Abstract
BACKGROUND
Atherosclerosis is accepted as an inflammatory disease. Evidence suggests that inflammation evoked by injury plays a pathogenic role in all stages of atherosclerosis. This study aimed to investigate whether the high-mobility group box-1 (HMGB1) a proinflammatory cytokine/nuclear protein, which is derived from both injured endothelium and activated macrophages/monocytes, could contribute to the progression of atherosclerosis and other cardiovascular diseases.
METHODS
This study was designed as case-control. A total of 135 patients who referred to the hospital due to angina pectoris had the diagnosis of unstable angina and were candidates of angiography were recruited in this study. Forty patients who had coronary artery disease confirmed by angiography were considered as case group and control group consists of 40 persons who had no plaque, and 55 persons were excluded according to the exclusion criteria. At first, a questionnaire was filled for each patient including demographic factors and their medical history. Then a blood sample was taken to assess the level of HMGB1. Data were analyzed using SPSS, Student’s independent t-test, and chi-square tests.
RESULTS
The mean plasma level of HMGB1 in the case group was 27.1 ± 2.9 ng/ml, while it was 19.6 ± 1.9 ng/ml in control groups (P = 0.03). The odds ratio for coronary artery plaque associated with high (> 15.03 ng/ml) levels of HMGB1 was 2.50 (95% confidence interval, 1.02-6.17, P = 0.03).
CONCLUSION
Increased plasma HMGB1 concentration may be associated with an increased risk of coronary atherosclerosis.
Keywords: High-Mobility Group Box-1, Coronary Artery Diseases, Inflammation, Biomarkers
Introduction
The cardiovascular disease has long been an issue that causes severe loss in population, especially those conditions associated with arterial malfunction, being attributable to atherosclerosis, and subsequent thrombotic formation.1
Atherosclerosis is an inflammatory condition that affects the arterial wall and is characterized by progressive thickening due to the accumulation of lipids.2
Our understanding of the role of inflammation in the initiation and progression of atherosclerosis has evolved considerably in recent years. This has led to advances in both diagnostic and prognostic approaches, as well as a novel treatment modalities targeting inflammatory and immune mediators.3
The high-mobility group box-1 (HMGB1) protein, also known as amphoterin, expressed in almost all eukaryotic cells and recently identified as a potent proinflammatory mediator when present extracellularly.4,5
HMGB1 has been suggested to be involved in the pathogenesis of several vascular diseases such as systemic vasculitis and atherosclerosis.6
HMGB1 is expressed in endothelial cells, smooth muscle cells and microvessels of the adventitia.7 Previous studies have shown that in human atherosclerotic lesions from the aorta, carotid and coronary arteries the expression of HMGB1 is noticeably increased in the nuclei and in the cytoplasm of macrophages and smooth muscle cells localized near the intima.7,8 Intense HMGB1 expression has also been observed in areas adjacent to the necrotic core of atherosclerotic lesions and release of HMGB1 from necrotic cells, inducing local inflammation.7,9
HMGB1 may be released from several cell types in the atherosclerotic plaque including smooth muscle cells, endothelial cells, foam cells, macrophages, and activated platelets.10,11
HMGB1 promotes smooth muscle cells proliferation, migration to the intimal layer, their release of C-reactive protein as well as matrix metalloproteinases (MMPs) (MMP2, MMP3, and MMP9) in atherosclerotic plaques.8,10,11
In this study, by using HMGB1 measurements and angiographic investigation of coronary plaques in patients with unstable angina, we can consider the correlation of inflammation and plaque and then we can relate HMGB1 as an inflammatory marker with high-risk unstable patients that need more intensive medical or interventional follow-up.
Materials and Methods
This study was approved by Ethics Committee in Isfahan University of Medical Science (288294). In this case-control study, we recruit 135 consecutive patients fewer than 55 years old that referred to Chamran Hospital (Tehran, Iran) due to unstable angina and underwent coronary artery angiography in 2010. Unstable angina is defined as angina at rest with an accelerating pattern (with more frequency, higher intensity or longer duration), rest angina or an angina, which has newly developed.12
Patients with a history of myocardial infarction, surgery and/or percutaneous transluminal coronary angioplasty, valvular disease, anemia, fever, thyroid abnormalities, and renal failure, inflammatory and rheumatologic diseases or patients taking related medications including corticosteroid were excluded from the study. Letters of consent were taken from patients regarding all steps of study.
A questionnaire including information about demographic factors, history of above-mentioned diseases, drug consumption, and smoking was filled for those patients who met the inclusion criteria.
Finally, 40 patients who had coronary artery disease (CAD) confirmed by angiography were considered as case group and 40 patients with chest pain without any coronary complications in their angiography were selected as a control group.
History of diabetes was defined as history of twice fasting blood sugar of higher than 126 mg/dl or once plus a clinical sign hyperlipidemia was determined as low-density lipoprotein higher than 160 mg/dl or triglyceride higher than 150 (mg/dl) and hypertension was blood pressure higher than 140/90 mm Hg.
On the day of angiography, blood samples were collected after 10-12 h fasting. The serums were separated and were stored in −80° C in the freezer and angiography was done with Judkins standard.
HMGB1 level was measured simultaneously in all samples in Applied Physiology Research Center. The HMGB1 enzyme-linked immunosorbent assay (ELISA) kit was from immuno biological laboratory (IBL) International (Hamburg, Germany-Cat. Number: ST51011).13
HMGB1 ELISA is a Sandwich-enzyme immunoassay for the quantitative determination of HMGB1 in serum and plasma. The microtiter strips were coated with purified anti-HMGB1 antibody. HMGB1 in the sample binds specifically to the immobilized antibody and is recognized by a second enzyme marked antibody. After substrate reaction, the HMGB1 concentration was determined by the color intensity in 450 nm wavelength. HMGB1 concentration is obtained by optical density and standard curve.
In the last step, all patients underwent coronary artery angiography according to Judkins standard protocol. The degree of stenosis in each epicardial coronary arteries was evaluated by the cardiologist who was blind about laboratory test results of the patients. Atherothrombotic plaques were classified into simple and complex according to Ambrose et al. criteria.14
In order to determine the appropriate cut-off point of HMGB1 levels for identifying the risk of coronary plaque, the sensitivity, specificity, and likelihood ratio were calculated at different levels of HMGB1 using receiver operating characteristics (ROC) curve.
Data were analyzed using SPSS for Windows (version 18, SPSS Inc., Chicago, IL, USA) using Student’s independent t-test, and chi-square. The odds ratio (OR) was calculated from multivariate logistic regression.
Results
The demographic characteristics of patients have been illustrated in table 1. There was no significant difference in age, sex, body mass index and history of hypertension, hyperlipidemia diabetes, and smoking between groups (P < 0.05).
Table 1.
Basic characteristics of the study population
| Characteristics | Case | Control |
|---|---|---|
| Age (year) | 49.6 ± 6.6 | 48.4 ± 6.5 |
| Body mass index (kg/m2) | 27.0 ± 6.7 | 25.6 ± 5.1 |
| Male (%) | 62.5 | 46.2 |
| Hypertension (%) | 30.3 | 29.5 |
| Hyperlipidemia (%) | 50.7 | 44.1 |
| Diabetes (%) | 22.8 | 16.6 |
| Smoking (%) | 15.9 | 13.6 |
Values are expressed as mean ± SD or number (%); There was no significant difference between case and control groups (P < 0.05); SD: Standard deviation
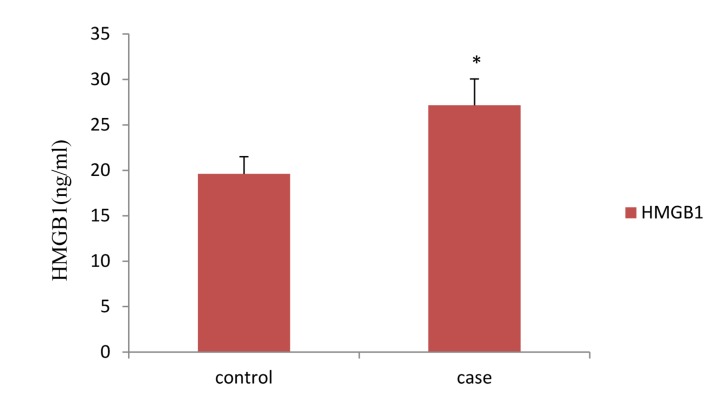
The HMGB1 levels was 19.6 ± 1.9 ng/ml in controls and 27.1 ± 2.9 ng/ml in cases, there is a significant difference between groups (P = 0.002) (Figure 1).
Figure 1.
Serum levels of high-mobility group box-1 protein in case and control group * Significantly different from control group (P = 0.002) HMGB1: High-mobility group box-1
The sensitivity, specificity, positive and negative predictive value of different levels of HMGB1 using ROC curve were 83.3%, 72.2%, 75.0%, and 71.3%, respectively.
The ROC curve analysis, applied to HMGB1 level, showed the best diagnostic profile with an area under the curve of 0.81 and 0.89, respectively. The best cut-off points were 15.03 ng/ml. The sensitivity, specificity, and accuracy were 88.9%, 83.3%, and 86.1%, respectively.
Crude odd ratio (ORs) for significant coronary artery plaque was calculated for HMGB1 above the 50th percentile. Estimated risk of association between significant coronary artery plaque associated with high (> 15.03 ng/ml) levels of HMGB1 was 2.50 (95% confidence interval, 1.02-6.17, P = 0.03).
Discussion
HMGB1 has been implicated in the pathogenesis of inflammatory vascular diseases including systemic vasculitis and atherosclerotic disease. Furthermore, HMGB1 is expressed in atherosclerotic lesions by several cell types contributing to the development of the atherosclerotic plaque. HMGB1 levels are significantly increased in patients with subclinical CAD and in those who develop acute ischemic events in cardiac and cerebral vascular beds. Experimental studies showed that HMGB1 has a significant effect, potentiating the inflammatory response as well as damage in the acute phase and participating in tissue remodeling during the late phase after ischemic injury. Targeting HMGB1 may be an attractive therapeutic modality for inflammatory vascular diseases.15
In our study, patients with CAD had upper plasma HMGB1 levels than control groups, and there were significant difference in HMGB1 levels between two groups.
Previous studies have been shown that HMGB1 stimulate vascular endothelial cells to express and secret cell adhesion molecules, monocyte chemotatic protein 1, and other inflammatory cytokines.16,17
In the other hand, study on apolipoprotein E-deficient mice that fed with a high-fat diet has been shown that HMGB1 has an importance role in the development of atherosclerosis.
Using antibodies against HMGB1 result in reduced inflammation and plaque progression in apolipoprotein E-deficient mice.18
Yang et al.19 and Haraba et al.20 showed that hyperlipidemia stimulates the extracellular release of the HMGB1 protein in hyperlipidemic hamsters. Furthermore, it has been shown that statins attenuate HMGB1-induced vascular endothelial activation.
In contrast, a beneficial effect of HMGB1 has been demonstrated after ischemic limb injury in diabetic and non-diabetic mice. HMGB1 expression was lower in ischemic limbs of diabetic mice, and this lower expression was associated with a diminished perfusion recovery after injury. Administration of HMGB1 significantly improved blood flow and capillary density in ischemic muscles of diabetic mice, and this beneficial effect was associated with an increased expression of vascular endothelial growth factor.21
Conclusion
The results of the present study show significantly higher levels of plasma HMGB1 in angiographically documented coronary plaque than healthy individuals. Increased plasma HMGB1 concentration may be associated with an increased risk of coronary atherosclerosis.
HMGB1 can reflect the coronary artery atherosclerosis in patients with unstable angina. Hence, it can be used as diagnostic factors, and as an independent factor for risk stratification in young patients with chest pain. However, future studies with larger sample size are needed in order to determine accurate cut-point value in CAD patients and healthy population.
Acknowledgments
We thank Isfahan University of Medical Sciences (IUMS) for funding this study vide Grant Number: 288294.
Footnotes
Conflicts of Interest
Authors have no conflict of interests.
REFERENCES
- 1.Wang W, Lee Y, Lee CH. Review: The physiological and computational approaches for atherosclerosis treatment. Int J Cardiol. 2013;167(5):1664–76. doi: 10.1016/j.ijcard.2012.09.195. [DOI] [PubMed] [Google Scholar]
- 2.Ross R. Atherosclerosis--an inflammatory disease. N Engl J Med. 1999;340(2):115–26. doi: 10.1056/NEJM199901143400207. [DOI] [PubMed] [Google Scholar]
- 3.Wong BW, Meredith A, Lin D, McManus BM. The biological role of inflammation in atherosclerosis. Can J Cardiol. 2012;28(6):631–41. doi: 10.1016/j.cjca.2012.06.023. [DOI] [PubMed] [Google Scholar]
- 4.Erlandsson HH, Andersson U. Mini-review: The nuclear protein HMGB1 as a proinflammatory mediator. Eur J Immunol. 2004;34(6):1503–12. doi: 10.1002/eji.200424916. [DOI] [PubMed] [Google Scholar]
- 5.Guo ZS, Liu Z, Bartlett DL, Tang D, Lotze MT. Life after death: targeting high mobility group box 1 in emergent cancer therapies. Am J Cancer Res. 2013;3(1):1–20. [PMC free article] [PubMed] [Google Scholar]
- 6.de Souza AW, Westra J, Limburg PC, Bijl M, Kallenberg CG. HMGB1 in vascular diseases: Its role in vascular inflammation and atherosclerosis. Autoimmun Rev. 2012;11(12):909–17. doi: 10.1016/j.autrev.2012.03.007. [DOI] [PubMed] [Google Scholar]
- 7.Kalinina N, Agrotis A, Antropova Y, DiVitto G, Kanellakis P, Kostolias G, et al. Increased expression of the DNA-binding cytokine HMGB1 in human atherosclerotic lesions: role of activated macrophages and cytokines. Arterioscler Thromb Vasc Biol. 2004;24(12):2320–5. doi: 10.1161/01.ATV.0000145573.36113.8a. [DOI] [PubMed] [Google Scholar]
- 8.Inoue K, Kawahara K, Biswas KK, Ando K, Mitsudo K, Nobuyoshi M, et al. HMGB1 expression by activated vascular smooth muscle cells in advanced human atherosclerosis plaques. Cardiovasc Pathol. 2007;16(3):136–43. doi: 10.1016/j.carpath.2006.11.006. [DOI] [PubMed] [Google Scholar]
- 9.Scaffidi P, Misteli T, Bianchi ME. Release of chromatin protein HMGB1 by necrotic cells triggers inflammation. Nature. 2002;418(6894):191–5. doi: 10.1038/nature00858. [DOI] [PubMed] [Google Scholar]
- 10.Porto A, Palumbo R, Pieroni M, Aprigliano G, Chiesa R, Sanvito F, et al. Smooth muscle cells in human atherosclerotic plaques secrete and proliferate in response to high mobility group box 1 protein. FASEB J. 2006;20(14):2565–6. doi: 10.1096/fj.06-5867fje. [DOI] [PubMed] [Google Scholar]
- 11.Degryse B, Bonaldi T, Scaffidi P, Muller S, Resnati M, Sanvito F, et al. The high mobility group (HMG) boxes of the nuclear protein HMG1 induce chemotaxis and cytoskeleton reorganization in rat smooth muscle cells. J Cell Biol. 2001;152(6):1197–206. doi: 10.1083/jcb.152.6.1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sadeghi M, Pourmoghaddas M, Tavasoli A, Roohafza H. Is there any relationship between C-reactive protein level and complex coronary plaques in patients with unstable angina? ARYA Atheroscler. 2010;6(1):31–4. [PMC free article] [PubMed] [Google Scholar]
- 13.Davis K, Banerjee S, Friggeri A, Bell C, Abraham E, Zerfaoui M. Poly (ADP-ribosyl) ation of high mobility group box 1 (HMGB1) protein enhances inhibition of efferocytosis. Mol Med. 2012;18:359–69. doi: 10.2119/molmed.2011.00203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ambrose JA, Tannenbaum MA, Alexopoulos D, Hjemdahl-Monsen CE, Leavy J, Weiss M, et al. Angiographic progression of coronary artery disease and the development of myocardial infarction. J Am Coll Cardiol. 1988;12(1):56–62. doi: 10.1016/0735-1097(88)90356-7. [DOI] [PubMed] [Google Scholar]
- 15.de Souza AW, Westra J, Limburg PC, Bijl M, Kallenberg CG. HMGB1 in vascular diseases: Its role in vascular inflammation and atherosclerosis. Autoimmun Rev. 2012;11(12):909–17. doi: 10.1016/j.autrev.2012.03.007. [DOI] [PubMed] [Google Scholar]
- 16.Fiuza C, Bustin M, Talwar S, Tropea M, Gerstenberger E, Shelhamer JH, et al. Inflammation-promoting activity of HMGB1 on human microvascular endothelial cells. Blood. 2003;101(7):2652–60. doi: 10.1182/blood-2002-05-1300. [DOI] [PubMed] [Google Scholar]
- 17.Treutiger CJ, Mullins GE, Johansson AS, Rouhiainen A, Rauvala HM, Erlandsson-Harris H, et al. High mobility group 1 B-box mediates activation of human endothelium. J Intern Med. 2003;254(4):375–85. doi: 10.1046/j.1365-2796.2003.01204.x. [DOI] [PubMed] [Google Scholar]
- 18.Kanellakis P, Agrotis A, Kyaw TS, Koulis C, Ahrens I, Mori S, et al. High-mobility group box protein 1 neutralization reduces development of diet-induced atherosclerosis in apolipoprotein e-deficient mice. Arterioscler Thromb Vasc Biol. 2011;31(2):313–9. doi: 10.1161/ATVBAHA.110.218669. [DOI] [PubMed] [Google Scholar]
- 19.Yang J, Huang C, Yang J, Jiang H, Ding J. Statins attenuate high mobility group box-1 protein induced vascular endothelial activation: a key role for TLR4/NF-kappaB signaling pathway. Mol Cell Biochem. 2010;345(1-2):189–95. doi: 10.1007/s11010-010-0572-9. [DOI] [PubMed] [Google Scholar]
- 20.Haraba R, Suica VI, Uyy E, Ivan L, Antohe F. Hyperlipidemia stimulates the extracellular release of the nuclear high mobility group box 1 protein. Cell Tissue Res. 2011;346(3):361–8. doi: 10.1007/s00441-011-1277-4. [DOI] [PubMed] [Google Scholar]
- 21.Biscetti F, Straface G, de Cristofaro R, Lancellotti S, Rizzo P, Arena V, et al. High-mobility group box-1 protein promotes angiogenesis after peripheral ischemia in diabetic mice through a VEGF-dependent mechanism. Diabetes. 2010;59(6):1496–505. doi: 10.2337/db09-1507. [DOI] [PMC free article] [PubMed] [Google Scholar]