Abstract
We evaluated 3T diffusion tensor imaging (DTI) for white matter injury in 76 adult mild traumatic brain injury (mTBI) patients at the semiacute stage (11.2±3.3 days), employing both whole-brain voxel-wise and region-of-interest (ROI) approaches. The subgroup of 32 patients with any traumatic intracranial lesion on either day-of-injury computed tomography (CT) or semiacute magnetic resonance imaging (MRI) demonstrated reduced fractional anisotropy (FA) in numerous white matter tracts, compared to 50 control subjects. In contrast, 44 CT/MRI-negative mTBI patients demonstrated no significant difference in any DTI parameter, compared to controls. To determine the clinical relevance of DTI, we evaluated correlations between 3- and 6-month outcome and imaging, demographic/socioeconomic, and clinical predictors. Statistically significant univariable predictors of 3-month Glasgow Outcome Scale-Extended (GOS-E) included MRI evidence for contusion (odds ratio [OR] 4.9 per unit decrease in GOS-E; p=0.01), ≥1 ROI with severely reduced FA (OR, 3.9; p=0.005), neuropsychiatric history (OR, 3.3; p=0.02), age (OR, 1.07/year; p=0.002), and years of education (OR, 0.79/year; p=0.01). Significant predictors of 6-month GOS-E included ≥1 ROI with severely reduced FA (OR, 2.7; p=0.048), neuropsychiatric history (OR, 3.7; p=0.01), and years of education (OR, 0.82/year; p=0.03). For the subset of 37 patients lacking neuropsychiatric and substance abuse history, MRI surpassed all other predictors for both 3- and 6-month outcome prediction. This is the first study to compare DTI in individual mTBI patients to conventional imaging, clinical, and demographic/socioeconomic characteristics for outcome prediction. DTI demonstrated utility in an inclusive group of patients with heterogeneous backgrounds, as well as in a subset of patients without neuropsychiatric or substance abuse history.
Key words: : axonal injury, computed tomography, diffusion tensor imaging, magnetic resonance imaging, traumatic brain injury
Introduction
Mild traumatic brain injury (mTBI) comprises 75% of the estimated 1.7 million patients who seek medical attention annually in the United States for acute head injury.1 The most widely accepted definitions of mTBI2–4 include patients with 1) nonpenetrating head trauma resulting in one or more of the following: confusion/disorientation; loss of consciousness (LOC) <30 min in duration, post-traumatic amnesia (PTA) <24 h in duration; and transient focal neurological signs or seizure and 2) Glasgow Coma Scale (GCS) score of 13–15 upon acute medical evaluation. Previous studies suggest that many mTBI patients have significant alterations in cognitive and/or behavioral functioning within weeks to months of injury, and approximately 15–20% have persistent measurable deficits at 1 year.5–12 There is also growing recognition that current classification schemes for mTBI/concussion based solely on GCS, PTA, and LOC are severely limited, with small mean effect sizes in long-term impairment obscuring differences among diverse subgroups of mTBI patients with very different prognoses.13,14 To date, there remains a need for practical, widely available clinical, laboratory, and/or imaging markers that identify patients who will experience persistent dysfunction after mTBI.
Many studies have reported changes in white matter diffusion tensor imaging (DTI) parameters in acute, subacute, and chronic time frames after mTBI.15–37 The clinical significance of acute traumatic intracranial findings on conventional computed tomography (CT) and magnetic resonance neuroimaging has also been explored.38,39 However, little is known about the relationship between conventional CT and magnetic resonance imaging (MRI) findings and DTI evidence of white matter injury within the mTBI spectrum. In addition, there has been little exploration of the use of acute or subacute DTI data for prediction of outcome in individual patients, after controlling for demographic, clinical, and CT and conventional MRI predictors. Although group differences in DTI parameters between mTBI patients and controls have been demonstrated, no consensus yet exists on the practical application of these techniques to outcome prediction in the individual patient. Finally, nearly all previous studies of DTI in mTBI have excluded patients with any history of substance abuse or other neuropsychiatric disorder, and the generalizability of their results to the general mTBI population is uncertain.
In this study, we used both whole-brain voxel-wise and region-of-interest (ROI) analyses to assess for an association between CT and conventional MRI abnormalities and early DTI measures of white matter integrity after mTBI. To determine the clinical relevance, if any, of DTI measures to outcome in mTBI, we then assessed for correlations between DTI measures and 3- and 6-month outcome. We compared the strengths of these correlations to those between outcome and conventional imaging, demographic, and clinical predictors previously found to influence outcome, based on the assumption that any utility of DTI in outcome prediction would require a differential increase in predictive power over predictors that are routinely assessed in current practice. To our knowledge, this is the first study to compare the relative strengths of DTI features in individual mTBI patients to conventional MRI, CT, clinical, demographic, and socioeconomic features for the prediction of 3- and 6-month outcome. In order to maximize the generalizability of study conclusions, we analyzed both an inclusive sample of 76 mTBI patients with very few exclusion criteria, as well as a subset of 37 patients with no significant drug, alcohol, or neuropsychiatric history.
Methods
Study population
mTBI patients were enrolled at San Francisco General Hospital (SFGH; San Francisco, CA) as part of the prospective multi-center TRACK-TBI (Transforming Research and Clinical Knowledge in Traumatic Brain Injury) pilot study.40 The primary inclusion criterion for the TRACK-TBI pilot study was performance of noncontrast head CT to assess for evidence of acute TBI within 24 h of injury, based on criteria from the American College of Emergency Physicians/Centers for Disease Control (ACEP/CDC) evidence-based joint practice guideline (Supplementary Table S1) (see online supplementary material at http://www.liebertpub.com).41 The TRACK-TBI pilot study exclusion criteria were limited and consisted of nonfluency in English, contraindication to MRI, pregnancy, and current incarceration/legal detention or placement on psychiatric hold.40
For the current study of DTI of mTBI, additional inclusion criteria were GCS 13–15 upon emergency department (ED) arrival, LOC <30 min, PTA duration <24 h, and age 18–55 years (inclusive); an additional exclusion criterion was any reported history of earlier TBI resulting in LOC >5 min. Of 190 mTBI patients in the 18- to 55-year age range enrolled at SFGH for the TRACK-TBI pilot study, 87 patients did not undergo brain MRI. Of the remaining 103 patients, 18 reported a history of earlier TBI with LOC >5 min or of unknown duration; 5 had a technically inadequate brain MRI exam (because of motion or, in 1 case, because of severe susceptibility artifact resulting from a metallic shunt valve within the scalp); 1 patient had an extensive area of encephalomalacia likely the result of an earlier TBI; 1 had an acute large-territory infarct resulting from acute traumatic arterial dissection; and 2 were excluded because their performance on the Trail Making Test (TMT) B and other outcome measures were extreme outliers, despite a GCS of 15 upon ED arrival, no LOC or PTA, and no CT or conventional MRI evidence of traumatic intracranial injury. The final patient group for the current study therefore consisted of 76 mTBI patients enrolled at SFGH who underwent brain MRI on a single 3T MRI scanner within 3 weeks of TBI. In addition, a control group consisted of 50 healthy subjects, ages 18–55 years, with no self-reported history of drug or alcohol abuse, neuropsychiatric illness, or earlier TBI, who underwent brain MRI on the same 3T scanner over the same time period, employing the same MRI protocol and software version. All study protocols were approved by the University of California at San Francisco Institutional Review Board, and all patients and control subjects or their legal representatives gave written informed consent.
Table 1 summarizes demographic, socioeconomic, and clinical characteristics of participants and control subjects. We assessed for statistically significant differences in demographic, socioeconomic, and clinical features at p<0.05 among the following groups: 1) CT/MRI-positive patients, defined as patients with any acute traumatic intracranial lesion or depressed skull fracture on day-of-admission CT or semiacute 3T MRI; 2) CT/MRI-negative patients, defined as patients without any such abnormality; and 3) control subjects. We used analysis of variance (ANOVA) for scale variables without significant deviation from a normal distribution, and Mann-Whitney U test for ordinal and non-normal variables. Differences in nominal variables were assessed by chi-square (χ2) test for independence or by Fisher's exact test for nominal variables with an expected count of fewer than 5 subjects in any cell. All statistical analyses were performed using SPSS Statistics (version 21; SPSS, Inc., Chicago, IL).
Table 1.
Demographic, Socioeconomic, and Clinical Predictors for 76 mTBI Patients and 50 Control Subjects
| Predictors | CT/MRI-negative mTBI (no acute traumatic intracranial abnormality or depressed skull fracture on CT and/or conventional MRI) (44 subjects) | CT/MRI-positive mTBI (acute traumatic intracranial abnormality and/or depressed skull fracture on CT and/or conventional MRI) (32 subjects) | Controls (50 subjects) | Analysis for group differences among CT/MRI-negative mTBI, CT/MRI-positive mTBI, and control subjects | |||
|---|---|---|---|---|---|---|---|
| Demographic and socioeconomic | |||||||
| Age (years, mean±standard deviation) | 31.2±9.5 | 33.9±12.0 | 28.7±9.2 |
F(2,123)=2.6; p=0.08 |
ANOVA | ||
| Education (years, mean±standard deviation) | 14.8±2.8 | 14.6±2.1 | 15.7±1.6 |
F (2,109)=2.6; p=0.08 |
|||
| Gender: male/female (% male) | 27/17 (61%) | 23/9 (72%) | 32/18 (64%) | χ2 (2; n=126)=0.9; p=0.65 |
χ2 test for independence | ||
| Unemployeda : yes/no (% yes) | 5/39 (11%) | 6/25 (19%) | Unknown | p=0.51 | Fisher's exact test | ||
| Handednessb (right/left/ambidextrous) | 39/4/1 | 27/4/1 | 48/1/0 | p=0.14 | |||
| Clinical | |||||||
| Neuropsychiatric history: yes/no (% yes) | 12/32 (27%)d | 6/26 (19%)d | 0/50 (0%)e | χ2 (2; n=126)=14.9; p=0.0004 |
χ2 test for independence | ||
| History of drug or alcohol problem: yes/no (% yes) | 21/23 (48%)f | 14/18 (44%)f | 0/50 (0%)g | χ2 (2; n=126)=32.0; p<10−6 |
|||
| LOC: yes, up to 30 min/no (% yes) | 28/16 (64%) | 23/9 (72%) | N/A | χ2 (1; n=76)=0.6; p=0.47 |
|||
| PTA: yes/no (% yes) | 26/18 (59%) | 25/7 (78%) | N/A | χ2 (1; n=76)=3.0; p=0.09 |
|||
| PTA durationc | None | 18 | None | 7 | N/A | CT/MRI-negative median PTA duration<1 min; CT/MRI-positive median PTA duration 1–29 min; U=440; z=−2.1; p=0.03 | Mann-Whitney U test |
| <1 min | 6 | <1 min | 1 | ||||
| 1–29 min | 14 | 1–29 min | 11 | ||||
| 30–59 min | 3 | 30–59 min | 5 | ||||
| 1–24 h | 3 | 1–24 h | 4 | ||||
| GCS (15/14/13) | 36/7/1 | 20/11/1 | N/A | p=0.13 | Fisher's exact test | ||
| Previous TBI with LOC up to 5 min: yes/no (% yes) | 15/29 (34%)h | 8/24 (25%)h | 0/50 (0%)i | p=0.000003 | |||
Gray shaded boxes indicate statistically significant difference at p<0.05.
One CT/MRI-positive mTBI patient with unknown employment status was not included in this analysis.
One control with unknown handedness was not included in this analysis.
Four CT/MRI-positive mTBI patients with PTA <24 h, but not otherwise specified, were not included in this analysis.
Each superscript denotes a subset of participants whose proportions do not significantly differ from each other at p<0.05 by Pearson's χ2 test (or Fisher's exact test when expected cell count <5).
mTBI, mild traumatic brain injury; ANOVA, analysis of variance; GCS, Glasgow Coma Scale; PTA, post-traumatic amnesia; LOC, loss of consciousness; N/A, not available.
CT and MRI protocols
CT was performed within 2 h 42 min±3 h 9 min of TBI. MRI was performed within 11.2±3.3 days (range, 5–18) postinjury. All CT exams were performed on a GE Lightspeed 64-row-detector CT scanner, and all MRI exams were performed on the same 3T GE Signa EXCITE scanner equipped with an eight-channel phased-array head radiofrequency coil (GE Healthcare, Waukesha, WI), using the same scanner software version. Whole-brain DTI was performed with a multi-slice single-shot spin echo echoplanar pulse sequence (echo time [TE]=63 ms; repetition time [TR]=14 sec) using 55 diffusion-encoding directions, isotropically distributed over the surface of a sphere with electrostatic repulsion, acquired at b=1000 sec/mm2, seven acquisitions at b=0 sec/mm2, 72 interleaved slices of 1.8-mm thickness each with no gap between slices, a 128×128 matrix, and a field of view (FOV) of 230×230 mm. Parallel imaging was employed using the array spatial sensitivity encoding technique (ASSET) with an acceleration factor of 2.
The following conventional 3T MRI sequences were also performed: 1) axial three-dimensional (3D) inversion recovery fast spoiled gradient recalled echo T1-weighted images (TE=1.5 ms; TR=6.3 ms; inversion time [TI]=400 ms; flip angle, 15 degrees) with 230-mm FOV, 156 contiguous partitions (1.0-mm) at 256×256 matrix; 2) axial T2-weighted fluid-attenuated inversion recovery images (TE=126 ms; TR=10 sec; TI=2200 ms) with 220 mm FOV, 47–48 contiguous slices (3.0-mm) at 256×256 matrix; and 3) axial magnetization-prepared gradient echo T2*-weighted images (TE=15 ms; TR=500 ms; flip angle 20 degrees) with 220×170 mm FOV and 47–48 contiguous slices (3.0-mm) at 256×192 matrix.
Neuroradiologist evaluation of CT and MRI studies for acute traumatic abnormalities
Each patient's head CT upon ED presentation and early brain MRI (11.2±3.3 days postinjury) was characterized using the TBI common data elements (TBI-CDE). The TBI-CDEs are consensus-based recommendations for data collection, data definitions, and best practices in TBI research established jointly by the National Institute of Neurological Disorders and Stroke (NINDS), Defense Centers of Excellence, National Institute on Disability and Rehabilitation Research, and Veterans Administration.42–44 Each CT and MRI was anonymized and reviewed by a board-certified neuroradiologist blinded to demographic, socioeconomic, and clinical data, except gender and age, and without concurrent access to the patient's other head imaging studies or 3- and 6-month outcome measures.
mTBI patients were divided into two subgroups: 1) CT/MRI positive, defined as patients with any acute traumatic intracranial lesion (epidural hematoma [EDH], subdural hematoma [SDH], subarachnoid hemorrhage [SAH], contusion, or evidence of traumatic axonal injury) and/or depressed skull fracture on either CT or MRI, and 2) CT/MRI negative, defined as patients without any such abnormality. Most previous studies of “complicated” mTBI, including Williams and colleagues,38 demonstrated poorer neuropsychiatric test performance based solely on CT findings (presence of any acute intracranial hemorrhage or depressed skull fracture). Our dichotomization of mTBI patients according to presence of abnormalities on either CT or MRI is based on more recent work that demonstrated poorer 3-month outcome associated with early MRI intracranial abnormalities, whether or not visible on CT.39
Diffusion tensor image processing
Nonbrain tissue was eliminated from the diffusion-weighted and 3D T1-weighted images using the Functional MRI of the Brain (FMRIB, Oxford University, Oxford, UK) Brain Extraction Tool.45 Diffusion-weighted images were corrected for eddy currents and registered to the b=0 sec/mm2 volume using the FMRIB Linear Image Registration Tool. A diffusion tensor model was constructed using the FMRIB DTIFit algorithm46 to yield fractional anisotropy (FA), mean diffusivity (MD), axial diffusivity (AD), and radial diffusivity (RD) at each voxel. Tract-based spatial statistics (TBSS)47 were used to align each subject's FA data to a white matter skeleton, after low FA values below a threshold of 0.25 were excluded to limit voxels to the white matter.
Voxel-wise nonparametric statistical comparison between 76 mTBI patients and 50 controls was performed using the FMRIB Software Library (FSL) randomise algorithm based on permutation testing, with corrections for multiple voxel-wise comparisons using threshold-free cluster enhancement (TFCE).48 Anatomic locations of voxel clusters with statistically significant differences in FA, MD, RD, or AD between mTBI and control groups at p<0.05 were determined. This analysis was also used to compare the subgroup of 32 CT/MRI-positive patients to the 50 controls and also the subgroup of 44 CT/MRI-negative mTBI patients to the 50 controls.
In addition to the whole-brain voxel-wise approach, we performed a complementary ROI analysis to address the possibility that a whole-brain, data-driven approach might not be sufficiently sensitive to reveal white matter injury because of possibly significant spatial heterogeneity of white matter injury across mTBI subjects. Twenty-seven white matter ROIs were delineated by the intersection of the Johns Hopkins University (Baltimore, MD) ICBM-DTI-81 White Matter Labeled Atlas49 and the reference white matter skeleton. These consisted of the anterior corona radiata, superior corona radiata, posterior corona radiata, anterior limb of internal capsule, posterior limb of internal capsule, external capsule, superior longitudinal fasciculus, sagittal striatum, ventral cingulum (parahippocampal gyrus), dorsal cingulum (cingulate gyrus), inferior fronto-occipital fasciculus, and superior fronto-occipital fasciculus, each on the left and right; and also the body, genu, and splenium of the corpus callosum. The FA, MD, AD, and RD within each of these 27 ROIs in each patient and control subject were determined. For each ROI, the mean and standard deviation (SD) of the FA within the group of 50 control subjects was calculated. Similarly, for each ROI, the mean and SD for each of the other DTI measures (MD, AD, and RD) in the group of 50 control subjects were calculated. For each of the 76 mTBI patients and 50 control subjects, an abnormal ROI was then defined as one in which a DTI measure (FA, MD, AD, or RD) was more than 2.2 SDs below or above the control-group mean, based on the distribution of the DTI measure within the 50 control patients alone.
Outcome measures
Outcome measures included the Extended Glasgow Outcome Scale (GOS-E) at 3 and 6 months postinjury, the Rivermead Postconcussion Symptoms Questionnaire (RPQ), California Verbal Learning Test–Second Edition (CVLT-II), Wechsler Adult Intelligence Scale–Fourth Edition, Processing Speed Index (WAIS-IV PSI), and Trail Making Tests A and B (TMT A and TMT B) at 6 months. The GOS-E was obtained at 3 and 6 months postinjury through structured interview with each participant by research assistants trained to uniformly assess the GOS-E. Modeled after the 5-point Glasgow Outcome Scale (GOS), the 8-point GOS-E provides better discrimination among more subtle aspects of disability within mild-to-moderate, rather than mild-to-severe, TBI and is a well-validated, widely employed measure of global function after mTBI.50 The TMT A and B are tests of visual attention, visual-motor coordination, task switching, and executive function.51,52 WAIS-IV PSI is a test of perceptual processing speed with additional contribution from working memory.53,54 The CVLT-II is a test of verbal learning and memory and was used in place of the TBI CDE Rey Auditory Verbal Learning Test because of recent revision of the CVLT with demonstration of improved psychometric properties.55,56 The RPQ consists of 16 symptoms frequently reported after mTBI.57,58 The first three symptoms, denoted RPQ-3, are more physical symptoms (headaches, dizziness, and nausea/vomiting) typically experienced immediately after the TBI event, whereas the other 13 symptoms (denoted RPQ-13) are more psychosocial in nature (hyperacusis, sleep disturbances, fatigue, irritability, depressed mood, frustration, forgetfulness, poor concentration, requiring longer times to think, blurred vision, light sensitivity, double vision, and restlessness) and have been shown to occur later in the clinical course after mTBI.59,60
We assessed for statistically significant group differences in each outcome measure between CT/MRI-positive and -negative mTBI patients. The CVLT-II, WAIS-IV PSI, and TMT A and B scores were converted to normative scores for age, and ANOVA was used to test for group differences in these variables between CT/MRI-positive and -negative mTBI patients at p<0.05. Mann-Whitney U test was used to assess for group differences in the 3-month GOS-E, 6-month GOS-E, RPQ-3, and RPQ-13 at p<0.05. All statistical analyses were performed using SPSS Statistics (version 21).
Spearman's correlation and ordinal logistic regression analyses
We calculated Spearman's correlation coefficients between each outcome measure and each of 11 demographic (age, gender), socioeconomic (employment status, number of years of formal education), and clinical (history of major neuropsychiatric diagnosis, history of drug or alcohol abuse, GCS upon ED arrival, any PTA, PTA duration, any LOC, any history of mTBI with LOC duration not exceeding 5 min) predictors, 5 noncontrast head CT features (calvarial or skull base fracture, EDH, SDH, SAH, contusion), and 3 brain MRI features (contusion, hemorrhagic axonal injury, or evidence of white matter injury on DTI ROI analysis). We used Spearman's correlation, rather than its parametric counterpart, Pearson's product-moment correlation, because of the nominal or ordinal nature and/or non-normal distribution of most of these variables. We then performed multivariable logistic or linear regression of each outcome measure upon all predictors with which the outcome measure had demonstrated a statistically significant pairwise Spearman's correlation. For both Spearman's correlation and the regression analyses, the CVLT-II, WAIS-IV PSI, and TMT A and B test scaled or z-scores, as well as binary outcome variables corresponding to performance worse or better than 2 SDs worse than the normative score as determined by previous studies,52,54,55 were included as outcome variables. For the ordinal logistic regression analyses, tests for parallel lines were performed and confirmed the proportional odds assumption for each analysis. These statistical analyses were performed using SPSS Statistics (version 21).
Results
Study population characteristics
Table 1 summarizes demographic, socioeconomic, and clinical characteristics of participants. There were no statistically significant differences among CT/MRI-positive, CT/MRI-negative, and control subjects in age, number of years of formal education, gender, or handedness. Employment status was unknown for control subjects, but there was no difference at p<0.05 between CT/MRI-positive and -negative patients. Among the clinical variables, rates of major neuropsychiatric diagnosis, history of drug or alcohol abuse, and history of previous mTBI with LOC up to 5 min were significantly higher in CT/MRI-negative and -positive mTBI patients than in control subjects, but were not statistically different between CT/MRI-negative and -positive patients. (Patients with a history of any previous TBI with LOC>5 min had been excluded from the study.) PTA duration was longer in CT/MRI-positive patients (median PTA duration, 1–29 min) than in CT/MRI-negative patients (median PTA duration,<1 min). There was no significant difference in GCS or LOC between CT/MRI-negative and -positive mTBI groups at p<0.05 (Table 1).
Conventional CT and MRI results
Table 2 shows that MRI identifies many more acute traumatic intracranial lesions than CT. TBI-CDE–defined pathoanatomic features observed on head CT upon ED presentation and early brain MRI in our study population consisted of the following: nondepressed skull fracture; EDH; SDH; SAH; brain contusion; and hemorrhagic axonal injury. Hemorrhagic axonal injury was observed on many brain MRI exams, but on only one head CT, in this study. Other TBI-CDE features, such as midline shift ≥5 mm and partial or complete basal cistern effacement that are more characteristic of moderate-to-severe TBI, were also not observed on any head CT or brain MRI in this study. In addition, no depressed skull fracture was observed in this study. As shown in Table 2, all 4 of 4 (100%) patients with CT evidence of contusion also had MRI evidence of contusion±hemorrhagic axonal injury. In contrast, 7 of 11 (64%) patients with MRI evidence of contusion and 25 of 27 (93%) with MRI evidence of hemorrhagic axonal injury had no CT evidence of any parenchymal injury. Three patients with nondepressed skull fractures had no CT or conventional MRI traumatic intracranial abnormality and were classified as CT/MRI-negative mTBI (analogous to the classification of patients with isolated nondepressed skull fracture and no acute intracranial hemorrhage as “uncomplicated” mTBI in previous literature38).
Table 2.
CT and Conventional MRI Findings in 76 mTBI Patients
| CT | |||||
|---|---|---|---|---|---|
| Normal | Nondepressed skull fracture only | Acute extraaxial hemorrhage (EDH, SDH, SAH) with no parenchymal injury | Contusion±extraaxial hemorrhage | Hemorrhagic axonal injury only | |
| MRI | |||||
| No parenchymal injury | 41 | 3 | 2 | 0 | 0 |
| Hemorrhagic axonal injury only | 17 | 0 | 1 | 0 | 1 |
| Contusion only | 0 | 0 | 0 | 3 | 0 |
| Both hemorrhagic axonal injury and contusion | 1 | 1 | 5 | 1 | 0 |
Gray shaded boxes comprise uncomplicated mTBI (no CT evidence of acute intracranial hemorrhage or depressed skull fracture).38
CT, computed tomography; MRI, magnetic resonance imaging; mTBI, mild traumatic brain injury; EDH, epidural hematoma; SDH, subdural hematoma; SAH, subarachnoid hemorrhage.
Whole-brain voxel-wise nonparametric statistical comparison of diffusion tensor imaging measures in mTBI (n=76) versus control subjects (n=50)
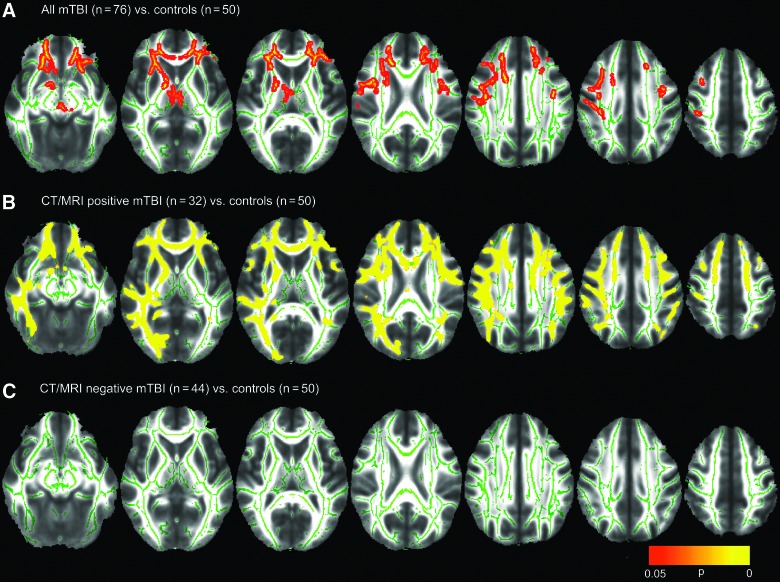
Figure 1A shows many statistically significant areas of reduced FA in the 76 mTBI patients, compared to the 50 control subjects, using TBSS and voxel-wise nonparametric statistical comparison implemented in the FSL randomise algorithm and corrected for multiple comparisons with TFCE. mTBI patients demonstrated significantly lower FA in the right internal and external capsules, genu of the corpus callosum, and uncinate fasciculi and anterior corona radiata bilaterally.
FIG. 1.
Voxel-wise nonparametric statistical comparison between mild traumatic brain injury (mTBI) patients and controls, with corrections for multiple voxel-wise comparisons using threshold-free cluster enhancement. This analysis was used to compare (A) 76 mTBI patients to 50 controls, (B) the subgroup of 32 computed tomography/magnetic resonance imaging (CT/MRI)-positive mTBI patients to the 50 controls, and (C) the subgroup of 44 CT/MRI-negative patients to the 50 controls. Voxel clusters with statistically significant differences in fractional anisotropy (FA) between mTBI and control groups at p<0.05 are shown in red/orange/yellow, with yellow denoting greater statistical significance. (A) shows that the 76 mTBI patients demonstrated significantly lower FA in the genu of the corpus callosum, uncinate fasciculi, and anterior corona radiata bilaterally as well as right internal and external capsules, compared to the 50 control subjects. (B) In a comparison of a much smaller subgroup of 32 CT/MRI-positive mTBI patients to the 50 controls, areas of reduced FA were even more extensive and attained much higher levels of statistical significance (yellow regions, corresponding to p<0.01) than in the comparison of 76 mTBI patients to the control group (mostly red/orange areas, corresponding to p<0.05, in [A]). (C) shows that this method demonstrated no evidence for white matter injury in 44 CT/MRI-negative mTBI patients, compared to the 50 controls. Color image is available online at www.liebertpub.com/neu
No voxel with significantly increased FA, and no significant group differences in MD, RD or AD, were found in mTBI patients, compared to the control group at p<0.05 using TBSS, randomise, and correction for multiple comparisons with TFCE.
Whole-brain voxel-wise nonparametric statistical comparison of diffusion tensor imaging measures in CT/MRI-positive mTBI (n=32) versus control subjects (n=50)
Figure 1B shows many highly statistically significant areas of reduced FA in the CT/MRI-positive subgroup of mTBI patients, compared to the control group. Despite the expected loss of statistical power for this comparison of a much smaller subgroup of 32 CT/MRI-positive mTBI patients to the control group, areas of reduced FA were even more extensive and attained higher levels of statistical significance (yellow regions, corresponding to p<0.01; Fig. 1B) than in the comparison of 76 mTBI patients to the control group (mostly red/orange areas, corresponding to p<0.05; Fig. 1A). mTBI patients demonstrated significantly lower FA in the genu and body of the corpus callosum, the external capsules, uncinate fasciculi, and anterior corona radiata bilaterally, the right internal capsule, and the right inferior longitudinal and inferior fronto-occipital fasciculi. Extensive areas of increased RD were also observed in the 32 CT/MRI-positive mTBI patients, relative to the control group, whereas none had been observed in the comparison of 76 mTBI patients to the control group. No voxel with increased FA or reduced RD was observed in CT/MRI-positive mTBI patients, relative to controls, at p<0.05. There were also no voxels in which MD or AD differed significantly between CT/MRI-positive mTBI and control groups at p<0.05.
Whole-brain voxel-wise nonparametric statistical comparison of diffusion tensor imaging measures in CT/MRI-negative mTBI (n=44) versus control subjects (n=50)
No significant group differences in FA (Fig. 1C), MD, RD, or AD were found between CT/MRI-negative mTBI and control groups at p<0.05.
Whole-brain voxel-wise nonparametric statistical comparison of diffusion tensor imaging measures in most highly educated versus least educated control subjects (n=50)
To exclude the possibility that the nonsignificant differences in educational level among CT/MRI-positive mTBI, CT/MRI-negative mTBI, and control groups (Table 1) could result in group differences in DTI parameters that could be erroneously attributed to mTBI, we assessed for group differences in DTI parameters between control subjects with the longest and shortest duration of education. The 50 control subjects were divided into two groups, one consisting of 25 patients with the most years of formal education and the other consisting of 25 patients with the fewest years of formal education. There were no statistically significant group differences in DTI parameters between these groups at p<0.05. This analysis was performed to exclude the possibility that the statistically significant group differences in FA shown in Figure 1A and 1B were attributable mostly to educational level or to other socioeconomic factors that might be correlated with educational level.
Region-of-interest analysis of individual mTBI subjects
Table 3 shows that abnormally low FA (FA more than 2.2 SDs below the control-group mean) was observed in ≥1 ROIs for 14 of 32 CT/MRI-positive mTBI (43.8%), 11 of 44 CT/MRI-negative mTBI (25.0%), and 5 of 50 (10.0%) control subjects. Pearson's χ2 test showed a highly significant difference between the proportions of CT/MRI-positive mTBI patients (43.8%) and control subjects (10.0%) with ≥1 abnormal ROIs (p=0.0006). There was a trend toward a significant difference between the proportions of CT/MRI-negative mTBI patients (25.0%) and controls (10.0%) with ≥1 abnormal ROIs (p=0.06). Finally, there was no significant difference between the proportions of CT/MRI-positive mTBI patients (43.8%) and CT/MRI-negative mTBI patients (25.0%) with ≥1 abnormal ROIs (p=0.14).
Table 3.
DTI Region-of-Interest (ROI) Analysis: Group Differences in Presence of One or More Abnormal ROIs among CT/MRI-Negative mTBI, CT/MRI-Positive mTBI, and Control Subjects
| CT/MRI-negative mTBI (no acute traumatic intracranial abnormality or depressed skull fracture on CT or conventional MRI) (44 subjects) | CT/MRI-positive mTBI (positive acute traumatic intracranial abnormality and/or depressed skull fracture on CT and/or conventional MRI) (32 subjects) | Controls (50 subjects) | |
|---|---|---|---|
| Number of subjects (proportion of subjects) | Number of subjects (proportion of subjects) | Number of subjects (proportion of subjects) | |
| One or more ROIs with FA more than 2.2 SDs below control-group mean | 11 (25.0%)a,b | 14 (43.8%)b | 5 (10.0%)a |
| One or more ROIs with FA more than 2.2 SDs above control group mean | 8 (18.2%)c | 5 (15.6%)c | 8 (16.0%)c |
Each superscript denotes a subset of participants whose column proportions do not differ significantly from one another, by Pearson's χ2 test with p < 0.05. Row 1: There was a statistically significant difference between CT/MRI-positive mTBI (43.8%) and control subjects (10.0%), with one or more ROIs with FA more than 2.2 SDs below the control group mean (p = 0.0006). There was no significant difference between CT/MRI-negative mTBI patients (25.0%) and controls (10.0%; p = 0.06). There was also no significant difference between CT/MRI-positive (43.8%) and CT/MRI-negative mTBI patients (25.0%; p = 0.14). Row 2: There was no significant difference among the proportions of CT/MRI-negative mTBI (18.2%), CT/MRI-positive mTBI (15.6%), and control subjects (16.0%) with one or more ROIs with FA more than 2.2 SDs above the control group mean (p = 0.96).
DTI, diffusion tensor imaging; ROI, region of interest; CT, computed tomography; MRI, magnetic resonance imaging; mTBI, mild traumatic brain injury; FA, fractional anisotropy; SD, standard deviation.
Table 3 also shows that there was no significant difference (p=0.93) among the proportions of CT/MRI-positive, CT/MRI-negative, and control subjects with ≥1 ROI with abnormally high FA (FA more than 2.2 SDs above the control-group mean).
Outcome measures
Table 4 summarizes 3- and 6-month outcome measures of participants. There were no statistically significant differences in any 3- or 6-month outcome measure between CT/MRI-negative and -positive mTBI groups at p<0.05. For the TMT A and B, the actual times for test completion, the corresponding TMT A and B z-scores adjusted for age,52 as well as the proportion of abnormal performances worse than 2 SDs from the age-adjusted mean, were compared between CT/MRI-positive and -negative mTBI groups, and none showed a statistically significant difference at p<0.05.
Table 4.
Group Differences in 3- and 6-Month Outcome Measures between 32 CT/MRI-Positive mTBI and 44 CT/MRI-Negative mTBI Patients
| CT/MRI-negative (no acute traumatic intracranial abnormality or depressed skull fracture on CT or conventional MRI) (44 subjects) | CT/MRI-positive (acute traumatic intracranial abnormality or depressed skull fracture on CT and/or conventional MRI) (32 subjects) | Analysis for group differences between CT/MRI negative, CT/MRI positive | ||||
|---|---|---|---|---|---|---|
| 3-month outcome measure | ||||||
| Score | Number of patients | Score | Number of patients | |||
| 3-month GOS-Ea | 4 | 1 | 4 | 0 | U=485; Z=−1.4; p=0.17 |
Mann-Whitney U test |
| 5 | 6 | 5 | 3 | |||
| 6 | 3 | 6 | 10 | |||
| 7 | 13 | 7 | 8 | |||
| 8 | 18 | 8 | 8 | |||
| 6-month outcome measures | ||||||
| 6-month GOS-Eb | 4 | 1 | 4 | 0 | U=459; z=−0.67; p=0.52 |
Mann-Whitney U test |
| 5 | 4 | 5 | 3 | |||
| 6 | 7 | 6 | 7 | |||
| 7 | 13 | 7 | 9 | |||
| 8 | 14 | 8 | 7 | |||
| RPQ-3b Median (25%, 75%) |
2.0 [0.0,4.0] | 1.5 [0.0,4.3] | U=467; z=−0.55; p=0.59 | Mann-Whitney U test | ||
| RPQ-13b Median (25%, 75%) |
7.0 [4.0,16.0] | 14.0 [3.3,21.0] | U=441; z=−0.89; p=0.38 | |||
| CVLT-II scaled scorec | 54±11 | 57±9 |
t(55)=0.91; p=0.37 |
Two-tailed t-test | ||
| WAIS IV PSId percentile | 58%±28% | 62%±27% |
t(57)=0.45; p=0.65 |
|||
| TMT Ae | ||||||
| • Time (sec) | 31±13 | 30±9 |
t(59)=−0.37; p=0.71 |
Two-tailed t-test | ||
| • Time (z-score) | 0.68±1.45 | 0.50±1.29 |
t(59)=−0.51; p=0.62 |
|||
| • TMT A >2 SDs above mean | Yes | 7 | Yes | 3 | U=417; z=−0.88; p=0.38 |
Mann-Whitney U test |
| No | 28 | No | 23 | |||
| TMT Be | ||||||
| • Time (sec) | 65±27 | 69±27 |
t(59)=0.51; p=0.61 |
Two-tailed t-test | ||
| • Time (z-score) | 0.93±1.75 | 1.09±1.94 |
t(59)=0.34; p=0.74 |
|||
| • TMT B >2 SDs above mean | Yes | 8 | Yes | 8 | U=419; z=−0.69; p=0.56 |
Mann-Whitney U test |
| No | 27 | No | 18 | |||
Three CT/MRI-negative mTBI and 3 CT/MRI-positive mTBI patients did not complete 3-month GOS-E evaluation.
Five CT/MRI-negative mTBI and 6 CT/MRI-positive mTBI patients did not complete 6-month GOS-E, RPQ-3, or RPQ-13.
Eleven CT/MRI-negative mTBI and 8 CT/MRI-positive mTBI patients did not complete 6-month CVLT-II.
Ten CT/MRI-negative mTBI and 7 CT/MRI-positive mTBI patients did not complete 6-month WAIS IV.
Nine CT/MRI-negative mTBI and 6 CT/MRI-positive mTBI patients did not complete 6-month TMT A or TMT B.
CT, computed tomography; MRI, magnetic resonance imaging; mTBI, mild traumatic brain injury; GOS-E, Glasgow Outcome Scale – Extended; CVLT-II, California Verbal Learning Test–Second edition; RPQ, Rivermead Postconcussion Symptoms Questionnaire; TMT, Trail Making Test; SD, standard deviation; WAIS IV PSI, Wechsler Adult Intelligence Scale–Fourth edition, Processing Speed Index.
Spearman's correlation
Table 5 shows the pair-wise Spearman's correlation coefficients between 3- and 6-month outcome measures and demographic, socioeconomic, clinical, CT, and MRI predictors. Gender, employment status, GCS at ED arrival, PTA, PTA duration, LOC, and history of previous TBI with LOC up to 5 min were not significantly correlated with any outcome variable, and these predictors were thus omitted from Table 5, for brevity. Similarly, worse outcomes, as measured by the 6-month TMT A (both age-adjusted z-score and the dichotomized score), TMT B (z-score), CVLT-II (both age-adjusted scaled score and dichotomized score), and WAIS-IV PSI (both age-adjusted scaled score and dichotomized score), were not significantly correlated with any imaging, clinical, demographic, or socioeconomic predictor (with the exception of modest correlations between CVLT-II scaled score and years of education and between age and TMT A z-score), and these outcome measures were thus also omitted from Table 5, for brevity.
Table 5.
Spearman's Correlation Coefficients (ρ) Between Outcome Measuresa and Demographic, Socioeconomic, Clinical, and Imaging Predictorsb in 76 mTBI Patients
| Demographic, clinical, socioeconomic | Day-of-injury head CT | Early brain MRI (11.2±3.3 days postinjury) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Age | Education (years) | Neuropsychiatric history | History of alcohol or drug problem | Nondepressed calvarial or skull base fracture | EDH | SDH | SAH | Any CT contusion | Any MRI contusion | Any MRI T2* evidence of hemorrhagic axonal injuryb | Any DTI axonal injury (≥1 ROI with FA >2.2 SDs below control-group mean) | |
| 3-month GOS-E (N=70) | −0.30* p=0.013 |
0.27* p=0.02 |
−0.27* p=0.03 (18 pos.) |
−0.12 p=0.34 (34 pos.) |
−0.12 p=0.33 (12 pos.) |
−0.08 p=0.54 (3 pos.) |
−0.23 p=0.06 (9 pos.) |
−0.28* p=0.02 (6 pos.) |
−0.22 p=0.07 (5 pos.) |
−0.36† p=0.003 (11 pos.) |
−0.12 p=0.34 (24 pos.) |
−0.34† p=0.004 (23 pos.) |
| 6-month GOS-E (N=65) | −0.18 p=0.16 |
0.31* p=0.011 |
−0.30* p=0.02 (17 pos.) |
−0.18 p=0.15 (31 pos.) |
−0.13 p=0.32 (10 pos.) |
0.01 p=0.97 (2 pos.) |
−0.17 p=0.18 (7 pos.) |
−0.20 p=0.11 (5 pos.) |
−0.19 p=0.14 (4 pos.) |
−0.19 p=0.12 (9 pos.) |
−0.03 p=0.84 (22 pos.) |
−0.25* p=0.04 (20 pos.) |
| Abnormal TMT B (>2 SDs above age-adjusted mean) at 6 months (N=61) | 0.11 p=0.42 |
−0.18 p=0.17 |
−0.02 p=0.90 (16 pos.) |
0.01 p=0.94 (30 pos.) |
−0.14 p=0.27 (9 pos.) |
−0.11 p=0.40 (2 pos.) |
0.02 p=0.88 (7 pos.) |
0.09 p=0.47 (5 pos.) |
−0.16 p=0.22 (4 pos.) |
0.07 p=0.61 (9 pos.) |
0.17 p=0.18 (22 pos.) |
0.32* p=0.011 (19 pos.) |
| 6-month RPQ-3 (N=65) | 0.23 p=0.07 |
−0.23 p=0.06 |
0.36† p=0.003 (17 pos.) |
0.25* p=0.045 (31 pos.) |
−0.12 p=0.32 (10 pos.) |
−0.21 p=0.09 (2 pos.) |
0.11 p=0.37 (7 pos.) |
0.01 p=0.93 (5 pos.) |
0.07 p=0.56 (4 pos.) |
0.03 p=0.84 (9 pos.) |
−0.10 p=0.45 (22 pos.) |
0.18 p=0.14 (20 pos.) |
| 6-month RPQ-13 (N=65) | 0.26* p=0.04 |
−0.28* p=0.02 |
0.31* p=0.013 (17 pos.) |
0.16 p=0.20 (31 pos.) |
0.02 p=0.85 (10 pos.) |
−0.07 p=0.60 (2 pos.) |
0.19 p=0.14 (7 pos.) |
0.16 p=0.21 (5 pos.) |
0.21 p=0.10 (4 pos.) |
0.12 p=0.34 (9 pos.) |
0.02 p=0.85 (22 pos.) |
0.29* p=0.02 (20 pos.) |
No statistically significant correlation was found between any imaging, demographic, socioeconomic, or clinical predictor and worse performance on 6-month TMT A (either z-score or dichotomized score), TMT B (z-score), CVLT-II (scaled score or dichotomized score), or WAIS-IV PSI (scaled score or dichotomized score), except for correlation of CVLT-II scaled score with years of education (ρ=0.27; p=0.04) and correlation of age with TMT A z- score (ρ=−0.33; p=0.0097). Thus, for brevity, these outcome measures are omitted from Table 5.
No statistically significant correlation was found between gender, unemployment, GCS at emergency department arrival, PTA, PTA duration, LOC, or history of previous TBI (with LOC not exceeding 5 min) and any outcome variable. Thus, for brevity, these predictors are omitted from Table 5. There was a trend toward significant correlation between 6-month GOS-E and unemployed status (ρ=−0.24; p=0.056).
p<0.05 (light-gray boxes); †p<0.01 (dark-gray boxes).
CT, computed tomography; MRI, magnetic resonance imaging; EDH,epidural hematoma; SDH, subdural hematoma; SAH, subarachnoid hemorrhage; DTI, diffusion tensor imaging; ROI, region of interest; SD, standard deviation; FA, fractional anisotropy; GOS-E, Glasgow Outcome Scale-Extended; TMT, Trail Making Test; RPQ, Rivermead Postconcussion Questionnaire; CVLT, California Verbal Learning Test; WAIS, Wechsler Adult Intelligence Scale; pos., positive.
Table 5 shows that among demographic, clinical, and socioeconomic predictors, previous history of neuropsychiatric disorder was the most consistent predictor of outcome, demonstrating statistically significant correlations with 3-month GOS-E (ρ=−0.27; p=0.03), 6-month GOS-E (ρ=−0.30; p=0.02), 6-month RPQ-3 (ρ=0.36; p=0.003), and 6-month RPQ-13 (ρ=0.31; p=0.013).
Among the imaging predictors, DTI evidence of one or more ROIs with abnormally reduced FA (>2.2 SDs below control-group mean) was the most consistent predictor of outcome, demonstrating statistically significant correlations with 3-month GOS-E (ρ=−0.34; p=0.004), 6-month GOS-E (ρ=−0.25; p=0.04), abnormal 6-month TMT B (ρ=0.32; p=0.011), and 6-month RPQ-13 (ρ=0.29; p=0.02). Among other imaging predictors, MRI evidence of contusion was significantly correlated with 3-month GOS-E (ρ=−0.36; p=0.003), as was CT evidence of SAH, though more weakly (ρ=−0.28; p=0.02).
Regression of 3- and 6-month outcome measures on demographic, clinical, and imaging predictors
Based on the results of Spearman's correlation analysis (Table 5), we constructed regression models of each of five outcome measures: 3-month GOS-E; 6-month GOS-E; 6-month TMT B (dichotomized score); 6-month RPQ-3; and 6-month RPQ-13. The predictive (independent) variables in the model for each outcome measure were limited to only those predictors that had demonstrated a statistically significant Spearman's correlation with that outcome measure in Table 5. This resulted in a multivariable regression model for four outcome measures (3- and 6-month GOS-E, 6-month RPQ-3, and 6-month RPQ-13) and a univariable regression model for one outcome measure (6-month TMT B dichotomized score). No regression model was constructed for any outcome measure that lacked a statistically significant Spearman's correlation with at least one predictor.
For the 3-month GOS-E, age, number of years of education, neuropsychiatric history, MRI evidence for contusion, and DTI evidence of one or more abnormal ROIs with FA more than 2.2 SDs below the control-group mean demonstrated statistically significant univariable odds ratios (ORs; Table 6A), compatible with the Spearman's correlation results from Table 5. The multivariable model for 3-month GOS-E, including all of these predictors, was also significant (pseudo-R2 of 34.5–36.9%; p=0.00002; Table 6A). Although CT evidence of SAH demonstrated a nearly statistically significant univariable OR (p=0.053), it was excluded from the multivariable model because of collinearity with MRI evidence of contusion. In particular, unstable ORs and a variance inflation factor >2 were observed for CT evidence of SAH and MRI evidence of contusion when both were simultaneously included in the multivariable model.
Table 6a.
Multivariable Ordinal Logistic Regression of 3- and 6-Month GOS-E Versus Statistically Significant Clinical, Demographic, Socioeconomic, CT, and MRI Predictors From Table 5
| Outcome variable | Predictor | Predictor values | Univariable odds ratio per unit decrease in GOS-E (95% CI), p value | Multivariable odds ratio of predictor per unit decrease in GOS-E (95% CI), p value | Multivariable model significance | Cox and Snell pseudo-R2 | Nagelkerke pseudo-R2 |
|---|---|---|---|---|---|---|---|
| 3-month GOS-E (N=70) | Age | 32.4±10.8 years | 1.07 per year (1.03, 1.1); p=0.002† |
1.07 per year (1.03, 1.1); p=0.002† |
p=0.00002§ | 34.5%§ | 36.9%§ |
| Education | 14.5±2.5 years | 0.79 per year (0.66, 0.94); p=0.0101* |
0.79 per year (0.65, 0.96); p=0.02* |
||||
| Neuropsychiatric history | Yes (18) No (52) |
3.3 (1.2, 8.8); p=0.02* |
1.9 (0.65, 5.3); p=0.25 |
||||
| CT subarachnoid hemorrhage | Yes (6) No (64) |
p=0.053 | Excluded because of collinearity (see text) | ||||
| MRI contusion present | Yes (11) No (59) |
4.9 (1.5, 16.4); p=0.0098† |
3.1 (0.87, 11.0); p=0.08 |
||||
| DTI axonal injury (≥1 ROI with FA>2.2 SD below control-group mean) | Yes (23) No (47) |
3.9 (1.5, 10.0); p=0.005† |
2.6 (0.94, 7.0); p=0.07 |
||||
| 6-month GOS-E (N=65) | Education | 14.8±2.5 years | 0.82 (0.68, 0.98); p=0.03* |
0.90 per year (0.74, 1.08); p=0.26 |
p=0.013* | 15.3%* | 16.3%* |
| Neuropsychiatric history | Yes (17) No (48) |
3.7 (1.3, 10.5); p=0.014* |
2.7 (0.92, 7.9) p=0 .07 |
||||
| Any DTI axonal injury (≥1 ROI with FA>2.2 SD below control-group mean) | Yes (20) No (45) |
2.7 (1.01, 7.1); p=0.048* |
2.5 (0.83, 6.1) p=0.11 |
For the 6-month GOS-E, years of education, neuropsychiatric history, and DTI evidence of one or more abnormal ROIs with FA more than 2.2 SDs below the control-group mean demonstrated statistically significant univariable ORs (Table 6A), compatible with Spearman's correlation results from Table 5. The multivariable model for 6-month GOS-E, including all of these predictors, was also significant (pseudo-R2 of 15.3–16.3%; p=0.013; Table 6A).
For 6-month RPQ-13, age, years of education, neuropsychiatric history, and DTI evidence of one or more abnormal ROIs with FA more than 2.2 SDs below the control group mean demonstrated statistically significant univariable ORs, consistent with Spearman's correlation results from Table 5. The multivariable linear regression model for 6-month RPQ-13, including all of these predictors was also significant (adjusted R2 of 23.7%; p=0.0004; Table 6B).
Table 6b.
Multivariable Linear Regression of 6-Month RPQ-13 Versus Statistically Significant Clinical, Demographic, Socioeconomic, CT, and MRI Predictors From Table 5
| Outcome variable | Predictor | Predictor values | Univariable standardized coefficient β, p value | Multivariable standardized coefficient β, p value | F (degrees of freedom) | Overall model significance | Adjusted R2 |
|---|---|---|---|---|---|---|---|
| 6-month RPQ-13 (N=65) | Age | 32.0±10.8 years | 0.32; p=0.009† | 0.26; p=0.02* | F(4,60)=6.0‡ | p=0.0004‡ | 23.7%‡ |
| Education | 14.8±2.5 years | −0.29; p=0.02* | −0.20; p=0.10 | ||||
| Neuropsychiatric history | Yes (17) No (48) |
0.36; p=0.003† | 0.22; p=0.07 | ||||
| Any DTI axonal injury (≥1 ROI with FA>2.2 SDs below control-group mean) | Yes (20) No (45) |
0.31; p=0.012* | 0.21; p=0.07 |
Because the 6-month TMT B was significantly correlated with only one predictor (Table 5), a univariable binary logistic regression model was constructed for this outcome measure. DTI evidence of one or more ROIs with abnormally reduced FA demonstrated a statistically significant univariable OR of 4.5 (p=0.014; Table 6C).
Table 6c.
Univariable Binary Logistic Regression of 6-Month TMT B Versus Statistically Significant Clinical, Demographic, Socioeconomic, CT, and MRI Predictors From Table 5
| Outcome variable | Predictor | Predictor values | Univariable odds ratio (95% CI), p value | Multivariable odds ratio (95% CI), p value | Multivariable model significance | Cox and Snell pseudo-R2 | Nagelkerke pseudo-R2 |
|---|---|---|---|---|---|---|---|
| 6-month TMT B>2 SDs above age-adjusted mean (N=61) | Any DTI axonal injury (≥1 ROI with FA>2.2 SDs below control-group mean) | Yes (19) No (42) |
4.5 (1.3, 15.1); p=0.014* |
4.5 (1.3, 15.1); p=0.014* |
p=0.015* | 9.5%* | 13.9%* |
CT, computed tomography; MRI, magnetic resonance imaging; GOS-E,Glasgow Outcome Scale-Extended; CI, confidence interval; DTI, diffusion tensor imaging; ROI, region of interest; FA, fractional anisotropy; SD, standard deviation; RPQ, Rivermead Postconcussion Questionnaire; TMT B, Trail Making Test B.
p≤0.05 (light-gray box) †p≤0.01 (medium-gray box) ‡p≤0.001 (dark-gray box) §p≤0.0001 (dark-gray box).
For 6-month RPQ-3, only neuropsychiatric history and history of drug or alcohol abuse demonstrated statistically significant univariable ORs. The multivariable ordinal logistic regression model for 6-month RPQ-3, including both of these predictors, was also statistically significant (pseudo-R2 of 9.5–13.9%; p=0.015).
Analysis of subset of patients without pre-existing neuropsychiatric or substance abuse history
Most previous studies of DTI in mTBI have excluded patients with history of neuropsychiatric disease or substance abuse on the grounds that DTI results could be influenced by one or both of these factors. We performed whole-brain voxel-wise nonparametric statistical comparison of FA in CT/MRI-negative patients with a positive history of neuropsychiatric disease or substance abuse (n=24), compared to those without (n=20). Many areas of reduced FA at p<0.25 (though not at p<0.05) were found. Therefore, to address the possibility that a previous history of substance abuse and/or neuropsychiatric disease could have influenced our results, we separately analyzed the subset of mTBI patients without such history. Supplementary Tables S2 and S3 (see online supplementary material at http://www.liebertpub.com) summarize demographic, socioeconomic, and clinical characteristics, and 3- and 6-month outcome measures, for this subset of 37 mTBI patients without history of substance abuse or neuropsychiatric disease.
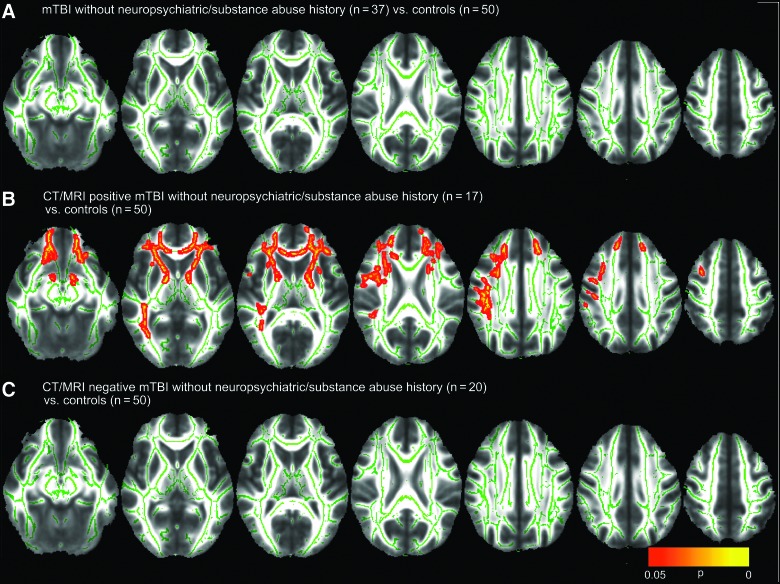
Figure 2A is analogous to Figure 1A, but compares only mTBI patients without history of neuropsychiatric disorder or substance abuse (n=37) to control subjects (n=50). Unlike Figure 1A, no significant group differences in FA (Fig. 2A), MD, RD, or AD were found.
FIG. 2.
Voxel-wise nonparametric statistical comparison between mild traumatic brain injury (mTBI) patients without previous history of substance abuse or other neuropsychiatric disorder and controls, with corrections for multiple voxel-wise comparisons using threshold-free cluster enhancement. This analysis was used to compare (A) 37 mTBI patients without pre-existing substance abuse or neuropsychiatric history to 50 controls, (B) the subgroup of 17 computed tomography/magnetic resonance imaging (CT/MRI)-positive mTBI patients to the 50 controls, and (C) the subgroup of 20 CT/MRI-negative patients to the 50 controls. Voxel clusters with statistically significant differences in fractional anisotropy (FA) between mTBI and control groups at p<0.05 are shown in red/orange/yellow, with yellow denoting greater statistical significance. (B) shows that CT/MRI-positive mTBI patients without substance abuse or neuropsychiatric history demonstrated significantly lower FA in the anterior and posterior limbs of the internal capsules, external capsules, uncinate fasciculi, genu of the corpus callosum, and anterior corona radiata bilaterally. In contrast, (C) shows that this method demonstrated no evidence for white matter injury in CT/MRI-negative mTBI. Color image is available online at www.liebertpub.com/neu
Analogous to Figure 1B, Figure 2B compares CT/MRI-positive mTBI patients without neuropsychiatric or substance abuse history (n=17) to controls (n=50). There are extensive areas of reduced FA in the CT/MRI-positive mTBI patients, despite the expected loss of statistical power for comparison of this small subgroup of only 17 CT/MRI-positive mTBI patients to controls. No region of increased FA, or of increased or reduced MD, AD, or RD, was observed in CT/MRI-positive mTBI patients, relative to controls, at p<0.05.
Finally, analogous to results in Figure 1C, no significant group differences in FA (Fig. 2C), MD, RD, or AD were found in CT/MRI-negative patients without neuropsychiatric or substance abuse history (n=20), compared to controls (n=50), at p<0.05.
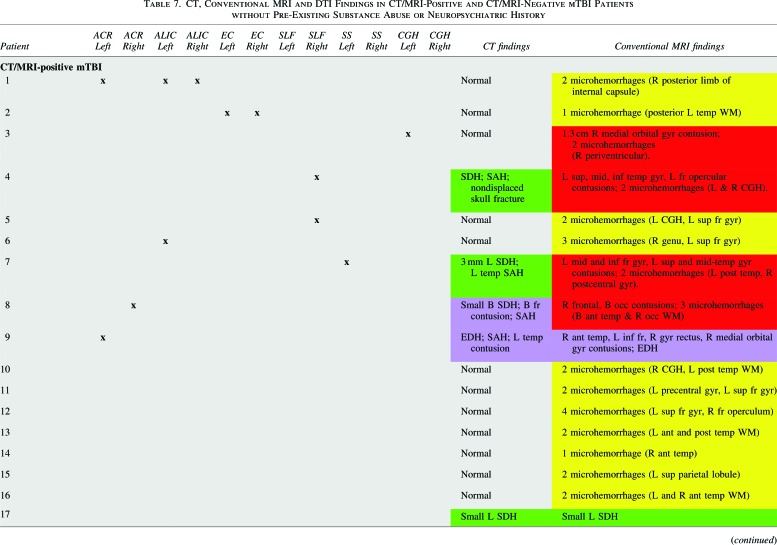
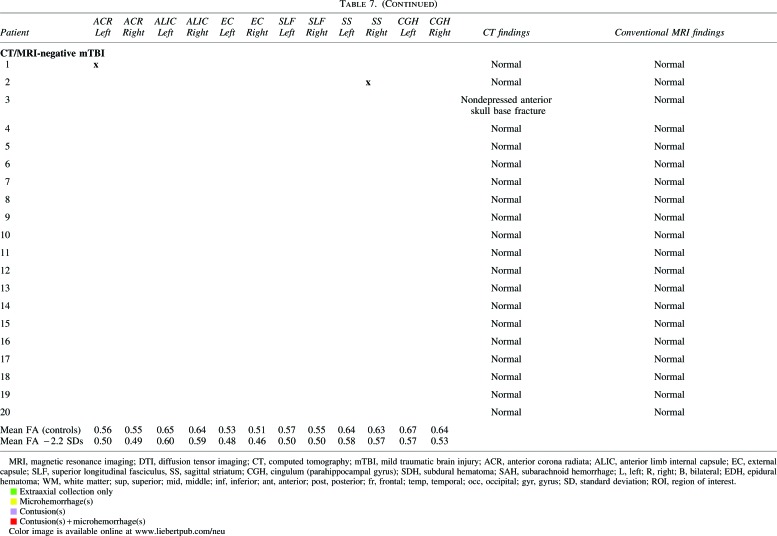
Table 7 shows that all 17 of 17 (100.0%) CT/MRI-positive mTBI patients without neuropsychiatric or substance abuse history had abnormal conventional MRI, but only 5 of 17 (24%) had abnormal head CT. One patient with a nondepressed anterior skull base fracture had no CT or MRI evidence of traumatic brain lesion or intracranial hemorrhage and was classified as CT/MRI-negative mTBI (analogous to the classification of isolated nondepressed skull fracture as uncomplicated mTBI in previous literature38). On conventional MRI sequences, most CT/MRI-positive mTBI patients (11 of 17; 64.7%) demonstrated isolated foci of hemorrhagic axonal injury without brain contusion; 4 of 17 (23.5%) demonstrated both hemorrhagic axonal injury and brain contusion; 1 of 17 (5.9%) demonstrated brain contusions and EDH; and 1 of 17 (5.9%) had isolated SDH.
Tables 7 and 8 also show results of ROI analysis of the 17 CT/MRI-positive and 20 CT/MRI-negative mTBI patients without a history of neuropsychiatric or substance abuse. Table 7 shows lesions with abnormally low FA (FA more than 2.2 SDs below the control-group mean) in individual patients. Table 8 shows that such lesions were observed in ≥1 ROIs for 9 of 17 CT/MRI-positive mTBI (52.9%), 2 of 20 CT/MRI-negative mTBI (10.0%), and 5 of 50 (10.0%) control subjects. Fisher's exact test showed a highly significant difference between the proportions of CT/MRI-positive mTBI patients (52.9%) and control subjects (10.0%) with ≥1 abnormal ROIs (p=0.0006). There was also a highly significant difference between the proportions of CT/MRI-positive mTBI patients (52.9%) and CT/MRI-negative mTBI patients (10.0%) with ≥1 abnormal ROIs (p=0.0097). However, there was no difference in the proportions of CT/MRI-negative mTBI patients (10.0%) and controls (10.0%) with ≥1 abnormal ROIs (p=1.0). Finally, there was no significant difference among CT/MRI-positive mTBI, CT/MRI-negative mTBI, and control subject groups in terms of the proportion of subjects with ≥1 ROI with abnormally high FA (p=0.75).
Table 8.
DTI Region-of-Interest (ROI) Analysis: Group Differences in Presence of One or More Abnormal ROIs among CT/MRI-Negative mTBI and CT/MRI-Positive mTBI without Neuropsychiatric or Substance Abuse History and Control Subjects
| CT/MRI-negative mTBI (20 subjects) | CT/MRI-positive mTBI (17 subjects) | Controls (50 subjects) | |
|---|---|---|---|
| Number of subjects (Proportion of subjects) | Number of subjects (Proportion of subjects) | Number of subjects (proportion of subjects) | |
| One or more ROIs with FA more than 2.2 SDs below control-group mean | 2 (10.0%)a | 9 (52.9%)b | 5 (10.0%)a |
| One or more ROIs with FA more than 2.2 SD above control-group mean | 3 (15.0%)c | 1 (5.9%)c | 5 (10.0%)c |
Each superscript denotes a subset of participants whose column proportions do not differ significantly from one another, by Fisher's exact test with p<0.05. Row 1: There was a significant difference between the proportions of CT/MRI-positive (52.9%) and CT/MRI-negative mTBI patients (10.0%) with one or more ROIs with FA more than 2.2 SDs below the control group mean (p=0.0097). There was also a highly significant difference between CT/MRI-positive mTBI patients (52.9%) and controls (10.0%; p=0.0006). However, there was no difference between CT/MRI-negative mTBI patients (10.0%) and controls (10.0%; p=1.0). Row 2: There was no significant difference among the proportions of CT/MRI-negative mTBI (15.0%), CT/MRI-positive mTBI (5.9%), and control subjects (10.0%) with one or more ROIs with FA more than 2.2 SDs above the control group mean (p=0.75).
CT, computed tomography; MRI, magnetic resonance imaging; DTI, diffusion tensor imaging; ROI, region of interest; mTBI, mild traumatic brain injury; FA, fractional anisotropy; SD, standard deviation.
Table 9 is analogous to Table 5 and shows the pairwise Spearman's correlations between 3- and 6-month outcome measures and demographic, socioeconomic, clinical, CT, and MRI predictors in patients without a history of neuropsychiatric or substance abuse. Except for an expected correlation52 of years of education with TMT B z-score (ρ=−0.50; p=0.007), and correlation of TMT A z-score with age (ρ=−0.39; p=0.04) and with PTA duration (ρ=0.48; p=0.014), no demographic, socioeconomic, or clinical variable (age, gender, employment status, GCS, PTA, PTA duration, LOC, or history of earlier TBI) was otherwise significantly correlated at p<0.05 with worse performance on any outcome measure; all demographic, socioeconomic, and clinical variables were thus excluded from Table 9 for brevity. Similarly, 6-month TMT A (both age-adjusted z-score and the dichotomized score), TMT B (z-score), CVLT-II (both age-adjusted scaled score and dichotomized score), and WAIS-IV PSI (both age-adjusted scaled score and dichotomized score) were also omitted from Table 9 because they demonstrated no other significant correlation with any other imaging, clinical, demographic, or socioeconomic predictor at p<0.05.
Table 9.
Spearman's Correlation Coefficients (ρ) between Outcome Measures and Early Neuroimaging Pathoanatomic Findings in 37 mTBI Patients without Previous history of Substance Abuse or Other Neuropsychiatric Disordera
| Day-of-injury head CT | Early brain MRI (10.9±3.6 days postinjury) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Nondepressed calvarial or skull base fracture | EDH | SDH | SAH | Any CT Contusion | Any acute traumatic intracranial CT finding | Any MRI contusion | Any MRI T2* hemorrhagic axonal injury | Any DTI axonal injury (≥1 ROI with FA >2.2 SDs below control-group mean) | Any conventional MRI and/or DTI lesion | |
| 3-month GOS-E (N=32) |
−0.15 p=0.40 (4 positive) |
−0.05 p=0.78 (1 positive) |
−0.24 p=0.19 (5 positive) |
−0.28 p=0.12 3 (positive) |
−0.28 p=0.12 (5 positive) |
−0.24 p=0.19 (5 positive) |
−0.47 p=0.006* (5 positive) |
−0.41 p=0.02 (12 positive) |
−0.50 p=0.004† (10 positive) |
−0.37 p=0.04* (14 positive) |
| 6-month GOS-E (N=30) |
−0.06 p=0.75 (3 positive) |
−0.06 p=0.76 (1 positive) |
−0.21 p=0.26 (3 positive) |
−0.08 p=0.67 (2 positive) |
−0.21 p=0.26 (2 positive) |
−0.21 p=0.26 (3 positive) |
−0.22 p=0.25 (4 positive) |
−0.29 p=0.12 (11 positive) |
−0.30 p=0.11 (7 positive) |
−0.39 p=0.03* (13 positive) |
| Abnormal TMT A (>2 SDs above mean) at 6 months (N=27) |
−0.14 p=0.50 N=2 (2 positive) |
−0.09 p=0.64 N=27 (1 positive) |
−0.17 p=0.40 N=27 (3 positive) |
−0.14 p=0.50 N=27 (2 positive) |
−0.14 p=0.50 N=27 (2 positive) |
−0.17 p=0.40 N=27 (3 positive) |
−0.20 p=0.32 (4 positive) |
0.01 p=0.97 (11 positive) |
0.15 p=0.45 (7 positive) |
0.11 p=0.57 (13 positive) |
| Abnormal TMT B (>2 SDs above mean) at 6 months (N=27) |
−0.17 p=0.40 (2 positive) |
−0.12 p=0.56 (1 positive) |
0.06 p=0.77 (3 positive) |
0.16 p=0.44 (2 positive) |
−0.17 p=0.40 (2 positive) |
0.06 p=0.77 (3 positive) |
0.23 p=0.25 N=27 (4 positive) |
0.20 p=0.32 N=27 (11 positive) |
0.42 p=0.03* N=27 (7 positive) |
0.28 p=0.16 N=27 (13 positive) |
| 6-month RPQ-3 (N=30) |
−0.10 p=0.60 (3 positive) |
−0.21 p=0.26 (1 positive) |
0.13 p=0.48 (3 positive) |
−0.02 p=0.90 (2 positive) |
0.03 p=0.87 (2 positive) |
0.13 p=0.48 (3 positive) |
0.27 p=0.15 (4 positive) |
0.32 p=0.09 (11 positive) |
0.12 p=0.54 (7 positive) |
0.23 p=0.22 (13 positive) |
| 6-month RPQ-13 (N=30) |
−0.06 p=0.74 (3 positive) |
−0.13 p=0.50 (1 positive) |
0.22 p=0.25 (3 positive) |
0.04 p=0.84 (2 positive) |
0.13 p=0.49 (2 positive) |
0.22 p=0.25 (3 positive) |
0.22 p=0.25 (4 positive) |
0.62 p=0.0003† (11 positive) |
0.40 p=0.03* (7 positive) |
0.61 p=0.0004† (13 positive) |
The only statistically significant pair-wise correlations between any demographic, clinical or socioeconomic predictor and worse performance on any outcome variable were between years of education and TMT B z-score (ρ=−0.50; p=0.007), age and TMT A z-score (ρ=−0.39; p=0.04), and PTA duration and TMT A z-score (ρ=0.48; p=0.014). Thus, for brevity, all demographic, clinical, and socioeconomic predictors (Supplementary Table 2) (see online supplementary material at http://www.liebertpub.com) are omitted from Table 9. Similarly, 6-month TMT A (both z-score and dichotomized score), TMT B (z-score), CVLT-II (scaled score and dichotomized score), and WAIS-IV PSI (scaled score and dichotomized score) were omitted from Table 9, because they demonstrated no other significant correlation with any imaging, clinical, demographic, or socioeconomic predictor at p<0.05. *p<0.05 (light-gray boxes); †p<0.01 (dark-gray boxes).
CVLT-II, California Verbal Learning Test–Second Edition; EDH,epidural hematoma; SDH, subdural hematoma; SAH, subarachnoid hemorrhage; DTI, diffusion tensor imaging; ROI, region of interest; SD, standard deviation; FA, fractional anisotropy; GOS-E, Glasgow Outcome Scale-Extended; TMT, Trail Making Test; RPQ, Rivermead Postconcussion Questionnaire; CVLT, California Verbal Learning Test; WAIS-IV PSI, Wechsler Adult Intelligence Scale–Fourth Edition, Processing Speed Index.
Table 9 shows that among the imaging predictors, no CT feature (CT evidence of nondepressed skull fracture, EDH, SDH, SAH, or contusion) was significantly correlated with any outcome measure at p<0.05. In contrast, several MRI features, including MRI evidence of contusion, MRI evidence of hemorrhagic axonal injury, and presence of abnormally reduced FA in at least one ROI, demonstrated statistically significant correlations with several outcome measures (3- and 6-month GOS-E, abnormal 6-month TMT B, and the 6-month RPQ-13).
Discussion
In the current study, white matter FA was significantly reduced in CT/MRI-positive, but not in CT/MRI-negative, mTBI patients, compared to healthy control subjects, on a group level. In addition, regions of reduced FA in individual mTBI patients were modest, but statistically significant, predictors of unfavorable 3- and 6-month outcome. These results held true for both the inclusive sample of 76 mTBI patients as well as the subset of 37 mTBI patients with no history of previous substance abuse or other neuropsychiatric disorder.
Previous studies have reported evidence of white matter injury on DTI in the acute-to-subacute time period after mTBI.15–18,20,23–25,27–31,34–36 In essentially all of these studies, patients with history of substance abuse or other neuropsychiatric disorders were excluded. In addition, in nearly all of these studies, the mTBI study population included a mixed group of both CT/MRI-positive and -negative mTBI, based on presence of intracranial abnormalities on CT alone, CT and 1.5T MRI, or CT and 3T MRI. Miles and colleagues31 found, using an ROI approach, reduced average FA and increased average MD within six ROIs in a group-wise comparison of 17 mTBI patients, studied within 10 days of injury at 1.5T MRI and with no evidence of microhemorrhages, to 29 age- and gender-matched controls. In contrast, Ling and colleagues24 found increased FA and decreased RD, within the callosal genu, in a mixture of 28 CT/MRI-negative and -positive mTBI patients who underwent MRI 15.6±4.3 days after injury. Messe and colleagues,30 using a whole-brain voxelwise approach to study a mixture of CT/MRI-negative and -positive mTBI patients, found higher MD values in poor-outcome patients, compared to good-outcome patients and controls, in the corpus callosum, right anterior thalamic radiations, superior longitudinal fasciculus, and inferior longitudinal and fronto-occipital fasciculi at 7–28 days after injury. Lange and colleagues,23 using an ROI approach, found no significant difference in FA or MD in the genu, body, or splenium of the corpus callosum in 60 CT/MRI-positive and -negative mTBI patients (on the more severe end of the mTBI spectrum), relative to 34 trauma controls. A smaller number of studies20,25,27,35 has reported statistically significant group-wise or individual FA differences in the acute-to-subacute time period in strictly CT/MRI-negative mTBI patients versus controls. For example, Lipton and colleagues, using a whole-brain voxelwise approach, found reduced FA in multiple white matter regions at 2–14 days postinjury in 20 CT/MRI-negative mTBI patients, compared to 20 age- and gender-matched controls.27 McAllister and colleagues56 found a statistically significant correlation between mean and maximum strain rate (based on measurements from instrumented helmets and finite element biomechanical simulation) and increased FA in the corpus callosum within the first 10 days after concussion in athletes with normal conventional brain MRI.
From the above, it is evident that DTI analysis techniques have varied between more data-driven, whole-brain voxel-wise analyses and hypothesis-driven ROI approaches. In addition, although nearly all studies have employed group-comparison designs, some investigators have chosen to compare mTBI patients to healthy controls (in some cases, matched by age, gender, and/or education), whereas others have compared mTBI subgroups with good versus poor outcome. These earlier studies, most of which are limited by small sample sizes, have also not analyzed DTI results in the context of important clinical, demographic, and socioeconomic factors relevant to TBI outcomes. Finally, there is a persistent and striking inconsistency across different DTI studies, in terms of the reported direction of changes in DTI measures after mTBI.
Whole-brain voxel-wise approaches may have limited sensitivity as a result of the heterogeneity of spatial distribution of white matter injury in mTBI; on the other hand, the ROI approach may be limited by failure to interrogate less-common areas of white matter injury. We employed both of these as complementary approaches in the current study and demonstrated that microstructural white matter injury severity does vary, on a group level, according to the presence of more-familiar macroscopic pathoanatomic lesions on CT and conventional MRI. It may not be surprising that the data show that CT/MRI-positive mTBI patients have more extensive white matter injury than CT/MRI-negative mTBI patients. However, such work is relevant because any utility of DTI in outcome prediction would be contingent on demonstration of a differential increase in diagnostic or prognostic accuracy beyond conventional CT and MRI as well as clinical, demographic, and socioeconomic predictors.
In this study of 76 mTBI patients and 50 control subjects, and using current DTI acquisition and postprocessing techniques, CT/MRI-positive mTBI patients demonstrated evidence of white matter injury when employing either whole-brain voxel-wise or ROI approaches. Indeed, we found no evidence for white matter injury, using either the whole-brain voxel-wise or ROI methods, in mTBI patients without lesions on CT or 3T MRI that included high-resolution 3D T1- and T2-weighted sequences as well as T2*-weighted gradient echo sequences. These findings held true in both the inclusive group of 76 mTBI patients, as well as the subset of 37 patients with no previous history of substance abuse or other neuropsychiatric disorders. There are several possible reasons for the discrepancy between our results with a few earlier studies demonstrating statistically significant FA differences on acute-to-subacute 3T DTI between strictly CT/MRI-negative mTBI patients and controls.20,25,27,35 Technical differences in DTI acquisition or DTI postprocessing techniques could always be an explanation for such differences. The effect size and incidence of white matter injury in CT/MRI-negative mTBI may be too small, or the severity and/or spatial distribution too variable among patients, to show statistically significant group differences based on the number of patients and analysis approach employed in the current study. The injury-to-MRI interval may be a critical factor; it has been postulated that a variety of different biological processes within injured white matter may vary not only according to injury severity, but also at different time intervals after injury, and that FA, in particular, may be abnormally increased within the first week of injury.16,18,29,35,36 Patients in the current study underwent MRI during the first 3 weeks after injury (11.2±3.3 days), when different biological processes and thus DTI parameters may still have been evolving. Finally, it is possible that our results differ because many cases of CT/MRI-positive mTBI in this study were placed in that group on the basis of very subtle MRI lesions at 3T, such as one or two subtle isolated foci of hemorrhagic axonal injury, and may have been classified as uncomplicated mTBI in other studies. This third explanation has the appeal of being compatible with earlier literature that reports DTI evidence of white matter injury in subjects classified as uncomplicated mTBI based on CT alone.15,16,18,36 Another main aim of this work was to investigate the utility of DTI parameters as predictors of individual outcome. We thus determined and compared ORs for a variety of demographic, socioeconomic, clinical, and imaging predictors, including DTI parameters. Our data suggest that MRI predictors, particularly MRI evidence of contusion and DTI evidence of one or more ROIs with reduced FA, and clinical and socioeconomic predictors, including education and previous history of neuropsychiatric disorder, surpass most CT features for prediction of most 3- and 6-month outcome measures.
Analysis of the subset of mTBI patients without a previous history of substance abuse and/or neuropsychiatric disease (Fig. 2; Tables 7–9 and Supplementary Tables 2 and 3) (see online supplementary material at http://www.liebertpub.com) is informative, because it addresses the problem of a possible strong confounding influence of these pre-existing conditions owing to their potential relationships with both DTI parameters and outcome. In this subset analysis, it was actually necessary to separate CT/MRI-positive from CT/MRI-negative mTBI patients to see any evidence of white matter injury using either the whole-brain voxel-wise or ROI approaches. Specifically, the whole-brain voxel-wise analysis (Fig. 2) and ROI analysis (Tables 7 and 8) both demonstrate differences between CT/MRI-positive and -negative mTBI patients that are even more striking and statistically significant than in the original analysis of the inclusive group of 76 mTBI patients. Table 8 shows a strikingly higher prevalence of abnormal ROIs with reduced FA in CT/MRI-positive patients without previous history of substance abuse or other neuropsychiatric disorders, relative to both the CT/MRI-negative mTBI patients (p=0.004) and the control group (p=0.0002); in contrast, the same prevalence of abnormal ROIs with reduced FA was observed in CT/MRI-negative patients (10.0%) and in the control group (10.0%).
It is noteworthy that both conventional MRI and DTI predictors demonstrated stronger correlation coefficients with 3- and 6-month outcome measures in the subset of 37 patients lacking any history of neuropsychiatric disease or substance abuse (Table 9) than in the larger inclusive sample of 76 patients (Table 5), despite the much smaller sample size of the former. We postulate that this is because correlations of pre-existing factors, such as neuropsychiatric disease, with the outcome measures (e.g., in Table 5) may have weakened the apparent influence or relevance of the imaging predictors.
It is also notable that there were generally much stronger correlations of MRI predictors with 3-month GOS-E than with 6-month GOS-E. This is plausible, because the MRI exams in this study were performed within 3 weeks after mTBI. Abnormal MRI features in the initial days after injury, which demonstrated a strong correlation with 3-month GOS-E, may be less relevant at 6 months, after a variable degree of recovery has taken place in different patients. The stronger correlation with the GOS-E at 3 months, compared to 6 months, is unlikely to be attributable solely to general overall improvement in the GOS-E over time: Though many individual patients' scores changed between the two time points, there was negligible change in the overall distribution of GOS-E scores at 3 versus 6 months (Table 4 and Supplementary Table 3) (see online supplementary material at http://www.liebertpub.com).
In this study, we sought to minimize the influence of confounding factors on group differences in DTI parameters between patient and control groups. Thus, we did not follow the approach of presorting patients according to an outcome measure, and thereafter assessing for group differences in DTI results according to good or poor outcome, because there are many potential confounding factors that could affect both DTI measures and outcome. Further, we analyzed, in addition to the original inclusive sample, the subset of patients lacking any significant reported substance abuse or other neuropsychiatric history, because these pre-existing conditions are heterogeneous by nature and thus difficult to control for in group comparisons and could act as confounding variables that could create or exacerbate group differences in DTI measures. Finally, because there was a nonsignificant, but noticeable, difference in number of years of education among CT/MRI-positive mTBI, CT/MRI-negative mTBI, and control groups, we explicitly demonstrated that there were no group differences in DTI measures, using either the DTI or ROI approach, between the most- and least-educated control subjects.
This study has several limitations. Alteration of DTI parameters in TBI has been linked to a variety of possible pathophysiological mechanisms, such as axonal disruption, axonal degeneration, and cytotoxic edema; recent work also suggests that DTI parameters, such as FA and MD, may be correlated with strain and strain rate in mTBI.56 Nevertheless, despite our attempt, in performing the subset analysis, to minimize or eliminate the influence of confounding factors that could account for both DTI lesions and poorer outcome, we acknowledge that lesions in the DTI ROI analysis are nonspecific and may reflect the patient's pre-existing brain structure, rather than a traumatic lesion.33 Second, a substantial unexplained variance in outcomes remains, even for our most inclusive models that were based on DTI, conventional neuroimaging, and other predictors (Table 6). Third, because the number of predictors we investigated was large, relative to the number of patients, this study should be regarded as exploratory and in need of confirmation in a larger study population. Finally, even for pathoanatomic findings, such as contusion and SAH, that can be definitively attributed to acute TBI based on their unique imaging appearance, the existence of any direct pathophysiological mechanism that accounts for their correlation with outcome remains uncertain.
In summary, this study provides evidence for the importance of individual pathoanatomic features on MRI, including DTI parameters, for prognosis after mTBI. Specifically, several MRI predictors, including DTI parameters, surpassed CT features for prediction of 3- and 6-month outcome measures. For the subset of patients lacking any significant neuropsychiatric or substance abuse history, MRI predictors, including DTI parameters, surpassed all clinical, demographic, socioeconomic, and CT features for prediction of 3- and 6-month outcome. Our results should be viewed as relevant primarily to mTBI patients who meet ACEP/CDC ED criteria for head CT and who thus generally have more severe injuries than mTBI patients who are not triaged to head CT. Our results support the potential utility of MRI and DTI in the acute/subacute stage of acute mTBI for better classification of injury severity. Effective, practical imaging markers that identify mTBI patients who will have unfavorable outcome are essential for clinical trials to evaluate treatments and for better triage to effective follow-up care.
Supplementary Material
Contributor Information
Collaborators: and the TRACK-TBI INVESTIGATORS including
Acknowledgments
This study was supported by National Institutes of Health (NIH) grants NS069409 and NS069409-02S1 (principal investigator [PI]: G.T.M.) and NS60776 (PI: P.M.) and Department of Defense United States Army Medical Research Acquisition Activity W81XWH-13-1-0441 (PI: G.T.M.). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NINDS or the NIH.
Author Disclosure Statement
No competing financial interests exist
References
- 1.Faul M., Xu L., Wald M.M., and Coronado V.G. (2010). Traumatic brain injury in the United States: emergency department visits, hospitalizations, and deaths. Centers for Disease Control and Prevention, National Center for Injury Prevention and Control: Atlanta, GA [Google Scholar]
- 2.Mild Traumatic Brain Injury Committee. Head Injury Interdisciplinary Special Interest Group of the American Congress of Rehabilitation Medicine. (1993). Definition of mild traumatic brain injury. J. Head Trauma Rehabil. 8, 86–87 [Google Scholar]
- 3.National Center for Injury Prevention and Control. (2003). Report to Congress on mild traumatic brain injury in the United States: steps to prevent a serious public health problem. Centers for Disease Control and Prevention: Atlanta, GA [Google Scholar]
- 4.Carroll L.J., Cassidy J.D., Holm L., Kraus J., and Coronado V.G.; WHO Collaborating Centre Task Force on Mild Traumatic Brain Injury. (2004). Methodological issues and research recommendations for mild traumatic brain injury: the WHO Collaborating Center Task Force on Mild Traumatic Brain Injury. J, Rehabil. Med. 43, Suppl., 113–125 [DOI] [PubMed] [Google Scholar]
- 5.Bernstein D.M. (1999). Recovery from mild head injury. Brain Inj. 13, 151–172 [DOI] [PubMed] [Google Scholar]
- 6.Lee H., Wintermark M., Gean A.D., Ghajar J., Manley G.T., and Mukherjee P. (2008). Focal lesions in acute mild traumatic brain injury and neurocognitive outcome: CT versus 3T MRI. J. Neurotrauma 25, 1049–1056 [DOI] [PubMed] [Google Scholar]
- 7.Hessen E., and Nestvold K. (2009). Indicators of complicated mild TBI predict MMPI-2 scores after 23 years. Brain Inj. 23, 234–242 [DOI] [PubMed] [Google Scholar]
- 8.Kashluba S., Hanks R.A., Casey J.E., and Millis S.R. (2008). Neuropsychologic and functional outcome after complicated mild traumatic brain injury. Arch. Phys. Med. Rehabil. 89, 904–911 [DOI] [PubMed] [Google Scholar]
- 9.Thornhill S., Teasdale G.M., Murray G.D., McEwen J., Roy C.W., and Penny K.I. (2000). Disability in young people and adults one year after head injury: prospective cohort study. BMJ 320, 1631–1635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dikmen S., Machamer J., Fann J.R., and Temkin N.R. (2010). Rates of symptom reporting following traumatic brain injury. J. Int. Neuropsychol. Soc. 16, 401–411 [DOI] [PubMed] [Google Scholar]
- 11.Carroll L.J., Cassidy J.D., Peloso P.M., Borg J., von Holst H., Holm L., Paniak C., and Pepin M. (2004). Prognosis for mild traumatic brain injury: results of the WHO Collaborating Center Task Force on Mild Traumatic Brain Injury. J. Rehabil. Med. 43, Suppl., 84–105 [DOI] [PubMed] [Google Scholar]
- 12.McMahon P.J., Hricik A.J., Yue J.K., Puccio A.M., Inoue T., Lingsma H.F., Beers S.R., Gordon W., Valadka A., Manley G.T., and Okonkwo D.O.; Track-TBI Investigators, Casey SS, Cooper SR, Dams-O'Connor K, Menon DK, Sorani MD, Yuh EL, Mukherjee P, Schnyer DM, Vassar MJ. (2014). Symptomatology and functional outcome in mild traumatic brain injury: results from the prospective TRACK-TBI Study. J. Neurotrauma 31, 26–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Iverson G.L. (2010). Mild traumatic brain injury meta-analyses can obscure individual differences. Brain Inj. 24, 1246–1255 [DOI] [PubMed] [Google Scholar]
- 14.Saatman K.E., Duhaime A.C., Bullock R., Maas A.I., Valadka A., and Manley G.T. (2008). Classification of traumatic brain injury for targeted therapies. J. Neurotrauma 25, 719–738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Arfanakis K., Haughton V.M., Carew J.D., Rogers B.P., Dempsey R.J., and Meyerand M.E. (2002). Diffusion tensor MR imaging in diffuse axonal injury. AJNR Am. J. Neuroradiol. 23, 794–802 [PMC free article] [PubMed] [Google Scholar]
- 16.Bazarian J.J., Zhong J., Blyth B., Zhu T., Kavcic V., and Peterson D. (2007). Diffusion tensor imaging detects clinically important axonal damage after mild traumatic brain injury: a pilot study. J. Neurotrauma 24, 1447–1459 [DOI] [PubMed] [Google Scholar]
- 17.Bazarian J.J., Zhu T., Blyth B., Borrino A., and Zhong J. (2012). Subject-specific changes in brain white matter on diffusion tensor imaging after sports-related concussion. Magn. Reson. Imaging 30, 171–180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chu Z., Wilde E.A., Hunter J.V., McCauley S.R., Bigler E.D., Troyanskaya M., Yallampalli R., Chia J.M., and Levin H.S. (2010). Voxel-based analysis of diffusion tensor imaging in mild traumatic brain injury in adolescents. AJNR Am. J. Neuroradiol. 31, 340–346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cubon V.A., Putukian M., Boyer C., and Dettwiler A. (2011). A diffusion tensor imaging study on the white matter skeleton in individuals with sports-related concussion. J. Neurotrauma 28, 198–201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim N., Branch C.A., Kim M., and Lipton M.L. (2013). Whole brain approaches for identification of microstructural abnormalities in individual patients: comparison of techniques applied to mild traumatic brain injury. PLoS One 8, e59382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kraus M.F., Susmaras T., Caughlin B.P., Walker C.J., Sweeney J.A., and Little D.M. (2007). White matter integrity and cognition in chronic traumatic brain injury: a diffusion tensor imaging study. Brain 130, 2508–2519 [DOI] [PubMed] [Google Scholar]
- 22.Kumar R., Gupta R.K., Husain M., Chaudhry C., Srivastava A., Saksena S., and Rathore R.K.S. (2009). Comparative evaluation of corpus callosum DTI metrics in acute mild and moderate traumatic brain injury: its correlation with neuropsychometric tests. Brain Inj. 23, 675–685 [DOI] [PubMed] [Google Scholar]
- 23.Lange R.T., Iverson G.L., Brubacher J.R., Madler B., and Heran M.K. (2012). Diffusion tensor imaging findings are not strongly associated with postconcussional disorder 2 months following mild traumatic brain injury. J. Head Trauma Rehabil. 27, 188–198 [DOI] [PubMed] [Google Scholar]
- 24.Ling J.M., Pena A., Yeo R.A., Merideth F.L., Klimaj S., Gasparovic C., and Mayer A.R. (2012). Biomarkers of increased diffusion anisotropy in semi-acute mild traumatic brain injury: a longitudinal perspective. Brain 135, 1281–1292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lipton M.L., Kim N., Park Y.K., Hulkower M.B., Gardin T.M., Shifteh K., Kim M., Zimmerman M.E., Lipton R.B., and Branch C.A. (2012). Robust detection of traumatic axonal injury in individual mild traumatic brain injury patients: intersubject variation, change over time and bidirectional changes in anisotropy. Brain Imaging Behav. 6, 329–342 [DOI] [PubMed] [Google Scholar]
- 26.Lipton M.L., Gellella E., Lo C., Gold T., Ardekani B.A., Shifteh K., Bello J.A., and Branch C.A. (2008). Multifocal white matter ultrastructural abnormalities in mild traumatic brain injury with cognitive disability: a voxel-wise analysis of diffusion tensor imaging. J. Neurotrauma 25, 1335–1342 [DOI] [PubMed] [Google Scholar]
- 27.Lipton M.L., Gulko E., Zimmerman M.E., Friedman B.W., Kim M., Gellella E., Gold T., Shifteh K., Ardekani B.A., and Branch C.A. (2009). Diffusion-tensor imaging implicates prefrontal axonal injury in executive function impairment following very mild traumatic brain injury. Radiology 252, 816–824 [DOI] [PubMed] [Google Scholar]
- 28.Mayer A.R., Ling J., Mannell M.V., Gasparovic C., Phillips J.P., Doezema D., Reichard R., and Yeo R.A. (2010). A prospective diffusion tensor imaging study in mild traumatic brain injury. Neurology 74, 643–650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McAllister T.W., Ford J.C., Ji S., Beckwith J.G., Flashman L.A., Paulsen K., and Greenwald R.M. (2012). Maximum principal strain and strain rate associated with concussion diagnosis correlates with changes in corpus callosum white matter indices. Ann. Biomed. Eng. 40, 127–140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Messe A., Caplain S., Paradot G., Garrigue D., Mineo J.F., Soto Ares G., Ducreux D., Vignaud F., Rozec G., Desal H., Pelegrini-Issac M., Montreuil M., Benali H., and Lehericy S. (2011). Diffusion tensor imaging and white matter lesions at the subacute stage in mild traumatic brain injury with persistent neurobehavioral impairment. Hum. Brain Mapp. 32, 999–1011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Miles L., Grossman R.I., Johnson G., Babb J.S., Diller L., and Inglese M. (2008). Short-term DTI predictors of cognitive dysfunction in mild traumatic brain injury. Brain Inj. 22, 115–122 [DOI] [PubMed] [Google Scholar]
- 32.Niogi S.N., Mukherjee P., Ghajar J., Johnson C., Kolster R.A., Sarkar R., Lee H., Meeker M., Zimmerman R.D., Manley G.T., and McCandliss B.D. (2008). Extent of microstructural white matter injury in postconcussive syndrome correlates with impaired cognitive reaction time: a 3T diffusion tensor imaging study of mild traumatic brain injury. AJNR Am. J. Neuroradiol. 29, 967–973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Niogi S.N., Mukherjee P., Ghajar J., Johnson C.E., Kolster R., Lee H., Suh M., Zimmerman R.D., Manley G.T., and McCandliss B.D. (2008). Structural dissociation of attentional control and memory in adults with and without mild traumatic brain injury. Brain 131, 3209–3221 [DOI] [PubMed] [Google Scholar]
- 34.Smits M., Houston G.C., Dippel D.W., Wielopolski P.A., Vernooij M.W., Koudstaal P.J., Hunink M.G., and van der Lugt A. (2011). Microstructural brain injury in post-concussion syndrome after minor head injury. Neuroradiology 53, 553–563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wilde E.A., McCauley S.R., Barnes A., Wu T.C., Chu Z., Hunter J.V., and Bigler E.D. (2012). Serial measurement of memory and diffusion tensor imaging changes within the first week following uncomplicated mild traumatic brain injury. Brain Imaging Behav. 6, 319–328 [DOI] [PubMed] [Google Scholar]
- 36.Wilde E.A., McCauley S.R., Hunter J.V., Bigler E.D., Chu Z., Wang Z.J., Hanten G.R., Troyanskaya M., Yallampalli R., Li X., Chia J., and Levin H.S. (2008). Diffusion tensor imaging of acute mild traumatic brain injury in adolescents. Neurology 70, 948–955 [DOI] [PubMed] [Google Scholar]
- 37.Wortzel H.S., Kraus M.F., Filley C.M., Anderson C.A., and Arciniegas D.B. (2011). Diffusion tensor imaging in mild traumatic brain injury litigation. J. Am. Acad. Psychiatry Law 39, 511–523 [PubMed] [Google Scholar]
- 38.Williams D.H., Levin H.S., and Eisenberg H.M. (1990). Mild head injury classification. Neurosurgery 27, 422–428 [DOI] [PubMed] [Google Scholar]
- 39.Yuh E.L., Mukherjee P., Lingsma H.F., Yue J.K., Ferguson A.R., Gordon W.A., Valadka A.B., Schnyer D.M., Okonkwo D.O., Maas A.I.R., Manley G.T., and Investigators T.-T. (2013). Magnetic resonance imaging improves 3-month outcome prediction in mild traumatic brain injury. Ann. Neurol. 73, 224–235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yue J.K., Vassar M.J., Lingsma H.F., Cooper S.R., Okonkwo D.O., Valadka A., Gordon W.A., Maas A.I.R., Mukherjee P., Yuh E.L., Puccio A.M., Schnyer D.M., Manley G.T., Casey S.S., Cheong M., Dams-O'Connor K., Hricik A.J., Knight E.E., Kulubya E.S., Menon D.K., Morabito D.J., Pacheco J.L., and Sinha T.K. (2013). Transforming research and clinical knowledge in traumatic brain injury (TRACK-TBI) pilot: multicenter implementation of the common data elements for traumatic brain injury. J. Neurotrauma 30, 1831–1844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jagoda A.S., Bazarian J.J., Bruns J.J., Cantrill S.V., Gean A.D., Howard P.K., Ghajar J., Riggio S., Wright D.W., Wears R.L., Bakshy A., Burgess P., Wald M.M., and Whitson R.R. (2008). Clinical policy: Neuroimaging and decisionmaking in adult mild traumatic brain injury in the acute setting. Ann. Emerg. Med. 52, 714–748 [DOI] [PubMed] [Google Scholar]
- 42.Duhaime A.C., Gean A.D., Haacke E.M., Hicks R., Wintermark M., Mukherjee P., Brody D., Latour L., and Riedy G.; Common Data Elements Neuroimaging Working Group Members, Pediatric Working Group Members. (2010). Common data elements in radiologic imaging of traumatic brain injury. Arch. Phys. Med. Rehabil. 91, 1661–1666 [DOI] [PubMed] [Google Scholar]
- 43.Haacke E.M., Duhaime A.C., Gean A.D., Riedy G., Wintermark M., Mukherjee P., Brody D.L., DeGraba T., Duncan T.D., Elovic E., Hurley R., Latour L., Smirniotopoulos J.G., and Smith D.H. (2010). Common data elements in radiologic imaging of traumatic brain injury. J. Magn. Reson. Imaging 32, 516–543 [DOI] [PubMed] [Google Scholar]
- 44.Whyte J., Vasterling J., and Manley G.T. (2010). Common data elements for research on traumatic brain injury and psychological health: current status and future development. Arch. Phys. Med. Rehabil. 91, 1692–1696 [DOI] [PubMed] [Google Scholar]
- 45.Smith S.M. (2002). Fast robust automated brain extraction. Hum. Brain Mapp. 17, 143–155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Behrens T.E.J., Woolrich M.W., Jenkinson M., Johansen-Berg H., Nunes R.G., Clare S., Matthews P.M., Brady J.M., and Smith S.M. (2003). Characterization and propagation of uncertainty in diffusion-weighted MR imaging. Magn. Reson. Med. 50, 1077–1088 [DOI] [PubMed] [Google Scholar]
- 47.Smith S.M., Jenkinson M., Johansen-Berg H., Rueckert D., Nichols T.E., Mackay C.E., Watkins K.E., Ciccarelli O., Cader M.Z., Matthews P.M., and Behrens T.E.J. (2006). Tract-based spatial statistics: voxelwise analysis of multi-subject diffusion data. Neuroimage 31, 1487–1505 [DOI] [PubMed] [Google Scholar]
- 48.Smith S.M., and Nichols T.E. (2009). Threshold-free cluster enhancement: addressing problems of smoothing, threshold dependence and localisation in cluster inference. Neuroimage 44, 83–98 [DOI] [PubMed] [Google Scholar]
- 49.Mori S., Oishi K., Jiang H., Jiang L., Li X., Akhter K., Hua K., Faria A.V., Mahmood A., Woods R., Toga A.W., Pike G.B., Neto P.R., Evans A., Zhang J., Huang H., Miller M.I., van Zijl P., and Mazziotta J. (2008). Stereotaxic white matter atlas based on diffusion tensor imaging in an ICBM template. Neuroimage 40, 570–582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Levin H.S., Boake C., Song J., McCauley S., Contant C.F., Diaz-Marchan P., Brundage S., Goodman H., and Kotra K.J. (2004). Validity and sensitivity to change of the Extended Glasgow Outcome Scale in mild to moderate traumatic brain injury. J. Neurotrauma 18, 575–584 [DOI] [PubMed] [Google Scholar]
- 51.Reitan R.M. (1955). The relation of the trail making test to organic brain damage. J. Consult. Psychol. 19, 393–394 [DOI] [PubMed] [Google Scholar]
- 52.Tombaugh T.N. (2004). Trail Making Test A and B: normative data stratified by age and education. Arch. Clin. Neuropsychol. 19, 203–214 [DOI] [PubMed] [Google Scholar]
- 53.Kennedy J.E., Clement P.F., and Curtiss G. (2003). WAIS-III processing speed index scores after TBI: the influence of working memory, psychomotor speed and perceptual processing. Clin. Neuropsychol. 17, 303–307 [DOI] [PubMed] [Google Scholar]
- 54.Lichtenberger E.O., and Kaufman A.S.Essentials of WAIS-IV Assessment. 2nd ed. Hoboken, NJ: John Wiley and Sons; 2013 [Google Scholar]
- 55.Delis D.C., Kramer J.H., Kaplan E., and Ober B.A. (2000). California Verbal Learning Test–Second Edition, Adult Version. The Psychological Corporation: San Antonio, TX [Google Scholar]
- 56.Stallings G., Boake C., and Sherer M. (1995). Comparison of the California Verbal Learning Test and the Rey Auditory Verbal Learning Test in head-injured patients. J. Clin. Exp. Neuropsychol. 17, 706–712 [DOI] [PubMed] [Google Scholar]
- 57.King N.S., Crawford S., Wenden F.J., Moss N.E., and Wade D.T. (1995). The Rivermead Post Concussion Symptoms Questionnaire: a measure of symptoms commonly experienced after head injury and its reliability. J. Neurol. 242, 587–592 [DOI] [PubMed] [Google Scholar]
- 58.Potter S., Leigh E., Wade D.T., and Fleminger S. (2006). The Rivermead Post Concussion Symptoms Questionnaire: a confirmatory factor analysis. J. Neurol. 253, 1603–1614 [DOI] [PubMed] [Google Scholar]
- 59.Sveen U., Bautz-Holter E., Sandvik L., Alvsåker K., and Roe C. (2010). Relationship between competency in activities, injury severity, and post-concussion symptoms after traumatic brain injury. Scand. J. Occup. Ther. 17, 225–232 [DOI] [PubMed] [Google Scholar]
- 60.Eyres S., Carey A., Gilworth G., Neumann V., and Tennant A. (2005). Construct validity and reliability of the Rivermead Post Concussion Symptoms Questionnaire. Clin. Rehabil. 19, 878–887 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.