Abstract
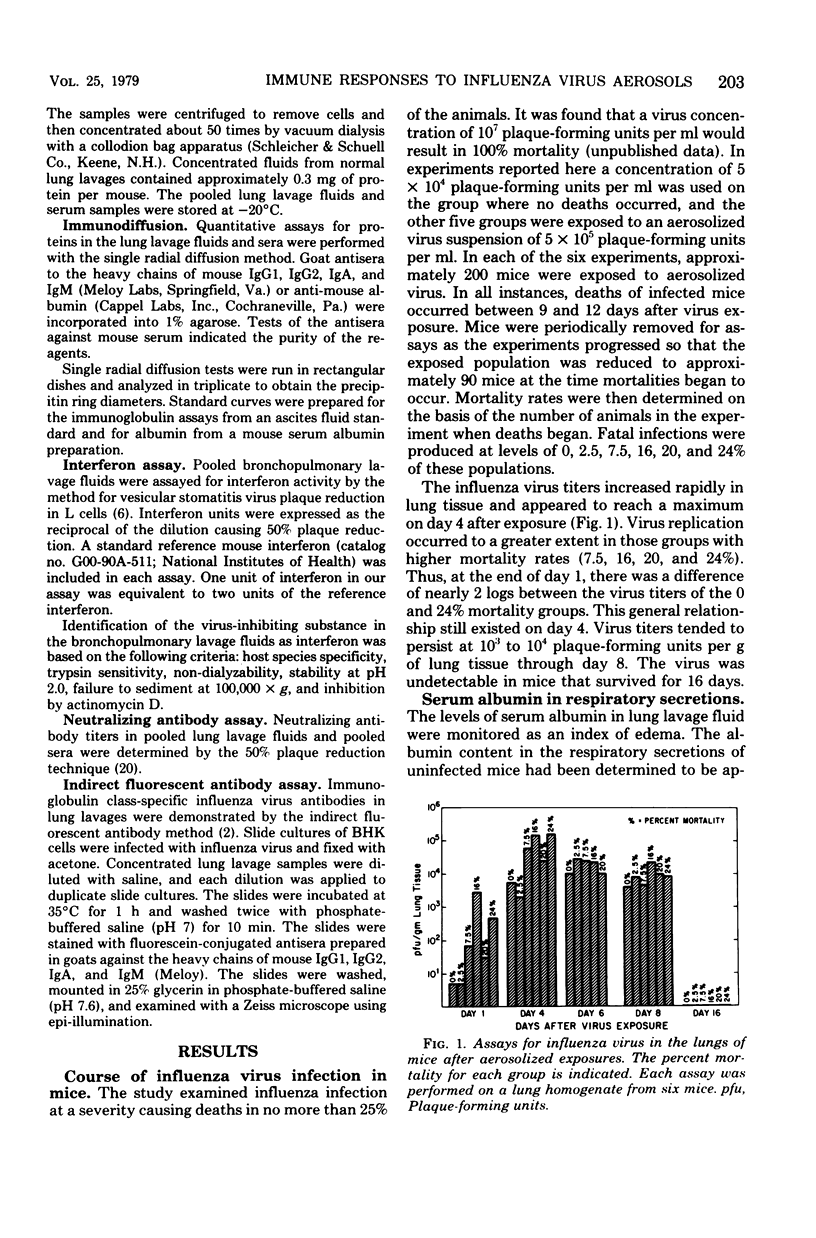
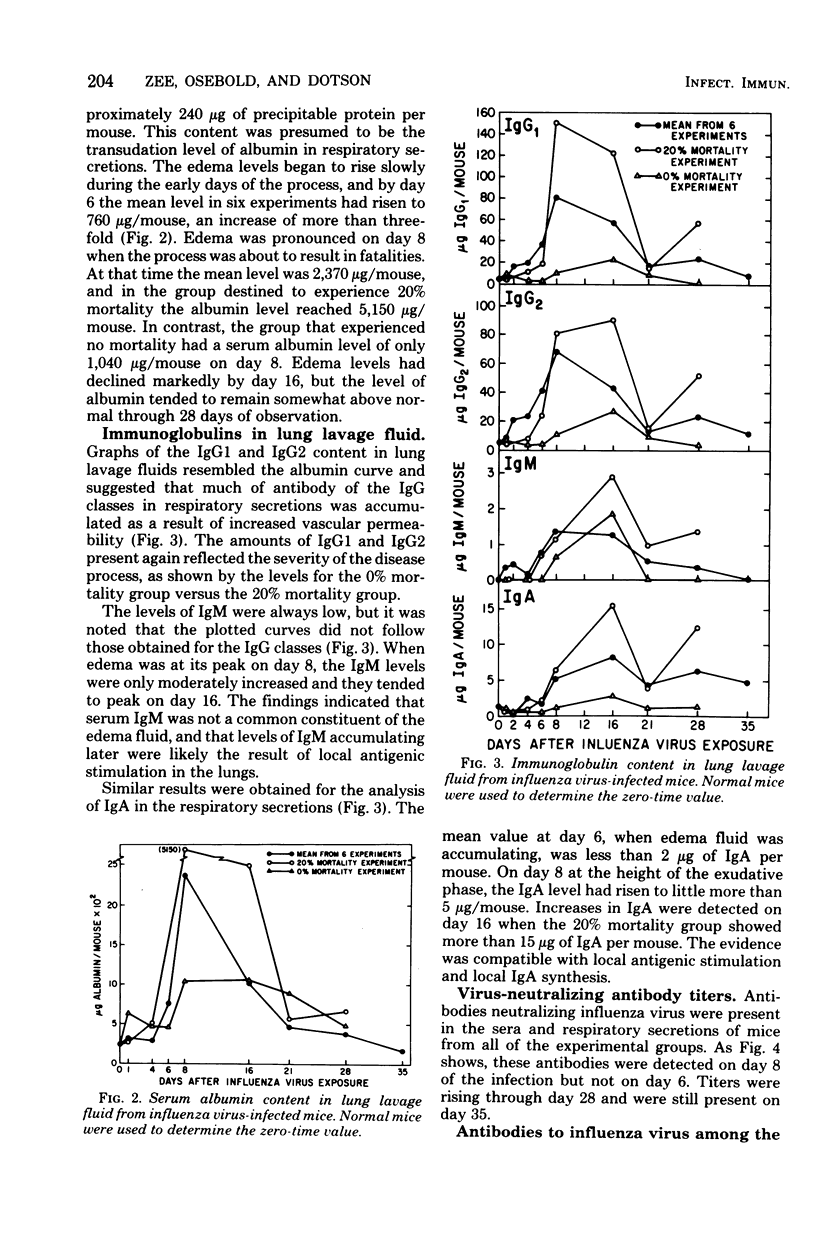
We studied the temporal appearance of immunoglobulins (immunoglobulins G1, G2, M, and A) and interferon in lung lavage fluids of mice after aerosol exposure to influenza virus in six animal groups in which mortality rates ranged from 0 to 24%. Immunoglobulin levels in the lung lavage fluids were markedly higher in mouse groups with higher mortality rates (16, 20, and 24%) than in those with low mortality rates (0, 2.5, and 7.5%). Analysis of serum albumin in the respiratory secretions as an index of edema indicated that increased immunoglobulin G levels during the early phase of infection were due to increased vascular permeability. The detection of virus-neutralizing antibodies and antibodies reactive with influenza virus antigens in the lavage fluids at 6 to 8 days postinfection suggested local immunoglobulin synthesis as a result of antigenic stimulation. Both systemic and local antibody productions contributed to immunoglobulin levels in the respiratory secretions after aerosolized influenza virus infection. Peak levels of interferon in the lavage fluids were reached before detection of significant levels of virus-neutralizing antibody in the serum or the lung lavage.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ANDERSON T. O., RIFF L. J., JACKSON G. G. Immunoelectrophoresis of nasal secretions collected during a common cold: observations which suggest a mechanism of seroimmunity in viral respiratory infections. J Immunol. 1962 Nov;89:691–697. [PubMed] [Google Scholar]
- Baublis J. V., Brown G. C. Specific response of the immunoglobulins to rubella infection. Proc Soc Exp Biol Med. 1968 May;128(1):206–210. doi: 10.3181/00379727-128-32979. [DOI] [PubMed] [Google Scholar]
- Butler W. T., Waldmann T. A., Rossen R. D., Douglas R. G., Jr, Couch R. B. Changes in IgA and IgG concentrations in nasal secretions prior to the appearance of antibody during viral respiratory infection in man. J Immunol. 1970 Sep;105(3):584–591. [PubMed] [Google Scholar]
- Cate T. R., Douglas R. G., Jr, Couch R. B. Interferon and resistance to upper respiratory virus illness. Proc Soc Exp Biol Med. 1969 Jun;131(2):631–636. doi: 10.3181/00379727-131-33941. [DOI] [PubMed] [Google Scholar]
- Choppin P. W. Replication of influenza virus in a continuous cell line: high yield of infective virus from cells inoculated at high multiplicity. Virology. 1969 Sep;39(1):130–134. doi: 10.1016/0042-6822(69)90354-7. [DOI] [PubMed] [Google Scholar]
- ISAACS A., HITCHCOCK G. Role of interferon in recovery from virus infections. Lancet. 1960 Jul 9;2(7141):69–71. doi: 10.1016/s0140-6736(60)91215-0. [DOI] [PubMed] [Google Scholar]
- Ibrahim A. L., Zee Y. C., Osebold J. W. The effects of ozone on the respiratory epithelium and alveolar macrophages of mice. I. Interferon production. Proc Soc Exp Biol Med. 1976 Sep;152(4):483–488. doi: 10.3181/00379727-152-39423. [DOI] [PubMed] [Google Scholar]
- Iwasaki T., Nozima T. Defense mechanisms against primary influenza virus infection in mice. I. The roles of interferon and neutralizing antibodies and thymus dependence of interferon and antibody production. J Immunol. 1977 Jan;118(1):256–263. [PubMed] [Google Scholar]
- Kasel J. A., Fulk R. V., Togo Y., Hornick R. B., Heiner G. G., Dawkins A. T., Jr, Mann J. J. Influenza antibody in human respiratory secretions after subcutaneous or respiratory immunization with inactivated virus. Nature. 1968 May 11;218(5141):594–595. doi: 10.1038/218594a0. [DOI] [PubMed] [Google Scholar]
- Link F., Blaskovic D., Raus J. Virus multiplication and interferon formation in lungs from mice infected with A2 influenza virus. Acta Virol. 1965 Jul;9(4):380–380. [PubMed] [Google Scholar]
- Medin N. I., Osebold J. W., Zee Y. C. A procedure for pulmonary lavage in mice. Am J Vet Res. 1976 Feb;37(2):237–238. [PubMed] [Google Scholar]
- OKUNO Y. FURTHER OBSERVATIONS ON IMMUNIZATION WITH LIVE ATTENUATED MEASLES VIRUS VACCINE BY INHALATION. Arch Gesamte Virusforsch. 1965;16:294–299. doi: 10.1007/BF01253825. [DOI] [PubMed] [Google Scholar]
- Osebold J. W., Medin N. I., Zee Y. C. Immunochemical analysis of serum-related proteins in the respiratory tract secretions of normal mice. Infect Immun. 1975 Nov;12(5):1141–1146. doi: 10.1128/iai.12.5.1141-1146.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkins J. C., Tucker D. N., Knope H. L., Wenzel R. P., Hornick R. B., Kapikian A. Z., Chanock R. M. Evidence for protective effect of an inactivated rhinovirus vaccine administered by the nasal route. Am J Epidemiol. 1969 Oct;90(4):319–326. doi: 10.1093/oxfordjournals.aje.a121076. [DOI] [PubMed] [Google Scholar]
- Reynolds H. Y., Newball H. H. Analysis of proteins and respiratory cells obtained from human lungs by bronchial lavage. J Lab Clin Med. 1974 Oct;84(4):559–573. [PubMed] [Google Scholar]
- Rossen R. D., Butler W. T., Cate T. R., Szwed C. F., Couch R. B. Protein composition of nasal secretion during respiratory virus infection. Proc Soc Exp Biol Med. 1965 Aug-Sep;119(4):1169–1176. doi: 10.3181/00379727-119-30406. [DOI] [PubMed] [Google Scholar]
- Scott G. H., Sydiskis R. J. Responses of mice immunized with influenza virus by serosol and parenteral routes. Infect Immun. 1976 Mar;13(3):696–703. doi: 10.1128/iai.13.3.696-703.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shifrine M., Smith J. B., Bulgin M. S., Bryant B. J., Zee Y. C., Osburn B. I. Response of canine fetuses and neonates to antigenic stimulation. J Immunol. 1971 Oct;107(4):965–970. [PubMed] [Google Scholar]
- Waldman R. H., Jurgensen P. F., Olsen G. N., Ganguly R., Johnson J. E., 3rd Immune response of the human respiratory tract. I. Immunoglobulin levels and influenza virus vaccine antibody response. J Immunol. 1973 Jul;111(1):38–41. [PubMed] [Google Scholar]
- Waldman R. H., Mann J. J., Small P. A., Jr Immunization against influenza. Prevention of illness in man by aerosolized inactivated vaccine. JAMA. 1969 Jan 20;207(3):520–524. doi: 10.1001/jama.207.3.520. [DOI] [PubMed] [Google Scholar]


