Abstract
A number of studies have revealed significant relationships between cognitive performance and average phenylalanine (Phe) levels in children with phenylketonuria (PKU), but only a few studies have been conducted to examine relationships between cognitive performance and variability (fluctuations) in Phe levels. In the current study, we examined a variety of indices of Phe control to determine which index best predicted IQ and executive abilities in 47 school-age children with early- and continuously-treated PKU. Indices of Phe control were mean Phe, the index of dietary control, change in Phe with age, and several indices of variability in Phe (standard deviation, standard error of estimate, and percentage of spikes). These indices were computed over the lifetime and during 3 developmental epochs (< 5, 5.0 – 9.9, and ≥ 10 yrs. of age). Results indicated that variability in Phe was generally a stronger predictor of cognitive performance than other indices of Phe control. In addition, executive performance was better predicted by variability in Phe during older than younger developmental epochs. These results indicate that variability in Phe should be carefully controlled to maximize cognitive outcomes, and that Phe control should not be liberalized as children with PKU age.
Keywords: phenylketonuria, phenylalanine, variability, stability, fluctuation, IQ, executive abilities
1. Introduction
Phenylketonuria (PKU) is a hereditary metabolic disorder associated with a deficiency in or absence of the phenylalanine hydroxylase (PAH) enzyme. As a result, the amino acid phenylalanine (Phe) is not properly metabolized, and blood Phe is elevated in individuals with PKU [1]. In turn, improper Phe metabolism disrupts the neurochemical cascade by which Phe is converted to tyrosine, a precursor of dopamine and other catecholaminergic neurotransmitters [2]. In addition to neurotransmitter deficiency, PKU is associated with widespread compromise of the white matter of the brain [3–6].
Untreated PKU typically results in profoundly lowered IQ and intellectual disability [7,8]. With early diagnosis and treatment to limit dietary Phe intake, however, individuals with PKU usually have IQs in the average range [9]. That said, individuals with early-treated PKU often have IQs that are lower than expected in comparison with peers and family members [10], and lower IQ has been associated with higher Phe levels [11–13]. Based on a meta-analysis of data from early-treated children with PKU and hyperphenylalaninemia who had Phe levels from 423 to 750 μmol/L, Waisbren et al. found that each 100 μmol/L increase in blood Phe predicted a reduction in IQ of 1.3 to 3.1 points [14]. Higher Phe has also been negatively associated with performance in specific areas of cognition such as executive abilities [9,15–18].
Previous studies have focused almost exclusively on relationships between cognition and central tendency measures of Phe (e.g., mean, median) at the time of cognitive evaluation or over specified periods (e.g., 1 month, 1 year, lifetime) prior to evaluation. In only a small number of studies have associations between cognition and variability (i.e., fluctuations) in Phe been investigated. Vilaseca et al. [19] and Burgard et al. [20] found that greater variability in Phe was related to lower IQ. In addition, although their results were inconclusive, Anastasoaie et al. [21] reported a statistical trend suggesting that greater variability in Phe may be associated with lower IQ in preschool and school-age children. Finally, in a small sample of preschool and early school-age children, Arnold et al. [22] demonstrated that greater variability in Phe was related to poorer performance on tests of executive abilities. In contrast with findings from these studies, Viau et al. [23] failed to find an association between variability in Phe and intelligence; however, in this study of children and adults, variability in Phe was examined only in relation to the verbal, processing speed, and perceptual reasoning subcomponents of IQ rather than overall IQ. Taken together, findings from the small number of studies conducted to date largely suggest that stability in Phe control is important for maximizing cognitive outcomes in individuals with PKU.
There are, however, issues that limit the interpretation of findings across these studies, such as the use of different indices of variability in Phe. Burgard et al. [20] and Vilaseca et al. [19] used the standard error of estimate (SEE Phe), whereas Anastasoaie et al. [21], Arnold et al. [22], and Viau et al. [23] used the standard deviation (SD Phe). Anastasoaie et al. [21] also reported the number of spikes in Phe but did not conduct statistical analyses on this index of variability. In addition, sample size was quite small in the Arnold et al. [22] study (n = 18), and in the study by Anastasoaie et al. [21] the relationship between SD Phe over the lifetime and IQ failed to reach statistical significance. Finally, to our knowledge, the association between variability in Phe and executive abilities has not been examined in children across the school-age range, a period during which executive abilities are rapidly developing. Thus, as pointed out in a recent review by Cleary et al. [24], additional research is needed, using multiple methods to assess variability in Phe to determine which index of Phe control is most strongly associated with cognitive outcomes.
To address this issue, we examined a number of indices of blood Phe control to determine which index best predicted IQ and various executive abilities (i.e., inhibitory control, working memory, strategic processing) in school-age children with early- and continuously-treated PKU. The indices representing average Phe included mean Phe (the most commonly reported indicator of Phe control) and the index of dietary control (IDC). A slope was also computed reflecting change in Phe as a function of age. Three indices were calculated to reflect variability in Phe, including the SD Phe, the SEE Phe, and the percentage of spikes in Phe (% spikes). Although our focus was on Phe over the lifetime, these 6 indices were also computed for 3 developmental epochs (< 5, 5.0 – 9.9, and ≥ 10 yrs. of age) prior to the time of cognitive evaluation.
2. Materials and methods
2.1. Participants and Phe levels
Children with PKU (n = 47; 22 male, 25 female) were recruited through metabolic clinics at Washington University in St Louis (n = 17), Oregon Health & Science University (n = 25), the University of Missouri (n = 3), New York Medical College (n = 1), and the University of Nebraska (n = 1). Although 62 children were initially considered for inclusion in the study, 15 were excluded due to gaps in available Phe levels of greater than 2 years at some point prior to cognitive evaluation. All children were diagnosed with PKU soon after birth and received early treatment through dietary management to limit Phe intake. Across the sample of 47 children, age ranged from 6 – 18 years (M = 11.9, SD = 3.6), and education ranged from 0 – 13 years (M = 6.1, SD = 3.3). No child had a reported history of major medical, psychiatric, or learning disorder unrelated to PKU, and no child was being treated with sapropterin dihydrochloride at the time of cognitive evaluation.
Data from all 47 children in our sample were included in analyses examining relationships between cognition and indices of Phe control across the lifetime. The number of blood Phe levels available across the lifetime ranged from 85 – 489 (M = 221.3, SD = 104.7), with 10,399 total Phe levels. Data from all 47 children were also available to examine relationships between cognition and indices of Phe control during the < 5 and 5.0 – 9.9 yrs. epochs, because all were at least 9.9 years of age at the time of cognitive evaluation. For the ≥ 10 yrs. epoch, data were available for only 29 children, because 18 children in the sample of 47 had not yet reached 10 years of age at the time of evaluation. The number of blood Phe levels available for the < 5, 5.0 – 9.9, and ≥ 10 yrs. developmental epochs ranged from 18 – 288 (M = 133.5, SD = 61.8), 14 – 154 (M = 62.4, SD = 35.9), and 2 – 214 (M = 41.6, SD = 54.9), respectively, with considerably more Phe levels obtained during the younger than older epochs.
2.2. Procedures
Approval to conduct this study was obtained from institutional review boards for the protection of human subjects at Washington University in St. Louis, Oregon Health & Science University, and the University of Missouri, the sites at which cognitive evaluations were completed. All participants and/or their guardians provided written informed consent prior to engagement in study procedures. Measures of IQ and executive abilities were administered in a quiet room as components of a larger study that included neuroimaging and additional measures of cognition that were not analyzed for this report; administration of all cognitive and neuroimaging procedures occurred during a single session lasting approximately 4 hours. The metabolic clinics from which children were referred provided blood Phe levels over the lifetime based on available medical records. Some cognitive data used in the current study have been published previously, but not in relation to variability in Phe.
2.3. Measures
2.3.1. Indices of Phe control
Six indices of blood Phe control were computed over the lifetime prior to cognitive evaluation; for secondary analyses these indices were computed for 3 developmental epochs (< 5, 5.0 – 9.9, and ≥ 10 yrs. of age). Two indices reflected average Phe: mean Phe and the IDC. Mean Phe was simply the mean of all available Phe levels for each child. To compute the IDC, median Phe for each year of age was calculated for each child; the mean of all median Phe levels was then calculated to determine the IDC for each child. This index was included because a greater number of Phe levels are typically obtained during earlier than later childhood, which results in a heavier weighting of earlier Phe levels when computing mean Phe. The IDC circumvents this issue because Phe levels from each year of age are given equal weight. We also obtained the slope from a regression function to represent change in Phe as a function of age.
To assess variability, 3 additional indices of Phe control were computed: SD Phe, SEE Phe, and % spikes in Phe. SD Phe indicated the degree of dispersion in Phe around the mean. SEE Phe indicated residual variation in Phe around a regression line, which reflected fluctuation in Phe that was not influenced by the mean or slope. Finally, similar to Anastasoaie et al. [19], spikes were counted as the number of Phe levels that were at least 600 μmol/L greater than either the preceding or succeeding Phe level. Unlike Anastasoaie et al. [21], however, we examined % spikes (rather than number of spikes), which represented the number of spikes in relation to the total number of Phe levels available. This approach was used because children with more available Phe levels had more opportunities for spikes to be detected, although as a percentage their spikes may have been equivalent to that of children with fewer available Phe levels.
2.3.2. IQ and executive abilities
The Wechsler Abbreviated Scale of Intelligence (WASI) [25] was used to estimate general intellectual ability (IQ) based on a composite of Vocabulary and Matrix Reasoning subtest scores. Administration was in accordance with test manual instructions, and age-referenced normative data from the test manual were used to generate an estimated IQ. In addition, we examined standard scores from Vocabulary and Matrix Reasoning subtests separately to determine their unique relationships with indices of Phe control. We were especially interested in Matrix Reasoning, as this subtest assesses strategic processing, which is an executive ability.
In addition to assessing strategic processing using Matrix Reasoning, two executive tasks were administered to assess working memory (n-back task) and inhibitory control (go/no-go task). Details of both tasks are provided in a prior publication [4].
Briefly, during the working memory n-back task, children were shown a series of letters that appeared at various locations on a computer monitor. In the letter condition, children pressed a target button when a letter appeared that was the same as the letter presented two trials earlier (regardless of location); in the location condition, children pressed a target button when a letter appeared in the same location as two trials earlier (regardless of which letter). The number of correct responses was averaged across location and letter conditions for analyses, as was the number of correct nonresponses, both of which were used in analyses.
During the inhibitory control go/no-go task, children were shown a series of shapes (i.e., circle, square, diamond, triangle) on a computer monitor. Children were asked to press a button as quickly as possible when any of 3 designated target shapes appeared (go condition). They were asked to do nothing when a fourth designated nontarget shape appeared (no-go condition), which required inhibition of the prepotent button press response. The number of errors made during no-go trials (i.e., commission errors) was used in analyses.
2.4. Data Analyses
We first determined the range, mean, and SD for each of our 6 indices of Phe control over the lifetime (see Table 1). Associations among these lifetime indices of Phe control were also computed using Pearson correlations (see Table 2). Given that a number of correlations were computed, statistical rigor was increased by considering relationships significant only if p < .05 and effect sizes were either medium or large. Following Cohen’s conventions [26], r = .1, r = .3, and r = .5 represented small, medium, and large effect sizes, respectively.
Table 1.
Indices of Phe control over the lifetime and during developmental epochs.
| Time Period | Average Phe
|
Change with Age
|
Variability in Phe
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean
|
IDC
|
Slope
|
SD
|
SEE
|
% Spikes
|
|||||||
| M | SD | M | SD | M | SD | M | SD | M | SD | M | SD | |
|
|
|
|
|
|
|
|
||||||
| Lifetime | 369.1 | 146.3 | 379.6 | 189.5 | 12.5 | 21.8 | 217.0 | 91.4 | 202.4 | 84.4 | 2.7 | 3.0 |
| < 5 yrs. | 332.8 | 117.0 | 309.3 | 136.3 | 2.0 | 42.3 | 213.3 | 95.1 | 207.4 | 92.8 | 2.8 | 3.3 |
| 5.0 – 9.9 yrs | 395.0 | 189.6 | 384.0 | 183.8 | 32.0 | 38.0 | 174.7 | 78.0 | 168.7 | 78.1 | 2.8 | 4.6 |
| ≥ 10 yrs. | 550.5 | 260.0 | 562.4 | 290.5 | 23.0 | 139.2 | 161.0 | 99.6 | 148.6 | 83.4 | 2.0 | 4.3 |
Table 2.
Correlations between indices of Phe control over the lifetime.
| Index
|
Mean
|
IDC
|
Slope
|
SD
|
SEE
|
|---|---|---|---|---|---|
| Mean | -- | -- | -- | -- | -- |
| IDC | .95* | -- | -- | -- | -- |
| Slope | .54* | .68* | -- | -- | -- |
| SD | .75* | .66* | .28 | -- | -- |
| SEE | .64* | .53* | .10 | .98* | -- |
| % Spikes | .50* | .38* | .00 | .83* | .89* |
Note:
p < .01 with medium or large effect sizes.
We next determined the range, mean, and SD for each of our 6 indices of Phe control across the 3 developmental epochs (< 5, 5.0 – 9.9, and ≥ 10 yrs.; see Table 1). Paired samples t-tests were used to identify possible differences in indices of Phe control across epochs. Given that a number of t-tests were performed, statistical rigor was again increased by considering differences significant only if p < .05 and effect sizes were either medium or large. Following Cohen’s convention [26], d = .2, .5, and .8 represented small, medium, and large effect sizes, respectively.
Turning to cognition, standard scores (mean = 100; SD = 15) based on normative data reflected IQ, Vocabulary, and Matrix Reasoning performance. Normative data were not available for the experimental working memory and inhibitory control tasks administered. However, to provide a similar context for the interpretation of results from these tasks, we computed standard scores (mean = 100; SD = 15) based on data that were previously collected in our laboratory from a group of 80 typically-developing healthy children with a comparable age range. Relationships between standard scores from each cognitive measure and indices of Phe control over the lifetime and across developmental epochs were then examined using Pearson correlations (see Table 3). As noted earlier, correlations were considered significant only if p < .05 and effect sizes were either medium or large.
Table 3.
Correlations between indices of Phe control and IQ and executive abilities.
| Lifetime (n = 47)
| ||||||
|---|---|---|---|---|---|---|
| Variable
|
Mean
|
IDC
|
Slope
|
SD
|
SEE
|
% Spikes
|
| IQ | −.17 | −.14 | −.14 | −.33* | −.33* | −.25 |
| Vocabulary | −.08 | −.03 | −.12 | −.16 | −.15 | −.03 |
| Matrix Reasoning | −.24 | −.23 | −.19 | −.39* | −.38* | −.35* |
| n-back # nonresponses | −.21 | −.18 | −.14 | −.39* | −.39* | −.38* |
| n-back # responses | −.32* | −.30* | −.15 | −.33* | −.31* | −.26 |
| Go/no-go # errors | .25 | .25 | .28 | .29 | .24 | .14 |
| < 5 yrs. (n = 47)
| ||||||
|---|---|---|---|---|---|---|
| Variable
|
Mean
|
IDC
|
Slope
|
SD
|
SEE
|
% Spikes
|
| IQ | −.17 | −.17 | −.17 | −.29 | −.29 | −.19 |
| Vocabulary | −.05 | −.05 | −.12 | −.12 | −.12 | .00 |
| Matrix Reasoning | −.19 | −.19 | −.22 | −.33* | −.33* | −.26 |
| n-back # nonresponses | −.22 | −.20 | −.15 | −.30* | −.29 | −.23 |
| n-back # responses | −.35* | −.39* | −.26 | −.34* | −.29 | −.14 |
| Go/no-go # errors | .23 | .22 | .34* | .25 | .25 | .07 |
| 5.0 – 9.9 yrs. (n = 47)
| ||||||
|---|---|---|---|---|---|---|
| Variable
|
Mean
|
IDC
|
Slope
|
SD
|
SEE
|
% Spikes
|
| IQ | −.19 | −.23 | −.13 | −.23 | −.24 | −.10 |
| Vocabulary | −.11 | −.17 | −.10 | −.10 | −.11 | −.01 |
| Matrix Reasoning | −.21 | −.23 | −.19 | −.30* | −.30* | −.18 |
| n-back # nonresponses | −.31* | −.27 | −.25 | −.45* | −.44* | −.40* |
| n-back # responses | −.29 | −.31* | −.02 | −.25 | −.27 | −.25 |
| Go/no-go # errors | .30* | −.30* | .03 | .13 | .12 | .17 |
| ≥ 10 yrs. (n = 29)
| ||||||
|---|---|---|---|---|---|---|
| Variable
|
Mean
|
IDC
|
Slope
|
SD
|
SEE
|
% Spikes
|
| IQ | −.23 | −.18 | .04 | −.37* | −.30 | −.05 |
| Vocabulary | −.03 | .06 | −.08 | −.06 | −.03 | −.25 |
| Matrix Reasoning | −.49* | −.49* | .15 | −.64* | −.54* | −.41* |
| n-back # nonresponses | −.44* | −.38* | −.23 | −.48* | −.41* | −.14 |
| n-back # responses | −.34 | −.34 | −.07 | −.26 | −.26 | −.25 |
| Go/no-go # errors | .49* | −.40* | .20 | .24 | .24 | .19 |
Note:
p < .05 with medium or large effect sizes.
Finally, hierarchical regression analyses were conducted to determine the degree to which SD Phe (reflecting variability in Phe) and mean Phe (reflecting average Phe) predicted losses in standard score points in IQ and executive abilities over the lifetime. Specifically, unstandardized regression coefficients (B) were used to determine the number of standard score points lost for every 100 μmol/L increase in SD Phe or mean Phe.
Given our particular interest in variability, we first conducted analyses in which only SD Phe was used as a predictor of IQ and executive abilities (see Table 4); these analyses were conducted using only those cognitive variables that were significantly correlated with SD Phe in earlier analyses. We then used hierarchical regression to examine both mean Phe and SD Phe as predictors of executive abilities (see Table 5); these analyses were conducted using only those cognitive variables that were significantly correlated with mean Phe in earlier analyses. Mean Phe was entered as an independent variable in the first step of the analyses, whereas SD Phe was entered in the second step. This approach allowed us to determine the degree to which mean Phe alone predicted performance, as well as the degree to which SD Phe predicted performance beyond that attributable to mean Phe.
Table 4.
Loss of standard score points in relation to 100 μmol/L increase in SD Phe.
| Time Period | IQ | Matrix Reasoning | n-back # nonresponses | n-back # responses |
|---|---|---|---|---|
|
|
|
|
|
|
| Lifetime | 3.8 | 4.7 | 6.4 | 5.3 |
| < 5 yrs. | nc | 3.8 | 4.6 | 5.3 |
| 5.0 – 9.9 yrs. | nc | 4.2 | 8.9 | nc |
| ≥ 10 yrs. | 3.8 | 6.8 | 6.1 | nc |
Note: nc = analysis not conducted due to lack of significant correlation between cognitive performance and SD Phe in earlier analyses; Vocabulary and go/no-go are not included in the table because neither had significant correlations with SD Phe.
Table 5.
Loss of standard score points in relation to 100 μmol/L increase in mean Phe and loss of standard score points in relation to 100 μmol/L increase in SD Phe after controlling for points lost in relation to mean Phe.
| Time Period | Matrix Reasoning | n-back # nonresponses | n-back # responses | Go/No-Go errors | ||||
|---|---|---|---|---|---|---|---|---|
|
|
|
|
||||||
| Mean Phe | SD Phe | Mean Phe | SD Phe | Mean Phe | SD Phe | Mean Phe | SD Phe | |
|
|
|
|
|
|
|
|
|
|
| Lifetime | --- nc --- | --- nc --- | 3.2 | ns | --- nc --- | |||
| < 5 yrs. | --- nc --- | --- nc --- | 4.4 | ns | --- nc --- | |||
| 5.0 – 9.9 yrs. | --- nc --- | 2.4 | 8.4 | --- nc --- | 2.4 | ns | ||
| ≥ 10 yrs. | 2.0 | 6.2 | 2.0 | ns | --- nc --- | 3.1 | ns | |
Note: nc = analysis not conducted due to lack of significant correlation between cognitive performance and mean Phe in earlier analyses; ns = not significant; IQ and Vocabulary are not included in the table because neither had significant correlations with mean Phe.
3. Results
3.1. Indices of Phe control over the lifetime
For illustrative purposes, in Figure 1 we provide three exemplar profiles of Phe control across the lifetime of three children in our sample. Panel (a) illustrates a profile in which average Phe (represented in the figure by mean Phe) over the lifetime was low, the slope representing change in Phe with age was flat (indicating little change in Phe as a function of age), and variability in Phe (represented in the figure by SD Phe) was relatively low. Phe control in this child was quite good in comparison with that of many children who participated in our study. Panel (b) demonstrates a similar profile in terms of a low average Phe and a flat slope, but variability in Phe was high. Based on only the average and slope, one might assume that Phe control in this child was quite good, but variability in Phe points to substantial instability. In sharp contrast, panel (c) shows a profile in which average Phe was high, the slope increased steeply (indicating poorer Phe control with increasing age), and variability in Phe was high. As such, Phe control in this child was quite poor in all regards.
Figure 1.
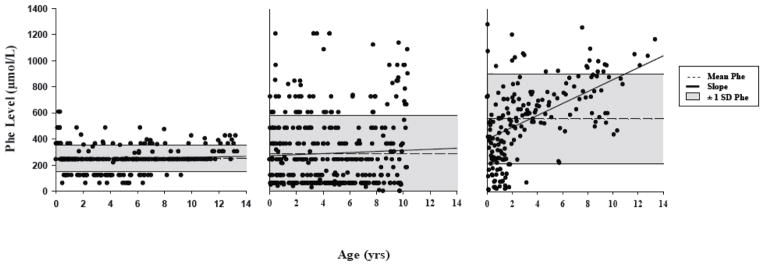
Profiles of Phe control over the lifetime from 3 children in our sample: (a) low average, flat slope, low variability; (b) low average, flat slope, high variability; (c) high average, steep slope, high variability.
Turning to our PKU group as a whole, the range of blood Phe over the lifetime was 0 – 2,742 μmol/L. Indices of Phe control over the lifetime are reported in Table 1 (as are indices for each developmental epoch, which will be discussed later). Inspection of these data revealed that average Phe (i.e., mean Phe, IDC) exceeded the recommended range of 120 – 360 μmol/L [27]. The positive slope indicated that Phe increased as children with PKU aged. Indices of variability (i.e., SD Phe, SEE Phe, % spikes) indicated considerable instability in Phe over the lifetime (in absolute terms, lifetime number of spikes ranged from 0 – 28; M = 6.1, SD = 7.4).
As shown in Table 2, a significant correlation was found between mean Phe and the IDC. In addition, significant correlations were found between both mean Phe and the IDC, and the slope, SD Phe, SEE Phe, and % spikes; in other words, higher Phe levels on average were associated with greater variability in Phe over the lifetime and a greater increase in Phe with age. Our indices of variability in Phe were significantly correlated with one another. The slope was not significantly correlated with any index of variability.
3.2. Indices of Phe control during developmental epochs
The range of blood Phe for the < 5, 5.0 – 9.9, and ≥ 10 yrs. epochs was 0 – 2742, 0 – 1580, and 30 – 1689 μmol/L, respectively. Indices of Phe control for each developmental epoch are reported in Table 1. Inspection of these data revealed that, with the exception of the youngest epoch, mean Phe and the IDC exceeded the recommended range of 120 – 360 μmol/L [27].
Results of paired samples t-tests showed that mean Phe was significantly higher during the ≥ 10 yrs. epoch than during either the < 5 [t(28) = 5.76, p < .001, d = .96] or 5.0 – 9.9 [t(28) = 5.13, p <. 001, d = .51] yrs. epochs. The pattern was identical for the IDC, which was significantly higher during the ≥ 10 yrs. epoch than during either the < 5 [t(28) = 6.16, p < .001, d = .96] or 5.0 – 9.9 [t(28) = 4.48, p <. 001, d = .51] yrs. epochs. With regard to slope, the increase in Phe with age was significantly greater during the 5.0 – 9.9 than < 5 yrs. epoch [t(46) = 3.62, p < .001, d = .75].
In terms of variability in Phe, there were no significant differences across developmental epochs for SD Phe, although SEE Phe was significantly greater during the < 5 than ≥ 10 yrs. epoch [t(28) = 3.69, p < .001, d = .57]. In absolute terms, spikes in Phe decreased across developmental epochs. Specifically, the number of spikes for the < 5, 5.0 – 9.9, and ≥ 10 yrs. epochs ranged from 0 – 17 (M = 3.9, SD = 4.9), 0 – 11 (M = 1.6, SD = 2.4), and 0 – 8 (M = 1.0, SD = 2.1), respectively. Although these absolute values are of interest, no significant differences were found in % spikes across any of the developmental epochs.
Overall, these findings point to a general pattern in which average Phe and change in Phe with age were greater during older than younger epochs. In contrast, with a single exception, there were no significant differences in indices of variability in Phe across developmental epochs.
3.3. IQ and executive abilities
IQ for the children with PKU in our sample ranged from 75 – 122 (M = 101.3, SD = 10.6). Scores from the Vocabulary and Matrix Reasoning subtests ranged from 72 – 126 (M = 102.2, SD = 13.3) and 75 – 118 (M = 99.6, SD = 11.0), respectively. Although the descriptive range of scores across individual children was considerable (borderline to superior), at the group level IQ and subtest scores were average.
For the working memory n-back task, the number of correct responses and nonresponses ranged from 18 – 32 (M = 26.3, SD = 3.3) and 27 – 64 (M = 52.7, SD = 9.7), respectively; standard scores ranged from 53 – 116 (M = 92.5, SD = 14.8) and 17 – 116 (M = 86.8, SD = 26.1), respectively. For the inhibitory control go/no-go task, number of commission errors for children with PKU ranged from 0 – 15 (M = 5.1, SD = 3.8), with standard scores ranging from 34 – 122 (M = 92.2, SD = 22.4). Overall, the range of scores across individual children was quite broad on the working memory and inhibitory control tasks; at the group level, however, performance was low average to average in relation to that of our typically-developing sample.
3.4. IQ and executive abilities in relation to indices of Phe control
As shown in Table 3, correlations between IQ and indices of Phe control were significant only for SD Phe and SEE Phe over the lifetime, as well as SD Phe during the ≥ 10 yrs. epoch. Performance on the Vocabulary subtest was not significantly correlated with any index of Phe control. Matrix Reasoning performance was of particular interest due to its relevance to executive strategic processing. For this subtest, significant correlations were found between performance and SD Phe and SEE Phe over the lifetime and during all developmental epochs; particularly robust correlations with large effect sizes were found during the ≥ 10 yrs. epoch. In addition, Matrix Reasoning performance was significantly correlated with % spikes over the lifetime and during the ≥ 10 yrs. epoch. With regard to average Phe, the only significant relationships for Matrix Reasoning performance were with mean Phe and the IDC during the ≥ 10 yrs. epoch.
Standard scores for the working memory and inhibitory control tasks were also examined in relation to indices of Phe control. As shown in Table 3, the pattern of relationships between correct nonresponses on the n-back task and SD Phe and SEE Phe was particularly striking, with significant correlations over the lifetime and across all development epochs with one exception (SEE Phe during the < 5 yrs. epoch). Nonresponses were also significantly correlated with our final index of variability in Phe, % spikes, over the lifetime and during the 5.0 – 9.9 yrs. epoch. Correct responses on the n-back task were significantly correlated with SD Phe and SEE Phe over the lifetime, as well as with SD Phe during the < 5 yrs. In terms of average Phe, number of correct nonresponses was significantly correlated with mean Phe during the 5.0 – 9.9 and ≥ 10 yrs. epochs, as well as with the IDC during the ≥ 10 yrs. epoch. Number of correct responses was significantly correlated with mean Phe and the IDC over the lifetime and during the < 5 yrs. epoch, as well as with the IDC during the 5.0 – 9.9 yrs. epoch.
Turning to inhibitory control, as shown in Table 3, number of commission errors on the go/no-go task was not significantly correlated with any index of Phe control over the lifetime. Commission errors were, however, significantly correlated with slope during the < 5 yrs. epoch and with mean Phe and the IDC during the 5.0 – 9.9 and ≥ 10 yrs. epochs.
3.5. Indices of Phe control as predictors of IQ and executive abilities
For the sake of parsimony, only SD Phe and mean Phe were included in these analyses because, as noted earlier, indices of variability in Phe (SD Phe, SEE Phe, % spikes) were highly correlated, indices of average Phe (mean Phe, IDC) were highly correlated, and there were few significant findings related to slope. We first conducted analyses in which only SD Phe was used as a predictor of IQ and executive abilities. As shown in Table 4, the decrease in standard score points for every 100 μmol/L increase in SD Phe over the lifetime ranged from 3.8 – 6.4. Extrapolating from the lifetime SD Phe of 217 μmol/L in our sample, standard scores for IQ, Matrix Reasoning, and number of n-back nonresponses and responses decreased by 8.2, 10.2, 13.9, and 11.5 points in relation to lifetime variability in Phe. Across developmental epochs, there were a number of significant results. Findings from Matrix Reasoning and n-back nonresponses were especially interesting, with more standard score points lost in relation to SD Phe during the older than youngest epochs.
We next conducted analyses in which both mean Phe and SD Phe were used as predictors of executive performance. As shown in Table 5, the only cognitive variable with which mean Phe was significantly correlated over the lifetime was number of correct n-back responses, with a decrease of 3.2 standard score points for every 100 μmol/L increase in mean Phe. Extrapolating from the lifetime mean Phe of 369 μmol/L in our sample, the standard score for n-back responses decreased by 11.8 points in relation to mean Phe over the lifetime. SD Phe was not a significant predictor of n-back responses after accounting for mean Phe in the regression model.
Turning to developmental epochs, mean Phe was a significant predictor of Matrix Reasoning performance during the ≥ 10 yrs. epoch. There was a loss of 2.0 points for every 100 μmol/L increase in mean Phe; after accounting for the contribution of mean Phe, SD Phe remained a significant predictor, with 6.2 points lost for every 100 μmol/L increase in SD Phe. A similar pattern was observed for number of n-back nonresponses during the 5.0 – 9.9 yrs. epoch. In this instance, there was a loss of 2.4 points for every 100 μmol/L increase in mean Phe; after accounting for the contribution of mean Phe, SD Phe remained a significant predictor, with 8.4 points lost for every 100 μmol/L increase in SD Phe. Additional significant results for mean Phe were found in relation to go/no-go errors during the 5.0 – 9.9 and ≥ 10 yrs. epochs, n-back responses during the < 5 yrs. epoch, and n-back nonresponses during the ≥ 10 yrs. epoch, with no additional contribution of SD Phe after accounting for mean Phe.
4. Discussion
A number of studies have shown that cognition is compromised in children with early- and continuously-treated PKU, and higher average Phe has been associated with poorer cognition [11–13]. In only a small number of studies have associations between variability in Phe and cognition been examined [19–23], and interpretation of findings across these studies has left questions unanswered due to a number of issues (e.g., inconsistency in indices of variability used). In addition, cognition has primarily been examined within only the broadest context (i.e., IQ), and relationships between variability in Phe and executive abilities in children across the school-age range have not been explored.
The current research was conducted to thoroughly evaluate the contribution of variability in Phe to cognitive outcomes in school-age children with early- and continuously-treated PKU. To do so, we examined IQ and executive abilities in relation to 6 indices of Phe control reflecting average Phe (mean Phe and IDC), change in Phe with age (slope), and variability in Phe (SD Phe, SEE Phe, and % spikes). All indices were calculated over the lifetime and during 3 developmental epochs (< 5, 5.0 – 9.9, and ≥ 10 yrs. of age).
Overall, our findings were most clearly reflected by single indices of average Phe (mean Phe) and variability in Phe (SD Phe) over the lifetime. These are also the most commonly and easily computed indices of Phe control. As such, this discussion is focused on correlations between these indices of Phe control and cognition.
Our findings indicated that performance on IQ and executive abilities tasks was more often correlated with variability in Phe than average Phe. Across the IQ and executive tasks administered, SD Phe over the lifetime was significantly correlated with 4 of 6 cognitive variables examined. Mean Phe over the lifetime, however, was significantly correlated with only 1 of 6 cognitive variables examined. In addition, performance on IQ and executive abilities tasks was more negatively affected by variability in Phe than average Phe over the lifetime. Standard scores on the IQ, Matrix Reasoning, and working memory n-back tasks decreased by an average of 5 points for every 100 μmol/L increase in SD Phe over the lifetime. Extrapolating from the lifetime SD Phe of 217 μmol/L of our sample, standard scores decreased by an average of 11 points in relation to variability in Phe.
There was a single instance in which mean Phe over the lifetime predicted a significant loss in standard score points; for n-back responses, 3.2 points were lost for every 100 μmol/L increase in mean Phe. In this instance, SD Phe did not make a significant contribution after accounting for the points lost due to mean Phe. However, it is interesting to keep in mind that 5.3 points were lost for every 100 μmol/L increase in SD Phe when this index was used as the sole predictor of n-back responses, whereas only 3.2 points were lost for every 100 μmol/L increase in mean Phe when it was used as the sole predictor.
Briefly turning to developmental epochs, in general, variability in Phe during the older epochs was more robustly correlated with executive performance than during the youngest epoch. In turn, a greater loss of standard score points was associated with increases in SD Phe during the older epochs than during the youngest epoch. The pattern of performance on the strategic Matrix Reasoning task was particularly striking in this regard, with every 100 μmol/L increase in SD Phe during the youngest and oldest epochs associated with losses of 3.8 and 6.8 points, respectively. These findings suggest that liberalization of Phe control as children with PKU age is unwise, as variability in Phe in older children may be particularly detrimental to executive abilities. These findings also suggest that Matrix Reasoning may be useful as a screening tool to evaluate the effects of variability in Phe on cognition.
Taken together, our findings clearly indicate that avoiding variability in Phe is important for optimizing cognitive outcomes in children with early- and continuously-treated PKU. We are not, however, arguing that average Phe is unimportant. Recall that in our study mean Phe and SD Phe were strongly correlated; in other words, higher average Phe was accompanied by greater variability in Phe. This raises the possibility that children with PAH mutations resulting in more severe PKU have greater difficulty maintaining stable Phe levels. Although genotypes were not available for analysis in our study, in support of this notion, Burgard et al. [20] described a robust correlation between predicted residual PAH activity (based on genotype) and variability in Phe (SEE Phe) in children with PKU. It should also be noted that it is quite possible that the rigorous level of statistical significance required in our study prevented us from identifying additional relationships between mean Phe and cognitive performance. Nonetheless, it is unlikely that our general pattern of findings, in which SD Phe was a more powerful predictor of cognition than mean Phe, would change with less statistical rigor.
Finally, from an explanatory perspective, it is unclear why greater variability in Phe is associated with poorer cognition in individuals with PKU. There are, however, other disorders in which physiological instability has been related to cognitive compromise. For example, fluctuations in glucose in patients with diabetes have been associated with poorer cognitive outcomes [28]. Although the underlying mechanisms are likely quite different for PKU and diabetes, it is clear that additional research is needed to understand the effects of physiological instability on cognition in children with PKU.
5. Conclusions
The current study included multiple indices of Phe control, a large number of blood Phe levels, measures of both IQ and executive abilities, and a relatively large sample of children with PKU. Findings strongly indicated that both variability in Phe and average Phe should be carefully monitored and controlled to maximize cognitive outcomes in children with PKU. Findings also indicated that Phe monitoring and control should not be liberalized as children with PKU age, as executive performance was better predicted by variability in Phe at older than younger ages.
Highlights.
6 indices of Phe control used to predict IQ and executive abilities in children with PKU
Indices of variability in Phe were stronger predictors than indices of average Phe
Variability in Phe during older childhood best predicted executive performance
Variability in Phe should be carefully controlled to maximize cognitive outcomes
Phe control should not be liberalized as children with PKU age
Acknowledgments
This research was supported by a National Institute of Child Health and Human Development grant (R01HD044901), by an Investigator Sponsored Trial grant from BioMarin Pharmaceutical Inc., and by the Human Clinical Core of the Washington University Intellectual and Developmental Disabilities Research Center which is supported by the National Institute of Child Health and Human Development (P30HD062171) and the James S. McDonnell Foundation. Drs. White, Grange, and Christ have served as consultants to and/or received research funding from BioMarin Pharmaceutical Inc. Dr. White serves as a consultant to Merck Serono S.A. The content of this article has not been influenced by these relationships. The authors wish to thank those who participated in our research for their contributions. We also thank Suzin Blankenship and Laurie Sprietsma for their contributions to study management, as well as the physicians, faculty, and staff of Washington University, Oregon Health & Science University, University of Missouri, New York Medical College, and University of Nebraska who generously contributed to the study through recruitment and phenylalanine monitoring.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.De Groot MJ, Hoeksma M, Blau N, Reijngoud DJ, Van Spronsen FJ. Pathogenesis of cognitive dysfunction in phenylketonuria: review of hypotheses. Mol Genet Metab. 2010;99:S86–S89. doi: 10.1016/j.ymgme.2009.10.016. [DOI] [PubMed] [Google Scholar]
- 2.Scriver CR. The PAH gene, phenylketonuria, and a paradigm shift. Hum Mutat. 2007;28:831–845. doi: 10.1002/humu.20526. [DOI] [PubMed] [Google Scholar]
- 3.Anderson PJ, Wood SJ, Francis DE, Coleman L, Anderson V, Boneh A. Are neuropsychological impairments in children with early-treated phenylketonuria (PKU) related to white matter abnormalities or elevated phenylalanine levels? Dev Neuropsychol. 2007;32:645–668. doi: 10.1080/87565640701375963. [DOI] [PubMed] [Google Scholar]
- 4.Antenor-Dorsey JAV, Hershey T, Rutlin J, Shimony JS, McKinstry RC, Grange DK, et al. White matter integrity and executive abilities in individuals with phenylketonuria. Mol Genet Metab. 2013 doi: 10.1016/j.ymgme.2013.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.White DA, Antenor-Dorsey JAV, Grange DK, Hershey T, Rutlin J, Shimony JS, et al. White matter integrity and executive abilities following treatment with tetrahydrobiopterin (BH4) in individuals with phenylketonuria. Mol Genet Metab. 2013 doi: 10.1016/j.ymgme.2013.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Peng H, Peck D, White DA, Christ SE. Tract-based evaluation of white matter damage in individuals with early-treated phenylketonuria. J Inherit Metab Dis. 2013:1–7. doi: 10.1007/s10545-013-9650-y. [DOI] [PubMed] [Google Scholar]
- 7.Mitchell JJ, Trakadis YJ, Scriver CR. Phenylalanine hydroxylase deficiency. Genet Med. 2011;13:697–707. doi: 10.1097/GIM.0b013e3182141b48. [DOI] [PubMed] [Google Scholar]
- 8.Paine RS. The variability in manifestations of untreated patients with phenylketonuria (phenylpyruvic aciduria) Pediatrics. 1957;20:290–302. [PubMed] [Google Scholar]
- 9.Brumm VL, Azen C, Moats RA, Stern AM, Broomand C, Nelson MD, et al. Neuropsychological outcome of subjects participating in the PKU adult collaborative study: a preliminary review. J Inherit Metab Dis. 2004;27:549–566. doi: 10.1023/b:boli.0000042985.02049.ff. [DOI] [PubMed] [Google Scholar]
- 10.Ris MD, Williams SE, Hunt MM, Berry HK, Leslie N. Early-treated phenylketonuria: adult neuropsychologic outcome. J Pediatr. 1994;124:388–392. doi: 10.1016/s0022-3476(94)70360-4. [DOI] [PubMed] [Google Scholar]
- 11.Azen C, Koch R, Friedman E, Wenz E, Fishier K. Summary of findings from the United States Collaborative Study of children treated for phenylketonuria. Eur J Pediatr. 1996;155:S29–S32. doi: 10.1007/BF03036507. [DOI] [PubMed] [Google Scholar]
- 12.Burgard P. Development of intelligence in early treated phenylketonuria. Eur J Pediatr. 2000;159:S74–S79. doi: 10.1007/pl00014388. [DOI] [PubMed] [Google Scholar]
- 13.Smith I, Beasley MG, Ades AE. Intelligence and quality of dietary treatment in phenylketonuria. Arch Dis Child. 1990;65:472–478. doi: 10.1136/adc.65.5.472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Waisbren SE, Noel K, Fahrbach K, Cella C, Frame D, Dorenbaum A, et al. Phenylalanine blood levels and clinical outcomes in phenylketonuria: A systematic literature review and meta-analysis. Mol Genet Metab. 2007;92:63–70. doi: 10.1016/j.ymgme.2007.05.006. [DOI] [PubMed] [Google Scholar]
- 15.Albrecht J, Garbade SF, Burgard P. Neuropsychological speed tests and blood phenylalanine levels in patients with phenylketonuria: A meta-analysis. Neurosci Biobehav Rev. 2009;33:414–421. doi: 10.1016/j.neubiorev.2008.11.001. [DOI] [PubMed] [Google Scholar]
- 16.Christ SE, Huijbregts SCJ, de Sonneville LMJ, White DA. Executive function in early-treated phenylketonuria: Profile and underlying mechanisms. Mol Genet Metab. 2010;99(Supple):S22–S32. doi: 10.1016/j.ymgme.2009.10.007. [DOI] [PubMed] [Google Scholar]
- 17.Janzen D, Nguyen M. Beyond executive function: non-executive cognitive abilities in individuals with PKU. Mol Genet Metab. 2010;99:S47–S51. doi: 10.1016/j.ymgme.2009.10.009. [DOI] [PubMed] [Google Scholar]
- 18.Leuzzi V, Pansini M, Sechi E, Chiarotti F, Carducci C, Levi G, et al. Executive function impairment in early-treated PKU subjects with normal mental development. J Inherit Metab Dis. 2004;27:115–125. doi: 10.1023/B:BOLI.0000028781.94251.1f. [DOI] [PubMed] [Google Scholar]
- 19.Vilaseca MA, Lambruschini N, Gomez-Lopez L, Gutiérrez A, Fusté E, Gassió R, et al. Quality of dietary control in phenylketonuric patients and its relationship with general intelligence. Nutr Hosp. 2010;25:60–66. [PubMed] [Google Scholar]
- 20.Burgard P, Rupp A, Konecki DS, Trefz FK, Schmidt H, Lichter-Konecki U. Phenylalanine hydroxylase genotypes, predicted residual enzyme activity and phenotypic parameters of diagnosis and treatment of phenylketonuria. Eur J Pediatr. 1996;155:S11–S15. doi: 10.1007/pl00014222. LA – English. [DOI] [PubMed] [Google Scholar]
- 21.Anastasoaie V, Kurzius L, Forbes P, Waisbren S. Stability of blood phenylalanine levels and IQ in children with phenylketonuria. Mol Genet Metab. 2008;95:17–20. doi: 10.1016/j.ymgme.2008.06.014. [DOI] [PubMed] [Google Scholar]
- 22.Arnold GL, Kramer BM, Kirby RS, Plumeau PB, Blakely EM, Cregan LS, et al. Factors affecting cognitive, motor, behavioral and executive functioning in children with phenylketonuria. Acta Paediatr. 1998;87:565–570. doi: 10.1080/08035259850158308. [DOI] [PubMed] [Google Scholar]
- 23.Viau KS, Wengreen HJ, Ernst SL, Cantor NL, Furtado LV, Longo N. Correlation of age-specific phenylalanine levels with intellectual outcome in patients with phenylketonuria. J Inherit Metab Dis. 2011;34:963–971. doi: 10.1007/s10545-011-9329-1. [DOI] [PubMed] [Google Scholar]
- 24.Cleary M, Trefz F, Muntau AC, Feillet F, van Spronsen FJ, Burlina A, et al. Fluctuations in phenylalanine concentrations in phenylketonuria: a review of possible relationships with outcomes. Mol Genet Metab. 2013 doi: 10.1016/j.ymgme.2013.09.001. [DOI] [PubMed] [Google Scholar]
- 25.Wechsler D. Wechsler abbreviated scale of intelligence. The Psychological Corporation; San Antonio, TX: 1999. [Google Scholar]
- 26.Cohen J. Statistical power analysis for the behavioral sciences. Lawrence Erlbaum; 1988. [Google Scholar]
- 27.N.I. of Health, Consensus Development Panel. Pediatrics; National Institutes of Health Consensus Development Conference Statement: phenylketonuria—screening and management; October 16–18, 2000; 2001. pp. 972–982. [DOI] [PubMed] [Google Scholar]
- 28.Rizzo MR, Marfella R, Barbieri M, Boccardi V, Vestini F, Lettieri B, et al. Relationships between daily acute glucose fluctuations and cognitive performance among aged type 2 diabetic patients. Diabetes Care. 2010;33:2169–2174. doi: 10.2337/dc10-0389. [DOI] [PMC free article] [PubMed] [Google Scholar]


