Abstract
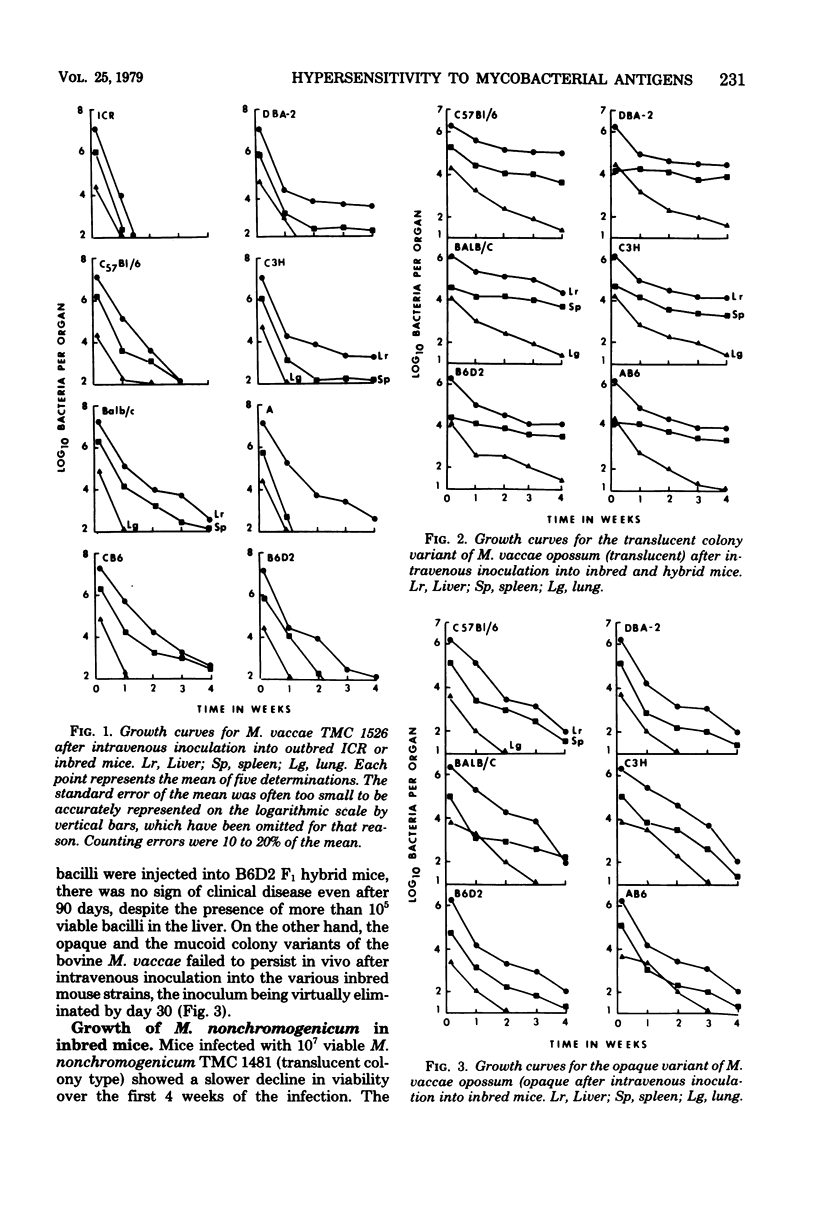
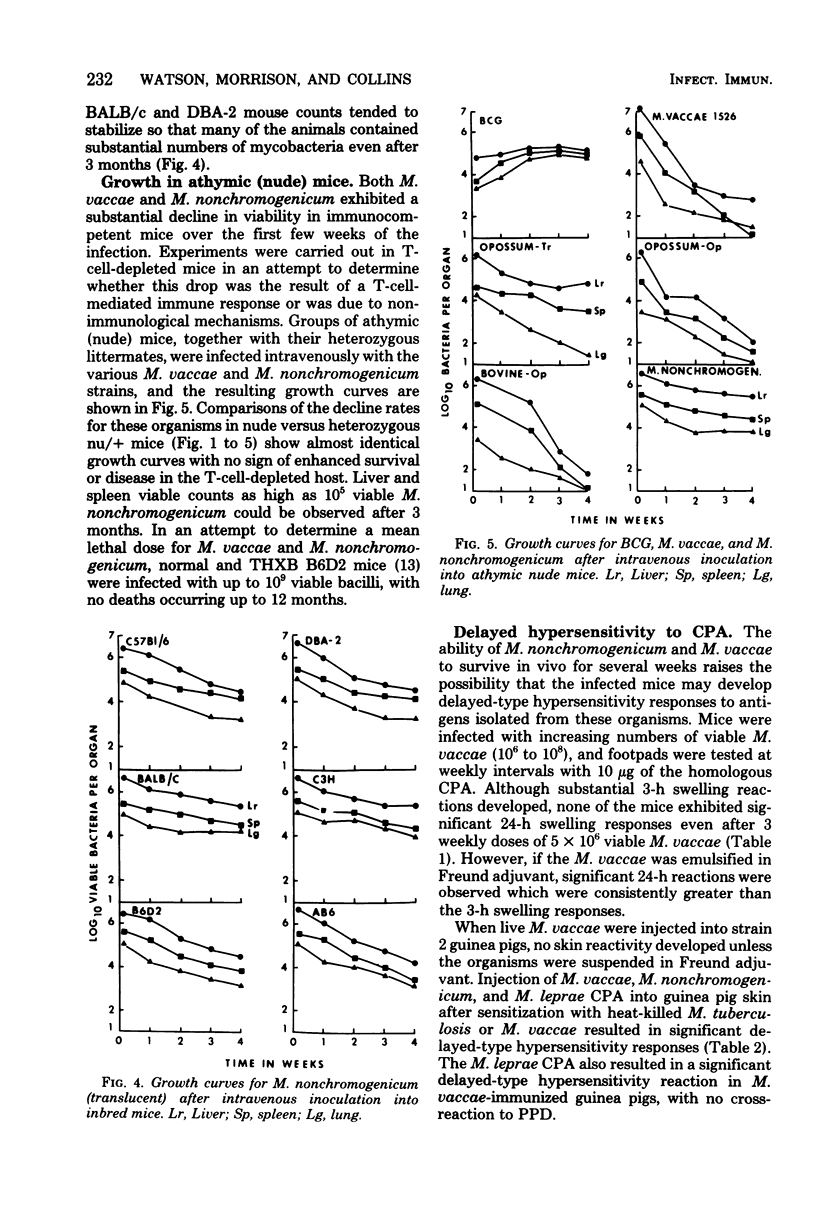
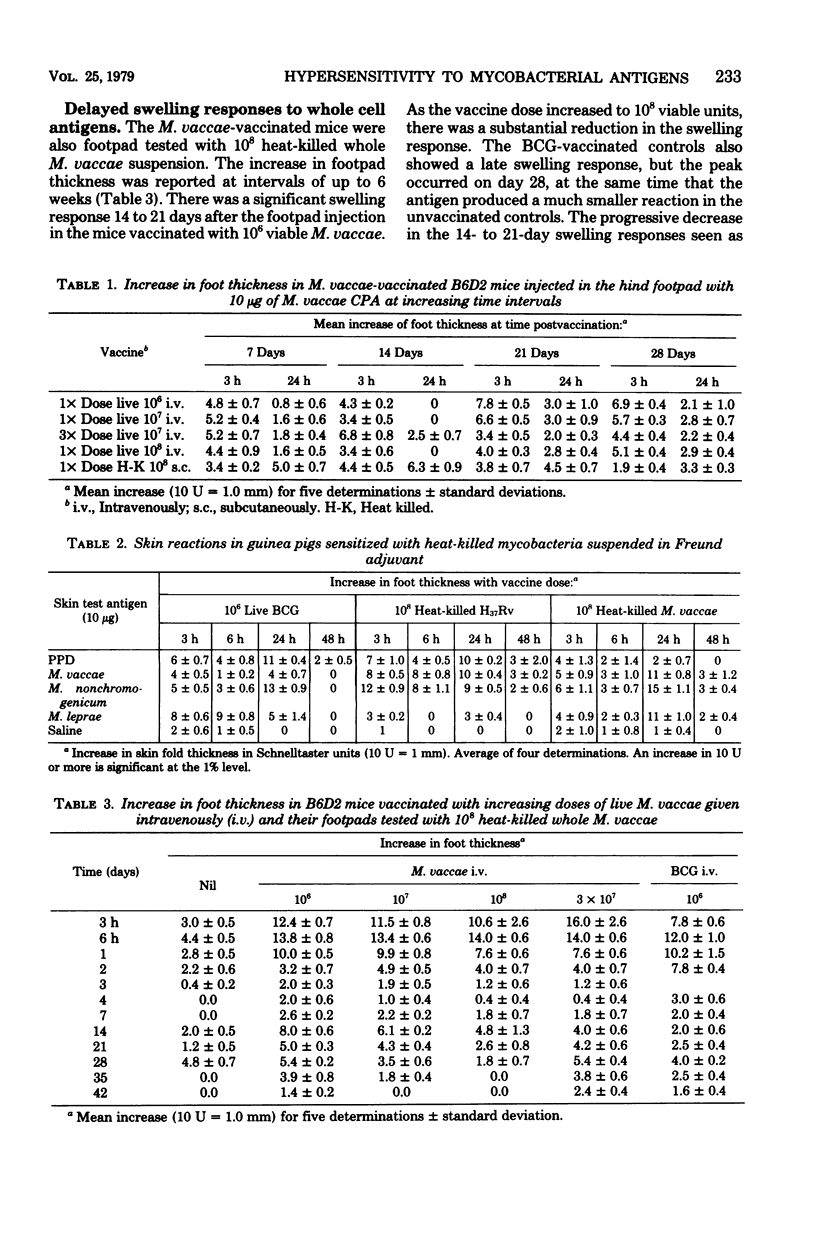
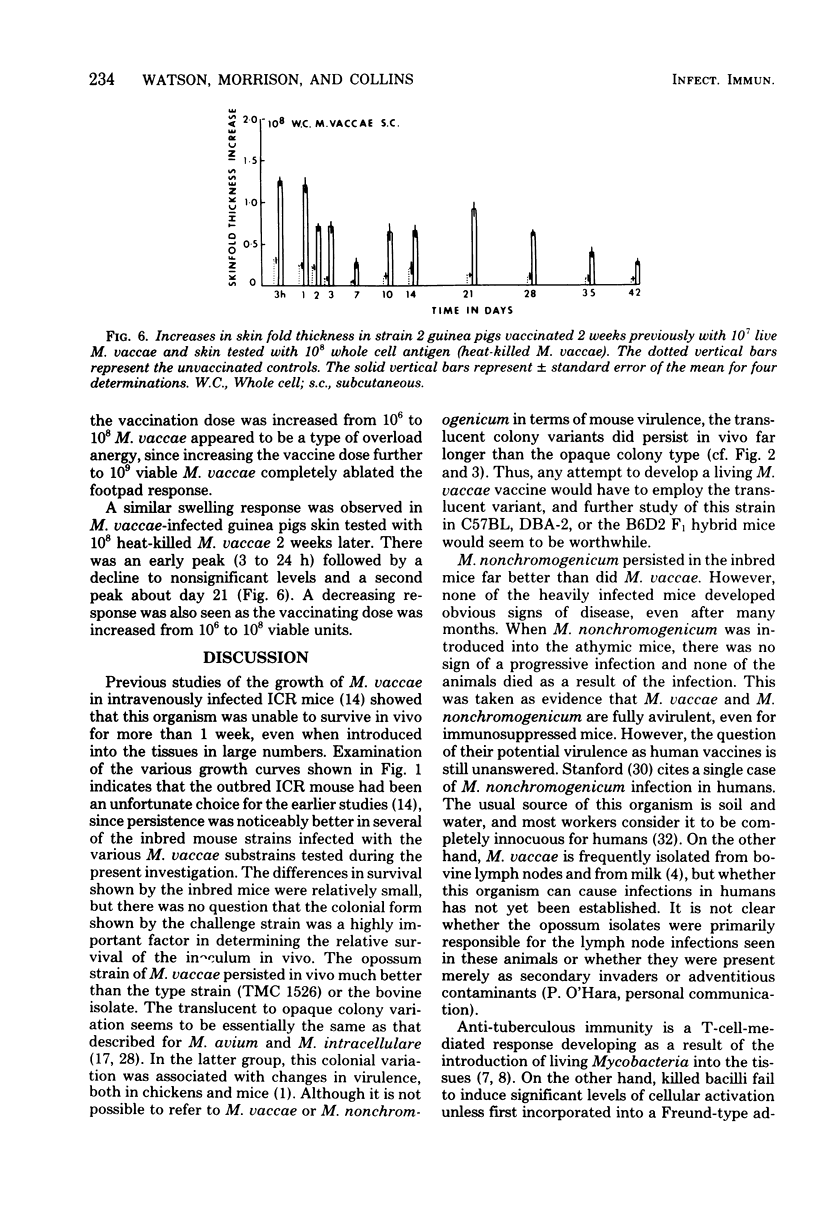
Antigenic relationships between Mycobacterium vaccae, M. nonchromogenicum, and M. leprae were examined in mice and guinea pigs injected with M. vaccae or M. nonchromogenicum suspensions. The growth of both organisms in outbred ICR and four inbred mouse strains was followed up to 30 days. M. nonchromogenicum persisted in the livers and spleens of the inbred mice substantially better than did the M. vaccae population in the same mouse strains. A translucent colony variant of M. vaccae isolated from the opossum survived in vivo better than the opaque colony isolated from opossums and cattle. Persistence of M. vaccae and M. nonchromogenicum was not markedly increased in T-cell-depleted (nude) mice. Normal mice infected with increasing numbers of M. vaccae did not develop delayed-type hypersensitivity to the homologous M. vaccae cytoplasmic protein antigen. When heat-killed M. vaccae were incorporated into Freund adjuvant, both mice and guinea pigs developed delayed hypersensitivity to cytoplasmic antigens prepared from M. vaccae, M. nonchromogenicum and M. vaccae vaccines cross-sensitized guinea pigs to the M. leprae cytoplasmic antigens.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anz W., Meissner G. Vergleichende Virulenzprüfungen am Huhn von transparenten und opaken Kolonien aus Stämmen der aviaären Mykobakteriengruppe verschiedener Serotypen. Zentralbl Bakteriol Orig A. 1972 Aug;221(3):334–342. [PubMed] [Google Scholar]
- BOENICKSE R., JUHASZ E. BESCHREIBUNG DER NEUEN SPECIES MYCOBACTERIUM VACCAE N. SP. Zentralbl Bakteriol Orig. 1964 Feb;192:133–135. [PubMed] [Google Scholar]
- Bechelli L. M., Garbajosa P. G., Gyi M. M., Uemura K., Sundaresan T., Martínez Domínguez V., Matejka M., Tamondong C., Quagliato R., Engler V. BCG vaccination of children against leprosy: seven-year findings of the controlled WHO trial in Burma. Bull World Health Organ. 1973;48(3):323–334. [PMC free article] [PubMed] [Google Scholar]
- Blanden R. V., Lefford M. J., Mackaness G. B. The host response to Calmette-Guérin bacillus infection in mice. J Exp Med. 1969 May 1;129(5):1079–1107. doi: 10.1084/jem.129.5.1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown J. A., Stone M. M., Sutherland I. B.C.G. vaccination of children against leprosy in Uganda: results at end of second follow-up. Br Med J. 1968 Jan 6;1(5583):24–27. doi: 10.1136/bmj.1.5583.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Closs O., Haugen O. A. Experimental murine leprosy. 2. Further evidence for varying susceptibility of outbred mice and evaluation of the response of 5 inbred mouse strains to infection with Mycobacterium lepraemurium. Acta Pathol Microbiol Scand A. 1974 Jul;82(4):459–474. [PubMed] [Google Scholar]
- Collins F. M., Auclair L., Mackaness G. B. Mycobacterium bovis (BCG) infection of the lymph nodes of normal, immune, and cortisone-treated guinea pigs. J Natl Cancer Inst. 1977 Nov;59(5):1527–1535. doi: 10.1093/jnci/59.5.1527. [DOI] [PubMed] [Google Scholar]
- Collins F. M. Cellular antimicrobial immunity. CRC Crit Rev Microbiol. 1978;7(1):27–91. doi: 10.3109/10408417909101177. [DOI] [PubMed] [Google Scholar]
- Collins F. M., Mackaness G. B. The relationship of delayed hypersensitivity to acquired antituberculous immunity. I. Tuberculin sensitivity and resistance to reinfection in BCG-vaccinated mice. Cell Immunol. 1970 Sep;1(3):253–265. doi: 10.1016/0008-8749(70)90047-x. [DOI] [PubMed] [Google Scholar]
- Collins F. M., Mackaness G. B. The relationship of delayed hypersensitivity to acquired antituberculous immunity. II. Effect of adjuvant on the allergenicity and immunogenicity of heat-killed tubercle bacilli. Cell Immunol. 1970 Sep;1(3):266–275. doi: 10.1016/0008-8749(70)90048-1. [DOI] [PubMed] [Google Scholar]
- Collins F. M., Montalbine V. Relative immunogenicity of streptomycin-sensitive and -resistant strains of BCG. Infect Immun. 1973 Sep;8(3):381–387. doi: 10.1128/iai.8.3.381-387.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins F. M., Montalbine V. Relative immunogenicity of streptomycin-susceptible and -resistant strains of BCG. II. Effect of the route of inoculation on growth and immunogenicity. Am Rev Respir Dis. 1975 Jan;111(1):43–51. doi: 10.1164/arrd.1975.111.1.43. [DOI] [PubMed] [Google Scholar]
- Collins F. M., Morrison N. E., Montalbine V. Immune response to persistent mycobacterial infection in mice. Infect Immun. 1978 May;20(2):430–438. doi: 10.1128/iai.20.2.430-438.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins F. M., Volkman A., McGregor D. D. Transfer of delayed and Arthus sensitivity with blood plasma from x-irradiated guinea-pigs. Immunology. 1970 Sep;19(3):501–509. [PMC free article] [PubMed] [Google Scholar]
- Convit J., Pinardi M. E., Avila J. L., Aranzazu N. Specificity of the 48-hour reaction to Mitsuda antigen. Use of a soluble antigen from human and armadillo lepromin. Bull World Health Organ. 1975;52(2):187–191. [PMC free article] [PubMed] [Google Scholar]
- Engel H. W. Pathogenicity as the main differentiating character within the avium-intracellulare complex. Ann Soc Belg Med Trop. 1973;53(4):275–286. [PubMed] [Google Scholar]
- GRAY D. F. The relative natural resistance of rats and mice to experimental pulmonary tuberculosis. J Hyg (Lond) 1961 Dec;59:471–477. doi: 10.1017/s0022172400039164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godal T. The rationale behind a leprosy vaccine research program. Int J Lepr Other Mycobact Dis. 1977 Jan-Mar;45(1):61–63. [PubMed] [Google Scholar]
- Kronvall G., Closs O., Bjune G. Common antigen of Mycobacterium leprae, M. lepraemurium, M. avium, and M. fortuitum in comparative studies using two different types of antisera. Infect Immun. 1977 May;16(2):542–546. doi: 10.1128/iai.16.2.542-546.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kronvall G., Stanford J. L., Walsh G. P. Studies of mycobacterial antigens, with special reference to Mycobacterium leprae. Infect Immun. 1976 Apr;13(4):1132–1138. doi: 10.1128/iai.13.4.1132-1138.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Myrvang B., Godal T., Ridley D. S., Fröland S. S., Song Y. K. Immune responsiveness to Mycobacterium leprae and other mycobacterial antigens throughout the clinical and histopathological spectrum of leprosy. Clin Exp Immunol. 1973 Aug;14(4):541–553. [PMC free article] [PubMed] [Google Scholar]
- Myrvang B. Immune responsiveness to Mycobacterium leprae of healthy humans. Application of the leucocyte migration inhibition test. Acta Pathol Microbiol Scand B Microbiol Immunol. 1974 Oct;82B(5):707–714. doi: 10.1111/j.1699-0463.1974.tb00239.x. [DOI] [PubMed] [Google Scholar]
- Paul R. C., Stanford J. L., Carswell J. W. Multiple skin testing in leprosy. J Hyg (Lond) 1975 Aug;75(1):57–68. doi: 10.1017/s0022172400047069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaefer W. B., Davis C. L., Cohn M. L. Pathogenicity of transparent, opaque, and rough variants of Mycobacterium avium in chickens and mice. Am Rev Respir Dis. 1970 Oct;102(4):499–506. doi: 10.1164/arrd.1970.102.4.499. [DOI] [PubMed] [Google Scholar]
- Shepard C. C., McRae D. H. A method for counting acid-fast bacteria. Int J Lepr Other Mycobact Dis. 1968 Jan-Mar;36(1):78–82. [PubMed] [Google Scholar]
- Stanford J. L. Editorial: A vaccine for leprosy. Lepr Rev. 1976 Jun;47(2):87–91. [PubMed] [Google Scholar]
- Stanford J. L., Rook G. A., Convit J., Godal T., Kronvall G., Rees R. J., Walsh G. P. Preliminary taxonomic studies on the leprosy bacillus. Br J Exp Pathol. 1975 Dec;56(6):579–585. [PMC free article] [PubMed] [Google Scholar]



