Abstract
Background
The aim of this work is to study the possible association between retinal nerve fiber layer (NFL) thickness and driving ability.
Methods
Thirty-eight drivers including 22 HIV-positive (HIV+) and 16 age-matched HIV-negative controls participants underwent a full ophthalmologic evaluation, including assessment of retinal NFL thickness. In the undilated state with standard optical correction and under standard illumination they also completed a computer-based, wide field-of-view driving simulation in which they were to obey traffic laws, engage in crash avoidance, and pass slower automobiles. Crashes, speeding and traffic light tickets, and off-road excursions contributed to a weighted score of driving errors.
Results
HIV-seropositive participants had a significantly higher weighted error score than control participants (18.4 [9.2] vs. 11.1 [4.5], p=0.006). NFL thickness was significantly correlated with driving errors (r=−0.51, p=0.025); there was a trend for participants with a CD4 nadir <100 to have more errors than those with a nadir >100 (29.7 [13.2] vs. 19.3 [8.4], p = 0.056). The highest number of driving errors occurred in individuals with both CD4 <100 and NFL thickness <80.
Conclusions
Driving ability may be impacted by reductions in retinal nerve fiber layer thickness. Physicians should consider the potential impact that more complex ophthalmologic conditions in HIV-infected patients may have on driving performance.
Keywords: HIV infection, Retinal nerve fiber layer thinning, Automobile driving, Vision function, Driving simulation
Introduction
Previous research has shown decreased visual functioning in HIV patients without retinitis, with patients performing more poorly on visual field and perimetry tests compared to control subjects [1–3]. We have previously shown that 40% of HIV patients manifest visual field abnormalities. Other tests of visual function such as color vision and color contrast sensitivity tests have also revealed that HIV positive patients without retinitis have lower color detection ability than control patients [4–7]. In recent studies, we tested retinal nerve fiber layer integrity using scanning laser polarimetry and optical coherence tomography and found significant retinal nerve fiber layer thinning in low CD4 HIV-positive patients (CD4 nadir <100 at least for six consecutive months) without retinitis when compared to high CD4 patients (CD4 nadir >200) and control patients without HIV [8, 9]. Inner retinal damage may be due to retinal ischemia or accumulated cotton wool spots damage. Indeed, we have recently demonstrated permanent inner retinal changes after CWS in HIV patients [10]. Although there has been extensive research exploring the ocular problems associated with HIV, no studies have examined how these ocular problems relate to the ability to perform tasks of daily living such as driving. HIV patients with neuropsychological impairment have problems in driving, assessed via driving simulators or on-road evaluations [11–13]. However, the studies did not evaluate the possible role of visual defects associated with HIV infection. The current study strives to determine if there is any association between HIV-associated reductions in nerve fiber layer thickness and driving ability of HIV patients without infectious retinitis. We hypothesized that advanced disease would be associated with reduced performance on the driving simulator, and this would be most pronounced in cases with reduced nerve fiber layer thickness.
Subjects and methods
The study consisted of 38 patients including 22 HIV positive and 16 age-matched HIV-negative subjects without ocular disease. All participants were active drivers. From June 2009 to October 2009, all patients were seen at the University of California San Diego (UCSD) Jacobs Retina Center and had full ophthalmologic examinations to rule out infectious retinitis. All patients had full eye examinations including best-corrected visual acuity, slit-lamp biomicroscopy of eye anterior segment including anterior one third of vitreous, intraocular pressure (IOP), and indirect ophthalmoscopy after dilation. The patients with IOP higher than 21 mmHg or evidence of glaucoma, media opacities, or other identified retinal abnormalities were excluded. HIV history and laboratories were obtained to determine the lowest CD4 T cell count in the patient's history. We performed measurement of the participants’ nerve fiber layer thickness using optical coherence tomography (Stratus OCT3, v.4.0, Zeiss Meditec, Dublin, CA). The study was approved by the UCSD Institutional Review Board and patient consent was obtained before entering the study.
A 10.2-mile simulated driving test (approximately 20 min) was performed by each participant using the STISIM driving simulator (Systems Technology Inc. Hawthorne, CA, USA), in the undilated state with standard optical correction and under standard illumination. The fully interactive simulator was comprised of three computer screens aligned to create a 135° field of view, a steering wheel and a reverse button, brake, and accelerator. Participants were instructed to drive as they normally would and to respect all traffic rules, including posted speed limits. The simulation includes four emergency situations (an unexpected road block, a jaywalking pedestrian, a nearby speeding car, a red truck that backs into the road) and a few more routine yet visually intensive situations (avoiding a bicyclist on the street, letting pedestrians cross at a stoplight, passing slow cars and stopped cars, navigating curved mountain roads). The number of driving errors, including total collisions with pedestrians and other automobiles, off road excursions (driving on the shoulder without having a crash), speeding tickets, and traffic light tickets were recorded. In addition to these individual metrics, similar to the approach used with on-road evaluations [14–16], we calculated a “weighted error score” in order to summarize the participants driving performance and give greater weight to more dangerous incidents. Crashes were assigned 3 points, running a traffic light 2 points, speeding tickets 1 point, and off-road excursions (without a crash) 0.5 points. Participants completed a brief training drive prior to the test in order to familiarize them with the task. A similar simulation was found to be predictive of on-road driving performance in HIV seropositive participants [12].
Statistical analysis
For each participant, each type of the recorded drive error was assigned a weighted score as described above and the scores were summed for each participant. The summed score was used as a response variable for regression analysis on NFL and HIV serology status. All of the analyses were conducted using JMP v. 8.0 (SAS).
Results
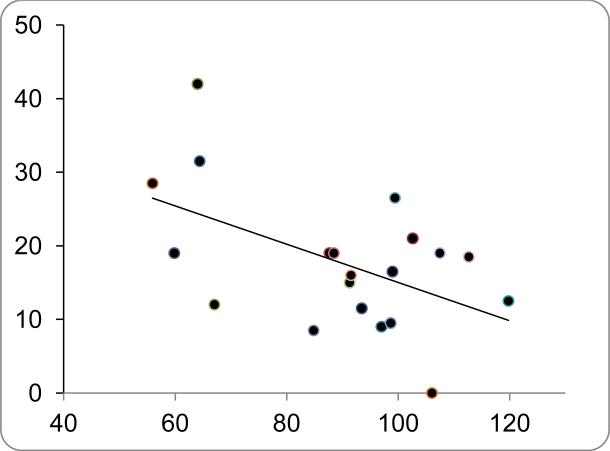
As seen in Table 1, the HIV + and control participants were similar with respect to age, gender, and ethnicity. The HIV + group represented a range of nadir CD4 cell counts, with 45% having a nadir less than 100. Central visual acuity in all subjects was 20/30 and better in both eyes and they did not have any signs of retinopathy at the time of examination. Mean nerve fiber layer thickness was significantly thinner for low CD4 group (Table 2). Compared to the control group, the HIV + group had a significantly higher number of crashes on the simulation, including collisions with pedestrians as well as other automobiles (Table 3). The HIV + group also had more off-road excursions. When these errors were combined into the weighted error score (Table 3), the HIV + performed significantly more poorly than the control group (18.4 [9.2] vs. 11.1 [4.5] errors; p=0.006). We used the weighted error score for all subsequent analyses. In order to determine predictors of poor driving within the HIV + group, we first examined whether demographic characteristics were predictive of the error score. Age, gender, and ethnicity were not associated with simulator errors. As a continuous variable, nadir CD4 cell count was not associated with driving performance (r=0.03; p=0.90), although there was a trend for participants with nadir CD4 <100 to have more errors than participants with CD4 >100 (22.9 [11.7] vs. 16.3 [7.3], p=0.12). The regression analysis found that NFL density was the significant predictor for driving errors (p= 0.0136). Within the HIV + group, NFL thickness was significantly correlated with driving errors (r=−0.51, p= 0.025; Fig. 1); this was not the case in the control group (p = 0.48). To further examine the relationship between immunosuppression, NFL thickness, and simulator performance, we stratified the groups into low/high CD4 (<100), and low/high NFL thickness (< 80). The low CD4/low NFL group had significantly higher driving error scores (30.3 [9.5]; n=4) than the low CD4/high NFL (13.2 [5.2]; n=3), high CD4/high NFL (15.2 [6.9]; n=3) and high CD4/low NFL (12.0; n=1) groups.
Table 1.
Demographics of the participants
| Demographics | HIV seronegative | HIV seropositive | p value |
|---|---|---|---|
| Age | 50.3 (9.4) | 52.0 (7.5) | 0.60 |
| Gender (% male) | 14 (87.5%) | 21 (95.5%) | 0.37 |
| Ethnicity (% white) | 14 (87.5%) | 17 (77.3%) | 0.41 |
Table 2.
Immune status and visual functioning
| CD4 nadir >100 | CD4 nadir ≤100 | p value | |
|---|---|---|---|
| Visual functioning | |||
| VAdec/VAlogMar | 1.16 ± 0.23/−0.05 ± 0.09 | 1.1 ± 0.17/−0.03 ± 0.07 | 0.6 |
| IOP | 13.45 ± 2.47 | 12.8 ± 2.4 | 0.52 |
| NFL thickness | 97.19 ± 12.82 | 74.62 ± 17.38 | 0.001 |
HIV human immunodeficiency virus, VA visual acuity, dec decimal, log logarithmic, IOP intraocular pressure, NFL nerve fiber layer
Table 3.
Participant driving performance
| HIV seronegative | HIV seropositive | p value | |
|---|---|---|---|
| Pedestrian collisions (A) | 0.63 (0.62) | 1.2 (0.80) | 0.025 |
| Automobile collisions (B) | 2.3 (10) | 3.5 (2.0) | 0.031 |
| Total collisions (A + B) | 2.9 (1.4) | 4.7 (2.6) | 0.019 |
| Off-road excursions | 3.4 (2.1) | 6.0 (2.1) | 0.023 |
| Traffic light tickets (n, %) | 0 (0%) | 2 (5.3%) | 0.50 |
| Speeding tickets | 0.56 (0.72) | 1.04 (0.95) | 0.098 |
| Weighted errors | 11.1 (4.5) | 18.4 (9.2) | 0.006 |
HIV human immunodeficiency virus
Figure 1.
Relationship between retinal nerve fiber layer thickness and weighted simulator errors for HIV-seropositive subjects. x - retinal nerve fiber layer thickness, y – weighted simulator errors.
Discussion
This study is the first to use a driving simulation to assess the potential impact of HIV-related visual pathology on driving performance. Compared to HIV-seronegative controls, HIV + individuals had significantly more crashes and were more likely to drive on the roadway shoulder; there was a trend for more speeding tickets. Within the HIV + participants, the worst driving performance was by individuals with both a low nadir CD4 cell count and significantly reduced retinal nerve fiber layer thickness. The relationship between NFL thinning and worse driving performance may in part be due to the reduced NFL causing decreased retinal sensitivity, as evidenced by subtle visual field defects [17]. Structural and functional damage in eyes without infectious retinitis occurs with CD4 count below 100 for a prolonged period of time [8, 9]. Neuropsychological impairment is also most common in individuals with advanced disease, and particularly in individuals with low nadir CD4 levels [18]. Previous studies have demonstrated that HIV-related cognitive impairments are associated with reduced performance on a simulator [11, 12], greater difficulty on a structured on-road evaluation [12], and real-world crashes [13]. Unfortunately, we were unable to examine the relationship between NFL thickness and cognitive functioning since we did not have neurocognitive testing on these individuals. This would be an area for future study. In addition to the lack of cognitive testing, there are other limitations to the present study. The sample size is small, and thus replication with larger samples is needed. We used a PC-based driving simulation as an estimate of driving ability, and it cannot be assumed that performance on the simulator translates to real world driving. However, it is worth noting that the simulation was designed to closely approximate real-life driving situations [12] and emulates visually demanding activities (e.g., changing lanes, making turns, passing other cars, avoiding pedestrians, and avoiding unexpected roadblocks [11]) that are consistent with documented structural and functional visual abnormalities associated with HIV, as shown through optical coherence tomography [8], scanning laser polarimetry [9], static perimetry [2, 3, 7, 17], and electroretinography [7, 17]. Supporting the validity of using simulations, a number of studies have demonstrated that similar driving simulations are predictive of on-road driving performance [19, 20], including in HIV-infected populations [12]. Despite these limitations, this preliminary study suggests that driving ability may be significantly impacted by reductions in retinal nerve fiber layer thickness that are associated with low CD4 nadir in HIV patients. Physicians should consider the potential impact that more complex ophthalmologic conditions in HIV-infected patients may have on driving performance.
Acknowledgments
Funding National Institutes of Health (NIH) Grant No. EY007366. NOT-OD-09-060-Recovery Act Administrative Supplement for undergraduate summer research fund. MH 62512 - HIV Neurobehavioral Research Center (HNRC).
References
- 1.Freeman WR, Van Natta ML, Jabs D, Sample PA, Sadun AA, Thorne J, Shah KH, Holland GN, Grp SR. Vision function in HIV-infected individuals without retinitis: report of the Studies of Ocular Complications of AIDS Research Group. Am J Ophthalmol. 2008;145(3):453–462. doi: 10.1016/j.ajo.2007.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Plummer DJ, Sample PA, Arevalo JF, Grant I, Quiceno JI, Dua R, Freeman WR. Visual field toss in HIV-positive patients without infectious retinopathy. Am J Ophthalmol. 1996;122(4):542–549. doi: 10.1016/s0002-9394(14)72115-4. [DOI] [PubMed] [Google Scholar]
- 3.Geier SA, Nohmeier C, Lachenmayr BJ, Klauss V, Goebel FD. Deficits in perimetric performance in patients with symptomatic human-immunodeficiency-virus infection or acquired-immunodeficiency-syndrome. Am J Ophthalmol. 1995;119(3):335–344. doi: 10.1016/s0002-9394(14)71177-8. [DOI] [PubMed] [Google Scholar]
- 4.Geier SA, Kronawitter U, Bogner JR, Hammel G, Berninger T, Klauss V, Goebel FD. Impairment of color contrast sensitivity and neuroretinal dysfunction in patients with symptomatic HIV infection or AIDS. Br J Ophthalmol. 1993;77(11):716–720. doi: 10.1136/bjo.77.11.716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shah KH, Holland GN, Yu F, Van Natta M, Nusinowitz S. Contrast sensitivity and color vision in HIV-infected individuals without infectious retinopathy. Am J Ophthalmol. 2006;142(2):284–292. doi: 10.1016/j.ajo.2006.03.046. [DOI] [PubMed] [Google Scholar]
- 6.Mueller AJ, Plummer DJ, Dua R, Taskintuna I, Sample PA, Grant I, Freeman WR. Analysis of visual dysfunctions in HIVpositive patients without retinitis. Am J Ophthalmol. 1997;124(2):158–167. doi: 10.1016/s0002-9394(14)70780-9. [DOI] [PubMed] [Google Scholar]
- 7.Sample PA, Plummer DJ, Mueller AJ, Matsubara KI, Sadun A, Grant I, Freeman WR. Pattern of early visual field loss in HIV-infected patients. Arch Ophthalmol. 1999;117(6):755–760. doi: 10.1001/archopht.117.6.755. [DOI] [PubMed] [Google Scholar]
- 8.Kozak I, Bartsch DU, Cheng LY, Kosobucki BR, Freeman WR. Objective analysis of retinal damage in HIV-positive patients in the HAART era using OCT. Am J Ophthalmol. 2005;139(2):295–301. doi: 10.1016/j.ajo.2004.09.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kozak I, Bartsch DUG, Cheng L, McCutchan A, Weinreb RN, Freeman WR. Scanning laser polarimetry demonstration of retinal nerve fiber layer damage in human immunodeficiency virus-positive patients without infectious retinitis. Retina. 2007;27(9):1267–1273. doi: 10.1097/IAE.0b013e31806463fb. [DOI] [PubMed] [Google Scholar]
- 10.Gomez ML, Mojana F, Bartsch DU, Freeman WR. Imaging of long-term retinal damage after resolved cotton wool spots. Ophthalmology. 2009;116(12):2407–2414. doi: 10.1016/j.ophtha.2009.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Marcotte TD, Heaton RK, Wolfson T, Taylor MJ, Alhassoon O, Arfaa K, Grant I. The impact of HIV-related neuropsychological dysfunction on driving behavior. J Int Neuropsychol Soc. 1999;5(7):579–592. doi: 10.1017/s1355617799577011. [DOI] [PubMed] [Google Scholar]
- 12.Marcotte TD, Wolfson T, Rosenthal TJ, Heaton RK, Gonzalez R, Ellis RJ, Grant I. A multimodal assessment of driving performance in HIV infection. Neurology. 2004;63(8):1417–1422. doi: 10.1212/01.wnl.0000141920.33580.5d. [DOI] [PubMed] [Google Scholar]
- 13.Marcotte TD, Lazzaretto D, Scott JC, Roberts E, Woods SP, Letendre S. Visual attention deficits are associated with driving accidents in cognitively-impaired HIV-Infected individuals. J Clin Exp Neuropsychol. 2006;28(1):13–28. doi: 10.1080/13803390490918048. [DOI] [PubMed] [Google Scholar]
- 14.Dobbs AR, Heller RB, Schopflocher D. A comparative approach to identify unsafe older drivers. Accid Anal Prev. 1998;30(3):363–370. doi: 10.1016/s0001-4575(97)00110-3. [DOI] [PubMed] [Google Scholar]
- 15.Baldock MR, Mathias JL, McLean AJ, Berndt A. Selfregulation of driving and its relationship to driving ability among older adults. Accid Anal Prev. 2006;38(5):1038–1045. doi: 10.1016/j.aap.2006.04.016. [DOI] [PubMed] [Google Scholar]
- 16.Kay L, Bundy A, Clemson L, Jolly N. Validity and reliability of the on-road driving assessment with senior drivers. Accid Anal Prev. 2008;40(2):751–759. doi: 10.1016/j.aap.2007.09.012. [DOI] [PubMed] [Google Scholar]
- 17.Kozak I, Sample PA, Hao J, Freeman WR, Weinreb RN, Lee TW, Goldbaum MH. Machine learning classifiers detect subtle field defects in eyes of HIV individuals. Trans Am Ophthalmol Soc. 2007;105:111–118. discussion 119–120. [PMC free article] [PubMed] [Google Scholar]
- 18.Heaton RK, Clifford DB, Franklin DR, Jr, Woods SP, Ake C, Vaida F, Ellis RJ, Letendre SL, Marcotte TD, Atkinson JH. HIVassociated neurocognitive disorders persist in the era of potent antiretroviral therapy: CHARTER Study. Neurology. 2010;75(23):2087–2096. doi: 10.1212/WNL.0b013e318200d727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shechtman OCS, Awadzi K, Mann W. Comparison of driving errors between on-the-road and simulated driving assessment: a validation study. Traffic Inj Prev. 2009;10(4):379–385. doi: 10.1080/15389580902894989. [DOI] [PubMed] [Google Scholar]
- 20.Lee H. The validity of driving simulator to measure on-road driving performance of older drivers. Transp Eng Aust. 2003;8(2):89–100. [Google Scholar]