Abstract
Objectives
To use multiphoton microscopy to image collagen fibers and matrix structure in nonfixed human keloid tissue and normal human facial skin obtained following surgery and to compare the findings to existing knowledge of normal skin and keloid morphology to determine if this technology is a suitable adjunct for conventional histology.
Methods
Epidermis was removed to expose the fibroblast- rich dermal layer that was then imaged using a multiphoton confocal microscope (Zeiss-Meta 510; Carl Zeiss, Jena, Germany). An 800-nm tunable titanium/sapphire femtosecond laser (Mai-Tai; Newport Co Spectra- Physics, Mountain View, California) was used to excite the tissue; second harmonic generation between 397 and 408 nm and autofluorescent signals were collected. Images were obtained using a Plan-Neofluar ×40 oil immersion objective lens and a Plan-Apochromat ×63 oil immersion lens.
Results
Compared with normal skin, keloids showed disorganized collagen fibers arranged in complex swirls and bundles 20 to 30 µm in diameter. Normal tissue showed collagen fibers as distinct, straight strands less than 10 µm in diameter. Differences between normal and keloid tissue were subtle but apparent.
Conclusions
The value of imaging living tissue is a significant benefit. Because keloids and hypertrophic scars result from altered collagen metabolism, the development of clinical multiphoton microscopy systems may allow examination of wound healing dynamics in vivo and potentially provides a means to monitor therapy without the need for biopsy or the risk of injury to tissue.
Keloids are disfiguring benign neoplasms that result from abnormal wound healing. They are characterized by their tendency to grow beyond the boundaries of the original injury and recur after surgical excision.1 Keloids occur equally in both sexes, and they have a propensity to develop in darker-skinned people in an autosomal dominant fashion with incomplete penetrance.2 Any tissue damage ranging from a severe burn to a minor scratch can result in keloid formation. Multiple treatment options are available once a keloid has formed including the following instances: surgical excision, corticosteroid injection, radiation therapy, pressure therapy, or laser therapy. None of these methods are completely effective, and all have a high recurrence rates when used alone.
Keloids are difficult to study and developing new treatment options is challenging for several reasons—the pathophysiology is not completely understood, there is no animal model,1 and access is limited to keloid specimens. The study of keloid architecture requires the use of formaldehyde or formalin to fix the tissue prior to imaging its collagen and elastin fibers using a trichrome stain.
Clinical management of keloids relies on serial examination of the injury or surgical site over time to ascertain whether the keloid recurs. Clinical observation is the only available monitoring technique. In general, this method does not identify keloid formation until the tumor is macroscopic. Multiphoton microscopy (MPM) is a nonlinear microscopy technique that may have value in imaging the dynamics of keloid formation and eventually treatment in these tissues. Visualizing the dynamic architecture of normal skin and keloid will provide a frame of reference for physicians to determine whether keloid therapy is working.
The main advantage of using multiple photons to image tissue is that photons of lower energy are used to penetrate deeper into tissue with less risk of photodamage. If a fluorophore is excited by a 400-nm photon, then it may also be excited by 2 near-infrared photons at 800 nm and will emit light in the visible spectrum. Endogenous fluorophores provide optical contrast by emitting light at varying wavelengths. For example, nicotinamide adenine dinucleotide phosphate is excited at approximately 360 nm and emits in the spectrum between 400 and 500 nm, flavoproteins are excited at approximately 450 nm and fluoresce between 500 and 600 nm, and crosslinks in elastin are excited by wavelengths between 340 and 370 nm and have an emission of 400 to 450 nm.3 The use of these and other fluorophores allow the imaging of living tissue without fixation, sectioning, or the use of exogenous dyes or stains. Thus, MPM permits the nondestructive and repetitive imaging of living tissue with diffraction-limited image resolution. Multiphoton microscopy and its applications have been reviewed by Emptage,4 White and Errington,5 and Halbhuber and König.6
Second harmonic generation (SHG) is a nonlinear optical microscopy technique that has been described previously. 7 This technique has been shown to produce images of collagen structure in the extracellular matrix by Mohler et al,8 Zoumi et al,9 and Zipfel et al.10 In this study, we used MPM and SHG to image nonfixed human keloid tissue and normal human facial skin and compared our findings with the existing knowledge of keloid and normal skin morphology to determine if this technology is a suitable adjunct for conventional histology.
METHODS
TISSUE PREPARATION
The research was conducted in accord with the guidelines of the institutional review board at the University of California, Irvine. Tissue samples were obtained from patients undergoing elective surgical procedures including facelift operations and keloid tumor removal. Five nonkeloid skin samples were acquired from white or Hispanic patients who ranged in age from 52 to 72 years and who underwent elective rhytidectomies. Five keloid samples were acquired from patients of African- American descent. Keloid specimens were sectioned obliquely and placed on a glass bottomed imaging dish with a No. 1 cover glass.
MPM IMAGING
Human keloid specimens were imaged using a multiphoton confocal microscope (Zeiss-Meta 510; Carl Zeiss, Jena, Germany). Images of collagen fiber orientation, distribution, and intrinsic fiber structure from the epidermis, dermis, and subcutaneous tissue were collected. An 800-nm tunable titanium/sapphire femtosecond laser (Mai-Tai; Newport Co Spectra-Physics, Mountain View, California) was used to excite the specimen. Second harmonic generation and autofluorescent signals for collagen were collected between 376 and 408 nm.
RESULTS
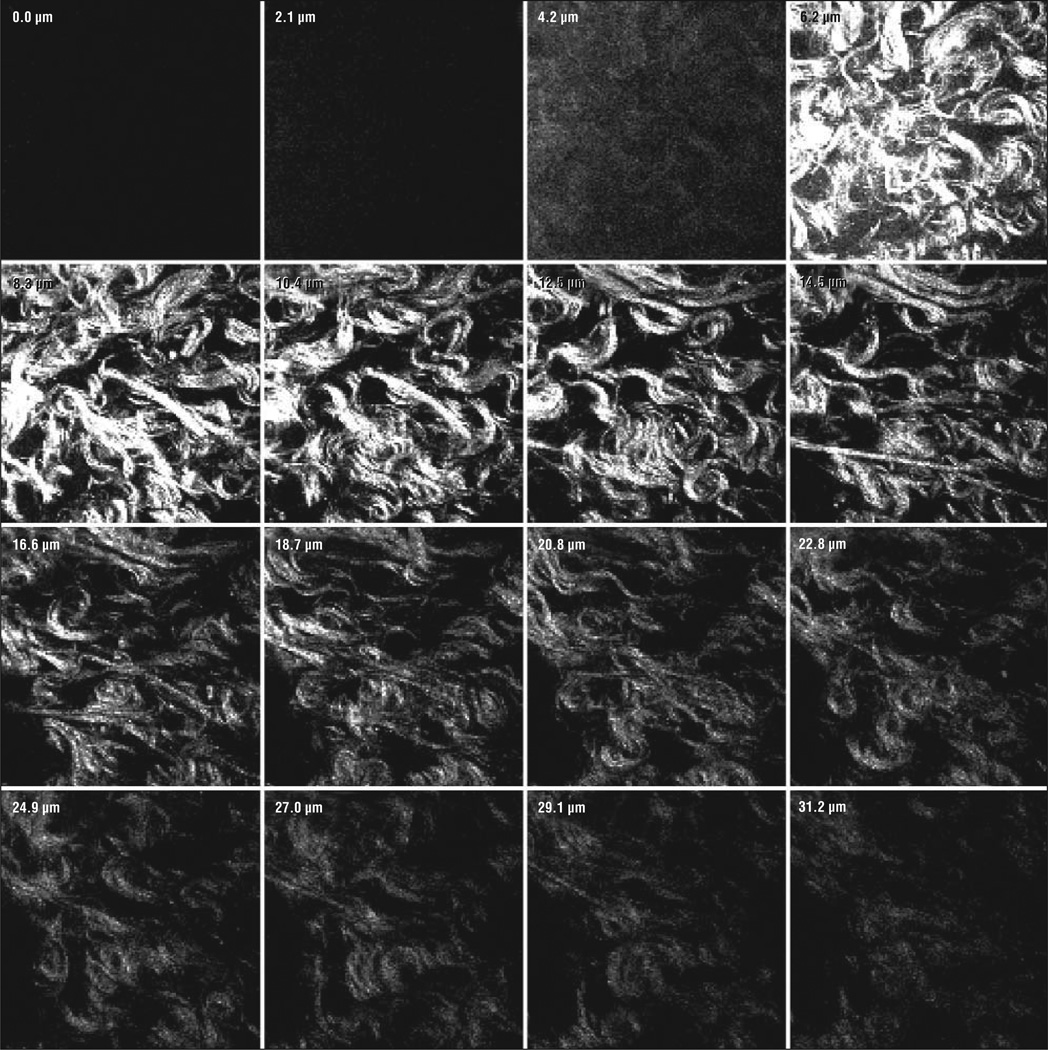
Collagen fibers were clearly seen in both normal human facial skin and human keloid samples. Compared with normal human facial tissue, the collagen structure of human keloids contains abnormally large and thick collagen bundle complexes Figure 1. The collagen fibers have also been observed to be organized in swirls composed of numerous fibrils closely bound together. This is in contrast to normal human facial skin that has straighter, thinner collagen bundle complexes with fewer fibrils.11,12 The MPM images in Figure 1 correlate well with these previously described histologic observations of keloids (Figure 2). The collagen yielded adequate signal for approximately 20 µm before the signal was lost. In those 20 µm, the collagen remains thick and wavy, reassuring us that our observations were not phenomena restricted to the surface of the tissue.
Figure 1.
Serial z-stack images of keloid tissue at a ×63 oil immersion objective lens. Sections are 2.1 µm each and extend 31.2 µm into the surface of the tissue.
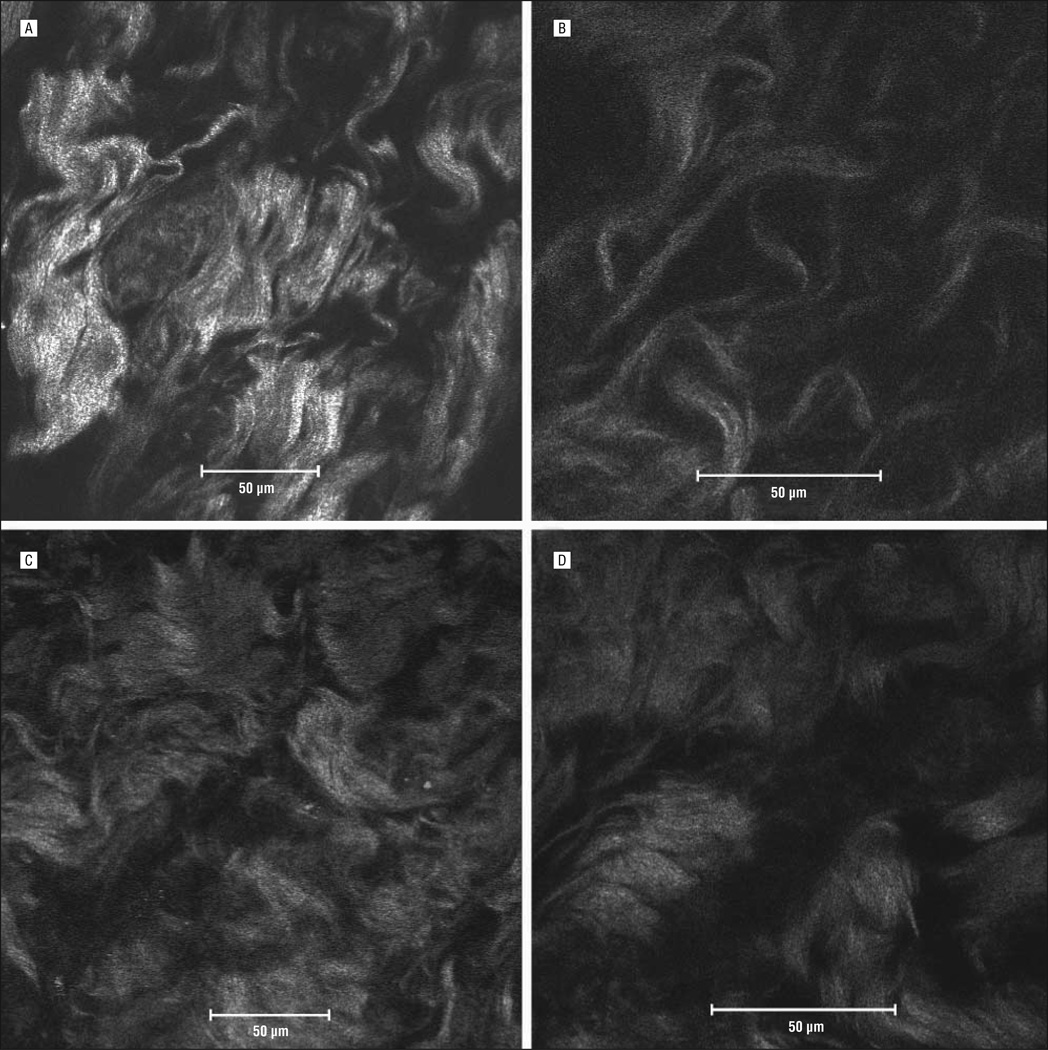
Figure 2.
Multiphoton confocal microscopic images from normal human facial skin (A and B) and human keloid tissue (C and D) shown under a ×40 oil immersion objective lens (A and C) and a ×63 oil immersion objective lens (B and D).
In the normal human facial skin images (Figure 2A and B), collagen fibers are largely orientated in the same direction, and they occur in small bundles (<10 µm). In Figure 2B, individual fibers are straighter than comparable keloid fibers. In the human keloid (Figure 2C and D), the fibers are not orientated with respect to one another. The collagen bundles are much thicker (20–30 µm in Figure 2D) and show the characteristic swirl pattern as described by Atiyeh et al.12
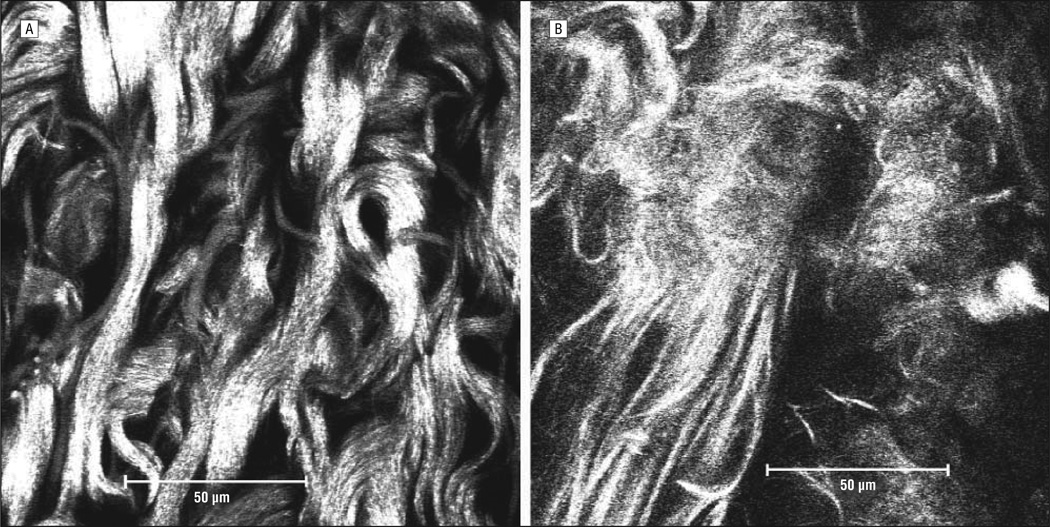
The MPM images of Figure 3 are comparable with those of Figure 2 showing the same differences in collagen fiber width and organization. In Figure 3A, the thick wavy collagen fibers are once again seen and are compared with the straight, thinner fibers seen in the left lower corner of Figure 3B. The abnormal collagen fibers of keloid tissue continue to persist throughout the tissue sample as seen in the z-stack (Figure 1). Serial MPM images were taken 2.1 µm apart for a total depth of 31.2 µm.
Figure 3.
Multiphoton confocal microscopic images at a ×63 oil immersion objective lens for human keloid tissue (A) and normal human facial skin (B).
COMMENT
Multiphoton microscopic and SHG imaging have been used to study living tissue including skin, cartilage, and the pathological changes in various diseases.7,10 On multiple occasions, the images produced by MPM demonstrated some of the same characteristic morphological findings of keloids that are seen using trichrome stain in conventional histology.
The structure of collagen has been well characterized as a polymerized fibrous protein that forms long chains. Three chains bind together in a triple helix to form a fibril that acquires strength from its orderly hydrogen bonding and the abundant presence of amino acids (proline and hydroxyproline). In turn, fibrils are organized in fibers or fascicles of varying sizes in a 3-dimensional lattice or interlocking network. Kischer13 identified these features using scanning and transmission electron microscopy, producing 3-dimensional images that have been essential in elucidating the pathogenesis of keloids and hypertrophic scars. However, conventional scanning and transmission electron microscopy do require tissue fixation and removal of tissue water that leads to artifact. As we have shown, MPM can also identify these collagen fibrils.
Perhaps the most significant advantage of MPM is the ability to observe samples in vivo without fixatives, dyes, or dehydration. Most forms of microscopy require samples to be dehydrated before imaging and this can alter the native shape of collagen, a complex molecule whose structure is determined by water. A collagen fibril is surrounded by an extracellular matrix composed largely of mucopolysaccharides that integrate water molecules into its structure through hydrogen bonding within the triple helix. Other water molecules bind to the polar surfaces of collagen fibrils and are more susceptible to dehydration. 14 It has been observed that dehydration of collagen in bone decreases the toughness of bone that is evident microscopically.15 It is possible that there is a correlative change in collagen structure when water is removed. Multiphoton microscopy can image collagen in its fully hydrated native state.
Electron microscopy has shown that the microanatomical unit of both the keloid and hypertrophic scar is the collagen nodule, a rodlike structure of uniformly aligned, straight collagen that is visible with the naked eye with a histologic appearance similar to that of load-bearing collagen. Also present in keloids and hypertrophic scars are increased numbers of active fibroblasts and microvessels.15 During pathogenesis, it has been postulated that the increased number of microvessels also has an increased number of endothelial cells that have a tendency to become occluded. The occlusion produces a local state of hypoxia that could stimulate a fibroblastlike cell to produce an excess amount of collagen.16 These occluded microvessels have only been observed with electron microscopy. As a hypertrophic scar matures and flattens over time, these occluded microvessels degenerate.13 The observation of the degeneration of these microvessels over time could provide a means to monitor therapy directed toward reducing microvessel growth and proliferation. Navarro et al3 have shown that MPM can be used to image blood vessels during wound healing after a full-thickness mechanical injury in a guinea pig. Through either direct observation of microvessels or indirect observation of associated collagen structure, the MPM could be used to monitor disease progression or therapy without the extensive preparation required for electron microscopy.
Keloid tumors have no ideal treatment and recurrence is common. The development of new therapies is limited owing to the scarcity of samples available for study and the fact that collected samples are usually fixed, stained, and sectioned for histopathologic evaluation. Recent work has been done by Meshkinpour et al,17 in which biopsy specimens of keloids and hypertrophic scars were obtained after treatment with a radiofrequency device. The biopsy specimens were observed using MPM, and they showed that the treatment can be monitored by MPM and accurately correlated with conventional histopathology. With the development of the technology, it may be possible for MPM to be used to image lesions in living tissue without necessitating a biopsy. This study has shown similarity between MPM images of keloids and well-known histopathologic observations using conventional methods. Our study, nevertheless, is limited in scope, and there is the possibility of observer bias. This study was not blinded, and the observers were aware of the structure of normal and keloid collagen when interpreting the MPM images.
Collagen structure in skin can vary greatly with age and ethnicity, and it was impossible to precisely account for these factors in the 2 study groups, which could be a source of error. Also, samples obtained from the keloid group were from excisional biopsy specimens without wide margins; therefore, it was impossible to compare the keloid tissue with normal tissue within the same patient. To reduce these sources of errors, future experiments must be done such as analysis of the normal skin of those patients from whom we obtained our keloid samples. When clinical MPM systems become available in the distant future, it may be possible to monitor treatment of keloids, as well as other skin and mucosal diseases. It is possible that this technology could become an integral component of any therapy aimed at modulated wound healing. The proliferation of multiphoton microscopes in research centers guarantees further developments of the technology and we expect to see the emergence of MPM as a superior alternative to traditional histology.
CONCLUSIONS
Keloid tumors have a unique collagen structure that is readily observed using well-known fixation and staining techniques. Using MPM and SHG to image keloid tissue yields images that are comparable to those obtained by conventional methods. We believe that once this technology becomes sufficiently compact and widespread, it will serve as a useful adjunct to conventional histology in analyzing the pathological changes found in keloids in live human subjects. Keloid management could one day be performed in an outpatient setting with repeated MPM imaging without the need for invasive biopsy to assess treatment efficacy.
Acknowledgments
Funding/Support: This study has been supported by grant DC005572, P41RR01192 from the National Institutes of Health and the US Department of Defense (Medical Free Electron Laser Program FA9550-04-1-0101).
Footnotes
Author Contributions: Study concept and design: Sun, Brown, and Wong. Acquisition of data: Da Costa and Wong. Analysis and interpretation of data: Da Costa, Wei, Lim, Sun, Brown, and Wong. Drafting of the manuscript: Wong. Critical revision of the manuscript for important intellectual content: Wong. Obtained funding: Wong. Administrative, technical, and material support: Sun. Study supervision: Wong.
Financial Disclosure: None reported.
Disclaimer: The views and conclusions contained herein are those of the authors and should not be interpreted as necessarily representing the official policies or endorsements, either expressed or implied, of the US Air Force Research Laboratory or the US government. The US government is authorized to reproduce and distribute reprints for governmental purposes notwithstanding any copyright notation.
Additional Contributions: Tatiana Krasieva, PhD, provided administrative, technical, material support, and helpful discussions; Zifu Wang, PhD, provided help and training in using the MPM.
REFERENCES
- 1.Al-Attar A, Mess S, Thomassen JM, Kauffman CL, Davison SP. Keloid pathogenesis and treatment. Plast Reconstr Surg. 2006;117(1):286–300. doi: 10.1097/01.prs.0000195073.73580.46. [DOI] [PubMed] [Google Scholar]
- 2.Marneros AG, Norris JE, Olsen BR, Reichenberger E. Clinical genetics of familial keloids. Arch Dermatol. 2001;137(11):1429–1434. doi: 10.1001/archderm.137.11.1429. [DOI] [PubMed] [Google Scholar]
- 3.Navarro FA, So PT, Nirmalan R, et al. Two-photon confocal microscopy: a nondestructive method for studying wound healing. Plast Reconstr Surg. 2004;114(1):121–128. doi: 10.1097/01.prs.0000128374.20913.4b. [DOI] [PubMed] [Google Scholar]
- 4.Emptage NJ. Fluorescent imaging in living systems. Curr Opin Pharmacol. 2001;1(5):521–525. doi: 10.1016/s1471-4892(01)00090-x. [DOI] [PubMed] [Google Scholar]
- 5.White N, Errington R. Multi-photon microscopy: seeing more by imaging less. Biotechniques. 2002;33(2):298–300. 302, 304–305. doi: 10.2144/02332bi01. [DOI] [PubMed] [Google Scholar]
- 6.Halbhuber KJ, König K. Modern laser scanning microscopy in biology, biotechnology and medicine. Ann Anat. 2003;185(1):1–20. doi: 10.1016/S0940-9602(03)80002-X. [DOI] [PubMed] [Google Scholar]
- 7.Williams RM, Zipfel WR, Webb WW. Multiphoton microscopy in biological research. Curr Opin Chem Biol. 2001;5(5):603–608. doi: 10.1016/s1367-5931(00)00241-6. [DOI] [PubMed] [Google Scholar]
- 8.Mohler W, Millard AC, Campagnola PJ. Second harmonic generation imaging of endogenous structural proteins. Methods. 2003;29(1):97–109. doi: 10.1016/s1046-2023(02)00292-x. [DOI] [PubMed] [Google Scholar]
- 9.Zoumi A, Yeh A, Tromberg BJ. Imaging cells and extracellular matrix in vivo by using second-harmonic generation and two-photon excited fluorescence. Proc Natl Acad Sci U S A. 2002;99(17):11014–11019. doi: 10.1073/pnas.172368799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zipfel WR, Williams RM, Christie R, Nikitin AY, Hyman BT, Webb WW. Live tissue intrinsic emission microscopy using multiphoton-excited native fluorescence and second harmonic generation. Proc Natl Acad Sci U S A. 2003;100(12):7075–7080. doi: 10.1073/pnas.0832308100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ehrlich HP, Desmoulière A, Diegelmann RF, et al. Morphological and immunochemical differences between keloid and hypertrophic scar. Am J Pathol. 1994;145(1):105–113. [PMC free article] [PubMed] [Google Scholar]
- 12.Atiyeh BS, Costagliola M, Hayek SN. Keloid or hypertrophic scar the controversy: review of the literature. Ann Plast Surg. 2005;54(6):676–680. doi: 10.1097/01.sap.0000164538.72375.93. [DOI] [PubMed] [Google Scholar]
- 13.Kischer CW. Contributions of electron microscopy to the study of the hypertrophic scar and related lesions. Scanning Microsc. 1993;7(3):921–930. [PubMed] [Google Scholar]
- 14.Nomura S, Hiltner A, Lando JB, Baer E. Interaction of water with native collagen. Biopolymers. 1977;16(2):231–246. doi: 10.1002/bip.1977.360160202. [DOI] [PubMed] [Google Scholar]
- 15.Nyman JS, Roy A, Shen X, Acuna RL, Tyler JH, Wang X. The influence of water removal on the strength and toughness of cortical bone. J Biomech. 2006;39(5):931–938. doi: 10.1016/j.jbiomech.2005.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chvapil M. Pharmacology of fibrosis and tissue injury. Environ Health Perspect. 1974;9:283–294. doi: 10.1289/ehp.749283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Meshkinpour A, Ghasri P, Pope K, et al. Treatment of hypertrophic scars and keloids with a radiofrequency device: a study of collagen effects. Lasers Surg Med. 2005;37(5):343–349. doi: 10.1002/lsm.20268. [DOI] [PubMed] [Google Scholar]