Abstract
Background
Laser light is known to stimulate nerves. This study investigated alternative wavelengths for nerve stimulation.
Materials and Methods
The sciatic nerves of rats were irradiated with four different lasers—a Ho:YAG (2100 nm), a Yb:glass fiber laser (1495 nm) and diode lasers (1450 nm and 1540 nm).
Results
All lasers evoked a visible leg twitch response, and electromyography confirmed muscle activation. The Yb:glass laser at 1495 nm delivered through a single mode fiber was found to be the most effective stimulus. The stimulation threshold for a 2 millisecond pulse from the Yb:glass laser was determined to be 3.7 ± 2.8 mJ/cm2.
Conclusions
The Yb:glass laser has the potential for use in neurostimulation, as an alternative to electrical stimulation.
Keywords: nerve stimulation, laser
INTRODUCTION
Laser light has been shown to be an effective nerve stimulus. For example, Wells et al. [1–4] have stimulated rat peripheral nerves, Izzo et al. [5–8] have stimulated gerbil cochlea and Fried et al. [9] have stimulated the rat cavernous nerve. Although electrical stimulation is the standard method of neural excitation, laser stimulation offers some significant advantages. Electrical stimulation requires an electrode to be in contact with the neuron, which may damage the tissue under investigation. Also, the electrical field will spread from the point of contact, so that neighboring nerves may also be stimulated and the stimulus may also be recorded by the detecting device. An optical stimulus does not have these issues—it is non-contact, spatially selective, and there is no stimulus artifact in neural recordings.
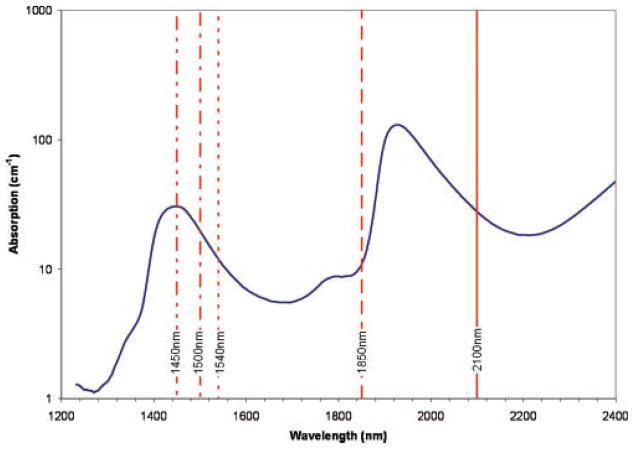
The thermal gradient created in the nerve fiber by absorption of the laser pulse is thought to stimulate the nerve. It has been suggested that the Ho:YAG laser emitting at 2.1 μm, has an ideal penetration depth in tissue for nerve stimulation [2]. This is based on a compromise between enough absorption to generate a thermal effect, but not so much as to damage the tissue. Aculight produces a nerve stimulator (Capella R-1850) emitting at 1.85 μm [7] which has similar absorption characteristics to a Ho:YAG laser. Assuming water to be the main chromophore, laser wavelengths in the region of 1.5 μm will result in comparable absorption, as can be seen in Figure 1. This article investigates 1,450, 1,495, and 1,540 nm lasers for nerve stimulation. The 1,450–1,540 nm wavelengths are absorbed more than 1.85 μm laser, but less than 2.1 μm, so offer alternative penetration depths in tissue. They, therefore, have the potential to provide a choice for matching the laser to the thickness of the nerve fiber requiring stimulation. The 1,500 nm region is commonly used in the telecommunications industry and, therefore, benefits from cheap and readily available technology which can be used for nerve stimulation procedures. The focus of this investigation is to determine the feasibility for stimulation of some of the lasers in this wavelength region. Direct comparison between lasers was not made since they had different power and delivery (beam diameter) characteristics. Indeed, the effect of pulse length or beam diameter on nerve stimulation is not systematically investigated in this article.
Fig. 1.
Water absorption spectrum, showing the wavelength of some relevant lasers [17].
MATERIALS AND METHODS
Sprague–Dawley rats (n =10, ~300 g) were used for in vivo sciatic nerve stimulation. All experiments were conducted in compliance with Institutional Animal Care and Use Committee (IACUC) guidelines. Prior to surgery, the rats were anesthetized by an intraperitoneal injection of ketamine (100 mg/kg) and xylazine (13 mg/kg). Additional doses were administered as needed to maintain sedation. The hair was shaved from the upper area of the hind legs. The rats were placed on their ventral surface and an incision made in the skin from the lumbar vertebral column in the midline in a lateral direction along the lateral aspect of the thigh. The sciatic nerve was exposed through a gluteal muscle splitting incision. Both hind legs were used to give a total sample size of n =20.
A stainless steel needle electrode (Jari Electrode Supply, Gilroy, CA) was inserted into the biceps femoris muscle and a ground surface electrode attached to the skin on the upper thigh. electromyograph (EMG) signals were recorded via a data acquisition module (NI-USB 6211, National Instruments, Austin, TX) with a 10 kHz sampling frequency and filtered between 20 and 4,999 Hz. EMG’s were recorded without laser firing, and with laser firing but not directed to the nerve, to ensure recorded signals had no artifacts. A holmium:YAG laser at 2,100 nm (Newstar, Irvine, CA), a ytterbium:glass fiber laserat 1,495 nm (IPG Photonics, Oxford, MA), and diode lasers at 1,540 and 1,450 nm (Candela, Wayland, MA) were used to irradiate the sciatic nerve.
The Ho:YAG laser, which has a 300 microsecond pulse length, was attenuated by a set of filters (40–80% transmission). A range of energies between 5 and 20 mJ was achieved with the filters. The beam was delivered through a 600 μm core 0.39 NA silica fiber (Thorlabs, Newton, NJ). Five millimeter from the fiber end, the beam diameter was measured with a Pyrocam III beamprofiler (Ophir-Spiricon, Logan, UT) to be 5.4 ± 0.6 mm. The sciatic nerve of a rat is on average 2 mm in diameter. To ensure all the energy is delivered to the nerve during stimulation, the fiber end was mounted 2 mm above the nerve, so that a 2 mm beam irradiates the nerve.
The Yb:glass laser had variable pulse length (1–50 millisecond) and had an output of up to 3 W. It was delivered via a single mode 28 μm core 0.14 NA optical fiber (Corning Incorporated, Corning, NY)—the distal end was either bare or had a lens to produce a 900 μm diameter collimated beam. Five millimeter from the bare fiber end, the diameter measured 1.1 ± 0.4 mm. During irradiation the fiber was mounted 2 mm above the nerve.
The diode lasers had variable pulse lengths (25–200 millisecond) and were delivered by a 1,000 μm core 0.39NA silica fiber (Thorlabs). Five millimeter from the fiber the beam diverged to 5.2 ± 0.6 mm. During irradiation the fiber was mounted 2 mm above the nerve. The 1,540 nm has an output power of up to 3 W. The 1,450 nm laser has an output power of up to 18 W.
The procedure was recorded on a Sony DV Handycam. Observation of a muscle, toe or lower leg/foot twitch indicated that the nerve had been stimulated. The observer was blind to the laser parameters being delivered.
A “staircase” method [10] was employed to determine stimulation threshold. Energy was increased pulse by pulse until a muscle twitch was observed, then decreased until there was no response, then increased again, but in smaller increments until threshold was found. For the lasers with variable pulse length, a short pulse length was chosen to begin and the staircase method employed up to the maximum power of the device. If this did not produce stimulation, the pulse length was increased and the procedure repeated. Results, therefore, quote the shortest pulse length that stimulation was possible with. The probability of stimulation over all animals was calculated for each laser fluence applied during this process.
At the end of the procedure, the rats were euthanized with an intracardiac injection of sodium pentabarbitol (1 ml/kg).
RESULTS
The minimum laser fluence and pulse length required to stimulate the sciatic nerve is shown in Table 1. The Ho:YAG 2,100 nm laser provides the lowest stimulation threshold.
TABLE 1.
Stimulation Threshold for Different Lasers
| Laser (nm) | Absorption coefficient (cm−1) | Pulse length (millisecond) | Beam diameter at tissue (mm) | Stimulation threshold (J/cm2) ± standard deviation (no. of samples) |
|---|---|---|---|---|
| 1,450 | 30 | 100 | 2.1 | 35 ± 11 (n = 3) |
| 1,495 (l) | 20 | 25 | 0.9 | 4.9 ± 1.6 (n = 6) |
| 1,495 (b) | 20 | 2 | 0.4 | 3.7 ± 2.8 (n = 4) |
| 1,540 | 12 | 200 | 2.1 | 8.9 ± 2.2 (n = 4) |
| 2,100 | 28 | 0.3 | 2.2 | 0.45 ± 0.05 (n = 3) |
n = 20 (10 animals using both legs). l, collimating lens; b, bare fiber end.
Figures 2–6 show the probability of muscle stimulation as a function of fluence for each of the laser devices.
Fig. 2.
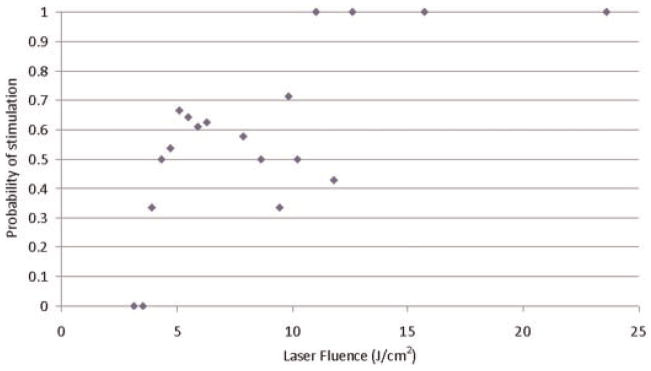
Probability of muscle stimulation for 1,495 nm Yb:glass laser with collimating lens producing a 0.9 mm diameter beam.
Fig. 6.
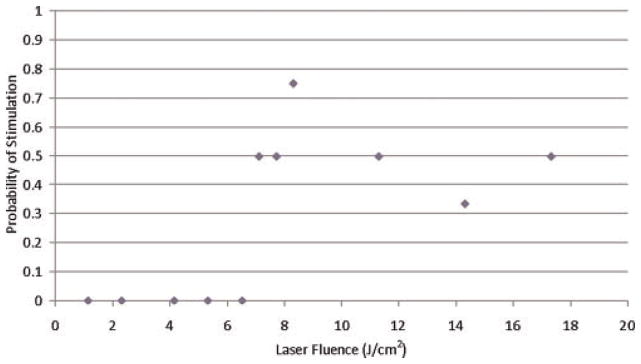
Probability of muscle stimulation for 1,540 nm diode laser.
The probability of stimulation increases with increasing laser fluence for the Ho:YAG 2,100 nm and Yb:glass 1,495 nm lasers. However, stimulation with the 1,450 and 1,540 nm diode lasers does not show a strong dependence on laser fluence.
Figure 7 shows the EMG signal recorded during stimulation with a 2.6 J/cm2 pulse from the Yb:glass laser firing at 2 Hz. The muscle twitches at the same frequency as the laser irradiation. The size of the EMG peak remained relatively constant (1.4 ± 0.2 × 10−4 V) for consecutive pulses.
Fig. 7.
EMG signal from muscle stimulated by 2.63 J/cm2 pulse from 1,495 nm laser firing at 2 Hz.
The size of the EMG peak was directly proportional to the laser fluence, as can be seen in Figure 8, which shows the EMG response from the 1,495 nm Yb:glass laser with 900 μm 25 millisecond output.
Fig. 8.
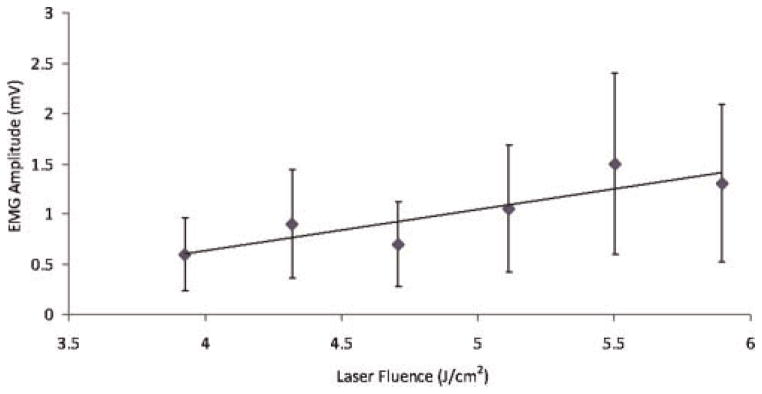
EMG spike magnitude as a function of collimated Yb:glass 1,495 nm laser fluence for one rat.
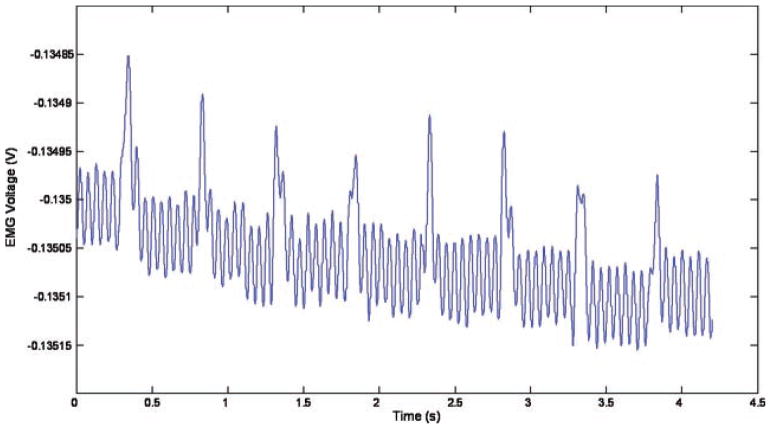
Figure 9 shows the EMG signal from the 1,450 nm laser firing at 33 J/cm2 for 100 millisecond at a repetition rate of 2 Hz. This EMG signal corresponds to three visible muscle twitches 0.5 second apart.
Fig. 9.
EMG signal from the 1,450 nm laser firing at 33 J/cm2 for 100 millisecond at a repetition rate of 2 Hz.
DISCUSSION
The Yb:glass laser proved to be an effective source for nerve stimulation. Trains of muscle twitches up to 30 in number at a repetition rate of 2 Hz were observed with this laser. Increasing laser fluence increased the probability of nerve stimulation. The Ho:YAG laser also provided an effective stimulus but it suffered from a lack of versatility in choosing power and frequency.
Although it was possible to stimulate the nerve with the 1,540 and 1,450 nm diode lasers, stimulation wasn’t consistent or easily reproducible. As can be seen in Figures 5 and 6 the probability of nerve stimulation has does not have a strong dependence on laser fluence. This may be because the long pulse used (100–200 millisecond) allowed heat to conduct out of the irradiated area during the laser pulse. Also the larger optical fiber diameter compared to the size of the nerve fiber makes it difficult to ensure that all of the laser beam is hitting and being absorbed by the nerve. The height of the fiber end above the nerve (± 0.5 mm) and the size of the nerve itself (±0.5 mm) are both variables which introduce an error when coupling the laser beam to the nerve. This could account for the large energy (35 J/cm2) required to stimulate the nerve with the diode lasers. The short pulse length and small diameter of the Yb:glass laser beam results in a much lower threshold, since all the energy is absorbed by the nerve and doesn’t have time to diffuse out of the irradiated area during the pulse.
Fig. 5.
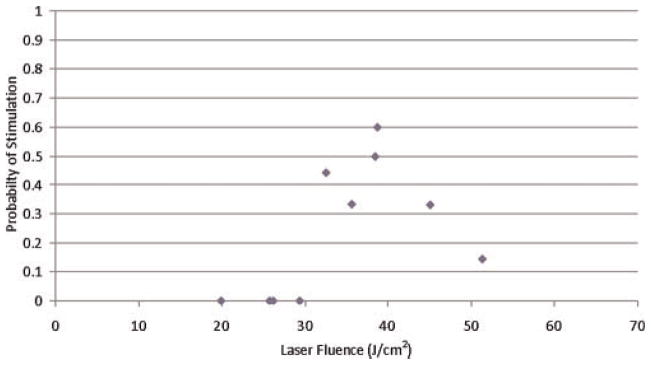
Probability of muscle stimulation for 1,450 nm diode laser.
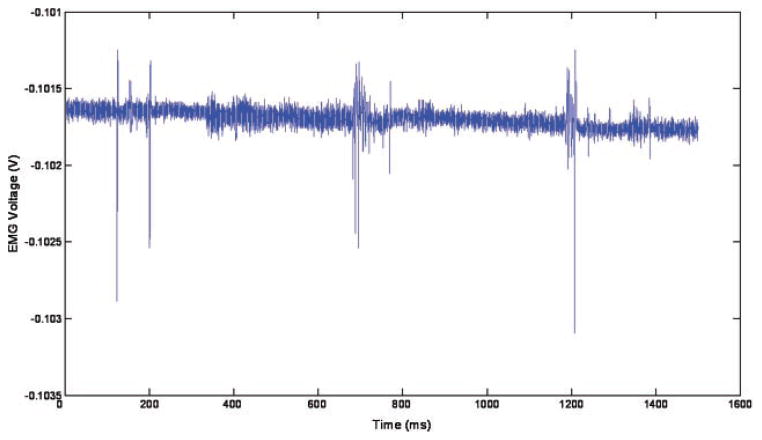
The long pulses were capable of evoking multiple stimulations per pulse, as can be seen in Figure 9—there are three distinct regions of muscle activity (at 200, 700, and 1,200 millisecond), corresponding to three laser pulses at 2 Hz, but within these regions (lasting approximately 100 millisecond, which matches the 100 millisecond laser pulse length) there is more than one EMG spike. These spikes are separated by a minimum of 6.5 millisecond, as can be seen in Figure 10, which gives an indication of the refractory period of the muscle.
Fig. 10.
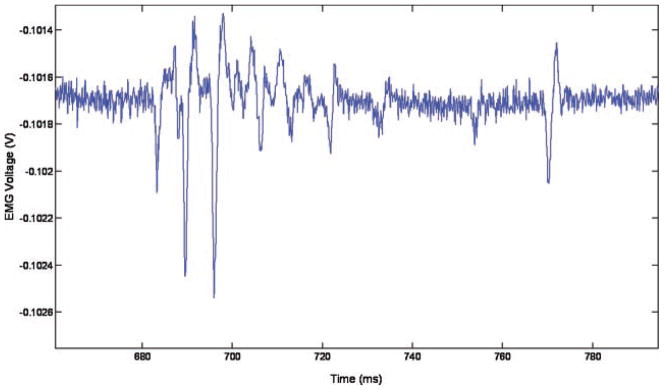
Close up of second muscle twitch from Figure 9.
The mechanism of laser nerve stimulation is thought to thermal in nature—the absorption of laser light in the nerve generates heat. Heat-gated ion channels have been identified which respond at specific temperatures. For example, the TRPV1 ion channel opens in response to temperatures above 43°C and the TRPV2 channel to temperatures above 53°C [11]. Hoffman et al. [12] demonstrated that peripheral nerve axons have thermosensory properties similar to cutaneous thermoreceptor nerves. The instantaneous temperature increase in the nerve tissue can be predicted by ΔT = ((μ0Φ exp (−μ0z))/(ρc)), where μ0 is the absorption coefficient, Φ the laser fluence, z the depth in the tissue, ρ the tissue density, and c the specific heat capacity. Assuming absorption through 150 μm of protective tissue surrounding the nerve [3] with the same absorption and thermal properties as water (nerve tissue is 63% water by mass [13], the stimulation threshold fluence (3.7 J/cm2) from the Yb:glass fiber laser (1,495 nm) will produce a temperature increase of 13°C at the nerve axon. This temperature increase is enough to activate a TPRV2 mediating channel.
The EMG signal was shown to be directly proportional to the laser power. However, the amplitude of an individual axon’s action potential is independent of the stimulus intensity—the all-or-nothing law [14]. Therefore, increasing the laser power must increase the number of individual axons stimulated, resulting in a cumulative effect in the muscle.
Temperature increases in tissue over a critical length of time can result in protein denaturation and irreversible tissue damage. No investigation of the damage threshold has been made in this study, but the risk for tissue damage should be considered when irradiating with a laser beam, by an Arrhenius model [15] and/or histological studies, to identify safe laser parameters.
Wells et al. [1–4] have already shown the Ho:YAG and 1.85 μm lasers are good sources for nerve stimulation. However, the protective covering on nerves (epineurium and perineurium) varies in thickness on different nerves throughout the body. Therefore, a range of penetration depths are required for effective stimulation. The 1,495 nm wavelength of the Yb:glass fiber lasers offers a greater penetration depth than Ho:YAG but is absorbed less than 1.85 μm, and therefore, can be a useful addition to the laser stimulation field.
In this study the 1,495 nm Yb:glass proved most effective for nerve stimulation. However, stimulation was shown to be possible with the 1,540 and 1,450 nm diode lasers. Focusing their output to smaller beams would provide greater fluence so that short pulse lengths could be used and delivery of the energy to the nerve is more efficient. This could improve their nerve stimulation ability. Future work includes investigating the effect of pulse length and beam diameter on stimulation threshold.
Optical stimulation has the potential to replace electrical stimulation in a number of routine neurological procedures, including diagnostics during surgery (electrical stimulation is used to identify and test the functionality of nerves, e.g., the facial nerve, but requires contact with the nerve which risks damage) or neurostimulation, for example, vagus nerve stimulation (VNS) to control epilepsy seizures. The spatial selectivity of laser stimulation would overcome one of the main side effects of electrical VNS, i.e. coughing and voice modulation resulting from stimulation of the superior and recurrent laryngeal nerves [16].
SUMMARY
Stimulation of the rat sciatic nerve has been shown with laser sources in the 1.5 μm wavelength range. The Yb:glass laser at 1,495 nm delivered through a single mode fiber was found to be an effective stimulus. Lower leg and foot twitches were observed and corresponding EMG signals recorded. This wavelength, already in common use for telecoms, offers an alternative source for neurostimulation.
Fig. 3.
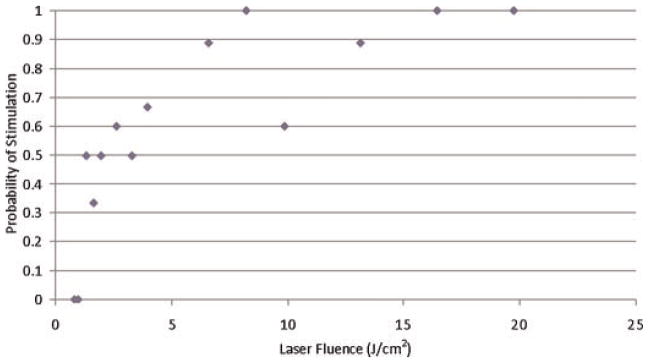
Probability of muscle stimulation for 1,495 nm Yb:glass laser with bare fiber end producing. Diameter (0.4 mm) beam on the nerve.
Fig. 4.
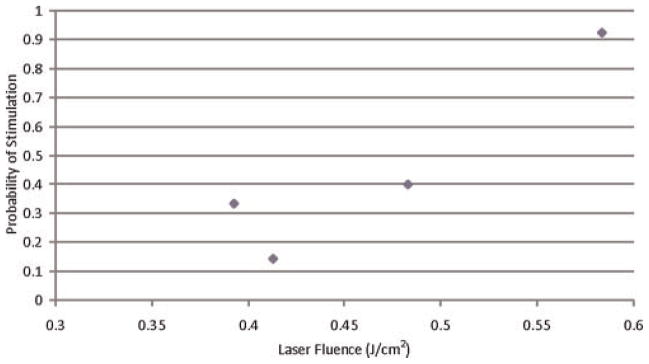
Probability of muscle stimulation for 2,100 nm Ho:YAG laser.
Acknowledgments
The authors would like to thank Laurie Newman for her help with the animal handling and preparation.
References
- 1.Wells J, Kao C, Konrad P, Milner T, Kim J, Mahadevan-Jansen A, Jansen ED. Biophysical mechanisms of transient optical stimulation of peripheral nerve. Biophys J. 2007;93(7):2567–2580. doi: 10.1529/biophysj.107.104786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wells J, Kao C, Jansen ED, Konrad P, Mahadevan-Jansen A. Application of infrared light for in vivo neural stimulation. J Biomed Opt. 2005;10(6):064003–064012. doi: 10.1117/1.2121772. [DOI] [PubMed] [Google Scholar]
- 3.Wells J, Konrad P, Kao C, Jansen ED, Mahadevan-Jansen A. Pulsed laser versus electrical energy for peripheral nerve stimulation. J Neurosci Methods. 2007;163(2):326–337. doi: 10.1016/j.jneumeth.2007.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wells JD, Thomsen S, Whitaker P, Jansen E, Kao C, Konrad P, Mahadevan-Jansen A. Optically mediated nerve stimulation: Identification of injury thresholds. Lasers Surg Med. 2007;39(6):513–526. doi: 10.1002/lsm.20522. [DOI] [PubMed] [Google Scholar]
- 5.Izzo AD, Suh E, Pathria J, Walsh JT, Whitlon DS, Richter C-P. Selectivity of neural stimulation in the auditory system: A comparison of optic and electric stimuli. J Biomed Opt. 2007;12(2):021008. doi: 10.1117/1.2714296. [DOI] [PubMed] [Google Scholar]
- 6.Izzo AD, Richter CP, Jansen ED, Walsh JT., Jr Laser stimulation of the auditory nerve. Lasers Surg Med. 2006;38(8):745–753. doi: 10.1002/lsm.20358. [DOI] [PubMed] [Google Scholar]
- 7.Izzo AD, Walsh JT, Jansen ED, Bendett M, Webb J, Ralph H, Richter CP. Optical parameter variability in laser nerve stimulation: A study of pulse duration, repetition rate, and wavelength. IEEE Trans Biomed Eng. 2007;54(6):1108–1114. doi: 10.1109/TBME.2007.892925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Izzo AD, Walsh JT, Jr, Ralph H, Webb J, Bendett M, Wells J, Richter C-P. Laser stimulation of auditory neurons: Effect of shorter pulse duration and penetration depth. Biophys J. 2008;94(8):3159–3166. doi: 10.1529/biophysj.107.117150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fried NM, Lagoda GA, Scott NJ, Su L-M, Burnett AL. Noncontact stimulation of the cavernous nerves in the rat prostate using a tunable-wavelength thulium fiber laser. J Endourol. 2008;22(3):409–414. doi: 10.1089/end.2008.9996. [DOI] [PubMed] [Google Scholar]
- 10.Cornsweet TN. Staircase-method in psychophysics. Am J Psychol. 1962;75:485–491. [PubMed] [Google Scholar]
- 11.Benham CD, Gunthorpe MJ, Davis JB. TRPV channels as temperature sensors. Cell Calcium. 2003;33(5–6):479–4487. doi: 10.1016/s0143-4160(03)00063-0. [DOI] [PubMed] [Google Scholar]
- 12.Hoffmann T, Sauer SK, Horch RE, Reeh PW. Sensory transduction in peripheral nerve axons elicits ectopic action potentials. J Neurosci. 2008;28(24):6281–6284. doi: 10.1523/JNEUROSCI.1627-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pace N, Rathburn EN. Studies on body compostion III. The body water chemically combined notrogen content in relation to fat content. J Biol Chem. 1945;158:685–691. [Google Scholar]
- 14.Martin EG. The application of the “all or nothing” principle of nervous conduction to the interpretation of vasomotor reflexes. Am J Physiol. 1922;59(1):400–412. [Google Scholar]
- 15.Wright NT. On a relationship between the Arrhenius parameters from thermal damage studies. J Biomech Eng. 2003;125(2):300–304. doi: 10.1115/1.1553974. [DOI] [PubMed] [Google Scholar]
- 16.Labiner DM, Ahern GL. Vagus nerve stimulation therapy in depression and epilepsy: Therapeutic parameter settings. Acta Neurol Scand. 2007;115(1):23–33. doi: 10.1111/j.1600-0404.2006.00732.x. [DOI] [PubMed] [Google Scholar]
- 17.Wieliczka DM, Weng S, Querry MR. Wedge shaped cell for highly absorbent liquids: Infrared optical constants of water. Appl Opt. 1989;28(9):1714. doi: 10.1364/AO.28.001714. [DOI] [PubMed] [Google Scholar]