Abstract
This paper describes the synthesis of three closely related families of mannopyranoside-containing dendrimers for the purpose of studying subtle structural parameters involved in the measurements of multivalent carbohydrate–protein binding interactions. Toward this goal, two trimers 5 and 9, three 9-mers 12, 17, 21, and one 27-mer 23, varying by the number of atoms separating the anomeric and the core carbons, were synthesized using azide–alkyne cycloaddition (CuAAc). Compound 23 was prepared by an efficient convergent strategy. The sugar precursors consisted of either a 2-azidoethyl (3) or a prop-2-ynyl α-D-mannopyranoside (7) derivative. The solvodynamic diameters of 9-mer 12, 17, and 21 were determined by pulsed-field-gradient-stimulated echo (PFG-STE) NMR experiments and were found to be 3.0, 2.5, and 3.4 nm, respectively.
Keywords: carbohydrates, click chemistry, dendrimers, glycodendrimers, lectins, multivalent glycosystems
Introduction
Multivalent carbohydrate–protein interactions are at the forefront of a wide range of biological events which have triggered a plethora of versatile synthetic methods for the design of potent inhibitors and glycomimetics [1–4]. Among the diverse strategies leading to efficient ligands, glycopolymers [1,5–7], glycodendrimers [7–14], and sugar rods [15–16] have retained most attention. An additional approach that has gained keen interest resides in the modifications of both the aglycon [17–19] and substituent residues [20–22] of the targeted sugar moieties through extensive studies of quantitative structure–activity relationships (QSARs). In most of the studies related to aglycon modifications, it was concluded that aromatic glycosides possessed improved binding properties due to the ubiquitous presence of aromatic amino acids in the cognate binding sites [23–25]. This is also supported by the recent findings that the sugar backbones themselves also possess a hydrophobic side that orients the sugars in appropriate aromatic amino acid rich pockets [26–28].
Unfortunately, due to the inherent complexity of studying multivalent binding interactions, researchers have used experimental conditions that often biased the intrinsic phenomena under investigations [29]. For instance, when evaluating thermodynamic parameters by isothermal calorimetry (ITC), scientists used either truncated versions of for instance, tetrameric lectins such as ConA, or diluted conditions to avoid precipitation of the complexes [30–31]. Alternatively, the application of surface plasmon resonance (SPR) also creates artificial situations not sufficiently related to the natural cellular events, thus requiring complex mathematical algorithms [32]. Most solid-phase immunoassays (ELLA, ELISA) also fall under the same criticism by providing unusually high (or too close) sugar densities. Also important and in spite of the two decades of glycodendrimer chemistry [7], there is still no general rule to allow predicting which structural parameters would be optimal for the binding interactions.
In order to gain more insight into this direction, we designed herein three families of closely related mannopyranoside clusters (glycodendrimers) aimed at evaluating their relative binding abilities against the hometetrameric leguminous lectin ConA from Canavalia ensiformis by inhibition of haemagglutination and by turbidimetry. The latter would allow us to measure relative kinetic factors involved in the cross-linking lattice formation using soluble partners.
Results and Discussion
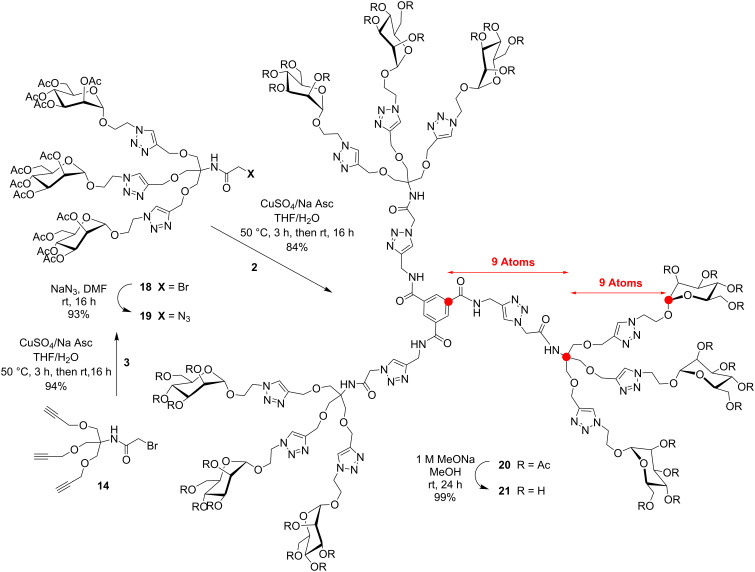
In order to critically evaluate the subtle structural parameters imparted by glycodendrimers in deciphering their relative thermodynamic and kinetic abilities towards multivalent lectins, we designed three families of closely related mannopyranoside dendrimers. Scheme 1 describes the preparation of trimers 5 and 9 built around benzene-1,3,5-tricarboxamide (BTA or trimesamide core) having respectively nine and ten atoms between the anomeric and the benzene carbon, hence differing by a distance of only ~1.5 Å. Schemes 2–4 illustrate the syntheses of 9-mers 12 and 21 using the same trimesic acid core, together with a phloroglucinol template to initiate the synthesis of homologue 17, but incorporating 2-amino-2-hydroxymethylpropane-1,3-diol as a branching unit (TRIS) at the G(1) level. Thus, compounds 12, 17, and 21 differ by having nine atoms between the anomeric carbon and the focal quaternary carbon of TRIS followed by two, four, and nine atoms to reach the benzene carbon, respectively (~4, 6, and 12 Å). Finally, the synthesis of a 27-mer mannosylated dendrimer 23 is shown in Scheme 5.
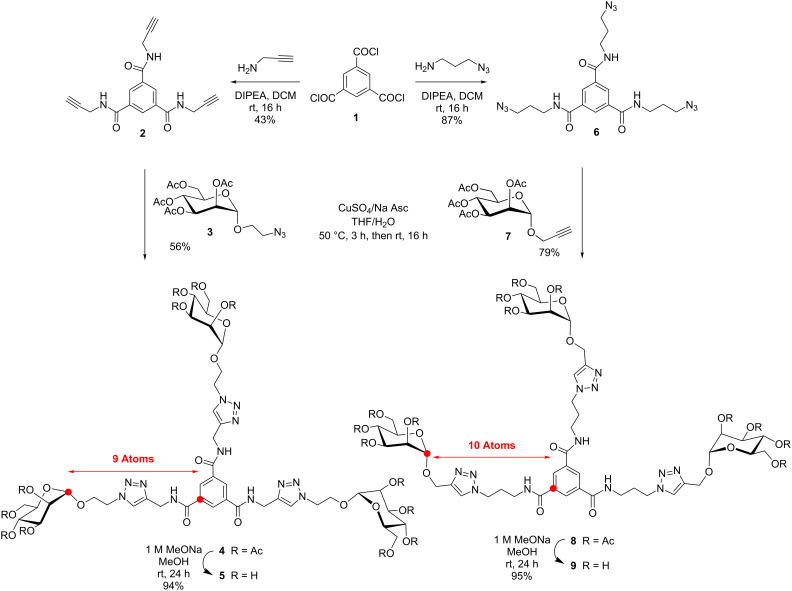
Scheme 1.
Synthesis of mannosylated trimers 5 and 9 using trimesic acid core transformed into propargylated (2) and azidopropylated (6) scaffolds and then coupled by “click chemistry” with either 2-azidoethyl (3) or propargyl (7) mannopyranosides.
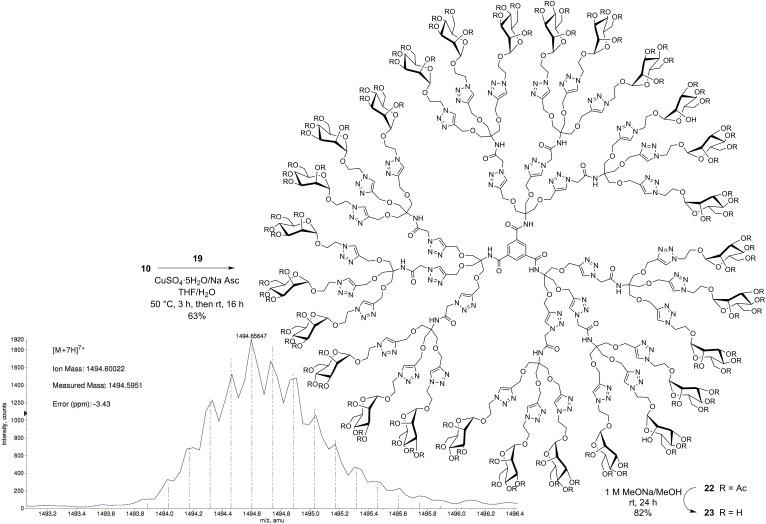
Scheme 5.
Convergent assembly of 27-mer 23 using key propargylated scaffold precursors 10 and mannosylated azidodendron 19. Insert: Zoom section of HRMS (+TOF) spectrum for deprotected G(1)-mannodendrimer 23 illustrating observed and theoretical isotopic distributions for [M + 7H]7+ adduct.
The synthesis of 5 was accomplished starting from commercial trimesic acid chloride 1 which was readily transformed into known tripropargyl amide derivative 2 [33] using propargylamine according to Scheme 1. Amide 2 was conjugated to peracetylated 2-azidoethyl α-D-mannopyranoside 3 [34] under classical copper-catalyzed dipolar cycloaddition (CuAAc) to afford 4 in 56% yield. Structure 4 was readily characterized by the absence of acetylenic protons at δ 3.16 ppm, the appearance of identical triazole protons (3H) at δ 7.74 ppm relative to the anomeric signal (3H) at δ 4.81 ppm and corresponding HRMS data. Zemplén deprotection (NaOMe, MeOH) afforded 5 in 94% yield. Synthesis of the related homolog 9, prepared in 74% overall yield from known 6 [17] by an analogous click chemistry, is also described in Scheme 1. To this end, trichloride 1 was treated as above with 3-azido-1-propanamine to provide 6 in 87% yield. Azide–alkyne cycloaddition of 6 with prop-2-ynyl α-D-mannopyranoside 7 [35] gave 8 (79%) which was de-O-acetylated under Zemplén conditions (NaOMe, MeOH, 95%) to give 9.
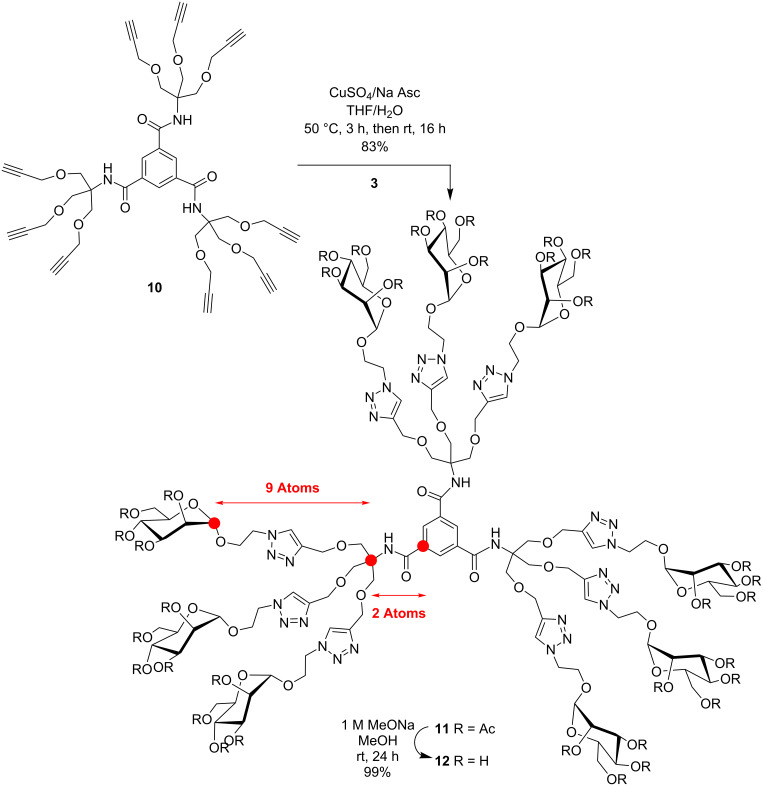
The syntheses of 9-mers 12, 17 and 21 are illustrated in Schemes 2–5 and follow a conceptually identical strategy to the one described above for trimers 5 and 9. Toward this goal, propargylated 9-mer scaffold 10 [17] was treated under the same CuAAc conditions with azide 3 to provide peracetylated 11 in 83% yield which upon Zemplén de-O-acetylation gave 12 in essentially quantitative yield (Scheme 2). Complete spectral characterization (1H, 13C NMR and HRMS) concluded for the aforementioned structure having twelve atoms in the linking arm (see Supporting Information File 1).
Scheme 2.
Divergent CuAAc “click reaction” between propargylated core 10 and azide 3 to afford 9-mer 12.
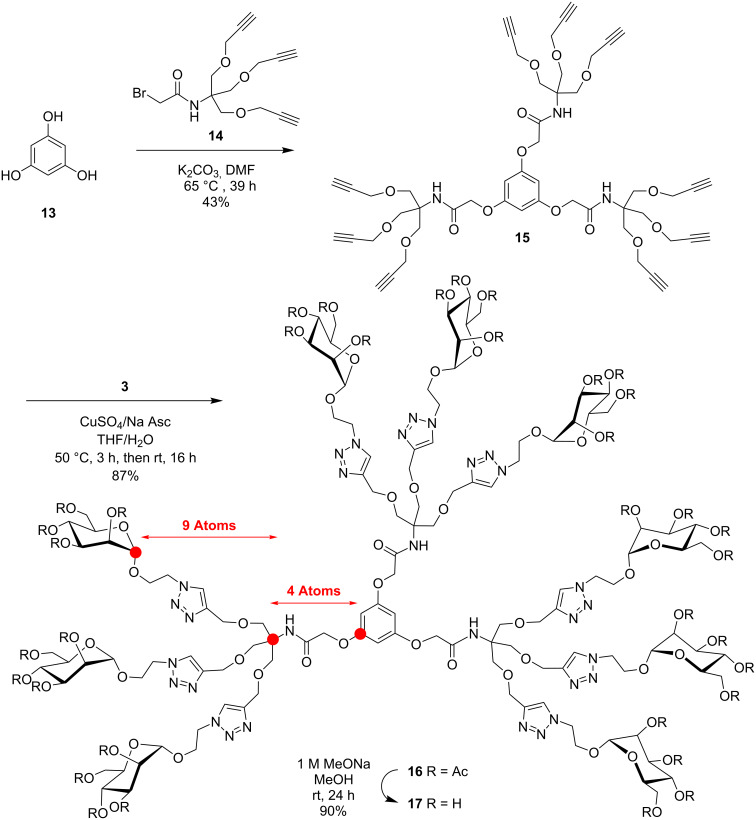
Analogously, the extended 9-mer glycodendrimer 17, possessing fourteen atoms between the anomeric carbon and the benzene carbon, was prepared according to Scheme 3. Thus, phloroglucinol (13) was carefully O-alkylated with the previously synthesized bromoacetylated TRIS derivative 14 [36] using K2CO3 in DMF to provide 15 in 43% yield. Again, the structural integrity of 15 was fully assessed by the simplicity of its 1H NMR symmetrical patterns that showed the characteristic singlets for the three amide protons at δ 6.85 ppm, relative to the three benzene protons (δ 6.17 ppm) and the six O-acyl protons at δ 4.36 ppm (core) compared with the peripheral acetylenic methylenes (18H), inner methylene of TRIS (18H), and the terminal alkyne protons (9H) at δ 4.16, 3.87, and 2.48 ppm, respectively.
Scheme 3.
Divergent CuAAc synthesis of “extended” 9-mer 17 using phloroglucinol (13) as core, bromoacylated TRIS as linker and mannopyranosylazide 3.
Toward the last and further extended 9-mer 21, a convergent strategy was rather adopted (Scheme 4). This strategy has the clear advantages of providing an easier purification process from partially substituted end-products together with a better assessment of complete surface group modifications. Hence, known 14 [36] was first cycloadded to mannosylazide 3 under the above CuAAc conditions. The “click reaction” proceeded exceptionally efficiently and provided bromoacylated dendron precursor 18 in 94% yield. Substitution of the bromide by azide also proceeded uneventfully (NaN3, DMF, rt, 16 h) to afford intermediate glycodendron 19 in 93% yield. Finally, coupling of the propargylated core 2 with azidodendron 19 under the typical CuAAc conditions gave peracetylated intermediate 20 which was readily deprotected to give 9-mer 21 in 84% overall yield. All spectral characteristics concurred to the expected structural integrity of 21 (see Supporting Information File 1).
Scheme 4.
Convergent synthesis of further “extended” 9-mer 21 using mannosylated bromoacyl dendron 18 transformed into azide 19 followed by CuAAc coupling to tripropargylated core 2.
Finally, a 27-mer mannosylated G(1)-dendrimer 23 was similarly prepared using an accelerated convergent strategy (Scheme 5). This time, the nonapropargylated scaffold 10 was “clicked” under CuAAc with trimeric azidodendron 19 to give 22 in an acceptable yield of 63% after silica gel column chromatography, corresponding to an excellent 95% yield per individual dendron’s incorporation. The complete disappearance of propargylic signals in the 1H NMR spectrum supported complete conversion. Note that working with peracetylated sugar precursors allows less tedious purification practices as opposed to working with unprotected sugars which often necessitate purification by cumbersome dialysis followed by HPLC treatment. Here again, the complete structural integrity of the final product can be readily confirmed from its characteristic spectral identification. Ultimately, dendrimer 23 was deprotected under the usual Zemplén conditions in 82% yield. Once again, all the relative integrations for each proton presented on the surface were in perfect agreement with those of the middle and internal regions. Interestingly, high resolution mass spectrometry (+TOF technique) resulted in the formation of multicharged adducts that matched the expected theoretical patterns, especially the one corresponding to [M + 7H]7+, as illustrated in Scheme 5 (insert).
NMR diffusion studies
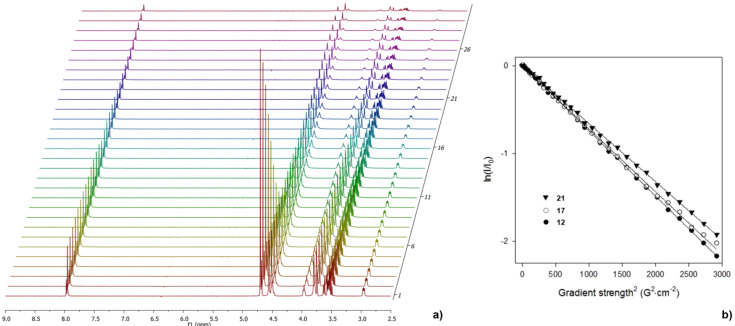
To accurately estimate the various structural factors involved in the intricate binding interactions between our synthetic multimeric mannosides and ConA, we determined their relative diffusivity measurements by NMR spectroscopy. In fact, diffusion NMR spectroscopy has recently become a method of choice to access information about sizes and shapes of macromolecular species by measuring their diffusion coefficients in a given solvent [17,37]. The size of nonavalent compounds 12, 17, and 21, and more particularly their solvodynamic radii, was thus estimated with the help of pulsed-field-gradient stimulated echo (PFG-STE) NMR experiments using bipolar pulse pairs-longitudinal-eddy-current delay (BPP-LED) in D2O at 25 °C. Stimulated echoes were used since they avoid signal attenuation due to transverse relaxation while bipolar gradient pulses reduce gradient artefacts [38]. The diffusion rates (D) were calculated from the decay of the signal intensity of the common H-5 proton (δ = 2.98 ppm) located on each epitope with increasing field gradient strength (Figure 1a). In all cases, monoexponential behavior was observed (Figure 1b), which was manifested as a linear decay of the logarithm of the signal intensity as a function of the gradient strength. This behavior is consistent with a spherical and unimolecular character of the glycodendrimers, confirming the absence of aggregation phenomena in aqueous solution under the working concentrations. The corresponding solvodynamic diameters (ds = 2 × rs) can be calculated using the Stokes–Einstein equation and the viscosity of pure D2O (Table 1).
Figure 1.
a) Decay of 1H signal for the nonavalent mannosylated compound 12 in D2O during the PFGSTE experiment. The gradient strength is increased linearly between 1.8 and 54.2 G·cm−1; b) characteristic echo decays of the H-5 resonances (δ = 2.98 ppm) as a function of squared gradient strength located in 12 (full circles) and 21 (full triangles) with δ = 4 ms and Δ = 50 ms (Δ = 40 ms for 17 (circles)). Notably, such linear behavior was also obtained for the decay of the signal intensities of other protons located either in internal regions of the conjugates on aromatic or branching sections, or in the peripheral saccharidic belt (results not shown).
Table 1.
Determination of diffusion data and solvodynamic diameters of nonavalent conjugates 12, 17, and 21 by diffusion NMR experiments.
| Entry | Compound | D [× 10−10 m2s−1]a,b,c | Solvodynamic diameter [ds, nm]d |
| 1 | 12 | 1.33 | 3.0 (2.9) |
| 2 | 17 | 1.62 | 2.5 (2.3) |
| 3 | 21 | 1.17 | 3.4 (2.9) |
aSee general procedures and Supporting Information File 1 for extraction of the diffusion rate and calibration of the gradient strength. D was determined from the decay of the H-5 resonance (δ = 2.98 ppm). bViscosity of D2O at 25 °C: ηD2O = 1.097 × 10−3 Pa s. cThe error associated with the measurement was estimated from repeated calculations of the diffusion coefficients to be below 10%. dResults in parentheses correspond to the average value calculated from the decays of 4 or 5 different proton signals.
As expected, nonavalent conjugates 12, 17, and 21 presented solvodynamic diameters in the range of roughly 3 nm when considering the decay of distinctive and common H-5 signals. These values remained consistent with similar congeners described earlier and harboring different epitopes [17]. The variation of the complexity of anchoring functionalities in the middle region with the incorporation of amide functions and triazole groups is responsible for a diameter enhancement for 21 when compared with 12, as expected. On the other hand, rather counter-intuitive tendencies were observed since the apparently slightly extended structure 17 was measured as the smallest molecule of the family in water. A specific spatial arrangement of the dendrons that emanate from 1,3,5-O-alkylations on the aromatic core in 17, compared to the one generated in BTAs-centered structures 12 and 21, could explain this observation. Also, these discrepancies might result from the general amphiphilic behavior of this kind of macromolecules [39]. In fact, these glycoclusters shared common structural factors with hydrophilic peripheral moieties and an aromatic central core but the introduction of distinct functionalized linkers may change the overall hydrophobic/hydrophilic balances of the structures. As such, they could engage supplementary intramolecular hydrogen bonding or hydrophobic interactions that could mediate their three-dimensional arrangement in aqueous media. Moreover, it is also reported that the relative spatial distribution of the branches around the C=O-centered BTAs strongly depends on the nature of the substituents [40]. This hypothesis can partly explain the discrepancy observed for the calculated diameter of 21 (Table 1, entry 3). In fact, diffusion data for 21 ranged from 1.61 × 10−10 m2s−1 for central CHar to 1.17 × 10−10 m2s−1 for H-5, indicating a heterogeneity in diffusivity depending on the proton location within the same molecule. As a consequence, the calculated ds value based on the utilization of an average value of diffusion data (D) extracted from signal decays of distinct protons located at different levels in the molecule differ from that obtained with the decay of peripheral H-5 signal only. This heterogeneity was less pronounced for 17 and absent for 12 that presented consistent values of D ranging from 1.51 to 1.33 × 10−10 m2s−1 for protons in the core or the periphery. Interestingly, calculation of the extended conformation (MM2, Chem3D) of the linkers in 12, 17, and 21 showed lengths of 14.8, 17.1, and 21.8 Å, respectively.
Conclusion
The syntheses of three related families of mannosylated glycoclusters and glycodendrimers were efficiently accomplished around a benzene core and using the CuAAc methods now routinely used in this field [9,41–42]. The targeted compounds were based on trimesic acid scaffold which is known to properly expose the surface sugar groups to tetrameric lectins such as ConA [43] and the LecA lectin from Pseudomonas aeruginosa [17]. With these closely related families of mannosylated dendrimers in hand, together with their known relative size in solution, we are now well positioned to evaluate their binding behavior against their cognate proteins and this work will be published in due course [44].
The study of subtle structural variations and the nature of anchoring functions, as observed in diffusivity experiments, could represent a first step towards rational interpretation to explain the differential kinetic behavior within a closely related family of glycoclusters.
Experimental
General remarks
All reactions in organic medium were performed in standard oven-dried glassware under an inert atmosphere of nitrogen using freshly distilled solvents. CH2Cl2 was distilled from CaH2 and DMF from ninhydrin, and kept over molecular sieves. Solvents and reagents were deoxygenated when necessary by purging with nitrogen. Water used for lyophilization of final dendrimers was nanopure grade, purified through Barnstead NANOPure II Filter with Barnstead MegOhm-CM Sybron meter. All reagents were used as supplied without prior purification unless otherwise stated, and obtained from Sigma-Aldrich Chemical Co. Ltd. Reactions were monitored by analytical thin-layer chromatography using silica gel 60 F254 precoated plates (E. Merck) and compounds were visualized by 254 nm light, a mixture of iodine/silica gel and/or mixture of ceric ammonium molybdate solution (100 mL H2SO4, 900 mL H2O, 25 g (NH4)6Mo7O24H2O, 10 g Ce(SO4)2) and subsequent development by gentle warming with a heat-gun. Purifications were performed by flash column chromatography using silica gel from Silicycle (60 Å, 40–63 µm) with the indicated eluent.
NMR, IR, and MS spectroscopy
1H NMR and 13C NMR spectra were recorded at 300 or 600 MHz and 75 or 150 MHz, respectively, on a Bruker spectrometer (300 MHz) and Varian spectrometer (600 MHz). All NMR spectra were measured at 25 °C in indicated deuterated solvents. Proton and carbon chemical shifts (δ) are reported in ppm and coupling constants (J) are reported in Hertz (Hz). The resonance multiplicity in the 1H NMR spectra are described as “s” (singlet), “d” (doublet), “t” (triplet), and “m” (multiplet) and broad resonances are indicated by “br”. Residual protic solvent of CDCl3 (1H, δ 7.27 ppm; 13C, δ 77.0 ppm (central resonance of the triplet)), D2O (1H, δ 4.79 ppm and 30.89 ppm for CH3 of acetone for 13C spectra of de-O-acetylated compounds), MeOD (1H, δ 3.31 ppm and 13C, δ 49.0 ppm). 2D Homonuclear correlation 1H-1H COSY together with 2D heteronuclear correlation 1H-13C HSQC experiments were used to confirm NMR peak assignments.
Fourier transform infrared (FTIR) spectra were obtained with Thermo-scientific, Nicolet model 6700 equipped with ATR. The absorptions are given in wavenumbers (cm−1). The intensity of the bands is described as s (strong), m (medium) or w (weak). Melting points were measured on a Electrothermal MEL-TEMP apparatus and are uncorrected.
Accurate mass measurements (HRMS) were performed on a LC–MSD–ToF instrument from Agilent Technologies in positive electrospray mode. Low-resolution mass spectra were performed on the same apparatus or on a LCQ Advantage ion trap instrument from Thermo Fisher Scientific in positive electrospray mode (Mass Spectrometry Laboratory (Université de Montréal), or Plateforme analytique pour molécules organiques (Université du Québec à Montréal), Québec, Canada). Either protonated molecular ions [M + nH]n+ or adducts [M + nX]n+ (X = Na, K, NH4) were used for empirical formula confirmation.
NMR diffusion measurements were performed at 25 °C on a Varian Inova Unity 600 spectrometer (Varian, Walnut Creek, CA, USA) operating at a frequency of 599.95 MHz for 1H using a 5 mm broadband z-gradient temperature-regulated probe. The temperature was calibrated with 1,2-ethanediol according to a standard procedure [38]. The diffusion experiment employed a bipolar pulse-field gradients stimulated echo sequence as proposed by Wu et al [45]. The gradient pulse duration δ was 4 ms and the diffusion times (Δ) were 40 to 50 ms to ensure that the echo intensities were attenuated by at least 80%. A complete attenuation curve was obtained by measuring 30 gradient strengths, which were linearly incremented between 1.8 and 54.2 Gcm−1. Hard 90° 1H pulses of 15 μs were used and 36 k data points were recorded with 16 scans acquired for each gradient’s strength. A recycle delay of 3.0 s was used. The gradient strength was calibrated by back calculation of the coil constant from diffusion experiments on H2O traces in D2O (D = 1.90 × 10−9 m2 s−1) [46].
Diffusion rates were extracted from the slope of the straight lines obtained by plotting ln(I) against the gradient-pulse power squared according to the following equation: ln(I) = −Dγ2G2δ2(Δ − δ/3 − τ/2) + ln(I0) where I is the relative intensity of a chosen resonance (I = I0exp−[Dγ2G2δ2(Δ − δ/3 − τ/2)]), G = gradient strength (T/m), γ = proton gyromagnetic ratio, D = diffusion rate (m2 s−1), δ = gradient duration, Δ = diffusion delay, and τ = pulse length for bipolar pulses. All diffusion spectra were processed in Mat NMR [47].
Glycodendrimer synthesis
Procedure A: multiple CuAAc couplings on polypropargylated cores
To a solution of polypropargylated core (1.00 equiv) and complementary azido synthon (1.25 equiv/propargyl) in a THF/H2O mixture (1:1) were added sodium ascorbate (0.30 equiv/propargyl) and CuSO4·5H2O (0.30 equiv/propargyl). The reaction mixture was stirred at 50 °C for 3 h then at room temperature for an additional 16 h period. Ethyl acetate (10 mL) was added and the resulting solution was poured in a separatory funnel containing 25 mL of EtOAc and 30 mL of a saturated aqueous solution of NH4Cl. Organics were washed with (2 × 25 mL) of saturated NH4Claq, water (2 × 20 mL) and brine (1 × 10 mL). The organic phase was then dried over MgSO4 and concentrated under reduced pressure. Column chromatography on silica (DCM/MeOH 100:0 to 90:10) afforded the desired glycocluster.
Procedure B: Zemplén de-O-acetylation procedure for insoluble hydroxylated derivatives
The acetylated compound was dissolved in anhydrous MeOH and a solution of sodium methoxide (1 M in MeOH, 5 µL every 20 min until precipitation) was added. An additional 100 µL was then injected and the heterogeneous reaction mixture was stirred at room temperature for 24 h. The solvent was then removed with a Pasteur pipette and a mixture of anhydrous MeOH/DCM (4:1, 5 mL) was added to the residual white foam. A vigorous agitation is maintained for an additional 15 min period. After removal of the solvents with a Pasteur pipette, the residue was dissolved in H2O (3 mL), and the pH was adjusted to 7 by the addition of ion-exchange resin (Amberlite IR 120 H+). After filtration, the solvent was removed under vacuum with a rotary evaporator and lyophilized to yield the fully deprotected glycocluster.
Synthesis of peracetylated trivalent derivative 8: To a solution of triazido core 6 (50.0 mg, 109 μmol, 1.00 equiv) and mannoside 7 (158 mg, 409 μmol, 3.75 equiv) in a THF/H2O mixture (1:1, 6 mL) were added sodium ascorbate (19.4 mg, 98.1 μmol, 0.90 equiv) and CuSO4·5H2O (24.5 mg, 98.1 μmol, 0.90 equiv). The reaction mixture was stirred at 50 °C for 3 h then at room temperature for an additional 16 h period. Ethyl acetate (10 mL) was added and the resulting solution was poured in a separatory funnel containing 35 mL of EtOAc and 30 mL of a saturated aqueous solution of NH4Cl. Organics were washed with (2 × 25 mL) of saturated NH4Claq, water (2 × 20 mL) and brine (1 × 10 mL). The organic phase was then dried over MgSO4 and concentrated under reduced pressure. Column chromatography on silica (DCM/MeOH 98:2 to 94:6) afforded the desired compound 8 (138 mg, 86.0 μmol, 79%) as a viscous oil. Rf 0.34 (95:5 DCM/MeOH); 1H NMR (600 MHz, CDCl3) δ (ppm) 8.27 (s, 3H, CHar), 7.79 (s, 3H, CHtriazole), 7.72 (t, J = 5.3 Hz, 3H, NH), 5.29–5.19 (m, 9H, H2, H3, H4), 4.92 (sapp, 3H, H1), 4.77–4.62 (2 × d, J = 12.4 Hz, 6H, OCH2), 4.54 (t, J = 6.4 Hz, 6H, NtriazoleCH2), 4.28 (dd, J = 12.4 Hz, J = 5.4 Hz, 3H, H6b), 4.11–4.03 (m, 6H, H5 + H6a), 3.55 (m, 6H, NHCH2), 2.28 (m, 6H, CH2CH2CH2), 2.12, 2.10, 2.02, 1.96 (4s, 36H, COCH3); 13C{1H} NMR (150 MHz, CDCl3) δ (ppm) 170.8, 170.1, 170.0, 169.7 (COCH3), 166.1 (CONH), 143.5 (Ctriazole), 134.9 (Carom), 128.5 (CHarom), 123.9 (CHtriazole), 96.7 (C1), 69.3 (C2), 69.0 (C3), 68.7 (C5), 65.9 (C6), 62.3 (C4), 60.7 (OCH2), 48.3 (CH2Ntriazole), 37.5 (NHCH2), 29.9 (CH2CH2CH2), 20.9, 20.8, 20.7, 20.7 (COCH3); MS (+TOF-MS, m/z): [M + H]+ calculated for C69H90N12O33, 1615.6; found, 1615.6.
Synthesis of nonapropargylated core 15: To a solution of phloroglucinol (13, 10.0 mg, 79.3 μmol, 1.00 equiv) in anhydrous DMF (3 mL) was added under nitrogen anhydrous K2CO3 (previously heated at 250 °C under vaccum, 39.5 mg, 285 μmol, 3.60 equiv). After 10 min of vigorous stirring, tripropargylated synthon 14 (93.0 mg, 285 μmol, 3.60 equiv) was added into the solution under inert atmosphere and the reaction mixture was allowed to stir at 65 °C for 39 h. In the end, the dark-brown heterogeneous reaction was poured in 30 mL of EtOAc and organics were washed with a saturated aqueous solution of NH4Cl (2 × 30 mL) then water (2 × 20 mL) and brine (10 mL). The organic phase was then dried over MgSO4 and concentrated under reduced pressure. Column chromatography on silica (EtOAc/hexane 40:60 to 50:50) afforded the desired compound 15 (32.0 mg, 33.8 μmol, 43%) as a colorless oil. Rf 0.27 (1:1 EtOAc/hexane); 1H NMR (300 MHz, CDCl3) δ (ppm) 6.85 (s, 3H, NH), 6.17 (s, 3H, CHar), 4.36 (s, 6H, OCH2CONH), 4.16 (m, 18H, OCH2C≡CH), 3.87 (br s, 18H, HNCqCH2O), 2.48 (m, 9H, OCH2C≡CH); 13C{1H} NMR (75 MHz, CDCl3) δ (ppm) 167.3 (CONH), 159.0 (CarOCH2), 95.8 (CHar), 79.4 (OCH2C≡CH), 74.9 (OCH2C≡CH), 68.3 (HNCqCH2O), 67.5 (OCH2CONH), 59.2 (Cq), 58.6 (OCH2C≡CH); HRMS (+TOF-HRMS, m/z): [M + H]+ calculated for C51H57N3O15, 952.3862; found, 952.3843 (Δ = −2.10 ppm); [M + Na]+: calculated for 974.3682; found, 974.3662 (Δ = −2.05 ppm).
Synthesis of bromoacylated dendron 18: To a solution of tripropargylated synthon 14 (140.0 mg, 393.0 μmol, 1.00 equiv) and mannoside 3 (616 mg, 1.48 mmol, 3.75 equiv) in a THF/H2O mixture (1:1, 6 mL) were added sodium ascorbate (70.0 mg, 354 μmol, 0.90 equiv) and CuSO4·5H2O (88.4 mg, 354 μmol, 0.90 equiv). The reaction mixture was stirred at 50 °C for 3 h then at room temperature for an additional 16 h period. Ethyl acetate (20 mL) was added and the resulting solution was poured in a separatory funnel containing 40 mL of EtOAc and 30 mL of a saturated aqueous solution of NH4Cl. Organics were washed with 2 × 35 mL of saturated NH4Claq, water (2 × 30 mL) and brine (20 mL). The organic phase was then dried over MgSO4 and concentrated under reduced pressure. Column chromatography on silica (DCM/MeOH 99:1 to 96:4) afforded the desired compound 18 (594 mg, 369.4 μmol, 94%) as a white solid. Rf 0.47 (94:6 DCM/MeOH); mp 68–72 °C (not corrected); 1H NMR (300 MHz, CDCl3) δ (ppm) 7.68 (br s, 3H, CHtriazole), 6.89 (br s, 1H, NH), 5.24–5.18 (m, 9H, H2, H3, H4), 4.80 (d, J = 1.3 Hz, 1H, H1), 4.61–4.58 (br s, 12H, OCH2Ctriazole + NtriazoleCH2), 4.17–4.00 (m, 11H, OCH2CH2 + H6a + BrCH2CONH), 3.94–3.78 (m, 9H, H6b + NHCqCH2O), 3.60 (m, 3H, H5), 2.12, 2.08, 2.03, 1.98 (4s, 36H, COCH3); 13C{1H} NMR (75 MHz, CDCl3) δ (ppm) 170.5, 169.9, 169.9, 169.5, (COCH3), 165.6 (CONH), 145.0 (Ctriazole), 123.7 (CHtriazole), 97.4 (C1), 69.1 (C2), 68.9 (C3), 68.8 (C5), 68.4 (NHCqCH2O), 66.2 (C6), 65.6 (C4), 64.6 (OCH2Ctriazole), 62.1 (OCH2CH2), 60.2 (Cq), 49.6 (CH2Ntriazole), 29.7 (CH2Br), 20.8, 20.7, 20.6, 20.6 (COCH3); IR (cm−1): 2956, 2937, 2361, 2337, 1751, 1734, 1540, 1370, 1218, 1045, 759; HRMS (+TOF-HRMS, m/z): [M + 2H]2+ calculated for C63H87BrN10O34, 804.2358; found, 804.2356 (Δ = −0.18 ppm); [M + H] + calculated for 1607.4642, found: 1607.4620 (Δ = −1.36 ppm); [M + Na]+ calculated for 1629.4462; found, 1629.4448 (Δ = −0.84 ppm).
Synthesis of azidoacylated dendron 19: To a stirring solution of brominated trivalent dendron 18 (121.0 mg, 75.2 μmol, 1.00 equiv) in dry DMF (1.5 mL) was added under a nitrogen atmosphere sodium azide (7.3 mg, 112 μmol, 1.50 equiv). After stirring overnight at room temperature, the solvent was removed under vaccum. Ethyl acetate (20 mL) was added and the resulting solution was poured in a separatory funnel containing 20 mL of EtOAc and 30 mL of a saturated aqueous solution of NH4Cl. Organics were washed with 2 × 30 mL of saturated NH4Claq, water (2 × 30 mL) and brine (20 mL). The organic phase was then dried over MgSO4 and concentrated under reduced pressure to furnish the desired compound 19 (110 mg, 69.9 μmol, 93%) as a white solid. Rf 0.47 (94:6 DCM/MeOH); mp 62–65 °C (not corrected); 1H NMR (300 MHz, CDCl3) δ (ppm) 7.68 (br s, 3H, CHtriazole), 6.69 (br s, 1H, NH), 5.27–5.18 (m, 9H, H2, H3, H4), 4.80 (d, J = 1.3 Hz, 1H, H1), 4.61–4.58 (br s, 12H, OCH2Ctriazole + NtriazoleCH2), 4.23–4.00 (m, 11H, OCH2CH2 + H6a + N3CH2CONH), 3.90–3.81 (m, 9H, H6b + NHCqCH2O), 3.60 (m, 3H, H5), 2.12, 2.08, 2.03, 1.98 (4s, 36H, COCH3); 13C{1H} NMR (75 MHz, CDCl3) δ (ppm) 170.4, 169.9, 169.8, 169.5, (COCH3), 166.7 (CONH), 144.9 (Ctriazole), 123.7 (CHtriazole), 97.4 (C1), 69.0 (C2), 68.8 (C3), 68.8 (C5), 68.4 (NHCqCH2O), 66.1 (C6), 65.6 (C4), 64.5 (OCH2Ctriazole), 62.1 (OCH2CH2), 59.9 (Cq), 52.5 (CH2N3), 49.5 (CH2Ntriazole), 20.7, 20.7, 20.6, 20.6 (COCH3); IR (cm−1): 2934, 2361, 2338, 2107 (N3), 1751, 1734, 1540, 1373, 1218, 1045, 761; HRMS (+TOF-HRMS, m/z): [M + H]+ calculated for C63H87N13O34, 1570.5551; found, 1570.5543 (Δ = −0.51 ppm); [M + Na]+ calculated for 1592.5371; found, 1592.5366 (Δ = −0.31 ppm).
Synthesis of peracetylated 27-mer derivative 22: To a solution of nonapropargylated core 10 (4.6 mg, 5.38 μmol, 1.00 equiv) and trimannosylated dendron 19 (95.0 mg, 60.5 μmol, 11.25 equiv) in a THF/H2O mixture (1:1, 3 mL) were added sodium ascorbate (2.9 mg, 15 μmol, 2.70 equiv) and CuSO4·5H2O (3.6 mg, 15 μmol, 0.90 equiv). The reaction mixture was stirred at 50 °C for 3 h then at room temperature for an additional 16 h period. Ethyl acetate (10 mL) was added and the resulting solution was poured in a separatory funnel containing 25 mL of EtOAc and 30 mL of a saturated aqueous solution of NH4Cl. Organics were washed with 2 × 25 mL of saturated NH4Claq, water (2 × 20 mL) and brine (10 mL). The organic phase was then dried over MgSO4 and concentrated under reduced pressure. Column chromatography on silica (DCM/MeOH 98:2 to 90:10) afforded the desired compound 22 (50.0 mg, 3.33 μmol, 63%) as a yellowish oil. Rf 0.72 (90:10 DCM/MeOH); 1H NMR (600 MHz, CDCl3) δ (ppm) 8.27 (m, 3H, CHar), 7.79 (s, 9H, CHint-triazole), 7.75 (s, 27H, CHext-triazole), 7.34–7.31 (m, 12H, NH), 5.23–5.18 (m, 81H, H2, H3, H4), 5.05 (br s, 18H, NtriazoleCH2CONH), 4.81 (sapp, 27H, H1), 4.62–4.53 (m, 126H, OCH2Ctriazole + NtriazoleCH2), 4.20–3.64 (m, 207H, OCH2 + H6 + NHCqCH2O + H5), 2.11, 2.08, 2.01, 1.96 (4s, 324H, COCH3); 13C{1H} NMR (150 MHz, CDCl3) δ (ppm) 170.6, 170.5, 170.0, 169.9, 169.9, 169.7, 169.6 (COCH3), 168.4 (CONH), 165.4 (CONH), 144.9 + 144.8 (Cext-triazole), 144.5 (Cint-triazole), 135.6 (Carom), 128.6 (CHarom), 124.9 (CHint-triazole), 124.0 (CHext-triazole), 97.5 (C1), 69.1 (C2), 69.0 (C3), 68.7 (C5), 68.4 (NHCqCH2O), 66.2 (C6), 65.6 (C4), 64.5 (OCH2Ctriazole), 62.1 (OCH2), 60.4 (Cq), 52.4 (NtriazoleCH2CONH), 49.5 (CH2Ntriazole), 20.8, 20.8, 20.7, 20.7 (COCH3); MS (+TOF-MS, m/z): [M + H]+ calculated for C615H834N120O318, 14995.8; found, 14995.9.
Synthesis of de-O-acetylated 27-mer derivative 23: Acetylated compound 22 (30.0 mg, 2.00 μmol) was dissolved in anhydrous MeOH (3 mL) and a solution of sodium methoxide (1 M in MeOH, 5 µL every 20 min until precipitation) was added. An additional 100 µL was then injected and the heterogeneous reaction mixture was stirred at room temperature for 24 h. The solvent was then removed with a Pasteur pipette and a mixture of anhydrous MeOH/DCM (4:1, 5 mL) was added to the residual white foam. A vigorous agitation is maintained for an additional 15 min period. After removal of the solvent with a Pasteur pipette, the residue was dissolved in 3 mL of H2O, and the pH was adjusted to 7 with addition of ion-exchange resin (Amberlite IR 120 H+). After filtration, the solvent was removed under vacuum with a rotary evaporator and lyophilized to yield the fully deprotected 27-mer 23 as a white solid (17.0 mg, 1.63 μmol) in 82% yield. 1H NMR (600 MHz, D2O) δ (ppm) 8.06 (m, 3H, CHar), 7.97 (s, 27H, CHext-triazole), 7.96 (s, 9H, CHint-triazole), 5.14 (br s, 18H, NtriazoleCH2CONH), 4.75 (s, 27H, H1), 4.59−4.51 (m, 126H, OCH2Ctriazole + NtriazoleCH2), 4.05–4.03 (m, 27H, OCHHCH2N), 3.83–3.80 (m, 72H, OCHHCH2N + H2 + NHCqCH2Oint), 3.71–3.57 (m, 162H, NHCqCH2Oext + H6 + H4 + H3), 3.01 (m, 27H, H5); 13C{1H} NMR (150 MHz, D2O) δ (ppm) 168.8 (CONHint), 167.5 (CONHext), 144.7 (Cext-triazole), 144.6 (Cint-triazole), 135.7 (Carom), 129.7 (CHarom), 127.0 (CHint-triazole), 126.1 (CHext-triazole), 100.2 (C1), 73.5 (C5), 71.1 (C3), 70.6 (C2), 68.2 (NHCqCH2O), 68.0 (NHCqCH2O), 67.0 (OCH2CH2Ntriazole), 66.1 (C4), 64.2 (OCH2Ctriazole), 61.3 (C6), 60.9 (Cq), 52.9 (NtriazoleCH2CONH), 50.7 (CH2Ntriazole), 35.7 (OCHNCH2Ctriazole); HRMS (+TOF-HRMS, m/z): [M + 7H]7+ calculated for C399H204N120O210, 1494.6002; found, 1494.5951 (Δ = −3.43 ppm).
Supporting Information
Experimental procedures, characterization data, NMR, IR and mass spectra and NMR diffusion experiments.
Acknowledgments
This work was supported by a discovery grant from the National Science and Engineering Research Council of Canada (NSERC) and by a Canadian Research Chair in Therapeutic Chemistry. YMC is grateful to the FQRNT (Québec) for a scholarship. We are thankful to Dr. A. Furtos (Université de Montréal) for HRMS determination.
This article is part of the Thematic Series "Multivalent glycosystems for nanoscience".
References
- 1.Roy R. Curr Opin Struct Biol. 1996;6:692–702. doi: 10.1016/S0959-440X(96)80037-6. [DOI] [PubMed] [Google Scholar]
- 2.Kiessling L L, Gestwicki J E, Strong L E. Angew Chem, Int Ed. 2006;45:2348–2368. doi: 10.1002/anie.200502794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Imberty A, Chabre Y M, Roy R. Chem–Eur J. 2008;14:7490–7499. doi: 10.1002/chem.200800700. [DOI] [PubMed] [Google Scholar]
- 4.Renaudet O, Roy R. Chem Soc Rev. 2013;42:4515–4517. doi: 10.1039/c3cs90029k. [DOI] [PubMed] [Google Scholar]
- 5.Kiessling L L, Grim J C. Chem Soc Rev. 2013;42:4476–4491. doi: 10.1039/c3cs60097a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bovin N V, Gabius H-J. Chem Soc Rev. 1995;24:413–421. doi: 10.1039/cs9952400413. [DOI] [Google Scholar]
- 7.Roy R. Trends Glycosci Glycotechnol. 2003;15:291–310. doi: 10.4052/tigg.15.291. [DOI] [Google Scholar]
- 8.Roy R, Zanini D, Meunier S J, Romanowska A. J Chem Soc, Chem Commun. 1993:1869–1872. doi: 10.1039/C39930001869. [DOI] [Google Scholar]
- 9.Chabre Y M, Roy R. Adv Carbohydr Chem Biochem. 2010;63:165–393. doi: 10.1016/S0065-2318(10)63006-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chabre Y M, Roy R. Curr Top Med Chem. 2008;8:1237–1285. doi: 10.2174/156802608785848987. [DOI] [PubMed] [Google Scholar]
- 11.Lindhorst T K. Top Curr Chem. 2002;218:201–235. doi: 10.1007/3-540-45010-6_7. [DOI] [Google Scholar]
- 12.Röckendorf N, Lindhorst T K. Top Curr Chem. 2001;217:201–238. doi: 10.1007/3-540-45003-3_6. [DOI] [Google Scholar]
- 13.Roy R. Polym News. 1996;21:226–232. [Google Scholar]
- 14.Pieters R J. Org Biomol Chem. 2009;7:2013–2025. doi: 10.1039/b901828j. [DOI] [PubMed] [Google Scholar]
- 15.Roy R, Das S K, Santoyo-González F, Hernández-Mateo F, Dam T K, Brewer C F. Chem–Eur J. 2000;6:1757–1762. doi: 10.1002/(SICI)1521-3765(20000515)6:10<1757::AID-CHEM1757>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 16.Roy R, Trono M C, Giguère D. ACS Symp Ser. 2005;896:137–150. doi: 10.1021/bk-2005-0896.ch008. [DOI] [Google Scholar]
- 17.Chabre Y M, Giguère D, Blanchard B, Rodrigue J, Rocheleau S, Neault M, Rauthu S, Papadopoulos A, Arnold A A, Imberty A, et al. Chem–Eur J. 2011;17:6545–6562. doi: 10.1002/chem.201003402. [DOI] [PubMed] [Google Scholar]
- 18.Sirois S, Giguère D, Roy R. Med Chem. 2006;2:481–489. doi: 10.2174/157340606778250252. [DOI] [PubMed] [Google Scholar]
- 19.Gerland B, Goudot A, Ligeour C, Pourceau G, Meyer A, Vidal S, Gehin T, Vidal O, Souteyrand E, Vasseur J-J, et al. Bioconjugate Chem. 2014;25:379–392. doi: 10.1021/bc4005365. [DOI] [PubMed] [Google Scholar]
- 20.van Hattum H, Branderhorst H M, Moret E E, Nilsson U J, Leffler H, Pieters R J. J Med Chem. 2013;56:1350–1354. doi: 10.1021/jm301677r. [DOI] [PubMed] [Google Scholar]
- 21.Öberg C T, Noresson A-L, Leffler H, Nilsson U J. Chem–Eur J. 2011;17:8139–8144. doi: 10.1002/chem.201003247. [DOI] [PubMed] [Google Scholar]
- 22.Giguère D, André S, Bonin M-A, Bellefleur M-A, Provencal A, Cloutier P, Pucci B, Roy R, Gabius H-J. Bioorg Med Chem. 2011;19:3280–3287. doi: 10.1016/j.bmc.2011.03.022. [DOI] [PubMed] [Google Scholar]
- 23.Sharon N. FEBS Lett. 1987;217:145–157. doi: 10.1016/0014-5793(87)80654-3. [DOI] [PubMed] [Google Scholar]
- 24.Arya P, Kutterer K M K, Qin H, Roby J, Barnes M L, Kim J M, Roy R. Bioorg Med Chem Lett. 1998;8:1127–1132. doi: 10.1016/S0960-894X(98)00182-6. [DOI] [PubMed] [Google Scholar]
- 25.Schierholt A, Hartmann M, Lindhorst T K. Carbohydr Res. 2011;346:1519–1526. doi: 10.1016/j.carres.2011.04.023. [DOI] [PubMed] [Google Scholar]
- 26.Asensio J L, Ardá A, Cañada F J, Jiménez-Barbero J. Acc Chem Res. 2013;46:946–954. doi: 10.1021/ar300024d. [DOI] [PubMed] [Google Scholar]
- 27.Chen W, Enck S, Price J L, Powers D L, Powers E T, Wong C-H, Dyson H J, Kelly J W. J Am Chem Soc. 2013;135:9877–9884. doi: 10.1021/ja4040472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lemieux R U. Acc Chem Res. 1996;29:373–380. doi: 10.1021/ar9600087. [DOI] [Google Scholar]
- 29.Reynolds M, Pérez S. C R Chim. 2011;14:74–95. doi: 10.1016/j.crci.2010.05.020. [DOI] [Google Scholar]
- 30.Dam T K, Roy R, Das S K, Oscarson S, Brewer C F. J Biol Chem. 2000;275:14223–14230. doi: 10.1074/jbc.275.19.14223. [DOI] [PubMed] [Google Scholar]
- 31.Dam T K, Roy R, Pagé D, Brewer C F. Biochemistry. 2002;41:1351–1358. doi: 10.1021/bi015830j. [DOI] [PubMed] [Google Scholar]
- 32.Munoz E M, Correa J, Fernandez-Megia E, Riguera R. J Am Chem Soc. 2009;131:17765–17767. doi: 10.1021/ja9074826. [DOI] [PubMed] [Google Scholar]
- 33.Giguère D, Patnam R, Bellefleur M-A, St-Pierre C, Sato S, Roy R. Chem Commun. 2006:2379–2381. doi: 10.1039/b517529a. [DOI] [PubMed] [Google Scholar]
- 34.Hayes W, Osborn H M I, Osborne S D, Rastall R A, Romagnoli B. Tetrahedron. 2003;59:7983–7996. doi: 10.1016/j.tet.2003.08.011. [DOI] [Google Scholar]
- 35.Touaibia M, Wellens A, Shiao T C, Wang Q, Sirois S, Bouckaert J, Roy R. ChemMedChem. 2007;2:1190–1201. doi: 10.1002/cmdc.200700063. [DOI] [PubMed] [Google Scholar]
- 36.Chabre Y M, Contino-Pépin C, Placide V, Shiao T C, Roy R. J Org Chem. 2008;73:5602–5605. doi: 10.1021/jo8008935. [DOI] [PubMed] [Google Scholar]
- 37.Cohen Y, Avram L, Frish L. Angew Chem, Int Ed. 2005;44:520–554. doi: 10.1002/anie.200300637. [DOI] [PubMed] [Google Scholar]
- 38.Berger S, Braun S. 200 and More NMR Experiments−A Practical Course. Weinheim: Wiley-VCH; 2004. pp. 145–148. [Google Scholar]
- 39.Chabre Y M, Roy R. Chem Soc Rev. 2013;42:4657–4708. doi: 10.1039/c3cs35483k. [DOI] [PubMed] [Google Scholar]
- 40.Cantekin S, de Greef T F A, Palmans A R A. Chem Soc Rev. 2012;41:6125–6137. doi: 10.1039/c2cs35156k. [DOI] [PubMed] [Google Scholar]
- 41.Aragão-Leoneti V, Campo V L, Gomes A S, Field R A, Carvalho I. Tetrahedron. 2010;66:9475–9492. doi: 10.1016/j.tet.2010.10.001. [DOI] [Google Scholar]
- 42.Finn M G, Fokin V V, editors. Click chemistry: function follows form. Chem Soc Rev. 2010;39:1221–1408. doi: 10.1039/C003740K. [DOI] [PubMed] [Google Scholar]
- 43.Corbell J B, Lundquist J J, Toone E J. Tetrahedron: Asymmetry. 2000;11:95–111. doi: 10.1016/S0957-4166(99)00589-3. [DOI] [Google Scholar]
- 44.Talga M L, Fan N, Fueri A L, Brown R K, Chabre Y M, Bandyopadhyay P, Roy R, Dam T K. Biochemistry. 2014 doi: 10.1021/6i5001307. [DOI] [PubMed] [Google Scholar]
- 45.Wu D H, Chen A D, Johnson C S., Jr J Magn Reson, Ser A. 1995;115:260–264. doi: 10.1006/jmra.1995.1176. [DOI] [Google Scholar]
- 46.Holz M, Weingärtner H. J Magn Reson. 1991;92:115–125. doi: 10.1016/0022-2364(91)90252-O. [DOI] [Google Scholar]
- 47.van Beek J D. J Magn Reson. 2007;187:19–26. doi: 10.1016/j.jmr.2007.03.017. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Experimental procedures, characterization data, NMR, IR and mass spectra and NMR diffusion experiments.