Abstract
Angiotensin II (Ang II) and nitric oxide (NO)/natriuretic peptide (NP) signaling pathways mutually regulate each other. Imbalance of Ang II and NO/NP has been implicated in the pathophysiology of many vascular diseases. cGMP functions as a key mediator in the interaction between Ang II and NO/NP. Cyclic nucleotide phosphodiesterase 5A (PDE5A) is important in modulating cGMP signaling by hydrolyzing cGMP in vascular smooth muscle cells (VSMC). Therefore, we examined whether Ang II negatively modulates intracellular cGMP signaling in VSMC by regulating PDE5A. Ang II rapidly and transiently increased PDE5A mRNA levels in rat aortic VSMC. Upregulation of PDE5A mRNA was associated with a time-dependent increase of both PDE5 protein expression and activity. Increased PDE5A mRNA level was transcription-dependent and mediated by the Ang II type 1 receptor. Ang II-mediated activation of extracellular signal-regulated kinases 1/2 (ERK1/2) was essential for Ang II-induced PDE5A upregulation. Pretreatment of VSMC with Ang II inhibited C-type NP (CNP) stimulated cGMP signaling, such as cGMP dependent protein kinase (PKG)-mediated phosphorylation of vasodilator-stimulated-phosphoprotein (VASP). Ang II-mediated inhibition of PKG was blocked when PDE5 activity was decreased by selective PDE5 inhibitors, suggesting that upregulation of PDE5A expression is an important mechanism for Ang II to attenuate cGMP signaling. PDE5A may also play a critical role in the growth promoting effects of Ang II because inhibition of PDE5A activity significantly decreased Ang II-stimulated VSMC growth. These observations establish a new mechanism by which Ang II antagonizes cGMP signaling and stimulates VSMC growth.
Keywords: Cyclic nucleotide phosphodiesterase, Vascular smooth muscle cell, Angiotensin II, cGMP, Cell growth
1. Introduction
Angiotensin II (Ang II) is the dominant effector of the renin–angiotensin system and regulates numerous responses in the cardiovascular system. In addition to vasoconstriction, Ang II regulates vascular smooth muscle cell (VSMC) growth, migration, apoptosis, and extracellular matrix deposition [1,2]. Ang II is of great interest in cardiovascular physiology and pathology because of the beneficial effects of Ang II-converting enzyme (ACE) inhibitors and Ang II receptor blockers in the treatment of cardiovascular diseases such as hypertension, atherosclerosis, heart failure, and stroke [3]. The Ang II type I (AT1) and type 2 (AT2) receptors are the main mediators of the actions of Ang II. In VSMC the vast majority of Ang II effects occur via the AT1 receptor, a G-protein-coupled seven-transmembrane domain receptor [2]. The signaling pathways stimulated by Ang II are complex and include the activation of phospholipase C, with resultant hydrolysis of membrane phospholipids, an increase in cytosolic calcium, and activation of protein kinase C (PKC), the activation of the mitogen-activated protein (MAP) kinases and several tyrosine kinases as well as induction of proto-oncogene expression such as c-fos and c-jun [4,5].
The cGMP-mediated signaling pathway also plays an important role in regulating smooth muscle contractility, growth, and survival [3]. cGMP is synthesized via soluble guanylyl cyclases (the NO receptors) and/or particulate guanylyl cyclases (the natriuretic peptide (NP) receptors). Phosphodiesterases (PDEs) play important roles in cGMP signaling by hydrolyzing cyclic nucleotides and thus turning off their signaling. PDEs are a superfamily of structurally and functionally related enzymes. PDE5A is the major isoform of PDE5 family in VSMC, which specifically hydrolyzes cGMP. PDE5A has been found in all types of vascular and visceral (uterus, small intestine) smooth muscle cells. The physiological importance of PDE5A in the regulation of smooth muscle contractility has been very well demonstrated by the successful clinical use of its inhibitor, sildenafil (Viagra), for the treatment of erectile dysfunction [6]. By inhibiting PDE5A activity, sildenafil increases accumulation of cGMP in response to NO, thus enhancing the erectile response [7]. Understanding the roles and regulation of PDE5 in other vascular events may expand the therapeutic usage of sildenafil in other vascular diseases.
There is an impressive body of evidence regarding the functional interaction between Ang II-stimulated and cGMP-mediated events [3,8]. For example, Ang II promotes VSMC growth, migration, and inflammation while cGMP inhibits these processes [3]. Thus, the antagonistic interplay may occur between Ang II and cGMP signaling pathways. Therefore, we hypothesized that Ang II may up-regulate cGMP-hydrolyzing PDE5 expression and activity in VSMC to attenuate cGMP accumulation and signaling. Here, we demonstrate that PDE5A is transcriptionally up-regulated by Ang II. Ang II upregulation of PDE5A expression is mediated by activation of ERK1/2. Finally, our findings suggest that PDE5A may be an important regulator in Ang II attenuation of cGMP signaling and Ang II-stimulated VSMC growth.
2. Materials and methods
2.1. Materials
Sildenafil was a kind gift from Pfizer. JNJ-10258859 was a kind gift from Johnson & Johnson [9]. Losartan was a kind gift from Merck. Ang II was purchased from ICN. PD098059 was purchased from Calbiochem®. The dominant negative MEK1 was MEK1 mutant with Serine 217 and 221 mutated to alanine, which has been previously shown to effectively block ERK1/2 activation in variety of cell types [10].
2.2. VSMC culture
Rat aortic VSMC were isolated from 250 to 300 g male Harlan Sprague–Dawley rats and maintained in 10% fetal bovine serum/Dulbecco’s modified eagle’s medium (DMEM, GIBCO BRL) as described previously [11]. Passages 5–10 VSMC at ≈70% confluence were growth-arrested by serum starvation (in DMEM containing 0.1% FBS) for 48 h before agonist Ang II treatment. Serum starvation reduces cellular growth to a minimum while maintaining VSMC in a synthetic phenotype.
2.3. Relative quantitative reverse transcription-polymerase chain reaction (RT-PCR)
Cellular RNA was extracted with Total RNA Isolation Kit (Qiagen) based on manufacturer’s protocol. First strand cDNA was synthesized from 5 μg of total RNA by use of random primers with SuperScript Pre-amplification System (GIBCO BRL) according to manufacturer’s protocol. Relative quantitative RT-PCR was performed as described previously using 18s rRNA as an internal control [12]. Ambion’s competimer technology (Quantrum RNA 18s Internal Standards, Ambion) was used to modulate the amplification of 18s rRNA in the same linear range as the RNAs under studies when amplified under the same condition. The numbers of PCR cycles that allowed for linear amplification of PDE5A as well as the optimal 18s primer: competimer ratio were determined empirically. PCR products were then run-on a 2% agarose gel, stained with ethidium bromide, and quantified by densitometry using image analysis software (NIH Image 1.60). Relative PDE5A mRNA levels were determined by normalizing to 18S rRNA or GAPDH mRNA.
2.4. Western blot analysis
Western blots were carried out as described previously [12]. Polyclonal antibody against VASP (Alexis) and monoclonal antibody against phospho-Ser239 VASP (Alexis) were used to detect total VASP and Ser239 phosphorylated VASP, respectively. A polyclonal PDE5 antibody, kind gift from Dr. Beavo (University of Washington, Seattle), was used to detect PDE5A protein. Total cellular ERK1/2 and phsophorylated ERK1/2 were detected by using anti-ERK1 plus anti-ERK2 antibodies (Santa Cruz) and anti-phospho-ERK1/2 antibody (Cell Signaling), respectively. Protein levels were quantified by densitometry using image analysis software (NIH Image 1.60).
2.5. PDE enzyme assay
Cultured VSMC were homogenized in 1 ml of cold homogenization buffer (40 mmol/l Tris–HCl, pH 7.5, 1 mmol/l EGTA, 0.1 mmol/l Na3VO4, 1 mmol/l DTT, 10 ug/ml aprotinin, 5 ug/ml pepstatin, 20 ug/ml leupeptin, 1 mmol/l benzamidine). PDE assays were carried out as described previously [12]. PDE5 activity was determined by the cGMP-hydrolyzing activity inhibited by PDE5-selective inhibitor JNJ-10258859 or sildenafil using 1 μM cGMP as the substrate. Protein concentrations were determined by the method of Bradford using commercially obtained reagent (Bio-Rad Laboratories).
2.6. [3H]-leucine or [3H]-thymidine incorporation
Subconfluent VSMC grown in 12-well plates were growth-arrested for 48 h and stimulated with Ang II in the presence or absence of the PDE inhibitors for 48 h. During the last 6 or 2 h of incubation, 2 μCi [3H]-leucine or [3H]-thymidine was added, respectively. Cells were then washed with ice-cold PBS and incubated in 1 ml of 5% trichloroacetic acid for 30 min at 0 °C. Cells were then solubilized in 1 mol/l NaOH for 30 min at 0 °C. After neutralization with HCl, solubilized proteins were counted by a scintillation counter.
2.7. Nuclear run-on assay
Nuclear run-on assays were performed as described by Greenberg [13]. Briefly, equal numbers of nuclei (5 × 107) from cells treated with or without Ang II were used for preparation of nascent transcripts. Nuclei were incubated in a reaction buffer containing ATP, GTP, CTP, and 100 μCi of [α-32P]UTP for 30 min at 30 °C. RNA was extracted using TRIzol reagent (Invitrogen) and hybridized with a Hybone-N+ membrane slot-blotted with 2 μg of mouse PDE5A, rat GAPDH, and pBluescript vector DNA. Results were visualized by autoradiography.
2.8. Data analysis and statistical evaluation
Values are expressed as mean ± S.E.M. of triplicate samples from an experiment. Comparisons were performed with Student’s t-test. Values of P < 0.05 were considered significant. At least three independent experiments were performed.
3. Results
3.1. Ang II up-regulates PDE5A mRNA, protein and activity in VSMC
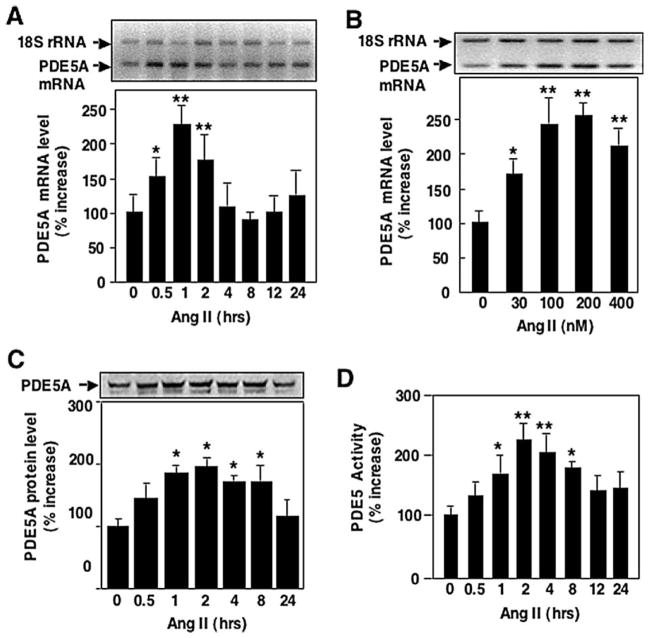
To determine if Ang II antagonizes cGMP signaling via the regulation of cGMP-hydrolyzing PDE5A expression in VSMC, we first assessed the effect of Ang II on steady-state PDE5A mRNA levels. VSMC were growth-arrested by serum starvation for 48 h before stimulation with Ang II. Total RNA was then isolated at various times and subjected to relative quantitative RT-PCR. VSMC stimulated with Ang II (200 nmol/l) showed a 225% ± 10% increase in PDE5A mRNA levels peaking at 1 h (Fig. 1A). Ang II-stimulated upregulation of PDE5A was in a dose-dependent manner (Fig. 1B).
Fig. 1.
Ang II up-regulates PDE5A expression in rat aortic VSMC. Growth-arrested rat aortic VSMCs were stimulated with 200 nmol/l Ang II for indicated time (A, C, and D) or various doses of Ang II for 1 h (B). mRNA levels of PDE5A (A and B) were measured by relative quantitative RT-PCR. 18s RNA was used as internal control. Protein levels of PDE5A (C) were measured by Western blot analysis. The activities of PDE5 (C) were measured by PDE assay using cGMP as a substrate in the absence or presence of sildenafil (30 nM). The sildenafil-inhibited cGMP-PDE activity is counted as PDE5 activity. Values are expressed as mean ± S.E.M. of triplicates from a representative experiment. Mean values for the percent change of PDE5A were calculated by comparing Ang II-stimulated samples versus samples collected at time 0, or without Ang II, which was arbitrarily set at 100% for each experiment. * P < 0.05, ** P < 0.01 compared with samples at time 0 or without Ang II. Similar results were obtained from at least three independent experiments.
To examine whether the increase in PDE5A mRNA stimulated by Ang II was associated with an increase in PDE5A protein level, western blot analysis with a PDE5A specific antibody was performed. PDE5A protein levels increased with Ang II stimulation, peaking at 2 h (195% ± 6%, which correlates with PDE5A mRNA levels), and returning to baseline by 24 h (Fig. 1C).
Finally, to determine whether the increase in PDE5A mRNA and protein stimulated by Ang II results in increased activity, PDE5 activity was determined. VSMC lysates stimulated with Ang II were assayed for cGMP-PDE activity with and without sildenafil, a PDE5-selective inhibitor, to determine the contribution of PDE5 to total cGMP-hydrolyzing phosphodiesterase activity. As shown in Fig. 1D, PDE5 activity increased by 220% at 2 h, consistent with the increase in the mRNA and protein levels shown in Fig. 1A, C.
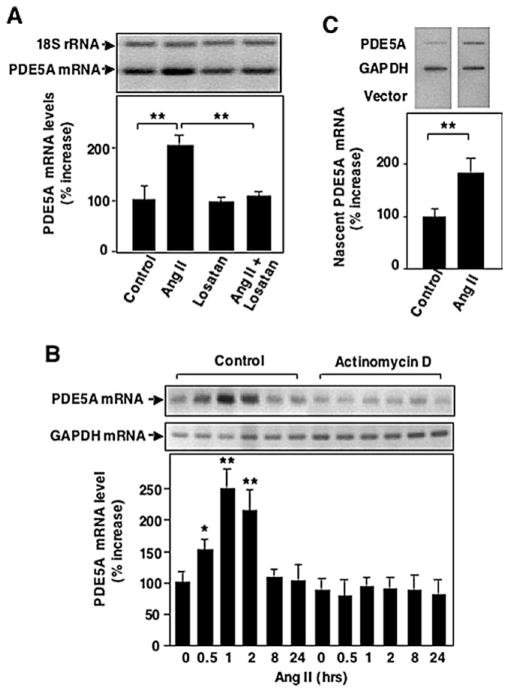
3.2. Ang II-induced PDE5A mRNA expression in VSMC is blocked by AT1 receptor blockade and is dependent on transcription
Most of the physiological and pathophysiological effects of Ang II in VSMC have been shown to occur via the AT1 receptor [2]. To determine whether Ang II-induced PDE5A expression is mediated by the AT1 receptor, VSMC were pre-treated with the AT1 receptor antagonists losartan or irbesartan (10 μmol/l). Both losartan (Fig. 2A) and irbesartan (data not shown) prevented the increase in PDE5A mRNA accumulation caused by Ang II. These data suggest that the induction of PDE5A expression by Ang II is mediated through AT1 receptors in VSMC. To investigate whether Ang II-induced increases in PDE5A mRNA levels were transcription-dependent, VSMC were pretreated with 10 μg/ml actinomycin D, an inhibitor of RNA polymerase II. In this case, we used GAPDH as an internal control because the 18S rRNA was altered by actinomycin D (Fig. 2B). Actinomycin D almost completely inhibited PDE5A mRNA induction elicited by Ang II (Fig. 2B). To further determine whether this was due to a change in PDE5A transcription, nuclear run-on analyses were performed. As shown in Fig. 2C, Ang II treatment significantly increased the amount of radiolabeled nascent PDE5A transcripts. In contrast, Ang II had no effects on transcription rate of the housekeeping gene, GAPDH. These results suggest that Ang II up-regulates PDE5A expression through a transcriptional mechanism.
Fig. 2.
Effects of losartan and actinomycin D on Ang II-induced PDE5 A ARNm expression. Growth-arrested rat aortic VSMCs were pretreated with the AT1 receptor blocker losartan (10 μmol/l) for 30 minutes (A) or actinomycin D (Act.D, 10 μg/ml) for indicated time (B) followed by 200 nmol/l Ang II stimulation for 1 heure. PDE5 A ARNm was measured by relative quantitative RT-PCR. GAPDH was used as internal control. (C) Growth-arrested rat aortic VSMCs were treated with or without Ang II (200 nmol/l) for 1 heure, followed by isolation of nuclei and nuclear run-on analysis. Specific bands were visualized by autoradiography and quantified by densitometry. Relative amount of nascent PDE5 A transcripts was determined by normalizing to GAPDH. Values are expressed as mean ± S.E.M. of triplicates from a representative experiment. Mean values for percentage changes of PDE5 A ARNm were calculated by comparing experimental samples versus control (treatment with vehicle) that was arbitrarily set at 100 % for each experiment. * P < 0.05, ** P < 0.01 compared with control. Similar results were obtained from at least three independent experiments.
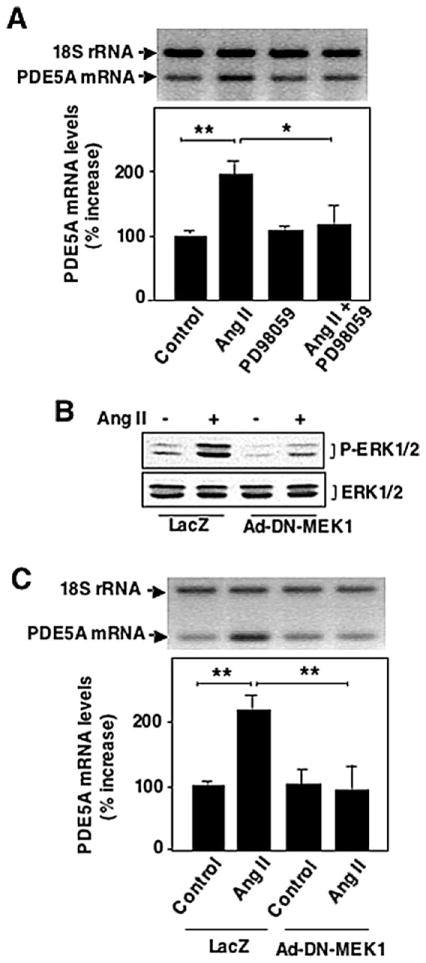
3.3. Ang II-induced PDE5A mRNA expression is ERK1/2 dependent
Ang II is a strong activator of the MAP kinase signaling pathways in rat aortic VSMC and Ang II-induced gene expression in VSMC is regulated by MAP kinases. To investigate whether activation of MAP kinase ERK1/2 is involved in Ang II-induced PDE5A mRNA expression, VSMCs were pre-treated with PD098059, the ERK1/2 kinase (MEK1/2) inhibitor, although PD098059 also inhibits ERK5 kinase MEK5. Pretreatment of VSMCs with 10 μmol/l PD098059 inhibited Ang II-induced PDE5A mRNA expression (Fig. 3A). To further specifically determine the role of ERK1/2 in Ang II-induced PDE5A expression, we overexpressed a dominant negative mutant of MEK1 (DN-MEK1) via adenovirus, which has been shown to specifically block ERK1/2 but not ERK5 activation [14]. As expected, overexpression of DN-MEK1 inhibited Ang II-stimulated ERK1/2 activation (Fig. 3B). Furthermore, the Ang II-mediated increase of PDE5A expression was completely diminished by DN-MEK1 (Fig. 3C). These observations suggest that activation of ERK1/2 signaling is essential for Ang II-induced PDE5A mRNA expression in VSMC.
Fig. 3.
Role of ERK1/2 in Ang II-induced PDE5 A ARNm expression. Effects of inhibition of ERK1/2 activation by PD098059 (A) and DN-MEK1 (B, C) on ERK1/2 activation or PDE5 A ARNm expression. (A) Growth-arrested rat aortic VSMCs were pretreated with PD098059 for 30 minutes, followed by 200 nmol/l Ang II stimulation for 1 heure. (B and C) Rat aortic VSMCs were treated with 100 MOI Ad-DN-MEK1 or Ad-LacZ for 2 heures and then changed to serum-free medium for 48 heures, followed by 200 nmol/l Ang II stimulation for 10 minutes (B) or 1 heure (C). The ARNm levels were measured by relative quantitative RT-PCR and ERK1/2 activation were determined by Western blot analysis. Values are expressed as mean ± S.E.M. of triplicates from a representative experiment. Mean values for percentage changes of PDE5 A ARNm were calculated by comparing experimental samples versus control (treatment with vehicle or LacZ) that was arbitrarily set at 100 % for each experiment. * P < 0.05, ** P < 0.01 compared with control. Similar results were obtained from at least three independent experiments.
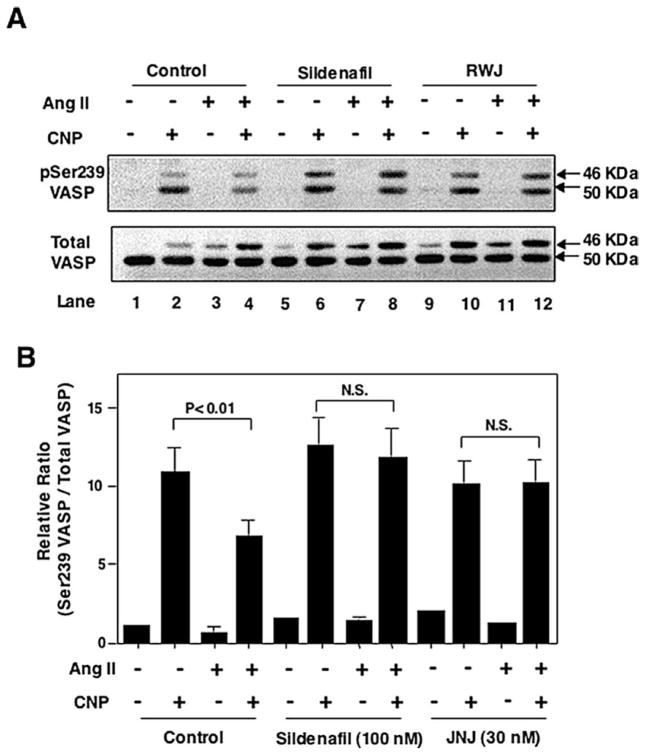
3.4. Upregulation of PDE5A by Ang II attenuates CNP-elicited cGMP signaling
The upregulation of PDE5 by Ang II suggests one mechanism by which Ang II regulates cGMP signaling in VSMC. To determine the effect of Ang II-mediated upregulation of PDE5A expression on intracellular cGMP signaling, we examined cGMP-dependent protein kinase (PKG) activity. PKG activity was measured by phosphorylation of VASP, a well-characterized substrate for PKG. VASP is a 46-kDa protein that is phosphorylated preferentially at Serine 239 (Ser239) by PKG and at Serine 157 (Ser157) by PKA. At high levels of cyclic nucleotides, both sites may be phosphorylated by either protein kinase [15]. Phosphorylation of Ser157 leads to a shift in the apparent molecular weight from 46 to 50 kDa on SDS-PAGE, which is detectable by Western blot analysis using anti-VASP antibody. Phosphorylation of Ser239 can be detected using a Ser239 phospho-specific VASP monoclonal antibody that recognizes the 46 kDa band phosphorylated on Ser239 alone, as well as the 50 kDa band phosphorylated on both Ser239 and Ser157. Therefore, we could determine the extent of VASP Ser239 phosphorylation by Western blot using combination of Ser239 phospho-specific VASP antibody and the total VASP antibody (Fig. 4A). Specifically, the relative amount of Ser239 phosphorylation was estimated based on the intensities of both 46 and 50 kDa bands detected using anti-phospho-Ser239 antibody normalized to the intensities of total VASP (Fig. 4B). As shown in Fig. 4, CNP significantly increased VASP Ser239 phosphorylation (lane 2 vs. 1) as we expected because CNP is a well known cGMP-elevating reagent. However, in cells pretreated with Ang II for 2 h, CNP-stimulated VASP Ser239 phosphorylation was significantly reduced (lane 4 vs. 2). The PDE5-selective inhibitor, sildenafil, diminished the effect of Ang II on CNP-stimulated VASP Ser239 phosphorylation (lane 8 vs. 6 compared with lane 4 vs. 2). A different PDE5-selective inhibitor, JNJ-10258859 [9], elicited a similar effect (lane 12 vs. 10 compared with lane 4 vs. 2). These observations suggest that the upregulation of PDE5 by Ang II may mediate the effect of Ang II to attenuate CNP-elicited cGMP signaling.
Fig. 4.
Role of PDE5 in Ang II attenuation of CNP-activated cGMP signaling. Growth-arrested rat aortic VSMCs were pretreated with 200 nmol/l Ang II for 2 heures. Ang II treated cells were then treated with or without PDE5 inhibitors sildenafil (100 nmol/l) or JNJ-10258859 (30 nmol/l) for 30 minutes before being stimulated with 100 nmol/l CNP for 1 minute Cell lysates were subjected to Western blot analysis using anti-phospho-Ser239 VASP antibody (A, top panel) or anti-VASP antibody (A, bottom panel). Ser239 phosphorylation of VASP was estimated by the relative ratio of Ser239 phosphorylated VASP (46 + 50 kDa) versus total VASP (46 + 50 kDa) (B). N.S.: no significant difference.
3.5. Inhibition of PDE5A activity attenuates Ang II-induced VSMC growth
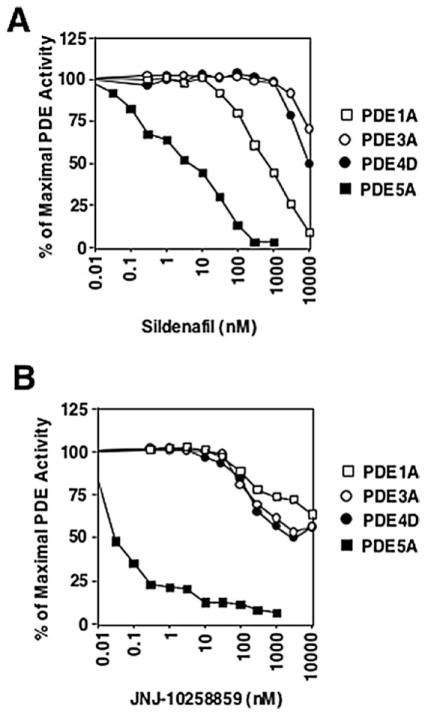
Ang II stimulates both VSMC hypertrophy [16,17] and hyperplasia [18]. cGMP and cGMP-elevating agonists such as NO and CNP have been shown to inhibit VSMC growth [19,20]. Thus, the upregulation of PDE5A and the resulting downregulation of cGMP signaling by Ang II may be one mechanism by which Ang II promotes VSMC growth. To test this hypothesis, we determined the role of PDE5 in Ang II-stimulated cell growth by evaluating Ang II-stimulated protein and DNA synthesis using two different PDE5-selective inhibitors, sildenafil and JNJ-10258859. To determine the specificity of sildenafil or JNJ-10258859 on PDE5, the inhibitory profiles of sildenafil or JNJ-10258859 on several other known PDEs present in rat aortic VSMCs have been examined (Fig. 5). Based on their inhibitory profiles, we believe that sildenafil at concentrations 10–100 nM or JNJ-10258859 at 10–30 nM should have no significant inhibitory effects on PDE1A, 3A, and 4D in vivo.
Fig. 5.
Specificity of PDE5 inhibitors sildenafil and JNJ-10258859. PDE1A1, PDE4D3, PDE3A1, and PDE5A1 were transiently expressed in CHO cells and assayed for PDE activity in cell lysates in vitro in the presence of various concentrations of sildenafil (A) or JNJ-10258859 (B).
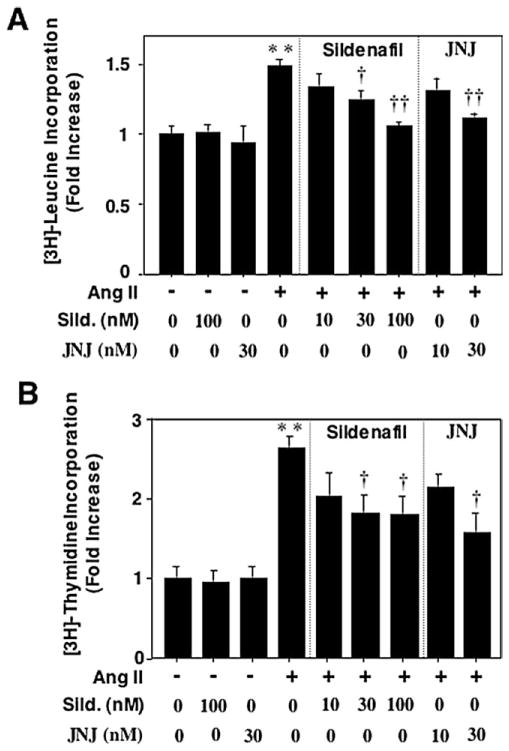
Ang II significantly increased protein synthesis in VSMC based on [3H]-leucine incorporation (Fig. 6A). Both PDE5A-selective inhibitors consistently decreased Ang II-stimulated protein synthesis in a concentration-dependent manner. Similarly, Ang II-stimulated DNA synthesis measured by [3H]-thymidine incorporation in VSMC was also significantly inhibited in the presence of either sildenafil or JNJ-10258859 (Fig. 6B). These results suggest that the upregulation of PDE5A may play an important role in Ang II-stimulated VSMC growth.
Fig. 6.
Role of PDE5 A in Ang II-induced rat aortic VSMC growth. Growth-arrested rat aortic VSMCs were pretreated with or without sildenafil (10–100 nmol/l) or JNJ-10258859 (10–30 nmol/l) for 30 minutes, followed by stimulation with 200 nmol/l Ang II for 48 heures. Protein synthesis (A) and ADN synthesis (B) were measured by [3H]-leucine and [3H]-thymidine incorporation, respectively. Values are expressed as mean ± S.E.M. of triplicates from a representative experiment. Mean values for fold changes were calculated by comparing experimental samples versus control (treatment with vehicle) that was arbitrarily set at 1. * P < 0.05, ** P < 0.01 compared with control. † P < 0.05, †† P < 0.01 compared with Ang II alone. Similar results were obtained from at least three independent experiments.
4. Discussion
The major finding of the present study is that Ang II transcriptionally up-regulates the expression of PDE5A via AT1 receptor mediated signaling. Upregulation of PDE5A expression by Ang II is dependent on the Ang II-mediated activation of ERK1/2. Importantly, PDE5A upregulation and activation appear to be required for Ang II attenuation of cGMP signaling and stimulation of VSMC growth. These observations suggest that upregulation of PDE5A expression represents a novel mechanism by which Ang II antagonizes cGMP signaling pathways and promotes VSMC growth. The roles of PDE5A in the regulation of cGMP signaling and VSMC growth were determined by two well known PDE5-selective PDE5 inhibitors sildenafil and JNJ-10258859. Because both structurally different PDE5 inhibitors elicit very similar effects on cGMP signaling and cell growth in VSMC, we believe that the biological effects of these two PDE5 inhibitors very likely reflect the consequences of PDE5A inhibition in VSMC.
4.1. Multiple mechanisms for Ang II to regulate cGMP signaling
In VSMC, Ang II stimulates a wide variety of intracellular signal transduction events, leading to diverse biological actions such as vascular smooth muscle contractility and growth. cGMP functions as an antagonist of Ang II action by countering Ang II signaling pathway at different steps [3]. For instance, cGMP has been shown to block Ang II-stimulated Ca2+ mobilization [21,22] and inhibit several protein kinases that are activated by Ang II [23]. Therefore, down-regulation of cGMP signaling is important for Ang II signaling to occur.
Ang II influences cGMP signaling in VSMC via regulating cGMP synthesis and breakdown [3]. For example, Ang II has been shown to rapidly inhibit sGC enzymatic activity [3]. The inhibitory effects of Ang II on sGC catalytic activity are likely mediated by the production of superoxide [3]. In addition to blocking cGMP production by inhibiting GC activity, Ang II also enhances cGMP breakdown by stimulating cGMP-phosphodiesterases. For example, we have previously shown that Ca2+/calmodulin-stimulated PDE1A1 is rapidly and transiently activated by Ang II in VSMC, probably via Ang II-mediated elevation of Ca2+, and plays an important role in the initial inhibitory effect of Ang II on A-type NP (ANP)-induced cGMP accumulation [12]. Similar observations have also been reported in mesangial cells [24] and in intact vessels [25]. These observations suggest that the rapid inactivation of GC and activation of PDE1A1 represent the earliest events following Ang II stimulation to attenuate cGMP accumulation, which may be important in Ang II signaling such as stimulating VSMC contraction.
Our finding that Ang II up-regulates cGMP-hydrolyzing PDE5A expression in VSMC suggests an additional key mechanism by which Ang II attenuates long term cGMP accumulation, which may be important for effects of Ang II, such as promoting VSMC growth. The fact that PDE5-selective inhibitors blocked Ang II-stimulated VSMC growth suggest that PDE5A-controlled cGMP pool functions as a critical inhibitor of Ang II-mediated VSMC growth (Fig. 5). Upregulation of PDE5A expression and downregulation of PDE5A-controlled cGMP pool might be an important mechanism for Ang II stimulating VSMC growth.
4.2. Molecular mechanisms of Ang II regulation of PDE5A expression
The majority of physiological and pathophysiological effects of Ang II in VSMC have been shown to occur via the AT1 receptor. Consistent with this, Ang II-mediated upregulation of PDE5A expression is also dependent on the AT1 receptor because the Ang II effect on PDE5A expression was completely blocked by the AT1 receptor antagonists losartan and irbesartan (Fig. 2). Stimulation of the AT1 receptor activates many protein kinases. Among them, MAP kinases are well known to be involved in Ang II-mediated VSMC hypertrophy and hyperplasia [5]. Using inhibitor PD098059 and DN-MEK1 adenovirus, we found that activation of ERK1/2 is important for Ang II upregulation of PDE5A expression (Fig. 3).
ERK1/2 can phosphorylate many proteins including transcription factors such as c-jun and p62TCF (also called elk-1 or SAP-1) [5]. The human PDE5A gene contains 21 exons, spanning at least 100-kb of chromosomal DNA [26]. Several potential transcription factor binding sequences have been found in the proximal promoter region of the PDE5A gene, such as sequences for AP1, Sp1, and SRF [27]. Some of these transcription factors have been shown to be regulated by Ang II. For example, it is very well known that AP-1 (c-fos/c-jun complex) is activated by Ang II. The role of Sp1 in Ang II-regulated gene expression has been suggested by the finding that Ang II activation of Sp1 was important for mediating Ang II-induced plasminogen-activator inhibitor type-1 gene expression [28]. Moreover, the CArG box, also referred to as Serum Response Element (SRE), is the binding site of serum response factor (SRF). Induction of the SRE occurs on the formation of a ternary complex with SRF, ternary complex factor (p62TCF), and SRE [29]. ERK1/2 has been shown to phosphorylate p62TCF, resulting in enhanced ternary complex formation [30]. Thus, some of these transcription factor binding sites are potentially involved in Ang II induction of PDE5A gene expression.
4.3. Role and mechanism of cGMP in Ang II-mediated regulation of VSMC growth
Changes in PDE5A expression and activity should alter intracellular cGMP concentration because PDE5A is a cGMP-specific enzyme in VSMC. Although the best known cGMP target molecule is PKG, cGMP also interacts with several other molecules in the cell, such as cGMP-gated ion channels and the guanine nucleotide exchange factor CNrasGEF [3]. In addition, the effects of cGMP could involve the modulation of cAMP levels and PKA activity via stimulation of cAMP-hydrolyzing PDE2 or inhibition of cAMP-hydrolyzing PDE3 activity [31]. High concentrations of cGMP may also directly stimulate PKA as well [32]. We have shown that inhibition of PDE5A activity by PDE5-delective inhibitors in VSMC significantly increases PKG phosphorylation of VASP (Fig. 4), suggesting that the PDE5-regulated VASP phosphorylation is mediated by PKG.
The anti-proliferative effects of increased cGMP in response to NO generation [19,33,34], NPs [35,36], or PDE inhibitor [34] have been known in VSMC for years, however, the role of PKG in cGMP-mediated inhibition of VSMC growth still controversial, probably due to the presence of multiple cGMP target molecules. Recently, a pro-atherogenic effect of PKG in VSMC was reported by Wolfsgruber et al. [37]. In transgenic mice in which type I PKG (PKGI) was selectively ablated postnatally in SMC, atherosclerotic lesions were significantly reduced, due to primarily the reduced number of PKGI-positive SMCs in the lesion areas [37]. Results from this study suggest that activation of PKGI in VSMCs promotes the phenotypic modulation of medial VSMCs to proliferate, which is somewhat contradictory to the current knowledge regarding the role of PKGI in anti-proliferating and maintaining the contractile (differentiated) phenotype of VSMCs [38,39].
Although the role of PKGI in VSMC growth and phenotypic change needs to be further addressed, the effect elicited by elevation of intracellular cGMP may not be fully explained by activation of PKG because cGMP can act on other target molecules as well. For example, we have recently found that cGMP raised by NO or CNP inhibits NF-kB-dependent transcription in rat aortic VSMCs via cGMP-dependent inhibition of PDE3, increase of cAMP, and activation of PKA [40]. It also has been shown that the inhibitory effects of organic nitrates and PDE5 inhibitor sildenafil on PDGF-induced DNA synthesis in VSMCs are mediated by activation of PKA probably via cGMP inhibition of PDE3 [34]. The findings presented in this study supports a potential role of PDE5A and cGMP in inhibiting Ang II-induced VSMC growth. Future studies are needed to determine whether the cGMP pool regulated by PDE5A acts via PKG or another effector to inhibit VSMC growth.
Acknowledgments
This work was supported by the American Heart Association (Grant #0030302T to C. Yan) and in part by the National Institutes of Health (Grant HL63462 to B.C. Berk). D. Kim was partially supported by Yonsei University, Seoul, Korea (Grant #2001-7). T. Aizawa was supported by a grant from the Japanese Heart Foundation, Bayer Yakuhin Research Grant Abroad. We thank Dr. Yuhong Qiu, Dr. Weiqin Jiang, Dr. Zhihua Sui, and Dr. Scott Lundeen (Johnson & Johnson) for kindly providing JNJ-10258859 and for their helpful comments on the manuscript. We also thank Dr. Joseph A. Beavo and Dr. James Surapisitchat (University of Washington) for kindly providing PDE5 antibody and their helpful comments on the manuscript.
References
- 1.Schmidt-Ott KM, Kagiyama S, Phillips MI. The multiple actions of angiotensin II in atherosclerosis. Regul Pept. 2000;93:65–77. doi: 10.1016/s0167-0115(00)00178-6. [DOI] [PubMed] [Google Scholar]
- 2.Touyz RM, Schiffrin EL. Signal transduction mechanisms mediating the physiological and pathophysiological actions of angiotensin II in vascular smooth muscle cells. Pharmacol Rev. 2000;52:639–72. [PubMed] [Google Scholar]
- 3.Yan C, Kim D, Aizawa T, Berk BC. Functional interplay between angiotensin II and nitric oxide: cyclic GMP as a key mediator. Arterioscler Thromb Vasc Biol. 2003;23:26–36. doi: 10.1161/01.atv.0000046231.17365.9d. [DOI] [PubMed] [Google Scholar]
- 4.Takahashi E, Berk BC. MAP kinases and vascular smooth muscle function. Acta Physiol Scand. 1998;164:611–21. doi: 10.1111/j.1365-201x.1998.tb10705.x. [DOI] [PubMed] [Google Scholar]
- 5.Duff JL, Marrero MB, Paxton WG, Schieffer B, Bernstein KE, Berk BC. Angiotensin II signal transduction and the mitogen-activated protein kinase pathway. Cardiovasc Res. 1995;30:511–7. [PubMed] [Google Scholar]
- 6.Boolell M, Gepi-Attee S, Gingell JC, Allen MJ. Sildenafil, a novel effective oral therapy for male erectile dysfunction. Br J Urol. 1996;78:257–61. doi: 10.1046/j.1464-410x.1996.10220.x. [DOI] [PubMed] [Google Scholar]
- 7.Ballard SA, Gingell CJ, Tang K, Turner LA, Price ME, Naylor AM. Effects of sildenafil on the relaxation of human corpus cavernosum tissue in vitro and on the activities of cyclic nucleotide phosphodiesterase isozymes. J Urol. 1998;159:2164–71. doi: 10.1016/S0022-5347(01)63299-3. [DOI] [PubMed] [Google Scholar]
- 8.Chrisman TD, Garbers DL. Reciprocal antagonism coordinates C-type natriuretic peptide and mitogen-signaling pathways in fibroblasts. J Biol Chem. 1999;274:4293–9. doi: 10.1074/jbc.274.7.4293. [DOI] [PubMed] [Google Scholar]
- 9.Qiu Y, Bhattacharjee S, Kraft P, John TM, Craig E, Haynes-Johnson D, et al. Characterization of a novel phosphodiesterase type 5 inhibitor: JNJ-10258859. Eur J Pharmacol. 2003;472:73–80. doi: 10.1016/s0014-2999(03)01868-5. [DOI] [PubMed] [Google Scholar]
- 10.Cowley S, Paterson H, Kemp P, Marshall CJ. Activation of MAP kinase kinase is necessary and sufficient for PC12 differentiation and for transformation of NIH 3T3 cells. Cell. 1994;77:841–52. doi: 10.1016/0092-8674(94)90133-3. [DOI] [PubMed] [Google Scholar]
- 11.Duff JL, Marrero MB, Paxton WG, Charles CH, Lau LF, Bernstein KE, et al. Angiotensin II induces 3CH134, a protein-tyrosine phosphatase, in vascular smooth muscle cells. J Biol Chem. 1993;268:26037–40. [PubMed] [Google Scholar]
- 12.Kim D, Rybalkin SD, Pi X, Wang Y, Zhang C, Munzel T, et al. Upregulation of phosphodiesterase 1A1 expression is associated with the development of nitrate tolerance. Circulation. 2001;104:2338–43. doi: 10.1161/hc4401.098432. [DOI] [PubMed] [Google Scholar]
- 13.Greenberg M. Identification of newly transcribed RNA. In: Ausubel FM, Brent R, Kingston RE, Moore DD, Smith JA, Seidman JG, et al., editors. Current protocols in molecular biology. New York: Wiley; 1987. pp. 4.10.1–4.10.11. [DOI] [PubMed] [Google Scholar]
- 14.Kato Y, Kravchenko VV, Tapping RI, Han J, Ulevitch RJ, Lee JD. BMK1/ERK5 regulates serum-induced early gene expression through transcription factor MEF2C. EMBO J. 1997;16:7054–66. doi: 10.1093/emboj/16.23.7054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Smolenski A, Bachmann C, Reinhard K, Honig-Liedl P, Jarchau T, Hoschuetzky H, et al. Analysis and regulation of vasodilator-stimulated phosphoprotein serine 239 phosphorylation in vitro and in intact cells using a phosphospecific monoclonal antibody. J Biol Chem. 1998;273:20029–35. doi: 10.1074/jbc.273.32.20029. [DOI] [PubMed] [Google Scholar]
- 16.Geisterfer AAT, Peach MJ, Owens GK. Angiotensin II induces hypertrophy, not hyperplasia, of cultured rat aortic smooth muscle cells. Circ Res. 1988;62:749–56. doi: 10.1161/01.res.62.4.749. [DOI] [PubMed] [Google Scholar]
- 17.Berk BC, Vekshtein V, Gordon HM, Tsuda T. Angiotensin II-stimulated protein synthesis in cultured vascular smooth muscle cells. Hypertension. 1989;13:305–14. doi: 10.1161/01.hyp.13.4.305. [DOI] [PubMed] [Google Scholar]
- 18.Weber H, Taylor DS, Molloy CJ. Angiotensin II induces delayed mitogenesis and cellular proliferation in rat aortic smooth muscle cells. Correlation with the expression of specific endogenous growth factors and reversal by suramin. J Clin Invest. 1994;93:788–98. doi: 10.1172/JCI117033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cornwell TL, Arnold E, Boerth NJ, Lincoln TM. Inhibition of smooth muscle cell growth by nitric oxide and activation of cAMP-dependent protein kinase by cGMP. Am J Physiol. 1994;267:C1405–C1413. doi: 10.1152/ajpcell.1994.267.5.C1405. [DOI] [PubMed] [Google Scholar]
- 20.Porter JG, Catalano R, McEnroe G, Lewicki JA, Protter AA. C-type natriuretic peptide inhibits growth factor-independent synthesis in smooth muscle cells. Am J Physiol. 1992;263:C1001–C1006. doi: 10.1152/ajpcell.1992.263.5.C1001. [DOI] [PubMed] [Google Scholar]
- 21.Komalavilas P, Lincoln TM. Phosphorylation of the inositol 1,4,5-trisphosphate receptor by cyclic GMP-dependent protein kinase. J Biol Chem. 1994;269:8701–7. [PubMed] [Google Scholar]
- 22.Komalavilas P, Lincoln TM. Phosphorylation of the inositol 1,4,5-trisphosphate receptor. Cyclic GMP-dependent protein kinase mediates cAMP and cGMP dependent phosphorylation in the intact rat aorta. J Biol Chem. 1996;271:21933–8. doi: 10.1074/jbc.271.36.21933. [DOI] [PubMed] [Google Scholar]
- 23.Sauzeau V, Le Jeune H, Cario-Toumaniantz C, Smolenski A, Lohmann SM, Bertoglio J, et al. Cyclic GMP-dependent protein kinase signaling pathway inhibits RhoA-induced Ca2+ sensitization of contraction in vascular smooth muscle. J Biol Chem. 2000;275:21722–9. doi: 10.1074/jbc.M000753200. [DOI] [PubMed] [Google Scholar]
- 24.Haneda M, Kikkawa R, Maeda S, Togawa M, Koya D, Horide N, et al. Dual mechanism of angiotensin II inhibits ANP-induced mesangial cGMP accumulation. Kidney Int. 1991;40:188–94. doi: 10.1038/ki.1991.199. [DOI] [PubMed] [Google Scholar]
- 25.Molina CR, Andresen JW, Rapoport RM, Waldman S, Murad F. Effect of in vivo nitroglycerin therapy on endothelium-dependent and independent vascular relaxation and cyclic GMP accumulation in rat aorta. J Cardiovasc Pharmacol. 1987;10:371–8. doi: 10.1097/00005344-198710000-00001. [DOI] [PubMed] [Google Scholar]
- 26.Yanaka N, Kotera J, Ohtsuka A, Akatsuka H, Imai Y, Michibata H, et al. Expression, structure and chromosomal localization of the human cGMP-binding cGMP-specific phosphodiesterase PDE5A gene. Eur J Biochem. 1998;255:391–9. doi: 10.1046/j.1432-1327.1998.2550391.x. [DOI] [PubMed] [Google Scholar]
- 27.Lin CS, Chow S, Lau A, Tu R, Lue TF. Identification and regulation of human PDE5A gene promoter. Biochem Biophys Res Commun. 2001;280:684–92. doi: 10.1006/bbrc.2000.4220. [DOI] [PubMed] [Google Scholar]
- 28.Motojima M, Ando T, Yoshioka T. Sp1-like activity mediates angiotensin-II-induced plasminogen-activator inhibitor type-1 (PAI-1) gene expression in mesangial cells. Biochem J. 2000;349:435–41. doi: 10.1042/0264-6021:3490435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Herrera RE, Shaw PE, Nordheim A. Occupation of the c-fos serum response element in vivo by a multi-protein complex is unaltered by growth factor induction. Nature. 1989;340:68–70. doi: 10.1038/340068a0. [DOI] [PubMed] [Google Scholar]
- 30.Gille H, Sharrocks AD, Shaw PE. Phosphorylation of transcription factor p62TCF by MAP kinase stimulates ternary complex formation at c-fos promoter. Nature. 1992;358:414–7. doi: 10.1038/358414a0. [DOI] [PubMed] [Google Scholar]
- 31.Vandecasteele G, Verde I, Rucker-Martin C, Donzeau-Gouge P, Fischmeister R. Cyclic GMP regulation of the L-type Ca(2+) channel current in human atrial myocytes. J Physiol. 2001;533:329–40. doi: 10.1111/j.1469-7793.2001.0329a.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sausbier M, Schubert R, Voigt V, Hirneiss C, Pfeifer A, Korth M, et al. Mechanisms of NO/cGMP-dependent vasorelaxation. Circ Res. 2000;87:825–30. doi: 10.1161/01.res.87.9.825. [DOI] [PubMed] [Google Scholar]
- 33.Yu SM, Hung LM, Lin CC. cGMP-elevating agents suppress proliferation of vascular smooth muscle cells by inhibiting the activation of epidermal growth factor signaling pathway. Circulation. 1997;95:1269–77. doi: 10.1161/01.cir.95.5.1269. [DOI] [PubMed] [Google Scholar]
- 34.Osinski MT, Rauch BH, Schror K. Antimitogenic actions of organic nitrates are potentiated by sildenafil and mediated via activation of protein kinase A. Mol Pharmacol. 2001;59:1044–50. doi: 10.1124/mol.59.5.1044. [DOI] [PubMed] [Google Scholar]
- 35.Abell TJ, Richards AM, Ikram H, Espiner EA, Yandle T. Atrial natriuretic factor inhibits proliferation of vascular smooth muscle cells stimulated by platelet-derived growth factor. Biochem Biophys Res Commun. 1989;160:1392–6. doi: 10.1016/s0006-291x(89)80158-5. [DOI] [PubMed] [Google Scholar]
- 36.Hamad AM, Johnson SR, Knox AJ. Antiproliferative effects of NO and ANP in cultured human airway smooth muscle. Am J Physiol. 1999;277:L910–L918. doi: 10.1152/ajplung.1999.277.5.L910. [DOI] [PubMed] [Google Scholar]
- 37.Wolfsgruber W, Feil S, Brummer S, Kuppinger O, Hofmann F, Feil R. A proatherogenic role for cGMP-dependent protein kinase in vascular smooth muscle cells. Proc Natl Acad Sci USA. 2003;100:13519–24. doi: 10.1073/pnas.1936024100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lincoln TM, Dey N, Sellak H. Invited review: cGMP-dependent protein kinase signaling mechanisms in smooth muscle: from the regulation of tone to gene expression. J Appl Physiol. 2001;91:1421–30. doi: 10.1152/jappl.2001.91.3.1421. [DOI] [PubMed] [Google Scholar]
- 39.Munzel T, Feil R, Mulsch A, Lohmann SM, Hofmann F, Walter U. Physiology and pathophysiology of vascular signaling controlled by cyclic guanosine 3′,5′-cyclic monophosphate-dependent protein kinase. Circulation. 2003;108:2172–83. doi: 10.1161/01.CIR.0000094403.78467.C3. [DOI] [PubMed] [Google Scholar]
- 40.Aizawa T, Wei H, Miano JM, Abe J, Berk BC, Yan C. Role of phosphodiesterase 3 in NO/cGMP-mediated antiinflammatory effects in vascular smooth muscle cells. Circ Res. 2003;93:406–13. doi: 10.1161/01.RES.0000091074.33584.F0. [DOI] [PubMed] [Google Scholar]