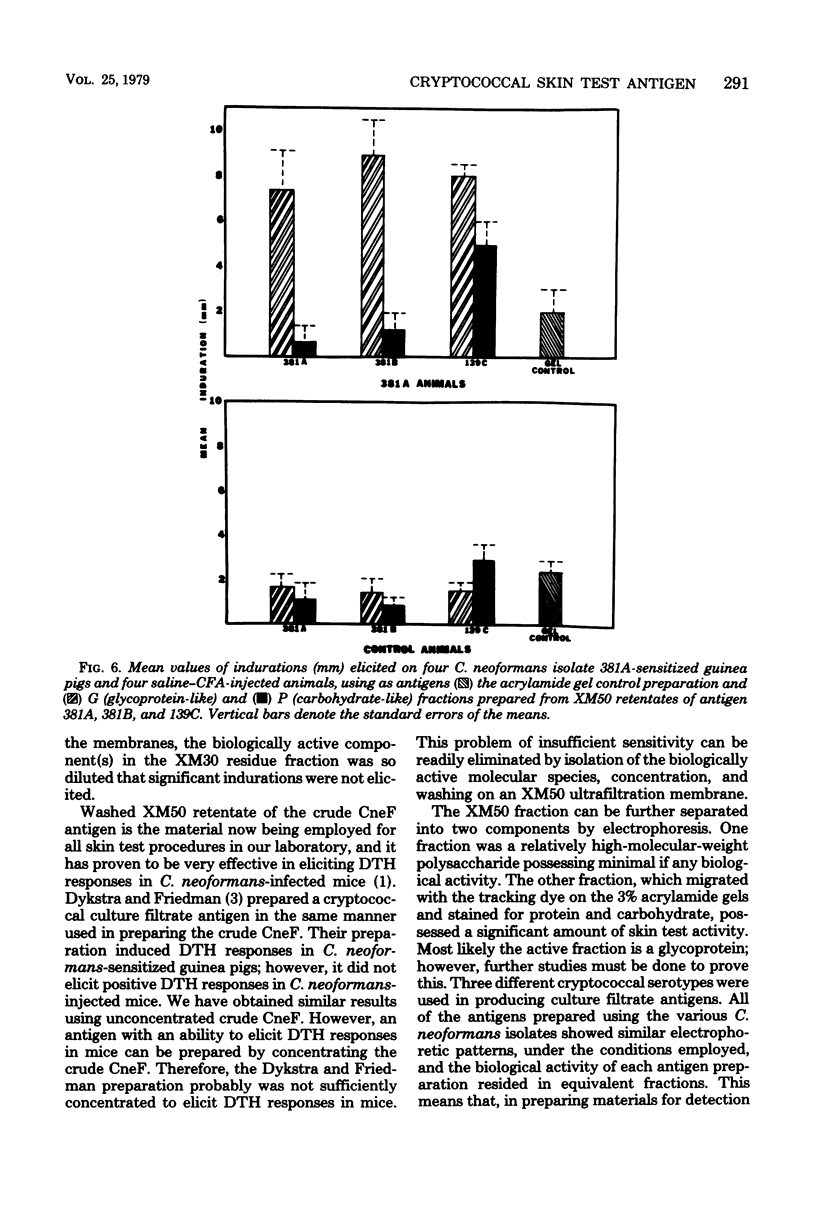
Abstract
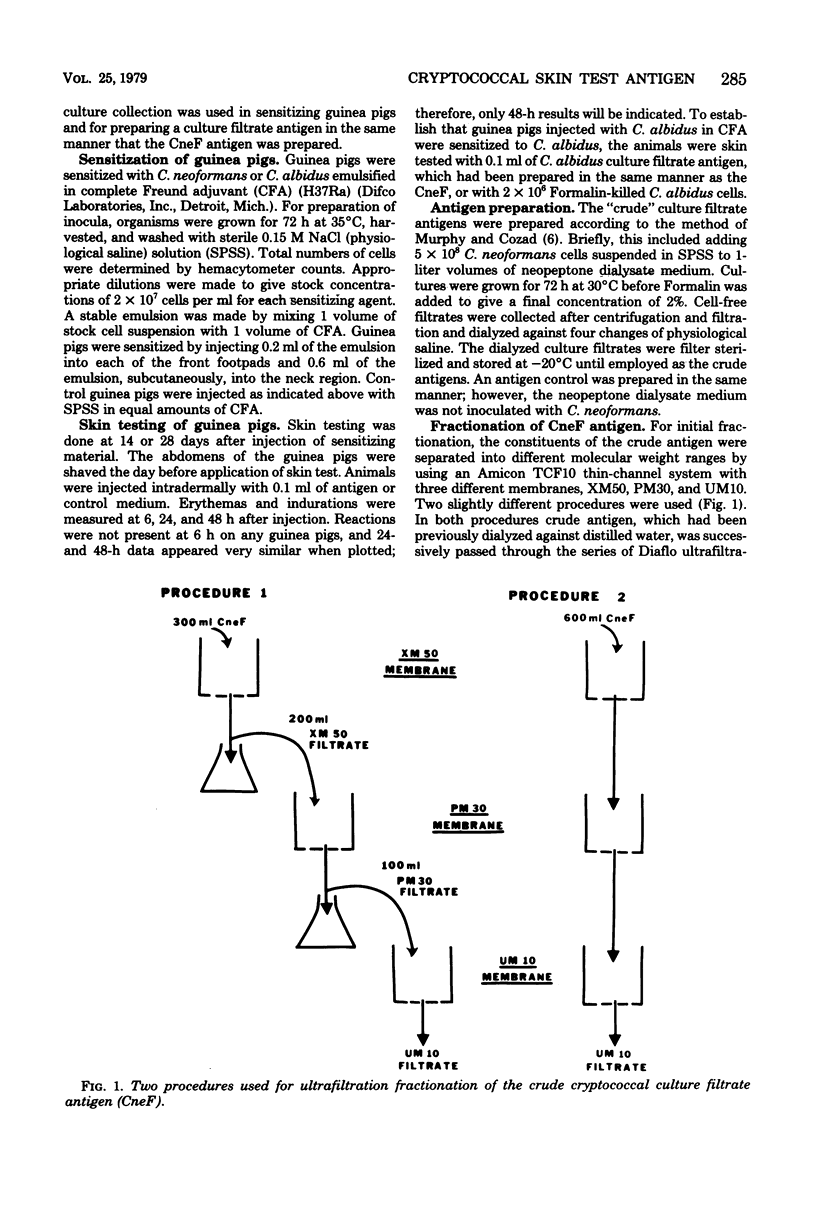
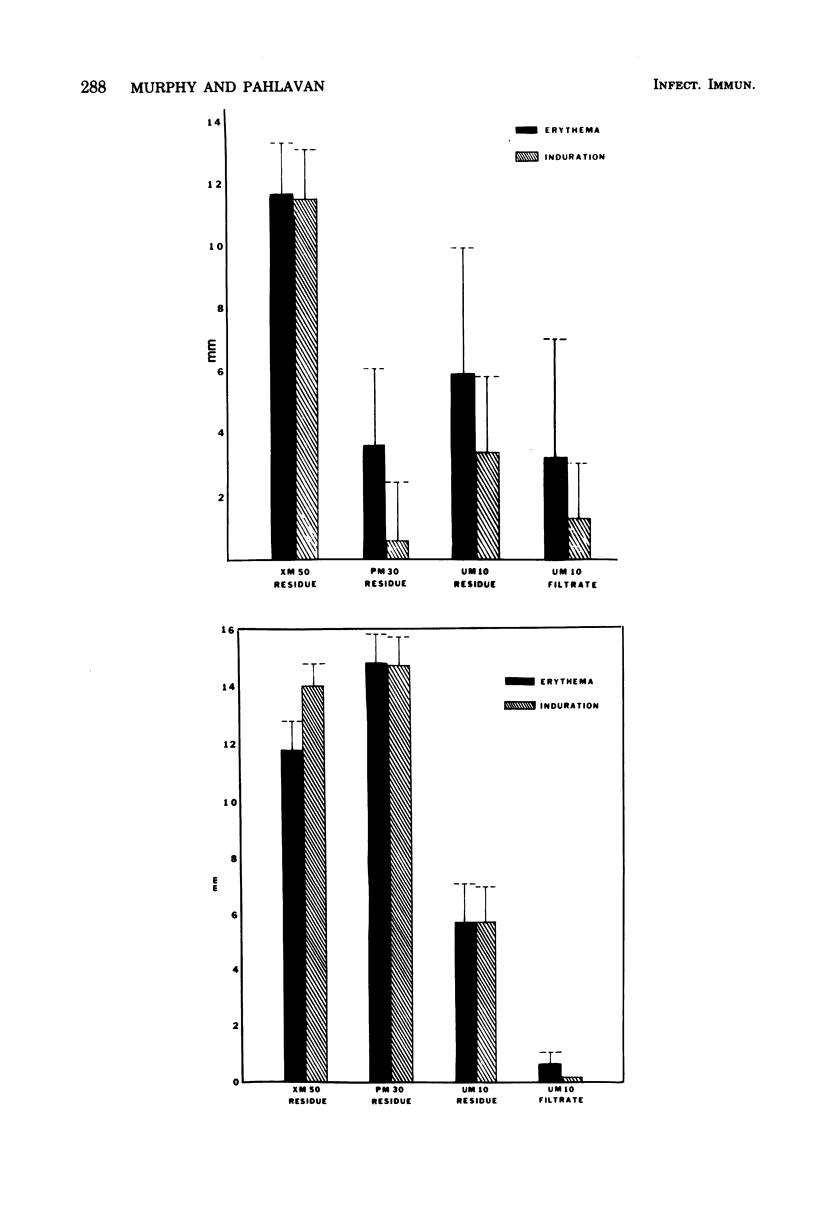
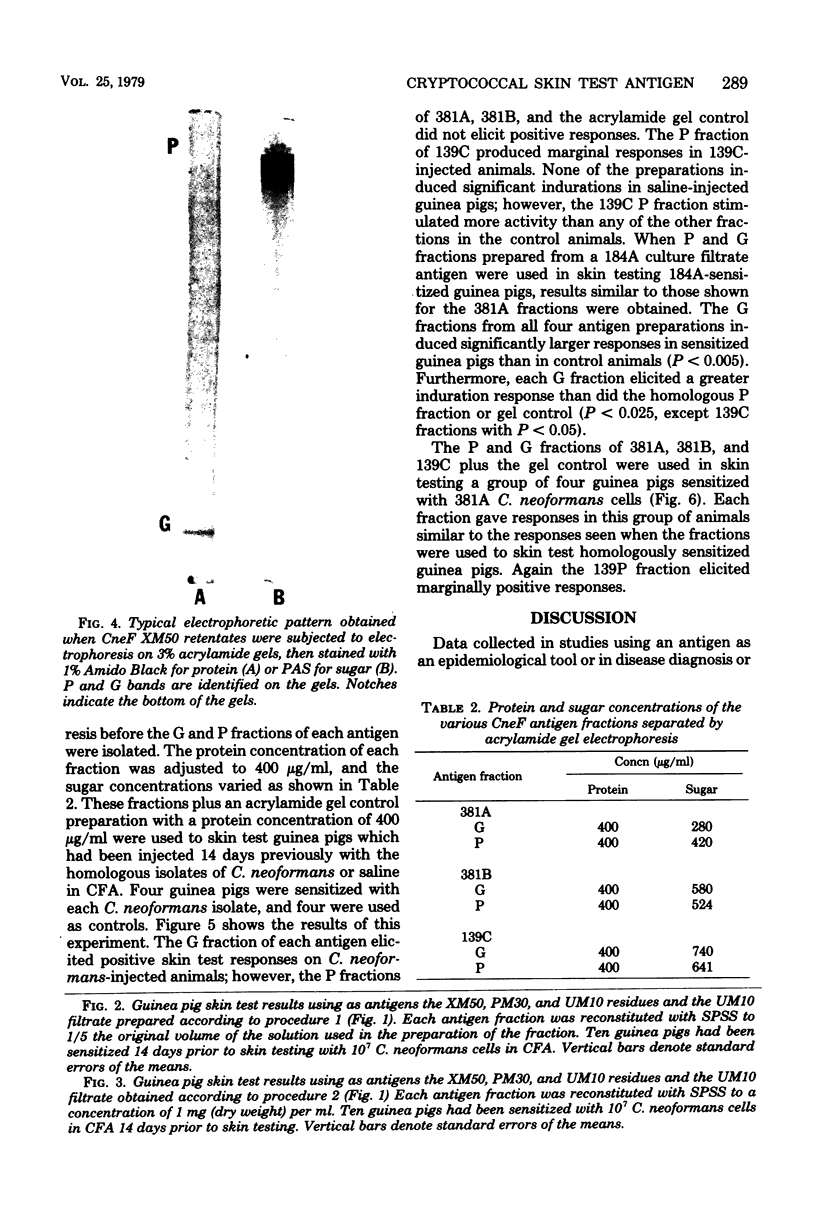
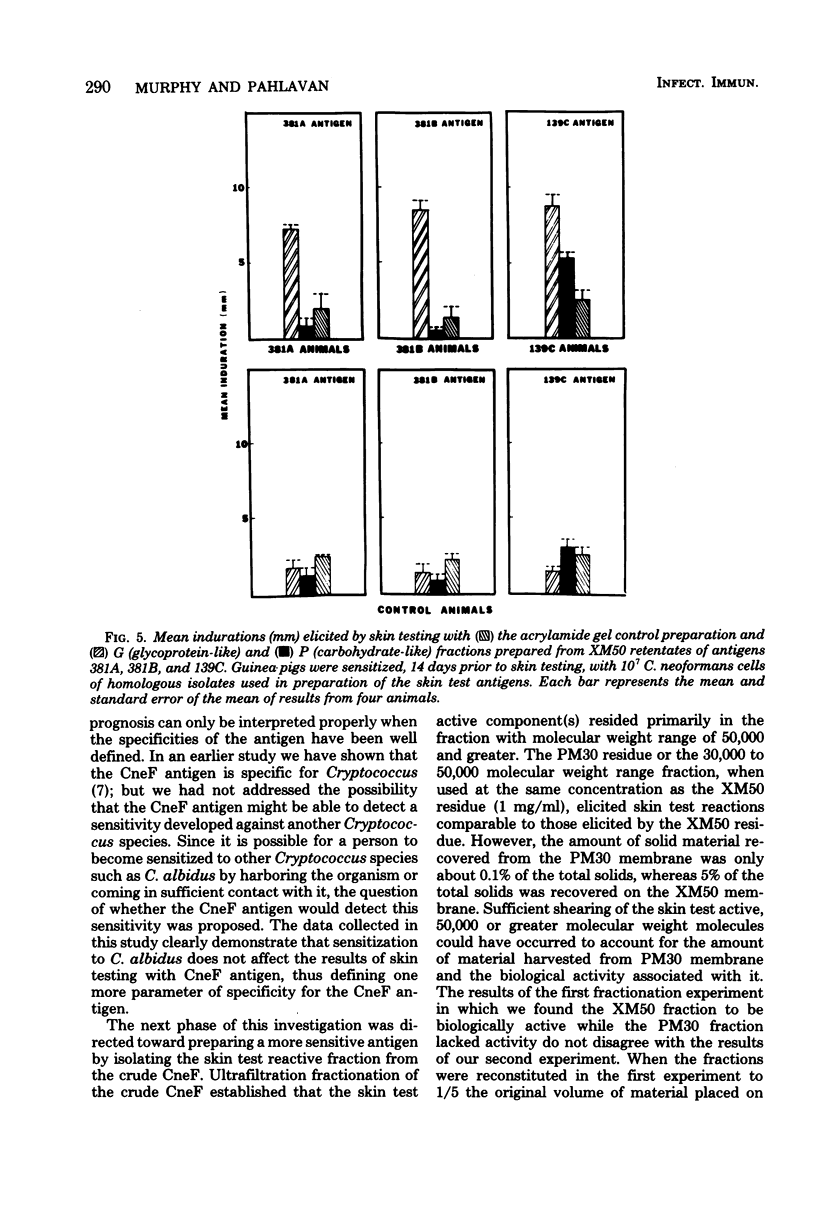
Previous studies on a cryptococcal culture filtrate (CneF) antigen have shown that the antigen is useful in detecting delayed-type hypersensitivity and that it is specific for Cryptococcus. This study further defined one more parameter of specificity, showing that the CneF antigen does not elicit delayed-type hypersensitivity responses in Cryptococcus albidus-sensitized guinea pigs. When the crude CneF antigen was subjected to ultrafiltration fractionation, the skin test active components were found to be in the 50,000 or greater molecular weight range fraction. The concentrated retentates of the XM50 ultrafiltration membrane were more sensitive antigens than the crude CneF antigens. Further fractionation of the XM50 retentate using 3% acrylamide gel electrophoresis separated the antigen into two bands. One band, the P fraction, migrated only a short distance into the gel; the fraction was carbohydrate-like and did not elicit significant skin test responses in sensitized guinea pigs. The other band, G fraction, appeared with the tracking dye, was glycoprotein-like, and elicited significantly positive skin tests in sensitized guinea pigs. G fractions prepared using three different serotypes of Cryptococcus neoformans elicited similar size indurations when used in skin testing guinea pigs sensitized with either the homologous serotype isolated of C. neoformans or the heterologous serotype isolate.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cauley L. K., Murphy J. W. Response of congenitally athymic (nude) and phenotypically normal mice to Cryptococcus neoformans infection. Infect Immun. 1979 Mar;23(3):644–651. doi: 10.1128/iai.23.3.644-651.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dykstra M. A., Friedman L. Pathogenesis, lethality, and immunizing effect of experimental cutaneous cryptococcosis. Infect Immun. 1978 May;20(2):446–455. doi: 10.1128/iai.20.2.446-455.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Land G. A., Vinton E. C., Adcock G. B., Hopkins J. M. Improved auxanographic method for yeast assimilations: a comparison with other approaches. J Clin Microbiol. 1975 Sep;2(3):206–217. doi: 10.1128/jcm.2.3.206-217.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy J. W., Cozad G. C. Immunological unresponsiveness induced by cryptococcal capsular polysaccharide assayed by the hemolytic plaque technique. Infect Immun. 1972 Jun;5(6):896–901. doi: 10.1128/iai.5.6.896-901.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy J. W., Gregory J. A., Larsh H. W. Skin testing of guinea pigs and footpad testing of mice with a new antigen for detecting delayed hypersensitivity to Cryptococcus neoformans. Infect Immun. 1974 Feb;9(2):404–409. doi: 10.1128/iai.9.2.404-409.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strauss E. G., Kaesberg P. Acrylamide gel electrophoresis of bacteriophage Q beta: electrophoresis of the intact virions and of the viral proteins. Virology. 1970 Oct;42(2):437–452. doi: 10.1016/0042-6822(70)90287-4. [DOI] [PubMed] [Google Scholar]
- Zacharius R. M., Zell T. E., Morrison J. H., Woodlock J. J. Glycoprotein staining following electrophoresis on acrylamide gels. Anal Biochem. 1969 Jul;30(1):148–152. doi: 10.1016/0003-2697(69)90383-2. [DOI] [PubMed] [Google Scholar]




