Entamoeba infections primarily involve the gastrointestinal tract and, although rare in North America, are common in the developing world. Infections can range from asymptomatic to severe or fatal invasions of multiple organ systems. Most cases in North America involve first-generation immigrant populations and returning international travellers. It can be difficult to differentiate Entamoeba histolytica-associated colitis from inflammatory bowel disease and invasive bacterial dysentery. Moreover, specific tests for E histolytica infection are not readily available in many centres, and stool studies and sigmoidoscopy can miss cases. Following two case presentations, this review discusses several aspects of these types of infection and stresses the importance of keeping E histolytica-associated colitis in differential diagnoses.
Keywords: Amoebic colitis, Entamoeba histolytica, Extraintestinal abscesses
Abstract
Entamoeba histolytica infections of the gastrointestinal tract are common in the developing world but rare in North America. The authors present two cases: one involving an individual who had not travelled to an endemic area and another involving an individual who was born in Bulgaria. Both presented with severe abdominal pain and diarrhea. Endoscopic assessment revealed scattered colonic ulcerations and one patient was found to have a liver abscess on imaging. Stool ova and parasite studies were negative in both cases and both were diagnosed on review of colonic biopsies. On review of all Entamoeba cases in the Calgary Health Zone (Alberta), ova and parasite analysis found an average of 63.7 Entamoeba cases per year and a pathology database review revealed a total of seven cases of invasive E histolytica (2001 to 2011). Both patients responded well to antibiotic therapy. E histolytica should be considered in new-onset colitis, especially in individuals from endemic areas.
Abstract
Les infections à Entamoeba histolytica des voies digestives sont courantes dans les pays en développement, mais rares en Amérique du Nord. Les auteurs présentent deux cas : l’un d’une personne qui ne s’était pas rendue dans une région endémique et l’autre, d’une personne née en Bulgarie. Toutes deux avaient eu des crampes abdominales importantes et de la diarrhée. L’évaluation endoscopique a révélé des ulcérations diffuses dans le colon, et l’imagerie a démontré la présence d’un abcès hépatique chez l’une d’entre elles. Les examens parasitologiques dans les selles étaient négatifs dans les deux cas, et tous deux ont été diagnostiqués à l’analyse des biopsies du côlon. À l’examen de tous les cas d’Entamoeba dans la zone de santé de Calgary, en Alberta, les examens parasitologiques ont permis de déterminer une moyenne de 63,7 cas d’Entamoeba par année et une analyse de la base de données pathologiques a révélé un total de sept cas d’E histolytica invasive entre 2001 et 2011. Les deux patients ont bien réagi à l’antibiothérapie. L’E histolytica devrait être envisagé en cas de colite de novo, particulièrement chez des personnes provenant de régions endémiques.
Entamoeba histolytica infections of the gastrointestinal (GI) tract are common in the developing world; however, in first-world countries, they are typically found in first-generation immigrant populations and returning international travellers. E histolytica is a parasitic protozoa that primarily infects the human bowel (1). It exists in two forms, a short-lived mobile trophozoite (10 μm to 20 μm in length) that can invade multiple organ systems, and a long-surviving cyst form that can colonize a patient (1). Diagnosis of a non-travel-related E histolytica infection in Canada is rare, with the most recent reported studies investigating cases in Inuit communities in Northern Labrador (2) and sexually transmitted cases in the homosexual population of Toronto, Ontario (3). Herein, we describe two cases of E histolytica colitis that presented to the Foothills Medical Centre, a large urban tertiary care centre located in Calgary, Alberta.
METHODS
The Calgary Zone, Alberta Health Services (CZ-AHS) serves a population of 1.2 million residents of Calgary and surrounding communities. All laboratory and pathology services for CZ-AHS are centralized and have searchable databases. The pathology database was searched for all reports containing the words “Entamoeba histolytica”, “Entamoeba” and “E. histolytica”, from 2001 to 2011. The microbiology database was searched for all positive stool studies consistent with Entamoeba. The microbiology database was searched from 2006 to 2011 for all positive stool ova and parasite (O&P) microscopic examinations that reported the presence of Entamoeba; data were only available from this period of time. Age (2007 to 2011) and sex (2006 to 2011), however, were the only variables that could be assessed due to privacy and ethics regulations. Unfortunately, E histolytica cannot be morphologically differentiated from Entamoeba dispar (a common non-invasive parasite) and Entamoeba moshkovskii (considered primarily to be a free-living amoeba); however, E dispar and E moshkovskii are generally believed to be nonpathogenic. Commercial ELISAs and molecular biological testing, such as polymerase chain reaction (PCR), are available to differentiate E histolytica from E dispar but they are not routinely used in the CZ-AHS due to the rarity of these infections in the region. Entamoeba serology testing can diagnose infection with E histolytica (both E dispar and E moshkovskii do not elicit an antibody response), although it also is not routinely ordered because it takes up to 12 weeks before results are available from the reference laboratory. Serology test results for most of the patients were, therefore, not available. No commercial molecular methods are currently available for distinguishing E moshkovskii, although PCR has been used to detect this parasite directly in stool samples during surveillance studies (4). Because serology testing is sent to a reference laboratory, these data were not searchable.
The CZ-AHS pathology database was searched from 2001 to 2011 to identify all cases of invasive E histolytica. Data are presented as mean ± SEM. Statistical analysis was performed using Graph Pad Prism (GraphPad, USA) using a parametric unpaired t test for age and nonparametric Mann-Whitney for sex. Ethics approval was obtained from the CZ-AHS for a limited data recovery as above. Permission to present the cases was obtained from both individuals.
RESULTS
Data were available for stool O&P analysis in the CZ-AHS from January 2006 to December 31, 2011. From 2006 to 2011, a mean (± SEM) of 63.7±2.3 cases of Entamoeba were diagnosed according to stool O&P examination (Figure 1A). Again, this would include E histolytica, E dispar and E moshkovskii. During the time period assessed, Entamoeba was more commonly diagnosed in men (39.0±2.4 cases/year) versus women (24.7±3.4 cases/year) (P<0.01). The average age of diagnosis was 31.7 years, with men being slightly older (33.2 years) than females (30.1 years) (P=0.25 [not significant]) (Figure 1B).
Figure 1).
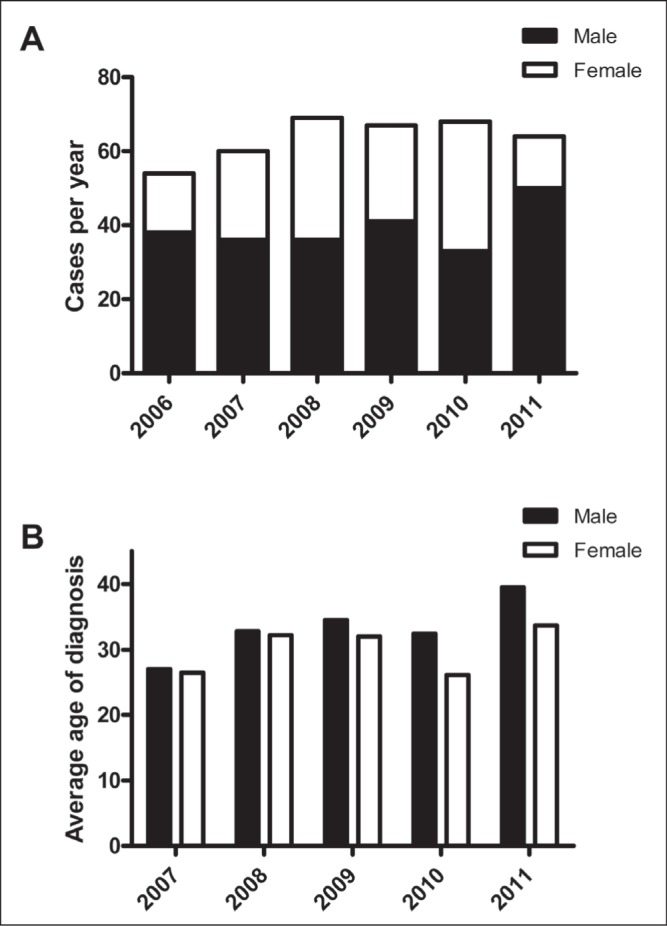
Cases of Entamoeba from stool ova and parasite analysis in the Calgary Health Region (Alberta) according to year (A) and age (B)
The CZ-AHS pathology database search from 2001 to 2011 revealed a total of seven cases, with three females and a mean age of 55±7.4 years (this includes the two cases reported below). In six cases, colitis with invasive E histolytica was only noted in the cecum and ascending colon and, in one case (patient 1 below), there was evidence of E histolytica throughout the colon involving the rectum to the cecum. Again, no further details of these cases could be obtained except for the two cases decribed below.
Patient 1
A 56-year-old heterosexual man presented to the emergency department with a 10-day history of abdominal pain, nausea and vomiting, and diarrhea. The patient denied taking any medication and had no history of recent travel. His medical history was also unremarkable and he denied having any previous homosexual partners. On physical examination, the patient had a temperature of 38.4°C, a heart rate of 110 beats/min and a blood pressure of 95/55 mmHg. Head and neck, respiratory and cardiovascular, and musculoskeletal examinations were all normal. The patient had a distended abdomen and identified marked right lower quadrant tenderness with guarding. Laboratory results revealed a hemoglobin level of 127 g/L (normal 127 g/L to 165 g/L), an increased white blood cell count of 18.2×109/L (normal 4.0×109/L to 11.0×109/L), lactate level of 8.2 mmol/L (normal 0.5 mmol/L to 2.2 mmol/L) and increased levels of alkaline phosphatase (232 U/L [normal 30 U/L to 145 U/L]) and gamma glutamyl-transferase (176 U/L [normal 11 U/L to 63 U/L]).
Computed tomography (CT) imaging of the abdomen and pelvis revealed severe colitis involving the cecum and ascending colon, and liver abscesses (Figures 2A and 2B). The liver abscesses were drained and the fluid was analyzed. Although microscopic examination of the fluid revealed an increased number of neutrophils, no organisms were visualized on Gram stain and the fluid cultures were negative. At this point, the differential diagnosis included infection, ischemia and new-onset inflammatory bowel disease (IBD). Blood and stool cultures, stool testing for O&P and Clostridium difficile were all negative. Serology for Entamoeba and Yersinia were also sent to the reference laboratory. The patient was initially treated with broad-spectrum antibiotics and conservative management.
Figure 2).
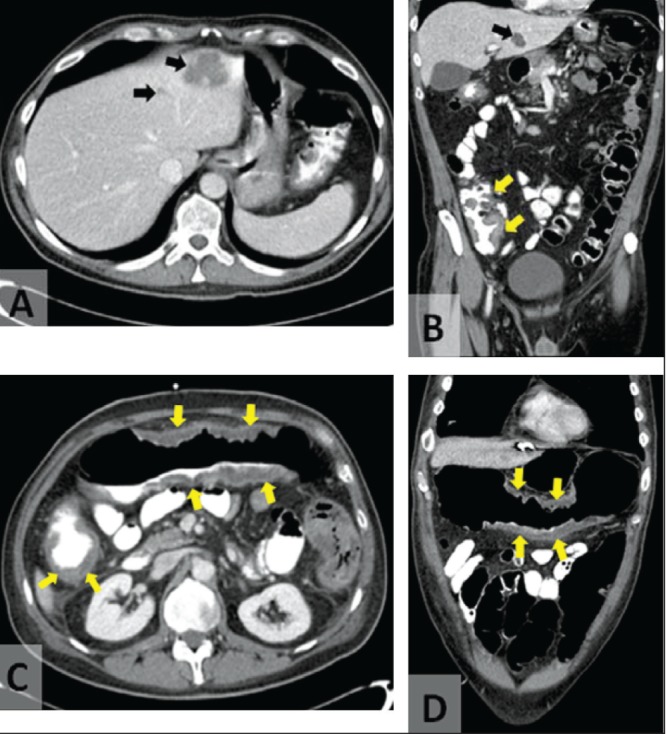
Transverse (A) and coronal (B) computed tomography images of the abdomen demonstrating liver abscesses (black arrows) and colonic wall thickening (yellow arrows) on initial presentation of patient 1. Computed tomography images of the abdomen demonstrating worsening colitis (arrows), pericolic stranding and fluid on day 6 of hospital admission of patient 1 (C and D)
Despite broad-spectrum antibiotics and conservative management, the patient deteriorated, developing more severe abdominal pain, with guarding and nausea. A repeat CT scan revealed worsening of colitis with increased bowel wall thickening, pericolic stranding and free fluid (Figures 2C and 2D). A colonoscopy was performed and classic amoebic ulcers were visualized (Figures 3A and 3B) and biopsies were collected. Histological examination revealed classic features of E histolytica (Figures 4A and 4B). The patient was treated with 14 days of metronidazole (750 mg per oral three times per day) followed by seven days of paromomycin (500 mg per oral three times per day). His symptoms resolved rapidly; however, a colonoscopy performed three months later showed normal colonic mucosa with a mid-transverse colonic stricture. After six weeks, his serology result was available and was positive for E histolytica and negative for Yersinia. This stricture did not cause symptoms and it has gradually improved over three years of follow-up.
Figure 3).
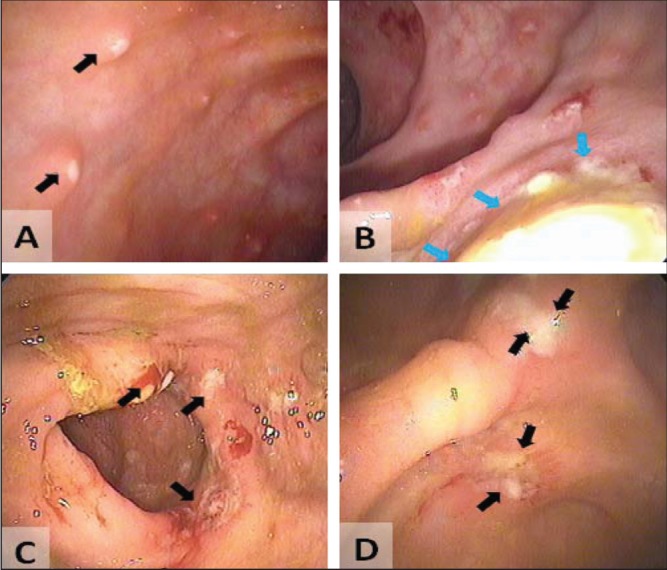
Colonoscopic imaging of patient 1 (A and B) and patient 2 (C and D) demonstrating classic amoebic ulceration in both patients (arrows)
Figure 4).
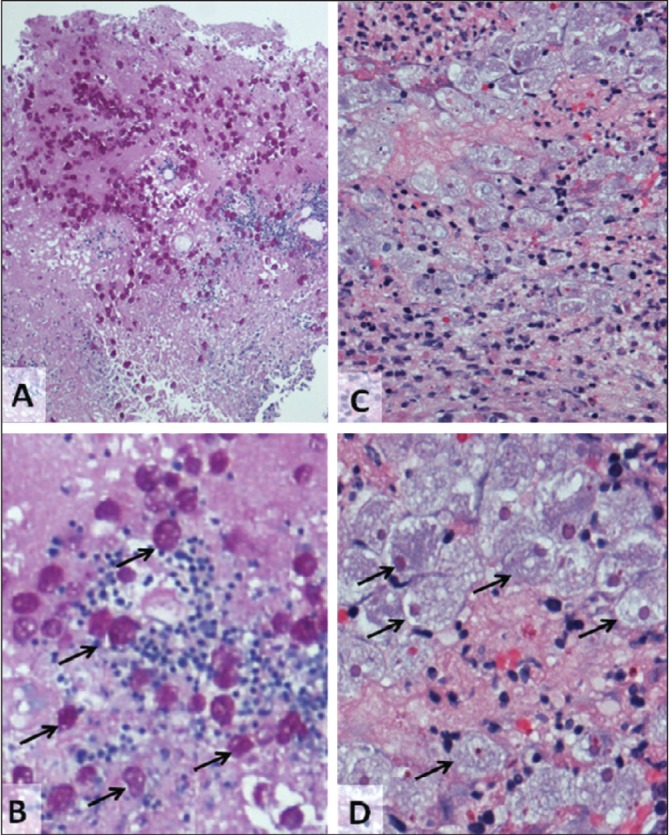
A and B Colon biopsies of patient 1. Alcian blue periodic acid Shiff (PAS) stain (A: original magnification ×40, B: higher magnification of A), arrows indicate Entamoeba histolytica trophozoites (many do not have arrows). C and D Patient 2 (hematoxylin and eosin stain C: original magnification ×100, D: higher magnification C, arrows indicate PAS-positive E histolytica trophozoites (many do not have arrows)
Patient 2
A 24-year-old heterosexual man presented to the outpatient gastro-enterology clinic with a three-month history of intermittent diarrhea and rectal bleeding. He lived in Bulgaria until eight years of age before immigrating to Canada. The patient had returned to Bulgaria for five months approximately one year previously. He was never sick nor did he experience any GI issues during his stay. Approximately four months after his visit to Bulgaria, he developed bloody diarrhea (at most six bowel motions per day) and lost 5.85 kg (13 lbs), which was associated with abdominal pain, bloating and tenesmus. At that time, his hemoglobin level was normal but his platelet count was slightly elevated (408×109/L [normal 150×109/L to 400×109/L]), as was his white blood cell count (11.9×109/L [normal 4.0×109/L to 11.0×109/L]) and erythrocyte sedimentation rate (13 mm [normal 0 mm to 10 mm]). His electrolyte and thyroid stimulating hormone levels, liver function studies, celiac serology, HIV serology and stool studies (O&P, C difficile toxin, culture and sensitivities) were all normal. A colonoscopy was performed (Figures 3C and 3D) and biopsies were collected. There were many endoscopic features consistent with Crohn disease including skip segments, deep ulcers and some linear ulcers. The ileum was normal. Biopsies again revealed classic features of E histolytica (Figures 4C and 4D). A CT scan was performed after the diagnosis and revealed colonic inflammation but no liver abscesses (not shown). The patient had a complete and rapid response to a 10-day course of metronidazole (750 mg per oral three times per day) followed by seven days of paromomycin (500 mg per oral three times per day). Serology was not performed.
DISCUSSION
Locally acquired E histolytica infections are a very rare occurrence in urban Canada aside from travellers, recent immigrants and the male homosexual population (5). A recent study investigating the prevalence of intestinal parasites in the United States demonstrated that for individuals infected with a single GI parasite, <5% were caused by E histolytica or a similar asymptomatic species (E dispar) (6). In the United States, the annual incidence of amoebic liver abscess (occurring in <1% of E histolytica colitis cases) was 1.38 per million population and mostly occurred in Hispanic men in the western and southern states (7). Similar studies have not been undertaken across Canada; however, E histolytica infections have been reported in both humans and canines in Canadian northern Aboriginal communities (2,8). A recent study from Ontario (9) reported 29 cases of amoebic liver abscesses that presented to seven hospitals in Toronto over a 30-year period. Of these cases, 86% had recent travel to endemic areas and some patients were born in endemic areas (9). They did not report any cases that developed in Canada without recent travel, foreign birth or other risk factors (9). Because most studies from endemic areas report that <1% of individuals with E histolytica colitis develop a liver abscess and only 10% with E histolytica in their stool develop invasive disease, one could estimate that in the Toronto area (based on one E histolytica abscess per year) there are >100 cases of E histolytica colitis per year and approximately 1000 without invasive disease (carriers).
Our two sources of review of the CZ-AHS databases consisted of identifying positive stool studies and intestinal pathology. On average, 63.7 cases of Entamoeba were identified by stool studies per year (Figure 1). As noted above, these are based on light microscopy assessment of morphology and cannot differentiate E histolytica from the nonpathogenic E dispar and E moshkovskii. This is likely an underestimate of the incidence/prevalence of Entamoeba in the CZ-AHS due to the low sensitivity and specificity (discussed further below). In the Ontario study above, only 24% of cases of proven E histolytica were found to have positive stool studies (9). Seven (including our two cases) were identified on review of pathology over a 10-year period.
There are several risk factors for acquiring E histolytica infection other than recent travel to an endemic area, including men who have sex with men (MSM). In a study from Los Angeles (USA), 6% of MSM were seropositive for E histolytica; however, significantly higher rates have been reported in MSM populations in other parts of the world including Rome, Italy (21%), Mexico City, Mexico (25% of HIV-positive MSM) and South Africa (43% in HIV positive and 15% in HIV negative, and 69% in those 50 to 59 years of age) (10,11). Both individuals in our study denied ever engaging in sex with men.
E histolytica is a parasite that is transmissible by the oral-fecal route. Infections can range from asymptomatic to severe or fatal invasions of multiple organ systems. Asymptomatic infections are responsible for the continuous transmission of the parasite because numerous cysts are produced and passed in feces. If exocystation occurs, E histolytica trophozoites are produced and invade the intestinal wall leading to amoebic dysentery and resulting in amoebic ulcers (1). Trophozoites are capable of penetrating the intestinal wall and can lead to more severe complications including liver abscesses (the most common) and, in rare cases, can spread to the brain and/or lungs, which is often fatal (12). A typical treatment regimen for E histolytica infection is metronidazole for 10 to 14 days (500 mg to 750 mg three times per day) followed by a seven-day treatment of paromomycin (25 mg/kg to 35 mg/kg daily in three divided doses) to eliminate colonization (13).
Amoebic liver abscesses usually present with fever and pain in the right upper quadrant (13,14). Diagnosis of E histolytica is based on the patient’s history, imaging modalities, serological findings, stool studies, fecal antigen testing (via ELISA) as well as real-time PCR (9,14). Stool microscopic assessment (which is the most common test used in Canada) has a low sensitivity (10% to 50%) and cannot differentiate E histolytica from the noninvasive, nonpathogenic E dispar and E moshkovskii (both of our cases had negative stool O&P studies) (9,14,15). Typically, a patient’s history reveals recent travel from an endemic area or other risk factors; however, this was not the case in either of our two cases. Serological testing can differentiate E histolytica from E dispar and E moshkovskii (the latter two do not induce antibody responses) (9); the sensitivity and specificity ranges from 85% to 95% (9–11,15). It is important to differentiate E histolytica from E dispar because, even in asymptomatic individuals, E histolytica should be treated to prevent spread and invasive disease. Unfortunately, most diagnostic laboratories in most centres in Canada refer serological testing to an external site, which can take several weeks before results are available. Stool antigen and DNA tests are generally not available ‘in house’ at most Canadian centres and are discussed further below.
Our first case was unique because it occurred in an individual who was born in Alberta, had not recently travelled to endemic areas and had no other identifiable risk factors. Furthermore, the differential for both cases included IBD. Corticosteroids are contraindicated in E histolytica because, not surprisingly, it has been associated with more adverse outcomes (16). The second case could also have been locally acquired; however, because of his history of travel to Bulgaria for five months in the past year, it is highly possible that he acquired E histolytica infection abroad because Eastern Europe has significantly higher prevalence rates than North America (17). As noted above, many individuals who are infected with E histolytica are asymptomatic and only approximately 10% develop invasive disease. Thus, although this entity is rare in Canada, one should consider this diagnosis in patients with new symptoms of colitis, especially in those with recent travel to endemic areas.
It can be difficult to differentiate E histolytica-associated colitis from IBD and invasive bacterial dysentery. In general, those who present with E histolytica-associated colitis have a duration of symptoms >7 days, most will be fecal occult blood positive whereas only approximately 40% of those with invasive bacterial dysentery will be fecal occult positive and generally experience a shorter disease duration (18). Fever (>38°C) is common in invasive bacterial dysentery but is less common in individuals with uncomplicated IBD or E histolytica-associated colitis (<40%) (1) (although those with E histolytica liver abscesses are commonly febrile) (18). E histolytica-associated colitis more commonly presents with weight loss compared with those with invasive bacterial dysentery (18). More than 90% of patients with E histolytica-associated colitis present with diarrhea and tenesmus whereas frank blood in stools and fever are rare (18). In short, the history, stool studies and colonic biopsy assessment play critical roles in differentiating E histolytica-associated colitis from IBD and invasive bacterial dysentery. Unfortunately, E histolytica antigen and antibody tests are not readily available in most North American centres; most laboratories outsource these tests, with results taking seven to 21 days. These ELISA-based antibody tests have a sensitivity and specificity of 85% to 95% but are less useful in patients from endemic areas because they may have antibodies from previous exposure (15). Again, stool studies (microscopy and culture) can miss cases, with studies reporting 10% to 50% sensitivity. Because E histolytica trophozoites degenerate rapidly in unfixed fresh samples, fixation and multiple collections increase the yield (18,19). Again, microscopy cannot differentiate E histolytica from other Entamoeba species. The best tests at present are the PCR- and ELISA-based assays that detect E histolytica DNA or antigens in stool, and have sensitivities and specificities of 90% to 95% and 95% to 100%, respectively (15,18,20,21). With the increase in world travel and emigration, we may have to consider increasing our use of more rapid and accurate DNA/antigen-based stool studies.
Because E histolytica-associated colitis can be localized to the cecum and right colon, a sigmoidoscopy can miss cases (18). Endoscopically, E histolytica colitis is associated with mucosal thickening, multiple discrete ulcers separated by regions of normal-appearing mucosa, diffuse inflammation and erythema and, rarely, necrosis and perforation (18). Recently, Upadhyay et al (22) described E histolytica ulcers as having a ‘poached egg’ appearance. They describe a patient who had multiple large irregular ulcers with a white slough and yellowish necrotic material on the top of the white slough, giving a ‘poached egg’ appearance. Both of our cases had irregular ulcers with white slough but neither patient had ulcers with the ‘poached egg’ appearance (they were missing the yellowish necrotic material). The most feared complication of E histolytica-associated colitis is acute necrotizing colitis and the development of toxic megacolon. This is rare but has been reported in approximately 0.5% of cases and is associated with high mortality (18). E histolytica colitis can also rarely be associated with penetrating disease, causing enterocutaneous, rectovaginal and enterovesicular fistulas (18). E histolytica can also cause inflammation of the appendix and present as appendicitis; in addition, it can cause pronounced granulomatous inflammation resulting in a pseudotumour that can lead to bowel obstruction (18). Fewer than 1% of individuals with E histolytica infections develop extraintestinal features that can include pericarditis, lung abscesses, peritonitis and skin lesions; however, the most common is hepatic abscesses (18). Hepatic abscesses are more common in men (male:female ratio 3.3:1 [23], 7.2:1 [24]), with a peak age of incidence between 30 and 50 years (25), and appears to be associated with increased alcohol consumption (18). Interestingly, a laboratory-based study (26) found that testosterone increased the susceptibility of mice to E histolytica liver abscesses by decreasing interferon-gamma secretion by natural killer T cells (26).
SUMMARY
With increased travel and emigration, we must keep E histolytica-associated colitis in our differential diagnosis list. Because one of our patients had no risk factors for E histolytica, we should entertain this diagnosis when we encounter new cases of colitis and wait for biopsies and stool studies before starting corticosteroids for presumed IBD.
Acknowledgments
Dr Beck is an Alberta Innovates Health Solutions Clinical Scholar and has research grants from Canadian Institute of Health Research and Crohn’s and Colitis Foundation of Canada.
Footnotes
DISCLOSURES: The authors have no financial disclosures or conflicts of interest to declare.
REFERENCES
- 1.Adams EB, MacLeod IN. Invasive amebiasis. I. Amebic dysentery and its complications. Medicine (Baltimore) 1977;56:315–23. doi: 10.1097/00005792-197707000-00003. [DOI] [PubMed] [Google Scholar]
- 2.Sole TD, Croll NA. Intestinal parasites in man in Labrador, Canada. Am J Trop Med Hyg. 1980;29:364–8. doi: 10.4269/ajtmh.1980.29.364. [DOI] [PubMed] [Google Scholar]
- 3.Keystone JS, Keystone DL, Proctor EM. Intestinal parasitic infections in homosexual men: Prevalence, symptoms and factors in transmission. Can Med Assoc J. 1980;123:512–4. [PMC free article] [PubMed] [Google Scholar]
- 4.Ali IK, Hossain MB, Roy S, et al. Entamoeba moshkovskii infections in children, Bangladesh. Emerg Infect Dis. 2003;9:580–4. doi: 10.3201/eid0905.020548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Marcus VA, Ward BJ, Jutras P. Intestinal amebiasis: A diagnosis not to be missed. Pathol Res Pract. 2001;197:271–4. doi: 10.1078/0344-0338-00047. discussion 275–8. [DOI] [PubMed] [Google Scholar]
- 6.Amin OM. Evaluation of a new system for the fixation, concentration, and staining of intestinal parasites in fecal specimens, with critical observations on the trichrome stain. J Microbiol Methods. 2000;39:127–32. doi: 10.1016/s0167-7012(99)00108-6. [DOI] [PubMed] [Google Scholar]
- 7.Congly SE, Shaheen AA, Meddings L, Kaplan GG, Myers RP. Amoebic liver abscess in USA: A population-based study of incidence, temporal trends and mortality. Liver Int. 2011;31:1191–8. doi: 10.1111/j.1478-3231.2011.02562.x. [DOI] [PubMed] [Google Scholar]
- 8.Unruh DH, King JE, Eaton RD, Allen JR. Parasites of dogs from Indian settlements in northwestern Canada: A survey with public health implications. Can J Comp Med. 1973;37:25–32. [PMC free article] [PubMed] [Google Scholar]
- 9.Wuerz T, Kane JB, Boggild AK, et al. A review of amoebic liver abscess for clinicians in a nonendemic setting. Can J Gastroenterol. 2012;26:729–33. doi: 10.1155/2012/852835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hung CC, Chang SY, Ji DD. Entamoeba histolytica infection in men who have sex with men. Lancet Infect Dis. 2012;12:729–36. doi: 10.1016/S1473-3099(12)70147-0. [DOI] [PubMed] [Google Scholar]
- 11.Samie A, Barrett LJ, Bessong PO, et al. Seroprevalence of Entamoeba histolytica in the context of HIV and AIDS: The case of Vhembe district, in South Africa’s Limpopo province. Ann Trop Med Parasitol. 2010;104:55–63. doi: 10.1179/136485910X12607012373911. [DOI] [PubMed] [Google Scholar]
- 12.Stanley SL., Jr Amoebiasis. Lancet. 2003;361:1025–34. doi: 10.1016/S0140-6736(03)12830-9. [DOI] [PubMed] [Google Scholar]
- 13.Li E, Stanley SL., Jr Protozoa. Amebiasis. Gastroenterol Clin North Am. 1996;25:471–92. doi: 10.1016/s0889-8553(05)70259-4. [DOI] [PubMed] [Google Scholar]
- 14.Marn H, Ignatius R, Tannich E, Harms G, Schurmann M, Dieckmann S. Amoebic liver abscess with negative serologic markers for Entamoeba histolytica: Mind the gap! Infection. 2012;40:87–91. doi: 10.1007/s15010-011-0157-x. [DOI] [PubMed] [Google Scholar]
- 15.Fotedar R, Stark D, Beebe N, Marriott D, Ellis J, Harkness J. Laboratory diagnostic techniques for Entamoeba species. Clin Microbiol Rev. 2007;20:511–32. doi: 10.1128/CMR.00004-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kobayashi CI, Yamamoto G, Hayashi A, et al. Fatal amebic colitis after high-dose dexamethasone therapy for newly diagnosed multiple myeloma. Ann Hematol. 2011;90:225–6. doi: 10.1007/s00277-010-0984-3. [DOI] [PubMed] [Google Scholar]
- 17.Nowak P, Jochymek M, Pietrzyk A. [Occurrence of human intestinal parasites in selected populations of Cracow region in the years 2000–2006 on the basis of parasitological stool examinations performed in the Laboratory of Parasitology of the District Sanitary-Epidemiological Center] Wiad Parazytol. 2007;53:285–93. [PubMed] [Google Scholar]
- 18.Choudhuri G, Rangan M. Amebic infection in humans. Indian J Gastroenterol. 2012;31:153–62. doi: 10.1007/s12664-012-0192-2. [DOI] [PubMed] [Google Scholar]
- 19.Proctor EM. Laboratory diagnosis of amebiasis. Clin Lab Med. 1991;11:829–59. [PubMed] [Google Scholar]
- 20.Mirelman D, Nuchamowitz Y, Stolarsky T. Comparison of use of enzyme-linked immunosorbent assay-based kits and PCR amplification of rRNA genes for simultaneous detection of Entamoeba histolytica and E. dispar. J Clin Microbiol. 1997;35:2405–7. doi: 10.1128/jcm.35.9.2405-2407.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Haque R, Ali IK, Akther S, Petri WA., Jr Comparison of PCR, isoenzyme analysis, and antigen detection for diagnosis of Entamoeba histolytica infection. J Clin Microbiol. 1998;36:449–52. doi: 10.1128/jcm.36.2.449-452.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Upadhyay R, Gupta N, Gogia P, Chandra S. Poached egg appearance in intestinal amebiasis. Gastrointest Endosc. 2012;76:189–90. doi: 10.1016/j.gie.2012.03.171. [DOI] [PubMed] [Google Scholar]
- 23.Acuna-Soto R, Maguire JH, Wirth DF. Gender distribution in asymptomatic and invasive amebiasis. Am J Gastroenterol. 2000;95:1277–83. doi: 10.1111/j.1572-0241.2000.01525.x. [DOI] [PubMed] [Google Scholar]
- 24.Shandera WX, Bollam P, Hashmey RH, Athey PA, Greenberg SB, White AC., Jr Hepatic amebiasis among patients in a public teaching hospital. South Med J. 1998;91:829–37. doi: 10.1097/00007611-199809000-00005. [DOI] [PubMed] [Google Scholar]
- 25.Blessmann J, Van Linh P, Nu PA, et al. Epidemiology of amebiasis in a region of high incidence of amebic liver abscess in central Vietnam. Am J Trop Med Hyg. 2002;66:578–83. doi: 10.4269/ajtmh.2002.66.578. [DOI] [PubMed] [Google Scholar]
- 26.Lotter H, Helk E, Bernin H, et al. Testosterone increases susceptibility to amebic liver abscess in mice and mediates inhibition of IFNgamma secretion in natural killer T cells. PLoS One. 2013;8:e55694. doi: 10.1371/journal.pone.0055694. [DOI] [PMC free article] [PubMed] [Google Scholar]


