Abstract
Acute biliary obstruction leads to periductal myofibroblasts and fibrosis, the origin of which is uncertain. Our study provides new information on this question in mice and humans. We show that bile duct obstruction induces a striking increase in cholangiocyte αvβ6 integrin and that expression of this integrin is directly linked to fibrogenesis through activation of transforming growth factor beta (TGF-β). Administration of blocking antibody to αvβ6 significantly reduces the extent of acute fibrosis after bile duct ligation. Moreover, in β6-null mice subjected to the injury, fibrosis is reduced by 50% relative to that seen in wild-type mice, whereas inflammation occurs to the same extent. The data indicate that αvβ6, rather than inflammation, is linked to fibrogenesis. It is known that αvβ6 binds latent TGF-β and that binding results in release of active TGFβ. Consistent with this, intracellular signaling from the TGFβ receptor is increased after bile duct ligation in wild-type mice but not in β6−/− mice, and a competitive inhibitor of the TGFβ receptor type II blocks fibrosis to the same extent as antibody to αvβ6. In a survey of human liver disease, expression of αvβ6 is increased in acute, but not chronic, biliary injury and is localized to cholangiocyte-like cells.
Conclusion
Cholangiocytes respond to acute bile duct obstruction with markedly increased expression of αvβ6 integrin, which is closely linked to periductal fibrogenesis. The findings provide a rationale for the use of inhibitors of αvβ6 integrin or TGFβ for down-regulating fibrosis in the setting of acute or ongoing biliary injury.
The injury response in epithelia is highly focal or diffuse, depending on the nature of the injury factor and whether the process is acute or chronic. In the liver, the response may be localized to a portion of the lobule: the pericentral area (zone 3) in alcohol-induced injury, or the periportal area (zone 1) in chronic hepatitis C. Acute biliary injury, caused by mechanical obstruction of the common bile duct, autoimmune disease, and some toxins, is a particularly striking example of focality. The initial reaction is strictly periductal, consisting of ductular proliferation and the appearance of myofibroblasts and collagenous matrix that encircle the ducts.1 The pathogenesis of the acute periductal reaction is poorly understood.2 Studies of fibrosing liver injury have focused on the role of the stellate cell, and indeed activation of stellate cells does occur in biliary obstruction.3 However, this is a relatively late event and may be distinct and separate from the acute periductal reaction.
Of the numerous cytokines that modulate the injury response, transforming growth factor beta (TGF-β) is particularly prominent.4 It is produced as a precursor, part of which is a “latency-associated peptide” (LAP). The latter is cleaved from the precursor but remains associated noncovalently with mature TGF, blocking its activity. Thus, a key event in the regulation of TGF is liberating the active cytokine from the LAP complex. This has been a subject of considerable investigation. Although proteases have been implicated,5 other mechanisms exist.6 LAP has an RGD (arg-glyasp) domain that has been shown to associate with integrins, specifically α5β1, αvβ6, and αvβ8.7 In binding assays, LAP has relatively high affinity for αv/β6, and binding causes the peptide to separate from TGFβ, presumably through an allosteric conformational change. This occurs at the cell surface, potentially serving to confine the action of TGF-β to the cell displaying αvβ6.7
Expression of αvβ6 is limited to epithelial cells,8 particularly duct epithelium. It is present during development, becoming minimal in resting normal adult epithelia. It increases in acute duct-associated injury in the adult, subsiding as the injury resolves.9 These observations suggest that αvβ6 could play a role in ductal injury in the liver, although the question has not been examined. An early survey of various tissues failed to detect αvβ6 mRNA in liver.8 However, expression of this integrin in the basal state is expected to be low. Moreover, if it is present in a small subset of liver cells and not in hepatocytes, it may escape detection by northern blot because of the dominance of hepatocyte RNA in whole-liver extracts.
We report that after acute biliary obstruction, expression of αvβ6 is strikingly increased specifically on cholangiocytes in both murine and human liver. Moreover, studies of mice lacking this integrin show that its expression is directly involved in the fibrogenic response. Although inflammation is present in bile duct obstruction, it does not appear to have a primary role in regulating αvβ6. In αvβ6-null mice subjected to bile duct ligation, fibrosis was reduced whereas inflammation was unaffected. Also, in a transgenic mouse model of immunemediated biliary inflammation, there was minimal stimulation of αvβ6 expression and no periductal fibrosis. With regard to the origin of the periductal myofibroblasts, activated stellate cells have been implicated as the principal fibrogenic cell type in multiple forms of liver injury. However, our studies of biliary obstruction failed to show stellate cells migrating to the portal area during the acute phase of the injury. We did see histochemical change suggestive of epithelial-to-mesenchymal transition (EMT) of biliary epithelial cells, as a novel source of fibrogenic cells in this type of injury.
Materials and Methods
Mouse Models of Liver Injury
C57Bl/6 mice were used. Wild-type mice were purchased from Charles River Laboratories (Wilmington, MA). Mice lacking β6 integrin were a gift from Dean Sheppard (University of California San Francisco Lung Biology Center). Surgery was carried out with isofluorane anesthesia according to a protocol approved by the institutional committee on animal research. The common bile duct or the left hepatic duct was exposed, double ligated, and severed between the ties. Mice with duct obstruction were studied at intervals of 3 days to 28 days after the procedure. We are terming this “acute” biliary injury, based on the rat model in which the histological changes of duct obstruction were reversible after 4 weeks and largely reversible after 8 weeks.10
For the blocking αvβ6 antibody study, C57Bl/6 mice underwent selective bile duct ligation and then were given 1 of 3 reagents: a blocking antibody to αvβ6 (3G9, Biogen Idec, 4 μg/g),11 a TGF-β–soluble receptor type II-Fc fusion protein (R&D Systems, 2 μg/g), or isotype control immunoglobulin G (1E6, Biogen Idec, 4 μg/g). The proteins were given intraperitoneally at the time of surgery and every third day thereafter. Liver was harvested for analysis at 2 weeks. We also studied mice with immunemediated biliary injury. The mice are transgenic for an ovalbumin epitope (SIINFEKL), under the promoter for the sodium-dependent bile acid transporter, which directs the ovalbumin epitope to the surface of biliary epithelial cells. When ova-specific splenocytes are transferred to these mice, periductal inflammation ensues over a period of 8 weeks,12 However, in contrast to bile duct ligation, fibrosis in this model (OVA-BIL) is minimal.
Analytical Procedures
Liver was fixed in 4% paraformaldehyde for paraffin embedding or placed directly in ornithine carbamoyltransferase medium for frozen sectioning. Frozen sections were stained for αvβ6 using chimeric mouse-human-Fc monoclonal anti-β6 (Biogen Idec) with donkey anti-human Fc as the secondary antibody. Sections were fixed in acetone at −20°C for 5 minutes, washed 3 times in phosphate-buffered saline (PBS) at room temperature, then permeabilized with 0.2% Tri-tonX in PBS (10 minutes at room temperature). After PBS washes, the tissue was blocked with casein/thimerosal solution, washed again in PBS, and covered overnight at 4°C with primary antibody in PBS. After washes, secondary antibody was applied in the same diluent for 30 minutes at room temperature. Granulocytes in tissue were visualized with rat anti-mouse Ly-6G (BD Biosciences, catalog no. 550291). Frozen sections were air dried for 30 minutes at 25°C, then fixed in methanol at −20°C for 10 minutes. Endogenous peroxidase was blocked with 0.3% H2O2 in methanol for 30 minutes at 25°C. After washes with avidin-containing protein block and PBS, primary antibody diluted 1:50 in protein block and biotin was applied for 2 hours at 25°C. Biotinylated secondary antibody was applied for 30 minutes at 25°C. After washes, peroxidase staining was carried out with the avidin-biotin complex procedure (Vector Laboratories). To quantify the number of Ly6-G–positive cells, stained slides were examined with a 40× objective, and positively stained cells were counted from comparable portal areas based on the cross-section size of the portal vein.
For pSmad2 staining, sections from paraffin-embedded tissue were heated to 56°C for 60 minutes in water, then transferred to Retrievagen A (BD Biosciences) and processed according to the manufacturer's protocol. Peroxidase block was carried out with 3% H2O2 in water for 30 minutes at 25°C. Antibody to phospho Smad2 (Chemicon, AB3849) was diluted 1:2000 in protein block solution and biotin, then applied to sections at 4°C overnight. After washes, biotinylated secondary antibody was applied for 30 minutes at 25°C. Peroxidase staining was performed as described above. Sections were counter-stained for 1 minute with hematoxylin. Collagen in mouse liver was stained with Sirius red dye and examined using a Leica DMLB microscope with a 10× objective with crossed polarizers. Images of the entire section were obtained in a serpentine fashion starting at 1 end of the tissue and working toward the other. Collagen staining was quantified by image analysis using the National Institutes of Health Image software,13 which has been shown to correlate with chemical quantification of collagen in mouse liver after bile duct obstruction.14 Collagen associated with large veins and arteries was excluded. Alanine aminotransferase (ALT) in serum was determined using a COBAS autoanalyzer (Roche, Basel, Switzerland).
Human liver in paraffin was processed using standard methods, then stained using mouse monoclonal anti-β6 and anti-mouse secondary. Coverslips were applied, and the slides were stored at 4°C in the dark.
Results
Fibrosis After Acute Bile Duct Obstruction in the Mouse
Ligation of the common bile duct in the mouse leads to periductal accumulation of myofibroblast-like cells and excess collagen, as visualized with Sirius-red stain. The number of bile ductules increases concurrently. Total duct ligation also has systemic effects, as indicated by weight loss and significant mortality over a period of a few weeks. Evidence of global stress to the liver includes elevation of ALT as well as signs of cholestasis.15 We developed selective ligation of the left biliary duct (LBDL) as a means of reducing systemic injury and focusing on the direct response to biliary obstruction. The left bile duct is readily visualized and reliably ligated. In the obstructed lobe, the histological change is comparable to that seen after total bile duct ligation, with ductal proliferation and fibrosis (Fig. 1), whereas the right lobe is completely normal (not shown). In mice with LBDL, the change in serum ALT is substantially reduced compared with that following total bile duct ligation (Fig. 1), and excess mortality is eliminated. Unless otherwise indicated, the studies that follow were carried out with selective duct ligation, so as to isolate the effects of obstruction independent of systemic effects.
Fig. 1.

The histological response to selective ligation of the left bile duct (LBDL). Top left: Hematoxylin-eosin (HE) stain showing typical ductal proliferation and mesenchymal expansion in the portal area. Top right: The same area stained for collagen with Sirius red. The nonobstructed lobes from these mice were normal. Below: Hepatocellular injury, as reflected in serum ALT, following acute common (total) bile duct ligation (CBDL) or selective left bile duct ligation (LBDL).
Expression of αvβ6 After Biliary Injury in the Mouse
The αvβ6 integrin was examined by fluorescence immunohistochemistry. Normal mouse liver showed no expression except for faint staining of the biliary epithelium in some sections. Epithelial cells within portal areas were identified by staining for cytokeratin. Nine days after total biliary obstruction, αvβ6 expression was strikingly increased and localized exclusively to biliary epithelial cells (Fig. 2). The effect of selective LBDL was similar in the obstructed lobe and was evident as early as 3 days after the injury; the contralateral lobe was unaffected. We also studied mice with acute centrilobular injury from administration of carbon tetrachloride. Expression of αvβ6 in this model was undetectable by immunohistology (not shown). The findings indicate that induction of αvβ6 expression is a direct result of bile duct obstruction and precedes periductal fibrosis.
Fig. 2.

Expression of αvβ6 integrin in normal mouse liver (“control”) and at 9, 14, or 16 days after common bile duct ligation. The normal liver lacks detectable αvβ6: the arrowhead indicates a bile ductule. In the liver after bile duct ligation, red fluorescence indicates αvβ6, which is present exclusively in biliary epithelial cells. A pan-cytokeratin antibody (green fluorescence) identifies epithelial cells (hepatocytes and biliary epithelium), whereas a 4′,6-diamidino-2-phenylindole (DAPI) stain (blue) marks nuclei. In the ligated liver, the nuclei surrounding the β6-positive ductules represent injury-associated myofibroblasts (keratin-negative). The appearance is typical. Essentially every bile ductule in all sections examined was strongly positive for αvβ6.
Up-Regulation of αvβ6 Coincides with the Appearance of Smooth Muscle Actin–Positive Cells Around Bile Ductules
In liver from obstructed mice, bile ductules were closely invested with mesenchymal cells displaying α-smooth muscle actin (SMA), which is characteristic of myofibroblasts (Fig. 3). As to the origin of these cells, we considered 2 possibilities: (1) stellate cells may migrate from the parenchyma to portal areas; and (2) cholangiocytes may give rise to fibroblastic cells through an epithelial-to-mesenchymal transition (EMT). Activated stellate cells differ from smooth muscle cells and myofibroblasts in exhibiting glial fibrillary acidic protein (GFAP).16 When we stained LBDL liver for GFAP, we found no evidence of stellate cell migration out of the lobule to the area around bile ductules. The lobular distribution of GFAP-positive cells was similar to that in uninjured liver.16 The cells were not increased in the portal tract, and clearing of zones 1 and 2 parenchyma was not seen (Fig. 4). These observations were confirmed by staining sections for desmin, a smooth muscle cytokeratin that is present in stellate cells. The lobular distribution of positive cells was unchanged 4 days after total biliary obstruction (data not shown). We conclude that the SMA-positive cells surrounding ductules during the acute phase of biliary obstruction are not migrating stellate cells. We then proceeded to co-stain liver for markers of cholangiocytes (cytokeratin) and myofibroblasts (SMA). Early after obstructive bile duct injury, individual cholangiocytes in some ductules appear to be positive for SMA as well as cytokeratin, suggesting active EMT (Fig. 5).
Fig. 3.

Expression of smooth muscle actin (SMA) after bile duct ligation (day 9). SMA-positive cells are in red, and epithelial cells are marked with a pan-cytokeratin antibody (green). On the left is a portal tract of a normal liver. SMA is present in the artery and portal venule; a bile ductule (arrow) is entirely negative. On the right is liver after common bile duct ligation, showing a portion of a medium-sized bile ductule with a large collar of SMA-positive cells. The section shows a relatively large ductule but is representative of ductules of any size. The magnitude of the mesenchymal reaction varied but was uniformly present.
Fig. 4.

Lack of stellate cell migration acutely after bile duct ligation. Mice underwent LBDL. Two weeks later, liver was processed and stained for glial fibrillary acidic protein (GFAP). The section on the left is representative and shows, in the lower portion of the photo, an expanded portal area secondary to biliary obstruction. The stained cells have the typical morphology of stellate cells, which are in their normal position within the lobule and show no tendency to cluster in the portal area. The section on the right is a control, with normal immunoglobulin G in place of the primary antibody (anti-GFAP).
Fig. 5.

Reactive cholangiocytes express smooth muscle actin. The section is from a liver on day 16 after total bile duct ligation, stained with a pancytokeratin antibody (green) and an antibody to smooth muscle actin (SMA, red). The lumen of 3 bile ductules is marked with an asterisk (*). In the ductule with the largest cuff of SMA-positive cells, individual cholangiocytes display red staining in situ (arrows). Of note also, at this stage of the injury, the surrounding SMA-positive cells have an epithelioid shape rather than the spindle shape of typical myofibroblasts, further suggesting that they are cholangiocytes in transition to myofibroblast-like cells. The photomicrograph is representative in that only some cholangiocytes in a ductule appeared to express smooth-muscle actin, and some ductules were entirely negative.
We also evaluated expression of αvβ6 in human biliary injury. In acute obstructive biliary injury including 2 cases of biliary atresia, the integrin was strongly expressed and localized entirely to the biliary epithelium, as in the mouse model (Fig. 6). In end-stage liver disease, by contrast, detectable expression was rare. In 4 cases of primary sclerosing cholangitis, 4 of primary biliary cirrhosis and (as controls) 2 of hepatocellular carcinoma, positive staining was seen only once, in primary biliary cirrhosis, and was limited to a portion of 3 ductules (data not shown). Interestingly, it was negative also in cholangiocarcinoma (3 cases). The findings suggest that the presence of αvβ6 signifies acute or ongoing biliary obstruction and is not associated with bile duct neoplasia.
Fig. 6.

Expression of αvβ6 in human liver (see text). The specimen is from a 59-year-old man with a posttransplantation acute biliary obstruction. On the left, the peroxidase stain shows αvβ6, which stains proliferating bile ductules exclusively. On the right is the same sample treated with nonspecific primary antibody. The view is representative.
The Role of αvβ6 and Inflammation in Fibrogenesis
To assess more precisely the relationship between αvβ6 expression, inflammation, and fibrosis, we studied mice with deletion of this integrin. The mice are healthy and fertile but show reduced fibrosis after lung injury17 or kidney injury.18 We also examined OVA-BIL mice as a model of inflammation without fibrosis.12 Finally, we quantified Ly-6G (Gr-1)–positive cells (granulocytes and monocytes) in the portal area of normal and β6-null mice after bile duct ligation.
After LBDL, periductule fibrosis was examined by Sirius red staining; it was diminished in the β6-null liver relative to wild-type liver (Fig. 7). Interestingly, the increase in ductule profiles was the same, if not more prominent, in the obstructed knockout mice relative to the wild-type controls. Ductules were counted in randomly selected portal areas of similar size (based on portal vein diameter). The mean number per portal area in control (nonobstructed) liver was 1.1 ± 0.1; in wild-type liver after LBDL, 5.4 ± 0.9 ductules; and in β6 knockout liver after LBDL, 6.6 ± 0.7 ductules. In the OVA-BIL model of biliary inflammation, the liver was largely negative for expression of αvβ6; rarely a portion of a ductule was positive (not shown). For correlating expression of αvβ6 and fibrosis, collagen deposition in these 2 models of biliary injury was evaluated with staining of sections with Sirius red and quantifying the amount of stain by image analysis (Fig. 8). In the OVA-BIL model of inflammation, the amount of collagen was no different from control liver acutely and increased only slightly over several weeks of observation. The level after 4 weeks was similar to that of the β6 null liver after LBDL, which was approximately 50% of that occurring in wild-type liver (P < 0.01).
Fig. 7.
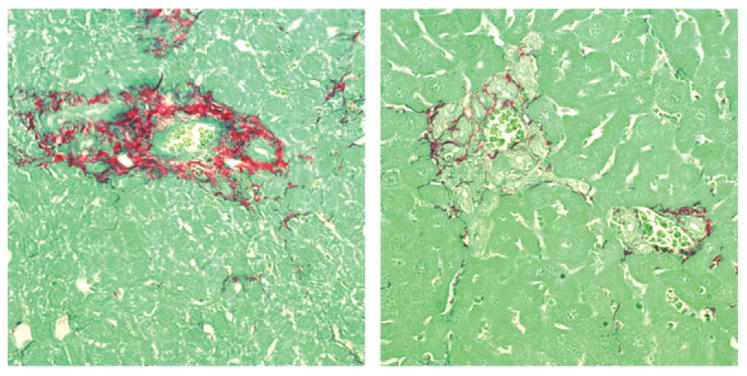
Periductular fibrosis in 6-null or wild-type mice subjected to selective left bile duct ligation (LBDL). The mice were sacrificed 3 weeks after the procedure, and liver sections were stained for collagen using Sirius red. In this representative view, collagen deposition is reduced in the 6-null liver (right panel) while ductule proliferation is increased.
Fig. 8.

Quantitation of collagen deposition in OVA-BIL, β6 null (KO) or wild-type mice. The OVA-BIL mice were studied at 0, 10, and 28 days after adoptive transfer of OVA-specific splenocytes (see Materials and Methods). The β6 null and wild-type mice were subjected to LBDL and evaluated 2 weeks later. Collagen on liver sections was quantified by Sirius red staining and image analysis. Collagen associated with vascular structures was excluded. N = 6 for all groups.
Biliary obstruction draws inflammatory cells,19 raising the question of whether fibrosis is driven by products of inflammation rather than expression of αvβ6. The lack of fibrosis in the OVA-BIL mice suggests that this is not the case12 We also examined the extent of inflammation after bile duct ligation in control and β6-null liver, respectively. Sections stained for Ly-6G, which identifies granulocytes, showed no difference in periductal inflammatory cells in response to bile duct ligation (Fig. 9). Taken together, the findings indicate that αvβ6 induction and fibrogenesis are linked, whereas the inflammatory response is independent.
Fig. 9.

Quantitation of periportal granulocytes in wild-type or αvβ6 null mice after selective bile duct ligation. Sections were stained with antibody to the granulocyte antigen Ly-6G as described in Materials and Methods.
Blockade of β6 or TGFβ Reduces Fibrosis After Bile Duct Ligation
As already noted, αvβ6 integrin has been proposed to mediate fibrogenesis by activating latent TGF. To verify this in bile duct–obstructed liver, we stained sections for phospho-Smad2 (pSmad2), an intermediate in the signaling pathway from the TGF receptor complex. Staining was prominent in biliary epithelial cells and in the reactive stroma (Fig. 10); it appeared to be concentrated mainly in the nucleus. By contrast, staining was essentially absent from αvβ6-null liver studied in parallel. We also examined the effect of blocking either αvβ6 or TGF on the fibrogenic response to biliary obstruction. Mice undergoing selective LBDL received either a neutralizing monoclonal antibody to 6 or TGF-soluble receptor20; controls received isotype-specific nonimmune immunoglobulin G. With either inhibitor, the reduction in periductal collagen was significant and comparable (Fig. 11). Moreover, there was no added effect of the TGF-soluble receptor in the αvβ6-null mice, consistent with a common site of action, namely, αvβ6-mediated activation of TGF. The inhibitor studies confirm the results with the αvβ6-null mouse, further linking this integrin to periductal fibrogenesis.
Fig. 10.

Immunohistological assessment of signaling from the TGF receptor after bile duct ligation. Wild-type and αvβ6-null mice, respectively, underwent selective bile duct ligation. Six days later, the liver was processed for phospho-Smad2 staining, as described in Materials and Methods. The periportal reaction includes ductule profiles (arrows) as well as an extensive periductal reaction. In liver from wild-type mice, both cholangiocytes and stromal cells express pSmad2 (brown stain), whereas the sections from αvβ6-null mice are negative. The view is representative: essentially all portal areas were positive in the wild-type liver after bile duct obstruction.
Fig. 11.

Effect of blocking antibody to β6 integrin or soluble TGF-β receptor on collagen deposition after bile duct ligation. Mice underwent selective bile duct ligation followed by administration of either a blocking antibody to αvβ6 integrin type II TGF-β soluble receptor, or a control antibody, as described in Materials and Methods. Blocking antibody to αvβ6 integrin and soluble type II TGF-β receptor both significantly reduced collagen deposition compared with control antibody (P = 0.040 and P = 0.036). The amount of reduction by soluble TGF-β receptor is similar to that in β6 knockout mice (n = 7 for anti-β6 group, n = 6 for sTBR and control antibody groups).
Discussion
Our studies indicate that the integrin αvβ6 increases dramatically after bile duct obstruction, is localized specifically to biliary epithelial cells, and is responsible for periductal fibrogenesis. Its expression appears to be unique to biliary injury in that centrilobular fibrosis from carbon tetrachloride administration was unaltered in mice lacking αvβ6 integrin (unpublished observation). Consistent with previous studies of lung injury, αvβ6 appears to act in part by converting latent TGF-β to its active form.7 Bile duct ligation activates signaling from the TGFβ receptor, as judged by increased expression of pSmad2 in proliferating bile ductules and surrounding stromal cells. Also, a competitive inhibitor of the TGF-β type II receptor and blocking antibody against αvβ6 are similarly effective (and not additive) in down-regulating peribiliary fibrosis. We show that the findings are likely relevant to human liver injury in that αvβ6 is prominent on the biliary epithelium during acute but not chronic injury, consistent with a role in mediating the fibrogenic response. Taken together, the data suggest that the response of the biliary epithelium to acute injury is distinct and different from that of lobular hepatocytes. It follows that effective treatment of biliary injury will target events unique to this epithelium.
The initial identification of αvβ6 was accomplished through homology polymerase chain reaction of RNA extracted from a human pancreatic carcinoma cell line.21 The integrin was shown to bind fibronectin, tenascin, and LAP via an arginine-glycine-aspartate (RGD) motif that is present in these matrix proteins.22-24 The human fetus expresses αvβ6 in several epithelia but prominently and often exclusively in the duct component of these tissues. In the normal adult, by contrast, αvβ6 is low or undetectable, present only on some ductular epithelia (at low levels) and in the secretory endometrial gland.9 In adult epithelial injury, expression increases acutely, and its localization mirrors that present in fetal tissues. The integrin is believed to regulate tissue remodeling by local activation of latent TGFβ, as suggested by a number of injury models. Cutaneous injury has been studied in some detail. After full-thickness injury to the skin, migrating keratinocytes express αvβ6.25 Mice lacking the integrin display a reduced number of hair follicles in the dermis and infiltration of mononuclear cells in the lungs but heal normally after cutaneous injury,17 suggesting that αvβ6 is not essential to wound repair. Conversely, when this integrin is overexpressed in skin, driven by the cytokeratin-14 promoter, the result is hypertrophic healing with abundant fibrosis.26 The cytokeratin promoter targets expression to all epithelial layers in skin,27 which results in prolonged expression after wounding, particularly in keratinocytes. The findings suggest that the timing and topology of αvβ6 expression are important to normal repair.
Studies of the role of αvβ6 in lung injury are consistent with those examining the skin. Intratracheal administration of bleomycin causes fibrosing bronchiolar injury. The response was significantly attenuated in animals lacking αvβ6, with a marked reduction in fibrosis but with little change in the degree of inflammation.28 The findings resembled those in the TGF-β1–null mouse, suggesting a possible link between αvβ6 and TGFβ.7 The LAP component of the latent TGF-β complex bound to αvβ6 through an RGD motif, similar to fibronectin and tenascin. Indeed, the affinity of αvβ6 for LAP was greater than for RGD-containing matrix molecules. The interaction of αvβ6 and LAP resulted in release of active TGF-β. This action was isoform-specific: TGF-β1 and TGF-β3 were activated but not TGF-β2.5,7 A truncation mutant of αvβ6 lacking the cytoplasmic domain was unable to activate TGF-β despite retaining binding affinity for LAP, suggesting a requirement for inside-out signaling in the TGF-β activating function.7 Recent studies have demonstrated functions of αvβ6 independent of its activation of TGF-β1, including growth regulation.29-31 In vivo, the integrin has been characterized as both a tumor suppressor32 and marker of tumor invasion.33 In our survey of human liver, it was associated with acute injury (ductal obstruction) but not with malignancy (cholangiocarcinoma).
The origin of the reactive and fibrogenic cell types in biliary obstruction is unknown. All glandular tissues have 2 distinct epithelial components, the parenchymal (acinar) epithelium and the duct epithelium. Functional and morphological differences between the 2 are well known. However, the analysis has not been extended to the injury response. Wake and Sato34 and Ballardini et al.35 have pointed out that “myofibroblasts” (SMA-positive cells) in the liver are heterogeneous, raising the possibility of several types of reactive fibrogenic cells in injury. Nonetheless in the liver, studies have focused almost entirely on stellate cells, which reside normally in the parenchyma. Because of their prominence in the lobular injury response, it has been widely assumed that in biliary injury, they migrate from the lobule proper to the portal area. Our findings dispute this assumption. Others have come to the same conclusion, postulating that the SMA-positive cells in biliary injury arise from portal “fibroblasts.”36-39 Beaussier and colleagues36 examined the SMA-positive mesenchymal population elicited by bile duct ligation in the rat. They found the majority of the periductal cells were negative for desmin; in contrast, activating stellate cells in the lobule over time were increasingly desmin positive. They conclude that the periductal fibrogenic cells that arise after biliary obstruction are distinct from stellate cells, arising possibly from portal mesenchymal cells.
Our findings support this general conclusion but suggest, in addition, that the fibrogenic cells arise from progenitor cells (known as oval cells), which are known to be present in obstructive duct injury, or from mature cholangiocytes in a so-called EMT. This type of event has been implicated in the neoplastic transformation of epithelial cells.40 In adult epithelia, EMT has been best documented in kidney injury.41,42 In the colon, αvβ6 has been proposed as a key mediator of EMT.43 TGF-β is thought to be a major driver of EMT, because it down-regulates E-cadherin, thereby loosening lateral cell–cell junctional complexes,44 which presumably facilitates migration of epithelial cells into the mesenchyme. These events, along with disruption of the basement membrane, allow epithelial cells to translocate and acquire a myofibroblast phenotype. Our studies of liver sections during acute biliary injury show cholangiocytes expressing in situ a myofibroblast marker, SMA In addition, the SMA-positive cells that surround the ductule are epithelioid rather than spindle-shaped in appearance, and many exhibit pSmad2, reflecting a response to TGFβ. The findings are suggestive rather than definitive—they require confirmation using mice with genetically tagged cholangiocytes that can be tracked into the periductal mesenchyme. Nonetheless, they point to the possibility that biliary epithelial cells are a source of periductal myofibroblasts in acute injury. The role of αvβ6 in activating latent TGFβ may be central to EMT in this type of injury.
Complete biliary obstruction produces rapid disease, with progression to cirrhosis within months, in contrast to the typically much more indolent course of parenchymal injury (chronic viral hepatitis, for example). It is treatable when the cause of obstruction is extrahepatic and is approachable with surgery or stents, and early fibrosis is reversed.45 Intrahepatic and end-ductule obstruction generally is not amenable to surgical or endoscopic intervention. An example is biliary atresia, which in many instances requires liver transplantation. The findings reported here suggest that inhibitors of αvβ6 integrin or TGF-β could be useful in modulating fibrogenesis and thus the course of acute biliary injury.
Acknowledgments
The Microscopy Core of the UCSF Liver Center (Sandra Huling) provided valuable service. We also thank Dean Sheppard (UCSF Lung Biology Center) for β6 knockout mice and helpful discussion.
Supported by a Research Training Fellowship from the Howard Hughes Medical Institute (to B.W.), R32 DK31198 (to D.M.B.), and P30 DK26743 (UCSF Liver Center).
Abbreviations
- ALT
alanine aminotransferase
- EMT
epithelial-to-mesenchymal transition
- GFAP
glial fibrillary acidic protein
- LAP
latency-associated peptide
- LBDL
left bile duct ligation
- RGD
arginine-glycine-aspartate
- SMA
smooth muscle actin
- TGF-β
transforming growth factor beta
Footnotes
Potential conflict of interest: Dr. Violette and Dr. Weinreb own stock in Biogen Idec.
References
- 1.Roskams T, Desmet V. Ductular reaction and its diagnostic significance. Semin Diagn Pathol. 1998;15:259–269. [PubMed] [Google Scholar]
- 2.Alpini G, McGill JM, Larusso NF. The pathobiology of biliary epithelia. Hepatology. 2002;35:1256–1268. doi: 10.1053/jhep.2002.33541. [DOI] [PubMed] [Google Scholar]
- 3.Jarnagin WR, Rockey DC, Koteliansky VE, Wang SS, Bissell DM. Expression of variant fibronectins in wound healing: cellular source and biological activity of the EIIIA segment in rat hepatic fibrogenesis. J Cell Biol. 1994;127:2037–2048. doi: 10.1083/jcb.127.6.2037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bissell DM. Chronic liver injury, TGF-beta, and cancer. Exp Mol Med. 2001;33:179–190. doi: 10.1038/emm.2001.31. [DOI] [PubMed] [Google Scholar]
- 5.Annes JP, Munger JS, Rifkin DB. Making sense of latent TGFbeta activation. J Cell Sci. 2003;116:217–224. doi: 10.1242/jcs.00229. [DOI] [PubMed] [Google Scholar]
- 6.Crawford SE, Stellmach V, Murphy-Ullrich JE, Ribeiro SM, Lawler J, Hynes RO, et al. Thrombospondin-1 is a major activator of TGF-beta1 in vivo. Cell. 1998;93:1159–1170. doi: 10.1016/s0092-8674(00)81460-9. [DOI] [PubMed] [Google Scholar]
- 7.Munger JS, Huang X, Kawakatsu H, Griffiths MJ, Dalton SL, Wu J, et al. The integrin alpha v beta 6 binds and activates latent TGF beta 1: a mechanism for regulating pulmonary inflammation and fibrosis. Cell. 1999;96:319–328. doi: 10.1016/s0092-8674(00)80545-0. [DOI] [PubMed] [Google Scholar]
- 8.Breuss JM, Gillett N, Lu L, Sheppard D, Pytela R. Restricted distribution of integrin beta 6 mRNA in primate epithelial tissues. J Histochem Cytochem. 1993;41:1521–1527. doi: 10.1177/41.10.8245410. [DOI] [PubMed] [Google Scholar]
- 9.Breuss JM, Gallo J, DeLisser HM, Klimanskaya IV, Folkesson HG, Pittet JF, et al. Expression of the beta 6 integrin subunit in development, neoplasia and tissue repair suggests a role in epithelial remodeling. J Cell Sci. 1995;108(Pt 6):2241–2251. doi: 10.1242/jcs.108.6.2241. [DOI] [PubMed] [Google Scholar]
- 10.Zimmermann H, Reichen J, Zimmermann A, Sagesser H, Thenisch B, Hoflin F. Reversibility of secondary biliary fibrosis by biliodigestive anastomosis in the rat. Gastroenterology. 1992;103:579–589. doi: 10.1016/0016-5085(92)90850-x. [DOI] [PubMed] [Google Scholar]
- 11.Weinreb PH, Simon KJ, Rayhorn P, Yang WJ, Leone DR, Dolinski BM, et al. Function-blocking integrin alphavbeta6 monoclonal antibodies: distinct ligand-mimetic and nonligand-mimetic classes. J Biol Chem. 2004;279:17875–17887. doi: 10.1074/jbc.M312103200. [DOI] [PubMed] [Google Scholar]
- 12.Buxbaum J, Qian P, Khuu C, Shneider BL, Daikh DI, Allen PM, et al. Novel model of antigen specific induction of bile duct injury. Gastroenterology. 2006;131:1899–1906. doi: 10.1053/j.gastro.2006.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pape L, Henne T, Offner G, Strehlau J, Ehrich JH, Mengel M, et al. Computer-assisted quantification of fibrosis in chronic allograft nephropaty by picosirius red-staining: a new tool for predicting long-term graft function. Transplantation. 2003;76:955–958. doi: 10.1097/01.TP.0000078899.62040.E5. [DOI] [PubMed] [Google Scholar]
- 14.Bergheim I, Guo L, Davis MA, Duveau I, Arteel GE. Critical role of plasminogen activator inhibitor-1 in cholestatic liver injury and fibrosis. J Pharmacol Exp Ther. 2006;316:592–600. doi: 10.1124/jpet.105.095042. [DOI] [PubMed] [Google Scholar]
- 15.Canbay A, Feldstein A, Baskin-Bey E, Bronk SF, Gores GJ. The caspase inhibitor IDN-6556 attenuates hepatic injury and fibrosis in the bile duct ligated mouse. J Pharmacol Exp Ther. 2004;308:1191–1196. doi: 10.1124/jpet.103.060129. [DOI] [PubMed] [Google Scholar]
- 16.Geerts A, Eliasson C, Niki T, Wielant A, Vaeyens F, Pekny M. Formation of normal desmin intermediate filaments in mouse hepatic stellate cells requires vimentin. Hepatology. 2001;33:177–188. doi: 10.1053/jhep.2001.21045. [DOI] [PubMed] [Google Scholar]
- 17.Huang XZ, Wu JF, Cass D, Erle DJ, Corry D, Young SG, et al. Inactivation of the integrin beta 6 subunit gene reveals a role of epithelial integrins in regulating inflammation in the lung and skin. J Cell Biol. 1996;133:921–928. doi: 10.1083/jcb.133.4.921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hahm K, Lukashev ME, Luo Y, Yang WJ, Dolinski BM, Weinreb PH, et al. Alphav beta6 integrin regulates renal fibrosis and inflammation in Alport mouse. Am J Pathol. 2007;170:110–125. doi: 10.2353/ajpath.2007.060158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Saito JM, Bostick MK, Campe CB, Xu J, Maher JJ. Infiltrating neutrophils in bile duct-ligated livers do not promote hepatic fibrosis. Hepatol Res. 2003;25:180–191. doi: 10.1016/s1386-6346(02)00247-4. [DOI] [PubMed] [Google Scholar]
- 20.George J, Roulot D, Koteliansky VE, Bissell DM. In vivo inhibition of rat stellate cell activation by soluble transforming growth factor beta type II receptor: a potential new therapy for hepatic fibrosis. Proc Natl Acad Sci U S A. 1999;96:12719–12724. doi: 10.1073/pnas.96.22.12719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sheppard D, Rozzo C, Starr L, Quaranta V, Erle DJ, Pytela R. Complete amino acid sequence of a novel integrin beta subunit (beta 6) identified in epithelial cells using the polymerase chain reaction. J Biol Chem. 1990;265:11502–11507. [PubMed] [Google Scholar]
- 22.Huang X, Wu J, Spong S, Sheppard D. The integrin alphavbeta6 is critical for keratinocyte migration on both its known ligand, fibronectin, and on vitronectin. J Cell Sci. 1998;111:2189–2195. doi: 10.1242/jcs.111.15.2189. [DOI] [PubMed] [Google Scholar]
- 23.Prieto AL, Edelman GM, Crossin KL. Multiple integrins mediate cell attachment to cytotactin/tenascin. Proc Natl Acad Sci U S A. 1993;90:10154–10158. doi: 10.1073/pnas.90.21.10154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Weinacker A, Chen A, Agrez M, Cone RI, Nishimura S, Wayner E, et al. Role of the integrin alpha v beta 6 in cell attachment to fibronectin: heterologous expression of intact and secreted forms of the receptor. J Biol Chem. 1994;269:6940–6948. [PubMed] [Google Scholar]
- 25.Haapasalmi K, Zhang K, Tonnesen M, Olerud J, Sheppard D, Salo T, et al. Keratinocytes in human wounds express alpha v beta 6 integrin. J Invest Dermatol. 1996;106:42–48. doi: 10.1111/1523-1747.ep12327199. [DOI] [PubMed] [Google Scholar]
- 26.Hakkinen L, Koivisto L, Gardner H, Saarialho-Kere U, Carroll JM, Lakso M, et al. Increased expression of beta6-integrin in skin leads to spontaneous development of chronic wounds. Am J Pathol. 2004;164:229–242. doi: 10.1016/s0002-9440(10)63113-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Moll R, Franke WW, Schiller DL, Geiger B, Krepler R. The catalog of human cytokeratins: patterns of expression in normal epithelia, tumors and cultured cells. Cell. 1982;31:11–24. doi: 10.1016/0092-8674(82)90400-7. [DOI] [PubMed] [Google Scholar]
- 28.Huang X, Wu J, Zhu W, Pytela R, Sheppard D. Expression of the human integrin beta6 subunit in alveolar type II cells and bronchiolar epithelial cells reverses lung inflammation in beta6 knockout mice. Am J Respir Cell Mol Biol. 1998;19:636–642. doi: 10.1165/ajrcmb.19.4.3293. [DOI] [PubMed] [Google Scholar]
- 29.Dalvi N, Thomas GJ, Marshall JF, Morgan M, Bass R, Ellis V, et al. Modulation of the urokinase-type plasminogen activator receptor by the beta6 integrin subunit. Biochem Biophys Res Commun. 2004;317:92–99. doi: 10.1016/j.bbrc.2004.02.178. [DOI] [PubMed] [Google Scholar]
- 30.Janes SM, Watt FM. Switch from alphavbeta5 to alphavbeta6 integrin expression protects squamous cell carcinomas from anoikis. J Cell Biol. 2004;166:419–431. doi: 10.1083/jcb.200312074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Niu J, Dorahy DJ, Gu X, Scott RJ, Draganic B, Ahmed N, et al. Integrin expression in colon cancer cells is regulated by the cytoplasmic domain of the beta6 integrin subunit. Int J Cancer. 2002;99:529–537. doi: 10.1002/ijc.10397. [DOI] [PubMed] [Google Scholar]
- 32.Ludlow A, Yee KO, Lipman R, Bronson R, Weinreb P, Huang X, et al. Characterization of integrin beta6 and thrombospondin-1 double-null mice. J Cell Mol Med. 2005;9:421–437. doi: 10.1111/j.1582-4934.2005.tb00367.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Westernoff TH, Jordan RC, Regezi JA, Ramos DM, Schmidt BL. Beta-6 Integrin, tenascin-C, and MMP-1 expression in salivary gland neoplasms. Oral Oncol. 2005;41:170–174. doi: 10.1016/j.oraloncology.2004.08.002. [DOI] [PubMed] [Google Scholar]
- 34.Wake K, Sato T. Intralobular heterogeneity of perisinusoidal stellate cells in porcine liver. Cell Tissue Res. 1993;273:227–237. doi: 10.1007/BF00312824. [DOI] [PubMed] [Google Scholar]
- 35.Ballardini G, Groff P, Badiali dGL, Schuppan D, Bianchi FB. Ito cell heterogeneity: desmin-negative Ito cells in normal rat liver. Hepatology. 1994;19:440–446. [PubMed] [Google Scholar]
- 36.Beaussier M, Wendum D, Schiffer E, Dumont S, Rey C, Lienhart A, et al. Prominent contribution of portal mesenchymal cells to liver fibrosis in ischemic and obstructive cholestatic injuries. Lab Invest. 2007;87:292–303. doi: 10.1038/labinvest.3700513. [DOI] [PubMed] [Google Scholar]
- 37.Kinnman N, Francoz C, Barbu V, Wendum D, Rey C, Hultcrantz R, et al. The myofibroblastic conversion of peribiliary fibrogenic cells distinct from hepatic stellate cells is stimulated by platelet-derived growth factor during liver fibrogenesis. Lab Invest. 2003;83:163–173. doi: 10.1097/01.lab.0000054178.01162.e4. [DOI] [PubMed] [Google Scholar]
- 38.Tang L, Tanaka Y, Marumo F, Sato C. Phenotypic change in portal fibroblasts in biliary fibrosis. Liver. 1994;14:76–82. doi: 10.1111/j.1600-0676.1994.tb00051.x. [DOI] [PubMed] [Google Scholar]
- 39.Tuchweber B, Desmouliere A, Bochaton-Piallat ML, Rubbia-Brandt L, Gabbiani G. Proliferation and phenotypic modulation of portal fibroblasts in the early stages of cholestatic fibrosis in the rat. Lab Invest. 1996;74:265–278. [PubMed] [Google Scholar]
- 40.Thiery JP, Sleeman JP. Complex networks orchestrate epithelial-mesenchymal transitions. Nat Rev Mol Cell Biol. 2006;7:131–142. doi: 10.1038/nrm1835. [DOI] [PubMed] [Google Scholar]
- 41.Kalluri R, Neilson EG. Epithelial-mesenchymal transition and its implications for fibrosis. J Clin Invest. 2003;112:1776–1784. doi: 10.1172/JCI20530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Liu Y. Epithelial to mesenchymal transition in renal fibrogenesis: pathologic significance, molecular mechanism, and therapeutic intervention. J Am Soc Nephrol. 2004;15:1–12. doi: 10.1097/01.asn.0000106015.29070.e7. [DOI] [PubMed] [Google Scholar]
- 43.Bates RC, Bellovin DI, Brown C, Maynard E, Wu B, Kawakatsu H, et al. Transcriptional activation of integrin beta6 during the epithelial-mesenchymal transition defines a novel prognostic indicator of aggressive colon carcinoma. J Clin Invest. 2005;115:339–347. doi: 10.1172/JCI23183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Roberts AB, Tian F, Byfield SD, Stuelten C, Ooshima A, Saika S, et al. Smad3 is key to TGF-beta-mediated epithelial-to-mesenchymal transition, fibrosis, tumor suppression and metastasis. Cytokine Growth Factor Rev. 2006;17:19–27. doi: 10.1016/j.cytogfr.2005.09.008. [DOI] [PubMed] [Google Scholar]
- 45.Ramm GA, Carr SC, Bridle KR, Li L, Britton RS, Crawford DH, et al. Morphology of liver repair following cholestatic liver injury: resolution of ductal hyperplasia, matrix deposition and regression of myofibroblasts. Liver. 2000;20:387–396. doi: 10.1034/j.1600-0676.2000.020005387.x. [DOI] [PubMed] [Google Scholar]


