] [Google Scholar]
- Hewlett E. L., Guerrant R. L., Evans D. J., Jr, Greenough W. B., 3rd Toxins of Vibrio cholerae and Escherichia coli stimulate adenyl cyclase in rat fat cells. Nature. 1974 May 24;249(455):371–373. doi: 10.1038/249371a0. [DOI] [PubMed] [Google Scholar]
- Johnson G. S., Morgan W. D., Pastan I. Regulation of cell motility by cyclic AMP. Nature. 1972 Jan 7;235(5332):54–56. doi: 10.1038/235054a0. [DOI] [PubMed] [Google Scholar]
- Rivkin I., Becker E. L. Effect of exogenous cyclic AMP and other adenine nucleotides on neutrophil chemotaxis and motility. Int Arch Allergy Appl Immunol. 1976;50(1):95–102. doi: 10.1159/000231485. [DOI] [PubMed] [Google Scholar]
- Rivkin I., Rosenblatt J., Becker E. L. The role of cyclic AMP in the chemotactic responsiveness and spontaneous motility of rabbit peritoneal neutrophils. The inhibition of neutrophil movement and the elevation of cyclic AMP levels by catecholamines, prostaglandins, theophylline and cholera toxin. J Immunol. 1975 Oct;115(4):1126–1134. [PubMed] [Google Scholar]
- Schafer D. E., Lust W. D., Sircar B., Goldberg N. D. Elevated concentration of adenosine 3':5'-cyclic monophosphate in intestinal mucosa after treatment with cholera toxin. Proc Natl Acad Sci U S A. 1970 Oct;67(2):851–856. doi: 10.1073/pnas.67.2.851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willoughby D. A., Dunn C. J., Yamamoto S., Capasso F., Deporter D. A., Giroud J. P. Calcium pyrophosphate-induced pleurisy in rats: a new model of acute inflammation. Agents Actions. 1975 Feb;5(1):35–38. doi: 10.1007/BF02027156. [DOI] [PubMed] [Google Scholar]