Abstract
BACKGROUND
In Goodpasture’s disease, circulating autoantibodies bind to the noncollagenous-1 (NC1) domain of type IV collagen in the glomerular basement membrane (GBM). The specificity and molecular architecture of epitopes of tissue-bound autoantibodies are unknown. Alport’s post-transplantation nephritis, which is mediated by alloantibodies against the GBM, occurs after kidney transplantation in some patients with Alport’s syndrome. We compared the conformations of the antibody epitopes in Goodpasture’s disease and Alport’s post-transplantation nephritis with the intention of finding clues to the pathogenesis of anti-GBM glomerulonephritis.
METHODS
We used an enzyme-linked immunosorbent assay to determine the specificity of circulating autoantibodies and kidney-bound antibodies to NC1 domains. Circulating antibodies were analyzed in 57 patients with Goodpasture’s disease, and kidney-bound antibodies were analyzed in 14 patients with Goodpasture’s disease and 2 patients with Alport’s post-transplantation nephritis. The molecular architecture of key epitope regions was deduced with the use of chimeric molecules and a three-dimensional model of the α345NC1 hexamer.
RESULTS
In patients with Goodpasture’s disease, both autoantibodies to the α3NC1 monomer and antibodies to the α5NC1 monomer (and fewer to the α4NC1 monomer) were bound in the kidneys and lungs, indicating roles for the α3NC1 and α5NC1 monomers as autoantigens. High antibody titers at diagnosis of anti-GBM disease were associated with ultimate loss of renal function. The antibodies bound to distinct epitopes encompassing region EA in the α5NC1 monomer and regions EA and EB in the α3NC1 monomer, but they did not bind to the native cross-linked α345NC1 hexamer. In contrast, in patients with Alport’s post-transplantation nephritis, alloantibodies bound to the EA region of the α5NC1 subunit in the intact hexamer, and binding decreased on dissociation.
CONCLUSIONS
The development of Goodpasture’s disease may be considered an autoimmune “conformeropathy” that involves perturbation of the quaternary structure of the α345NC1 hexamer, inducing a pathogenic conformational change in the α3NC1 and α5NC1 subunits, which in turn elicits an autoimmune response. (Funded by the National Institute of Diabetes and Digestive and Kidney Diseases.)
Goodpasture’s disease is an organ-specific autoimmune disorder characterized by rapidly progressive glomerulonephritis, pulmonary hemorrhage, and glomerular pathological findings that include linear deposits of antibodies along the glomerular basement membrane (GBM) (Fig. 1A).1,2 (For this article we have studied Goodpasture’s disease, which describes the specific entity in which the cause of organ dysfunction is proven to be anti-GBM antibodies, in contrast with Goodpasture’s syndrome, which is a clinical term used to describe rapidly progressive glomerulonephritis and pulmonary hemorrhage.) Lerner and colleagues3 passively transferred Goodpasture anti-GBM antibodies in a primate model, inducing glomerulonephritis and thereby showing that an autoantibody itself can cause disease. The target GBM antigen for circulating antibodies was subsequently identified as the noncollagenous-1 (NC1) domain of the α3 chain of collagen IV4–6; further studies revealed that collagen IV is a family of six α-chains (α1 through α6).7 Immunization of laboratory animals indicated that the α3NC1 specifically induced severe proteinuria and glomerulonephritis, causally linking the self-antigen and antibody in Goodpasture’s disease.8–10
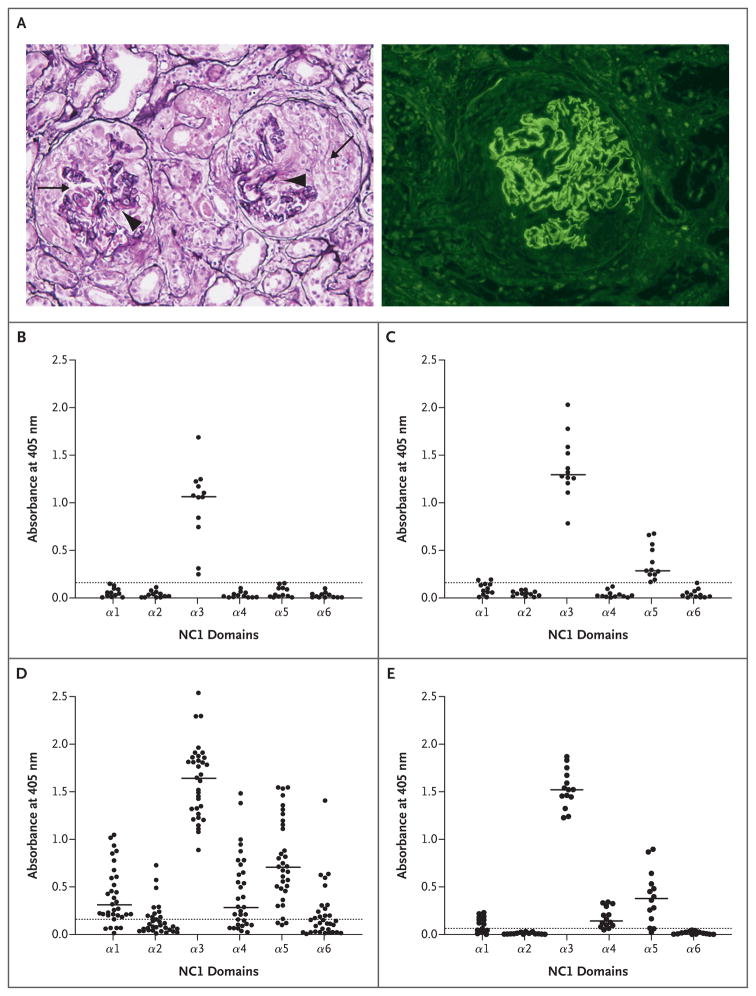
Figure 1. Classic Kidney Lesions in Goodpasture’s Disease, and the Immunoreactivity of Circulating and Kidney-Bound Goodpasture Autoantibodies to Six Noncollagenous-1 Domain Monomers of Human Collagen IV.
The specimen at left in Panel A (Jones’s silver stain) shows cellular crescents (arrows) and necrosis of glomerular tufts (arrowheads), features of glomerulonephritis mediated by anti–glomerular-basement-membrane (GBM) antibodies; the specimen at right shows a glomerulus with crescent and linear staining of the GBM with fluorescein-labeled antihuman IgG antibody. Panels B, C, and D show the reactivity of serum from a total of 57 patients with Goodpasture’s disease, grouped according to noncollagenous-1 (NC1) specificity. In Panel B, serum samples from 12 patients react only with α3NC1. In Panel C, samples from 12 different patients react with α3NC1 and α5NC1. In Panel D, samples from 33 different patients react with α1NC1, α3NC1, α4NC1, and α5NC1. These findings differed significantly from the findings in serum samples from 18 healthy volunteers, which showed non-reactivity (P<0.05). Panel E shows the binding of autoantibodies eluted from the kidneys of 14 patients with Goodpasture’s disease. Significant binding was detected only to the α3NC1, α5NC1, and α4NC1 domains, with less binding to the last than to the first two (P<0.05). Normal kidney eluates from 3 patients without Goodpasture’s disease were nonreactive with all NC1 domains. In Panels B through E, the circles indicate values in individual patients, the solid horizontal lines indicate medians, and the dotted horizontal lines indicate means plus 3 SD for normal samples.
The α3NC1 monomer is assembled into the collagen IV network through the association of the α3, α4, and α5 chains to form a triple helical protomer and through the oligomerization of α345 protomers by means of end-to-end associations and intertwining of triple helixes.7 Two protomers associate through C-terminal NC1 domains, forming an NC1 hexamer.11 The major cross-linked hexamer is reinforced by novel sulfilimine bonds that fasten two protomers12 and must be dissociated in order for autoantibody binding to occur.11,13 In contrast, the hexamer that is not cross-linked can be dissociated by the antibodies themselves, after which they bind to subunits.11
The α345 network is also a target for anti-GBM alloantibodies in Alport’s post-transplantation glomerulonephritis, which occurs in 3 to 5% of patients with Alport’s syndrome who receive kidney transplants; in most such patients, the development of Alport’s post-transplantation nephritis results in allograft loss.14 Alport’s post-transplantation nephritis is mediated by the deposition of alloantibodies to the α3NC1 and α5NC1 domains in response to the “foreign” α345 collagen network that is absent in the kidneys of patients with Alport’s syndrome but present in the renal allograft.15,16
Thus, the α345NC1 hexamer is targeted by antibodies that arise in both Goodpasture’s disease and Alport’s post-transplantation nephritis, but these antibodies have different binding properties. Alloantibodies bind epitopes exposed on the native hexamer, whereas in Goodpasture’s disease the autoantibodies require hexamer dissociation to unmask hidden epitopes.7,17 Our retrospective study investigated the molecular basis for these differences in antibody binding to provide insight into the pathogenic mechanisms of autoimmunity in Goodpasture’s disease.
METHODS
PROTEINS
We purified recombinant human monomers α1NC1 through α6NC1 and chimeras from the culture medium of stably transfected human embryonic kidney (HEK) 293 cells with the use of anti-FLAG agarose.10 To construct α5/α1 chimeras corresponding to the EA and EB regions of the α3NC1 domain, we used polymerase-chain-reaction mutagenesis (for details see the Supplementary Appendix, available with the full text of this article at NEJM.org). Collagen IV NC1 hexamers were isolated from bovine GBM with the use of collagenase digestion.13
SERUM AND TISSUE SAMPLES
Approval from local institutional ethics committees and written informed consent from patients were obtained before the collection of samples. Serum samples from 35 patients with anti-GBM glomerulonephritis were obtained from the serum bank of the Department of Nephrology at Lund University Hospital as a representative subgroup of samples from a larger cohort that were used in our previous study.18 An additional 22 serum samples were collected at the Scripps Research Institute, Kansas University, and the Vanderbilt University Medical Center from 1985 through 2008. Samples were collected before plasma exchange or immunosuppressive drug treatment was initiated. Serum samples from 18 healthy adult volunteers were used as normal controls. Tissue eluates were isolated from the kidneys of 13 patients with Goodpasture’s disease after they underwent nephrectomy at the Scripps Research Institute, as previously described.3,19 Serum and tissue samples obtained at the time of autopsy from one patient with anti-GBM glomerulonephritis who had undergone hemodialysis and immunosuppressive therapy for 3 months20 were snap-frozen, stored at −80°C, and processed later for elution of kidney- and lung-bound antibodies.
Alloantibodies were purified from the rejected kidney allografts of two previously described patients with X-linked Alport’s post-transplantation nephritis. Patient 1 was a 23-year-old man with renal insufficiency, proteinuria, and microscopic hematuria.15 Nephrectomy was performed on a second transplant after linear IgG staining of GBM and crescentic glomerulonephritis were revealed on renal biopsy. In Patient 2, end-stage kidney disease developed at 20 years of age; alloantibodies were eluted from the fourth allograft.16 Kidneys and lungs from normal donors were obtained from the National Disease Research Interchange in Philadelphia.
Tissue-bound antibodies were eluted with the use of 0.1 M glycine, pH 2.8 and 2.2, after homogenization in TRIS-buffered saline (pH 7.4) with protease inhibitors.21
AFFINITY PURIFICATION OF GOODPASTURE AUTOANTIBODIES
The recombinant domain α3NC1 or α5NC1 was coupled with Affi-Gel 10 (Bio-Rad Laboratories) at a concentration of 1 mg per milliliter.22 Plasmapheresis fluid from patients with Goodpasture’s disease was fractionated by means of sequential passing through α3NC1 and α5NC1 columns. Bound antibodies were eluted with 6 M urea in 50 mM sodium citrate (pH 4.0) diluted with TRIS-buffered saline (pH 7.4) and concentrated with the use of ultrafiltration.
IMMUNOASSAYS
Immunoassays of NC1 domains or chimeras were performed with the use of indirect and inhibition enzyme-linked immunosorbent assays.23
STATISTICAL ANALYSIS
All data sets were analyzed for normality with the use of the Kolmogorov–Smirnov test. To determine differences between groups, we used the Mann–Whitney U test or the Kruskal–Wallis analysis of variance on ranks for continuous variables and Fisher’s exact test for categorical variables. A P value of less than 0.05 was considered to indicate statistical significance.
RESULTS
CLINICAL DATA
For this retrospective study, we included serum samples from 57 patients with Goodpasture’s disease. The median age of the patients at the time of diagnosis was 59 years (range, 19 to 87); 44% of all patients were women. There was no significant difference in age distribution between male and female patients. In 3 patients, no further clinical data were available. Among the remaining 54 patients, 22 (41%) had positive results for myeloperoxidase antineutrophil cytoplasmic antibodies (ANCA). Clinical data on lung involvement were available for 46 patients, and 12 of these patients (26%) had overt lung hemorrhage. Follow-up information was available for 50 of the 57 patients at 6 months; 17 patients (34%) remained alive, with stable kidney function; 21 (42%) were being treated with dialysis; and 12 (24%) had died.
SPECIFICITY OF CIRCULATING AND KIDNEY-BOUND ANTIBODIES
Serum samples from all 57 patients with Goodpasture’s disease reacted strongly with the α3NC1 domain. There were three categories of specificity: 12 samples reacted only with the α3NC1 monomer (Fig. 1B), 12 reacted with both the α3NC1 and α5NC1 monomers (Fig. 1C), and the remaining 33 samples were immunoreactive to α3NC1, α5NC1, α1NC1, and α4NC1, with occasional binding to α2NC1 and α6NC1 (Fig. 1D). Overall, 72% of the samples from these patients reacted with the α5NC1 monomer. The antibodies eluted from the kidneys of all 14 patients with Goodpasture’s disease showed binding to α3NC1 and α5NC1 monomers in the majority of samples (11 of 14, or 79%) (Fig. 1E), with significantly lower binding to α4NC1.
CHARACTERIZATION OF CIRCULATING α3NC1 AND α5NC1 AUTOANTIBODIES
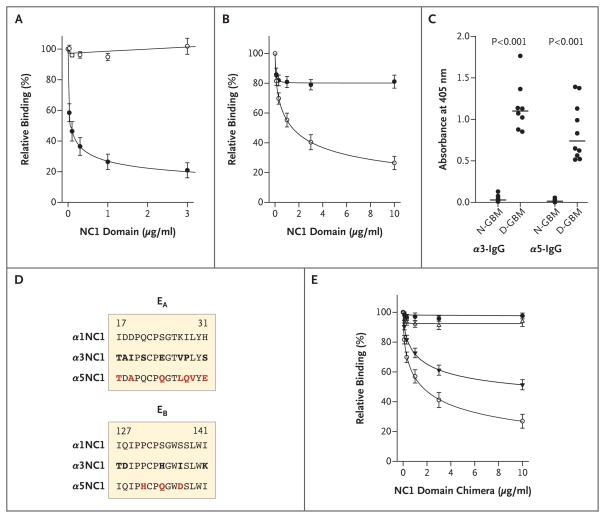
We purified antibodies from seven patients with Goodpasture’s disease, using α3NC1 and α5NC1 affinity columns. All purified antibodies belonged to the IgG subclass (data not shown). Binding of the α3NC1 antibodies to immobilized α3NC1 was strongly inhibited with soluble α3NC1 but not with the α5NC1 monomer (Fig. 2A). Potent α3NC1 inhibition (half-maximal inhibitory concentration [IC50], 0.05 μg per milliliter) indicates high affinity of α3NC1 antibodies (apparent dissociation constant [KD], 2×10−9 M). The α5NC1 IgG antibodies had lower affinity for the α5NC1 monomer (IC50, 1.3 μg per milliliter; apparent KD, 5×10−8 M) (Fig. 2B). The absence of cross-inhibitory effects of α5NC1 and α3NC1 shows that α3NC1 antibodies and α5NC1 antibodies are two distinct populations of circulating autoantibodies in Goodpasture’s disease.
Figure 2. Characterization and Epitope Mapping of Circulating Goodpasture Autoantibodies Specific to the α3 and α5 Noncollagenous-1 Domains.
Autoantibodies were preincubated with various concentrations of the monomer α3 or α5 noncollagenous-1 (NC1) domain, and binding to immobilized antigens α3NC1 and α5NC1 was measured with the use of an enzyme-linked immunosorbent assay (ELISA). Panels A and B show means (±SE) for relative binding, expressed as a percentage of binding in the absence of NC1 monomers in solution, for α3NC1 and α5NC1 IgG antibodies, respectively, from seven patients with Goodpasture’s disease. Binding of the α3NC1 IgG antibodies to immobilized α3NC1 was strongly inhibited in the presence of soluble α3NC1 (solid circles) but not α5NC1 (open circles) (Panel A). The α5NC1 IgG antibodies had a lower affinity for α5NC1 (Panel B). Panel C shows the extent of binding of α3NC1 and α5NC1 IgG antibodies to NC1 hexamers from native glomerular basement membrane (N-GBM) and dissociated GBM (D-GBM). IgG antibodies from individual serum samples from patients with Goodpasture’s disease are represented by circles and medians by horizontal lines. Panel D shows the alignment of the α1NC1 and α5NC1 amino acid sequences corresponding to the EA and EB regions of the α3NC1 domain. Residues that differ from those in α1NC1 (bold) and residues that were mutated in α5 chimeras (bold red) are shown. Panel E shows means (±SE) for the inhibition of the binding of circulating α5NC1-IgG antibodies from the seven patients with Goodpasture’s disease to the α5NC1 domain. EA-α5 chimeras are represented by solid triangles, and EB-α5 chimeras by open triangles. The monomers α5NC1 (open circles) and α1NC1 (solid circles) were included as positive and negative controls, respectively. In Panels A, B, and E, I bars denote standard errors for seven α5NC1 antibodies.
Reduction of the α5NC1 monomer completely inhibited binding of the purified α5NC1 antibodies (data not shown), indicating that the epitopes are conformational and dependent on a critical disulfide bond, analogous to that of α3NC1.13 Moreover, the α3NC1 and α5NC1 antibodies displayed negligible binding to native GBM NC1 hexamers, but the binding was greatly increased on dissociation of the hexamers into constituent subunits (Fig. 2C). We previously described this phenomenon for α3NC1 antibodies as cryptic (hidden) epitopes.13,22
EPITOPE MAPPING FOR CIRCULATING α5NC1 GOODPASTURE ANTIBODIES
We hypothesized that regions in the α5NC1 monomer that were homologous to the EA and EB regions of the α3NC1 monomer23,24 would harbor the epitopes for the α5NC1 antibodies. We created two α1/α5 chimeras by substituting unique amino acid residues in α1NC1, as a nonreactive scaffold, for those in α5NC1 (Fig. 2D). Preincubation with the EA-α5 chimera, but not with the EB-α5 chimera or a parental α1NC1 monomer, significantly inhibited binding of Goodpasture α5NC1 antibodies to α5NC1 in a dose-dependent manner (Fig. 2E). These results establish the EA region as a part of the epitope for circulating α5NC1 autoantibodies.
EPITOPE MAPPING FOR KIDNEY-BOUND AUTOANTIBODIES AND ALLOANTIBODIES
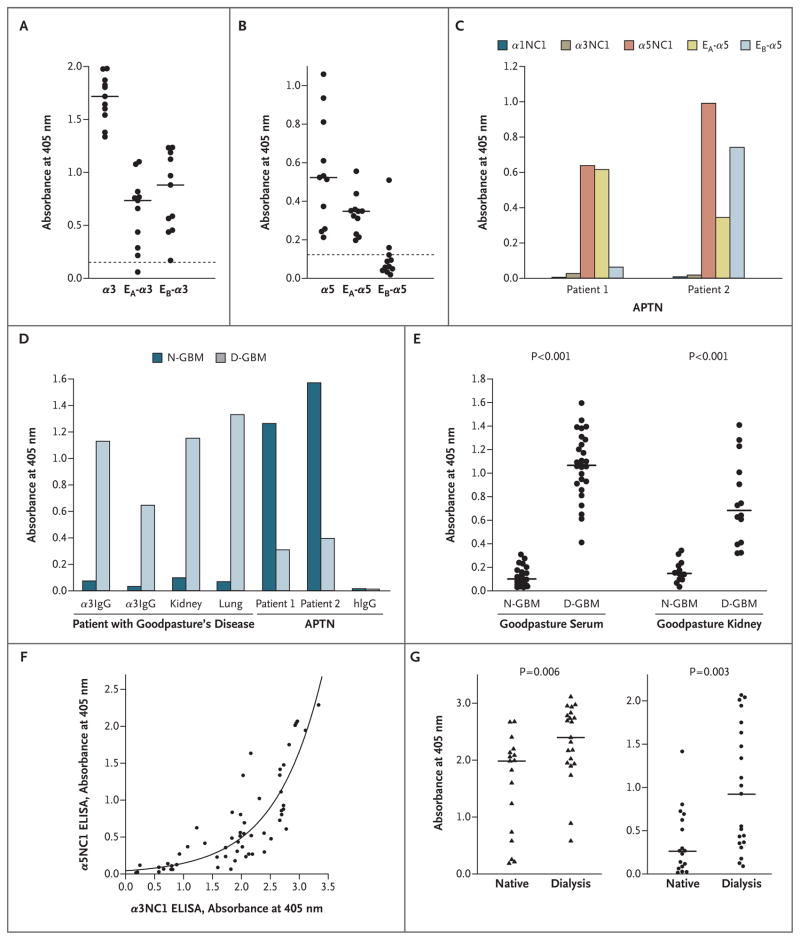
Both the EA and EB regions of the α3NC1 monomer were targets for kidney-bound antibodies in 11 patients with Goodpasture’s disease (Fig. 3A). All kidney eluates also targeted the EA region of the α5NC1 monomer, whereas only 1 patient had antibodies that were reactive to the EB region (Fig. 3B). Moreover, comparison of samples from a single patient with Goodpasture’s disease revealed that circulating antibodies and lung-bound and kidney-bound antibodies shared the same specificity, affinity, and epitopes (Fig. 1 in the Supplementary Appendix). In contrast, the alloantibodies in kidney eluates from the two patients with Alport’s post-transplantation nephritis (Patients 1 and 2) targeted the α5NC1 monomer but not the homologous α1NC1 or α3NC1 monomer (Fig. 3C), and both strongly bound the EA-α5 chimera, whereas the EB-α5 chimera reacted with alloantibodies from Patient 2. These unexpected findings indicate that the EA region of the α5NC1 monomer is a critical part of the epitopes in both Goodpasture’s disease and Alport’s post-transplantation nephritis.
Figure 3. Comparison of Kidney- and Lung-Bound Autoantibodies from Patients with Goodpasture’s Disease and Alloantibodies from Patients with Alport’s Post-Transplantation Nephritis.
Panels A and B show the extent to which kidney-bound Goodpasture autoantibodies bind to the EA and EB chimeras of the α3 noncollagenous-1 (NC1) and α5NC1 domains. Individual patients with Goodpasture’s disease are represented by circles, background binding to α1NC1 by dotted lines, and median values for groups that are different from the background by horizontal lines (P<0.05). Panel C shows the specificity of kidney-bound alloantibodies for the α3/α5NC1 monomer and epitope in samples from two patients with Alport’s post-transplantation nephritis (APTN). Panel D shows the binding of circulating, kidney-bound, and lung-bound autoantibodies to native glomerular basement membrane (N-GBM) and dissociated GBM (D-GBM) NC1 hexamers in samples from one patient with Goodpasture’s disease and two patients with APTN. Normal human IgG (hIgG) does not bind to NC1 hexamers. Panel E shows the binding of circulating antibodies from 27 patients with Goodpasture’s disease and kidney-bound autoantibodies from 14 patients with Goodpasture’s disease to N-GBM and D-GBM NC1 hexamers. Individual patients are represented by circles, and medians for each group by horizontal lines. Panel F shows the positive correlation between the immunoreactivity of the α3NC1 and α5NC1 monomers as revealed by simultaneous enzyme-linked immunosorbent assay (ELISA) for all 57 patients with Goodpasture’s disease (Spearman’s correlation coefficient, 0.852; P<0.001). Data points representing individual patients are fitted to the exponential curve. Panel G shows the levels of α3NC1 (triangles) and α5NC1 (circles) autoantibodies in serum from 17 patients with Goodpasture’s disease who had functioning native kidneys and 21 patients who were dependent on dialysis at 6-month follow-up. P values are based on the Mann–Whitney U test.
Furthermore, both the α3NC1 and α5NC1 autoantibodies were nonreactive to the normal α345NC1 hexamer until the hexamer was dissociated with protein denaturant. The induction of binding was observed for affinity-purified α3NC1 and α5NC1 antibodies from a single patient with Goodpasture’s disease (Fig. 3D), circulating antibodies from 27 patients with Goodpasture’s disease, and kidney eluates from 14 other patients with Goodpasture’s disease (Fig. 3E). Collectively, these findings indicate that circulating and tissue-bound α3NC1 and α5NC1 antibodies in Goodpasture’s disease have identical properties — that is, their respective epitopes arise only after the dissociation of the NC1 hexamer. In sharp contrast, the alloantibodies associated with Alport’s post-transplantation nephritis have a strong reaction to the normal hexamer, and binding is greatly decreased on dissociation of the hexamer (Fig. 3D).
ASSOCIATION OF α3NC1 AND α5NC1 AUTOANTIBODIES WITH DISEASE ACTIVITY
A strong positive correlation was found between titers for α3NC1 and α5NC1 antibodies among all serum samples from patients with Goodpasture’s disease (Fig. 3F). The presence of the α3NC1 antibodies in all samples and the gradual increase in α5NC1 reactivity suggest that α5NC1 autoantibodies may develop after α3NC1 autoantibodies.
Further analyses revealed no significant difference in age, sex, ANCA status, renal outcome, or serum reactivity to α3NC1 or α5NC1 monomers in patients with and those without lung hemorrhage. ANCA status was not associated with sex, presence or absence of lung involvement, renal outcome, or titers for α3NC1 and α5NC1 antibodies; however, patients with positive test results for ANCA were older than patients with negative test results (median age, 70 years vs. 58 years; P = 0.03). Patients with Goodpasture’s disease who were undergoing dialysis and those with preserved renal function at follow-up were of similar age (median, 45 years and 57 years, respectively), but patients who died were significantly older (median, 73 years; P<0.001) and were excluded from further analyses. Patients undergoing dialysis had higher titers of α3NC1 antibodies at presentation than did patients with stable kidney function (Fig. 3G), and had much higher titers for α5NC1 antibodies (median, 0.922 vs. 0.262). A serum sample from 1 of 21 patients with progressive disease requiring dialysis had reactivity that was restricted to the α3NC1 monomer; the majority of samples (from 20 of 21 patients) were reactive with α3NC1 and α5NC1 monomers. In contrast, samples from 6 of 17 patients with preserved renal function had restricted α3NC1 reactivity (P = 0.03 by Fisher’s exact test). Thus, our results support the possibility that increased titers of circulating α3NC1 and α5NC1 autoantibodies are associated with a poor renal outcome.
THREE-DIMENSIONAL STRUCTURE OF THE α345NC1 HEXAMER
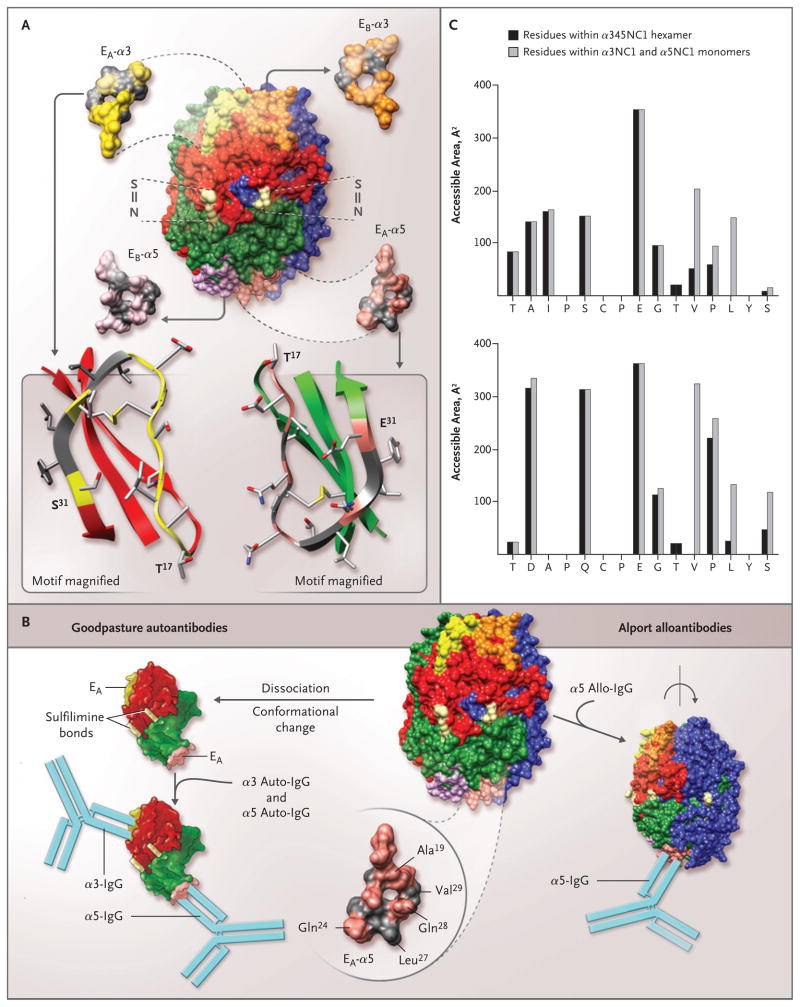
We analyzed the structure of the immunoreactive EA and EB regions in the α345NC1 hexamer model (see the Supplementary Appendix). The EA region of the α5NC1 subunit was not reactive to the Goodpasture autoantibodies in the α345NC1 hexamer cross-linked by sulfilimine bonds (Fig. 4A). This lack of reactivity is analogous to that of the EA and EB regions of the α3NC1 subunit.11,25 However, disruption of the hexamer quaternary structure after treatment with guanidine or by lowering pH leads to dissociation into α35 and α44 dimers and antibody binding (Fig. 4B). The dissociation is concomitant with conformational changes that unlock domain-swapping interactions26 and expose residues sequestered by neighboring subunits. The dissociation and conformational change are reversible, since Goodpasture antibodies do not bind the reassembled hexamer.11,25
Figure 4. Topology of the EA and EB Regions in the α345 Noncollagenous-1 Hexamer, Structural Determinants for the Binding of Alport Alloantibodies and Goodpasture Autoantibodies In Vitro, and Accessible Surface Area of the EA-α3 and EA-α5 Regions of the Noncollagenous-1 Hexamer.
The α345 noncollagenous-1 (NC1) hexamer is composed of two trimeric caps, each consisting of α3NC1 (red), α4NC1 (blue), and α5NC1 (green) subunits (Panel A). Two of the six sulfilimine bonds (S = N) that stabilize the trimer–trimer interface are shown (light yellow). The location and structure of the four homologous regions are also shown: EA (yellow) and EB (orange) in the α3NC1 subunit, and EA (pink) and EB (purple) in the α5NC1 subunit. Three regions, EA and EB in α3NC1 and EA in α5NC1, become critical parts of the neoepitopes for Goodpasture autoantibodies. The topology of the EA regions in α3NC1 and α5NC1 is similar, as indicated in the ribbon diagrams (Panel A, bottom), with the characteristic folding pattern of a β-sheet stabilized with a disulfide bond. Ala19, Gln24, and Gln28 (pink) within the EA region of α5NC1, exposed in the α345NC1 hexamer, are candidates for the binding of Alport alloantibodies (Panel B, bottom right). In contrast, Leu27 and Val29 (gray) are sequestered by their lateral interaction with the α4NC1 domain, and when exposed as a result of hexamer dissociation, they become critical to the binding of Goodpasture autoantibodies. Dissociation of the sulfilimine-cross-linked hexamer into α35 dimer subunits is concomitant with a conformational change that results in the formation of the neoepitopes encompassing the EA regions of the α5NC1 and α3NC1 monomers and the binding of their respective autoantibodies (Panel B, bottom left). The accessible surface area of the EA-α3 region (Panel C, top) and the EA-α5 region (Panel C, bottom) was calculated for a probe, which mimics the antibody molecule (radius, 9 Å); the area of individual residues in the α345NC1 hexamer (black bars) and the α3NC1/α5NC1 model monomers (gray bars) is shown. An increase in the surface area of the monomers indicates that residues are buried in the hexamer (Val27 and Leu29 in EA-α3 and Leu27 and Val29 in EA-α5). In contrast, residues with similar areas within the hexamer and monomers are exposed in the hexamer (Ala19, Gln24, and Gln28 in EA-α5).
Further evidence of conformational transition as a key step in neoepitope formation is provided by the differential effect of dissociating agents on the binding of Goodpasture and Alport post-transplantation nephritis antibodies to the EA region of the α5NC1 subunit. Goodpasture autoantibodies react only with the subunits of a dissociated hexamer, whereas Alport post-transplantation nephritis alloantibodies bind to the intact hexamer and lose binding on dissociation. Analysis of the accessible surface area of the EA-α5 residues within the α345 hexamer and in an α5NC1 monomer reveals that exposure of buried amino acid residues Leu27 and Val29 on hexamer dissociation transforms the EA-α5 region into a part of the Goodpasture neoepitope; likewise, homologous residues Val27 and Leu29 become exposed within the EA-α3 region (Fig. 4B and 4C). In contrast, Ala19, Gln24, and Gln28 are located on the hexamer surface and constitute a part of the alloepitope. The diminished binding of the alloantibodies indicates a conformational change in the EA-α5 region, which is concomitant with hexamer dissociation.
DISCUSSION
The immunoreactivity of circulating Goodpasture autoantibodies to several NC1 domains of collagen IV was reported previously,27–30 but the specificity of tissue-bound autoantibodies is unknown, except in a single patient, in whom the antibodies were reactive to the α3NC1 domain.31 We report here that α5NC1 autoantibodies, in addition to α3NC1 autoantibodies, are frequently present in the kidneys and lungs of patients with Goodpasture’s disease. The α5NC1 Goodpasture antibodies bind to a conformation-dependent epitope encompassing the EA region in the α5NC1 monomer. This region also encompasses the epitope for alloantibodies in patients with Alport’s post-transplantation nephritis.
In the α345NC1 hexamer, quaternary interactions reinforced by sulfilimine cross-links present key structural constraints against the transition of EA-α3 and EA-α5 regions to pathogenic conformation in Goodpasture’s disease. Disruption of hexamer structure changes the conformation of the EA regions of α3NC1 and α5NC1 and the EB region of α3NC1, transforming them into neoepitopes for autoantibodies. In the GBM, an additional level of constraint is provided by the triple helical domain tethered to the hexamer (conformer 1) (Fig. 5). In the absence of cross-links, quaternary constraints against conformational transition are diminished (conformer 2), shifting the equilibrium toward the trimers (conformer 3). The presence of such trimers in basement membranes has been confirmed on electron microscopy.32 Moreover, Goodpasture antibodies can induce a conformational change, dissociate conformer 3, and form an antigen–antibody complex that is consistent with binding to a non–cross-linked hexamer in vitro11 and in passive-transfer experiments.3
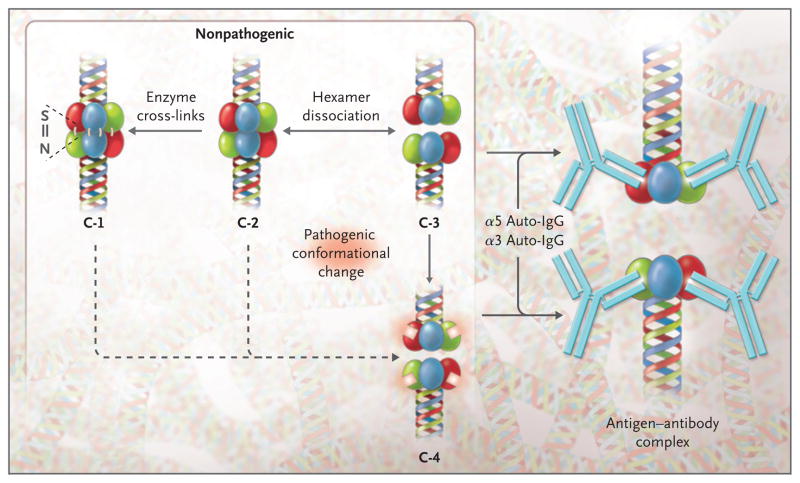
Figure 5. Conformational Diversity and Differential Reactivity of α345 Noncollagenous-1 Hexamers of the Glomerular Basement Membrane.
The diagram shows a portion of the collagen IV network with the α345 noncollagenous-1 (NC1) hexamer tethered to the triple-helical domain. The different possible NC1 conformers shown are the cross-linked form stabilized by sulfilimine bonds (conformer 1 [C-1]), the non-cross-linked form (C-2), and the form in which the NC1 hexamers are dissociated into trimers (C-3). In Goodpasture’s disease the latter may undergo a conformational change resulting in the formation of neoepitopes shown as white squares on the α3NC1 (red) and α5NC1 (green) subunits of C-4, eliciting antibody formation and subsequent binding to conformers C-3 and C-4. Conformers C-1 and C-2 have the potential to be transformed into the pathogenic conformer C-4.
We postulate that an early pivotal step of Goodpasture’s disease involves conformational transitions in subunits of non–cross-linked hexamers or trimers (conformers 2 and 3), forming pathogenic neoepitopes that elicit both antibody production and binding (conformer 4). The triggering event may be an individual factor or a combination of factors — such as enzymatic or nonenzymatic post-translational modifications (oxidation, nitrosylation, and glycation), a rise in body temperature, or proteolytic cleavage — that perturbs the quaternary structure of the hexamer. Indeed, cleavage of a disulfide bond in α3NC1 in a non–cross-linked hexamer (conformer 3) has been shown to enhance the binding of Goodpasture antibodies.33 Furthermore, environmental factors such as cigarette smoking or exposure to organic solvents could inhibit the putative enzyme that catalyzes formation of sulfilimine bonds and thereby increase the proportion of non–cross-linked hexamers (conformer 2).
Goodpasture’s disease may be considered an autoimmune “conformeropathy,” a designation that reflects the requirement for a conformational transition between two distinct NC1 conformers — a nonpathogenic conformer within the hexamer and a dissociated pathogenic conformer that elicits an autoimmune response. Grave’s disease and antiphospholipid autoimmune disease,34–38 which involve pathogenic conformational changes, and perhaps idiopathic membranous nephropathy39 may also be included in such a category. This conceptual framework reflects fundamental issues about the causes of autoimmune disease in molecular terms, answering questions about what triggers the conformational change.
Supplementary Material
Acknowledgments
Supported by a grant (DK18381-37) from the National Institute of Diabetes and Digestive and Kidney Diseases (to Dr. Hudson).
We thank Parvin Todd and Neonila Danylevych for their technical assistance, Drs. Julia Lewis and Ashton Byington for providing kidney and lung specimens from a patient with Goodpasture’s disease, Dr. Julie K. Hudson for critical reading of an earlier version of the manuscript, and Dr. Richard A. Lerner for coining the term “conformeropathy.”
Footnotes
Disclosure forms provided by the authors are available with the full text of this article at NEJM.org.
References
- 1.Wilson CB, Dixon FJ. Anti-glomerular basement membrane antibody-induced glomerulonephritis. Kidney Int. 1973;3:74–89. doi: 10.1038/ki.1973.14. [DOI] [PubMed] [Google Scholar]
- 2.Salama AD, Levy JB, Lightstone L, Pusey CD. Goodpasture’s disease. Lancet. 2001;358:917–20. doi: 10.1016/S0140-6736(01)06077-9. [DOI] [PubMed] [Google Scholar]
- 3.Lerner RA, Glassock RJ, Dixon FJ. The role of anti-glomerular basement membrane antibody in the pathogenesis of human glomerulonephritis. J Exp Med. 1967;126:989–1004. doi: 10.1084/jem.126.6.989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Saus J, Wieslander J, Langeveld JP, Quinones S, Hudson BG. Identification of the Goodpasture antigen as the alpha 3(IV) chain of collagen IV. J Biol Chem. 1988;263:13374–80. [PubMed] [Google Scholar]
- 5.Butkowski RJ, Langeveld JP, Wieslander J, Hamilton J, Hudson BG. Localization of the Goodpasture epitope to a novel chain of basement membrane collagen. J Biol Chem. 1987;262:7874–7. [PubMed] [Google Scholar]
- 6.Turner N, Mason PJ, Brown R, et al. Molecular cloning of the human Goodpasture antigen demonstrates it to be the alpha 3 chain of type IV collagen. J Clin Invest. 1992;89:592–601. doi: 10.1172/JCI115625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hudson BG, Tryggvason K, Sundaramoorthy M, Neilson EG. Alport’s syndrome, Goodpasture’s syndrome, and type IV collagen. N Engl J Med. 2003;348:2543–56. doi: 10.1056/NEJMra022296. [DOI] [PubMed] [Google Scholar]
- 8.Kalluri R, Gattone VH, II, Noelken ME, Hudson BG. The alpha 3 chain of type IV collagen induces autoimmune Goodpasture syndrome. Proc Natl Acad Sci U S A. 1994;91:6201–5. doi: 10.1073/pnas.91.13.6201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Abbate M, Kalluri R, Corna D, et al. Experimental Goodpasture’s syndrome in Wistar-Kyoto rats immunized with alpha3 chain of type IV collagen. Kidney Int. 1998;54:1550–61. doi: 10.1046/j.1523-1755.1998.00153.x. [DOI] [PubMed] [Google Scholar]
- 10.Sado Y, Boutaud A, Kagawa M, Naito I, Ninomiya Y, Hudson BG. Induction of anti-GBM nephritis in rats by recombinant α3(IV)NC1 and α4(IV)NC1 of type IV collagen. Kidney Int. 1998;53:664–71. doi: 10.1046/j.1523-1755.1998.00795.x. [Erratum, Kidney Int 1998;54:311.] [DOI] [PubMed] [Google Scholar]
- 11.Borza DB, Bondar O, Colon S, et al. Goodpasture autoantibodies unmask cryptic epitopes by selectively dissociating autoantigen complexes lacking structural reinforcement: novel mechanisms for immune privilege and autoimmune pathogenesis. J Biol Chem. 2005;280:27147–54. doi: 10.1074/jbc.M504050200. [DOI] [PubMed] [Google Scholar]
- 12.Vanacore R, Ham AJ, Voehler M, et al. A sulfilimine bond identified in collagen IV. Science. 2009;325:1230–4. doi: 10.1126/science.1176811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wieslander J, Langeveld J, Butkowski R, Jodlowski M, Noelken M, Hudson BG. Physical and immunochemical studies of the globular domain of type IV collagen: cryptic properties of the Goodpasture antigen. J Biol Chem. 1985;260:8564–70. [PubMed] [Google Scholar]
- 14.Kashtan CE. Renal transplantation in patients with Alport syndrome. Pediatr Transplant. 2006;10:651–7. doi: 10.1111/j.1399-3046.2006.00528.x. [DOI] [PubMed] [Google Scholar]
- 15.Hudson BG, Kalluri R, Gunwar S, et al. The pathogenesis of Alport syndrome involves type IV collagen molecules containing the alpha 3(IV) chain: evidence from anti-GBM nephritis after renal transplantation. Kidney Int. 1992;42:179–87. doi: 10.1038/ki.1992.276. [DOI] [PubMed] [Google Scholar]
- 16.Kang JS, Kashtan CE, Turner AN, Heidet L, Hudson BG, Borza DB. The allo-antigenic sites of alpha3alpha4alpha5(IV) collagen: pathogenic X-linked Alport alloantibodies target two accessible conformational epitopes in the alpha5NC1 domain. J Biol Chem. 2007;282:10670–7. doi: 10.1074/jbc.M611892200. [DOI] [PubMed] [Google Scholar]
- 17.Kalluri R, Sun MJ, Hudson BG, Neilson EG. The Goodpasture autoantigen: structural delineation of two immunologically privileged epitopes on alpha3(IV) chain of type IV collagen. J Biol Chem. 1996;271:9062–8. doi: 10.1074/jbc.271.15.9062. [DOI] [PubMed] [Google Scholar]
- 18.Segelmark M, Hellmark T, Wieslander J. The prognostic significance in Goodpasture’s disease of specificity, titre and affinity of anti-glomerular-basement-membrane antibodies. Nephron Clin Pract. 2003;94:c59–c68. doi: 10.1159/000072022. [DOI] [PubMed] [Google Scholar]
- 19.Marquardt H, Wilson CB, Dixon FJ. Isolation and immunological characterization of human glomerular basement membrane antigens. Kidney Int. 1973;3:57–65. doi: 10.1038/ki.1973.12. [DOI] [PubMed] [Google Scholar]
- 20.Clyne S, Frederick C, Arndt F, Lewis J, Fogo AB. Concurrent and discrete clinico-pathological presentations of Wegener granulomatosis and anti-glomerular basement membrane disease. Am J Kidney Dis. 2009;54:1116–20. doi: 10.1053/j.ajkd.2009.04.026. [DOI] [PubMed] [Google Scholar]
- 21.Saxena R, Bygren P, Butkowski R, Wieslander J. Specificity of kidney-bound antibodies in Goodpasture’s syndrome. Clin Exp Immunol. 1989;78:31–6. [PMC free article] [PubMed] [Google Scholar]
- 22.Borza DB, Netzer KO, Leinonen A, et al. The Goodpasture autoantigen: identification of multiple cryptic epitopes on the NC1 domain of the alpha3(IV) collagen chain. J Biol Chem. 2000;275:6030–7. doi: 10.1074/jbc.275.8.6030. [DOI] [PubMed] [Google Scholar]
- 23.Netzer KO, Leinonen A, Boutaud A, et al. The Goodpasture autoantigen: mapping the major conformational epitope(s) of alpha3(IV) collagen to residues 17–31 and 127–141 of the NC1 domain. J Biol Chem. 1999;274:11267–74. doi: 10.1074/jbc.274.16.11267. [DOI] [PubMed] [Google Scholar]
- 24.Hellmark T, Burkhardt H, Wieslander J. Goodpasture disease: characterization of a single conformational epitope as the target of pathogenic autoantibodies. J Biol Chem. 1999;274:25862–8. doi: 10.1074/jbc.274.36.25862. [DOI] [PubMed] [Google Scholar]
- 25.Vanacore RM, Ham AJ, Cartailler JP, et al. A role for collagen IV cross-links in conferring immune privilege to the Goodpasture autoantigen: structural basis for the crypticity of B cell epitopes. J Biol Chem. 2008;283:22737–48. doi: 10.1074/jbc.M803451200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Khoshnoodi J, Sigmundsson K, Cartailler JP, Bondar O, Sundaramoorthy M, Hudson BG. Mechanism of chain selection in the assembly of collagen IV: a prominent role for the alpha2 chain. J Biol Chem. 2006;281:6058–69. doi: 10.1074/jbc.M506555200. [DOI] [PubMed] [Google Scholar]
- 27.Hellmark T, Johansson C, Wieslander J. Characterization of anti-GBM antibodies involved in Goodpasture’s syndrome. Kidney Int. 1994;46:823–9. doi: 10.1038/ki.1994.338. [DOI] [PubMed] [Google Scholar]
- 28.Kalluri R, Wilson CB, Weber M, et al. Identification of the alpha 3 chain of type IV collagen as the common autoantigen in antibasement membrane disease and Goodpasture syndrome. J Am Soc Nephrol. 1995;6:1178–85. doi: 10.1681/ASN.V641178. [DOI] [PubMed] [Google Scholar]
- 29.Bondar O, Borza D-B, Todd P, Hudson BG. Characterization of a subpopulation of Goodpasture autoantibodies targeted to the non-collagenous domain of the α5(IV) chain of type IV collagen. J Am Soc Nephrol. 2002;13:173A. abstract. [Google Scholar]
- 30.Zhao J, Cui Z, Yang R, Jia XY, Zhang Y, Zhao MH. Anti-glomerular basement membrane autoantibodies against different target antigens are associated with disease severity. Kidney Int. 2009;76:1108–15. doi: 10.1038/ki.2009.348. [DOI] [PubMed] [Google Scholar]
- 31.Kalluri R, Melendez E, Rumpf KW, et al. Specificity of circulating and tissue-bound autoantibodies in Goodpasture syndrome. Proc Assoc Am Physicians. 1996;108:134–9. [PubMed] [Google Scholar]
- 32.Gunwar S, Noelken ME, Hudson BG. Properties of the collagenous domain of the alpha 3(IV) chain, the Goodpasture antigen, of lens basement membrane collagen: selective cleavage of alpha (IV) chains with retention of their triple helical structure and noncollagenous domain. J Biol Chem. 1991;266:14088–94. [PubMed] [Google Scholar]
- 33.Calvete JJ, Revert F, Blanco M, et al. Conformational diversity of the Goodpasture antigen, the noncollagenous-1 domain of the alpha3 chain of collagen IV. Proteomics. 2006;6(Suppl 1):S237–S244. doi: 10.1002/pmic.200500495. [DOI] [PubMed] [Google Scholar]
- 34.Chen CR, Pichurin P, Nagayama Y, Latrofa F, Rapoport B, McLachlan SM. The thyrotropin receptor autoantigen in Graves disease is the culprit as well as the victim. J Clin Invest. 2003;111:1897–904. doi: 10.1172/JCI17069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chazenbalk GD, Pichurin P, Chen CR, et al. Thyroid-stimulating autoantibodies in Graves disease preferentially recognize the free A subunit, not the thyrotropin holoreceptor. J Clin Invest. 2002;110:209–17. doi: 10.1172/JCI15745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schott M, Scherbaum WA, Morgenthaler NG. Thyrotropin receptor autoantibodies in Graves’ disease. Trends Endocrinol Metab. 2005;16:243–8. doi: 10.1016/j.tem.2005.05.009. [DOI] [PubMed] [Google Scholar]
- 37.Kasahara H, Matsuura E, Kaihara K, et al. Antigenic structures recognized by anti-beta2-glycoprotein I autoantibodies. Int Immunol. 2005;17:1533–42. doi: 10.1093/intimm/dxh330. [DOI] [PubMed] [Google Scholar]
- 38.de Laat B, Derksen RH, van Lummel M, Pennings MT, de Groot PG. Pathogenic anti-beta2-glycoprotein I antibodies recognize domain I of beta2-glycoprotein I only after a conformational change. Blood. 2006;107:1916–24. doi: 10.1182/blood-2005-05-1943. [DOI] [PubMed] [Google Scholar]
- 39.Beck LH, Jr, Bonegio RGB, Lambeau G, et al. M-type phospholipase A2 receptor as target antigen in idiopathic membranous nephropathy. N Engl J Med. 2009;361:11–21. doi: 10.1056/NEJMoa0810457. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.