C-type inactivation in K+ channels is enhanced by external Ca2+ or La3+, consistent with a mechanism which involves dilation of the outer pore.
Abstract
Many voltage-gated K+ channels exhibit C-type inactivation. This typically slow process has been hypothesized to result from dilation of the outer-most ring of the carbonyls in the selectivity filter, destroying this ring’s ability to bind K+ with high affinity. We report here strong enhancement of C-type inactivation upon extracellular addition of 10–40 mM Ca2+ or 5–50 µM La3+. These multivalent cations mildly increase the rate of C-type inactivation during depolarization and markedly promote inactivation and/or suppress recovery when membrane voltage (Vm) is at resting levels (−80 to −100 mV). At −80 mV with 40 mM Ca2+ and 0 mM K+ externally, ShBΔN channels with the mutation T449A inactivate almost completely within 2 min or less with no pulsing. This behavior is observed only in those mutants that show C-type inactivation on depolarization and is distinct from the effects of Ca2+ and La3+ on activation (opening and closing of the Vm-controlled gate), i.e., slower activation of K+ channels and a positive shift of the mid-voltage of activation. The Ca2+/La3+ effects on C-type inactivation are antagonized by extracellular K+ in the low millimolar range. This, together with the known ability of Ca2+ and La3+ to block inward current through K+ channels at negative voltage, strongly suggests that Ca2+/La3+ acts at the outer mouth of the selectivity filter. We propose that at −80 mV, Ca2+ or La3+ ions compete effectively with K+ at the channel’s outer mouth and prevent K+ from stabilizing the filter’s outer carbonyl ring.
INTRODUCTION
C-type inactivation is a (usually slow) gating phenomenon originally observed as a conductance decrease that occurs in many voltage-gated K+ channels after activating the channels with a depolarizing step (Hoshi et al., 1991). Results of experiments using mutant K+ channels show that C-type inactivation is associated with the extracellular mouth of the channel (Hoshi et al., 1991; López-Barneo et al., 1993; Baukrowitz and Yellen, 1995; Kurata and Fedida, 2006). Some mutants such as those containing W434F are constitutively almost completely C-type inactivated and generate only gating current under typical recording conditions (Perozo et al., 1993; Yang et al., 1997); the C-type inactivation “gate” is closed, regardless of the voltage and the position of the S4 helices that drive activation/deactivation of the V-gate located at the inner end of the channel (del Camino and Yellen, 2001; Jensen et al., 2012). C-type inactivation is quite distinct from N-type inactivation, which is usually more rapid, and occurs by a ball and chain mechanism that involves the entry of the N terminus of a channel peptide (or a moiety of a β subunit) into the channel’s inner mouth from the cytoplasmic side when the V-gate is open (Hoshi et al., 1990; Zagotta et al., 1990; Demo and Yellen, 1991; Zhou et al., 2001a). Functionally, C-type inactivation serves as a form of short-term memory, modifying the availability of the channel based on recent experience.
In the absence of N-type inactivation, ShB, a commonly used splice variant of Shaker K+ channels (Timpe et al., 1988), inactivates with a time constant of ∼1 s in normal extracellular solution (Hoshi et al., 1991; López-Barneo et al., 1993). Mutation of the threonine residue at position 449 (T449 using ShB numbering), located just external to the channel’s outer mouth, has a strong influence on C-type inactivation, which, for example, is virtually eliminated by the mutation of T449 to valine (T449V; López-Barneo et al., 1993). With the mutation W434F, C-type inactivation is essentially permanent. Interestingly, W434F channels (ShBΔN W434F) can be rescued from C-type inactivation by combining the T449V mutation with W434F (Yang, Y., Y. Yan, and F.J. Sigworth. 2002. Biophysical Society Annual Meeting. 1138-Pos Board #B190). Mutating T449 to alanine, glutamate, lysine, or serine shortens the time constant of inactivation to milliseconds (López-Barneo et al., 1993). Except in mutants such as W434F, recovery from C-type inactivation is facilitated by repolarization (Kurata and Fedida, 2006).
C-type inactivation has proven to be a challenge to define mechanistically (Kurata and Fedida, 2006) and has been traditionally explained as a constriction of the channel’s outer mouth (Yellen et al., 1994; Cuello et al., 2010). More recently, this inactivation has been proposed to result from a dilation of the channel’s outer mouth (Hoshi and Armstrong, 2013) that destroys the ability of the selectivity filter’s outermost site (Åqvist and Luzhkov, 2000; Morais-Cabral et al., 2001) to complex K+. This outermost site is called FS1 (filter site 1) hereafter. The crystal structure of a Shaker-like voltage-gated K+ channel (Kv1.2-2.1 chimera; Long et al., 2007) has been an enormous aid in understanding permeation and channel gating. However, the requirement for high concentrations of K+ in the crystallization mix has so far limited its usefulness in understanding this gating phenomenon, at least in voltage-activated channels, because high concentrations of K+ antagonize C-type inactivation (Choi et al., 1991; López-Barneo et al., 1993; Baukrowitz and Yellen, 1995; Kurata and Fedida, 2006). Aside from K+, many previous studies show that other ions also affect C-type inactivation (Choi et al., 1991; López-Barneo et al., 1993; Loboda et al., 2001; Kurata and Fedida, 2006). We report here the surprising effects of high concentrations of extracellular Ca2+ or low concentrations of extracellular La3+ on C-type inactivation; both Ca2+ and La3+ drastically enhance C-type inactivation when Vm is negative. Here we use these multivalent cation effects as tools for unraveling the mechanism of C-type inactivation.
MATERIALS AND METHODS
Channel expression
All experiments were performed in cells expressing various mutants of the Shaker K+ channel called ShBΔ6-46; i.e., a segment of the N terminus of the ShB channel was deleted to remove N-type inactivation (Hoshi et al., 1990). Mutants of this base channel (e.g., T449A, meaning that the threonine residue at position 449 was changed to alanine) were transiently expressed in human embryonic kidney (HEK) or mouse Neuro2a (N2a) cells by transfection with plasmid DNAs using FuGene6 (Roche) as described previously (Hoshi and Armstrong, 2012). GFP was used as the transfection marker.
Electrophysiology and data analysis
The electrophysiological measurements were performed at room temperature typically 24–48 h after transfection as described previously (Hoshi and Armstrong, 2012). The outside-out and whole-cell configurations of the patch-clamp method were used. Outside-out patch experiments were performed as described previously (Hoshi et al., 2013). Unless otherwise noted, the base external solution for these whole-cell experiments contained (mM) 157 NaCl, 2 CaCl2, 2 KCl, and 10 Tris, pH 7.0. External solutions used in the whole-cell current measurements are show in Table 1. The internal solution for whole-cell experiments contained (mM) 100 K glutamate, 30 KF, 20 KCl, and 10 Tris, pH 7.0. Other solutions used are described in the figure legends. Linear leak and capacitive currents were subtracted using a P/6 protocol in outside-out experiments or, for whole-cell experiments, by scaling membrane current for a step from −100 to −140 mV. Series resistance compensation of ∼60% and ∼70–90% was used in outside-out and whole-cell experiments, respectively. Series resistance error was at most 10 mV, which is not of serious concern in our qualitative measurement of outward current. The results presented here are illustrative of numerous experiments: >30 exposures to elevated Ca2+ and ∼10 to La3+. Kinetics of current inactivation was estimated by fitting a single exponential.
Table 1.
External solutions used in the whole-cell current measurements
| Solution name | [CaCl2] | [KCl] | [NaCl] | [LaCl3] |
| mM | mM | mM | mM | |
| 2Ca0K | 2 | 0 | 157 | |
| 2Ca2K | 2 | 2 | 155 | |
| 4Ca2K | 4 | 2 | 152 | |
| 10Ca0K | 10 | 0 | 145 | |
| 10Ca2KLa | 10 | 2 | 143 | 0.01 |
| 20Ca0K | 20 | 0 | 130 | |
| 28Ca0K | 28 | 0 | 118 | |
| 40Ca0K | 40 | 0 | 100 | |
| 40Ca2K | 40 | 2 | 98 |
Each solution also contained 10 mM Tris and pH 7.0.
Online supplemental material
Fig. S1 calculates the decrease in K+-binding affinity caused by moving a coordinating carbonyl group away from the K+ ion by 1 Å. Fig. S2 calculates the energy change when an empty carbonyl ring (K+ ion removed) increases or decreases in diameter by 1 Å. Online supplemental material is available at http://www.jgp.org/cgi/content/full/jgp.201411223/DC1.
RESULTS
C-type inactivation in high extracellular Ca2+
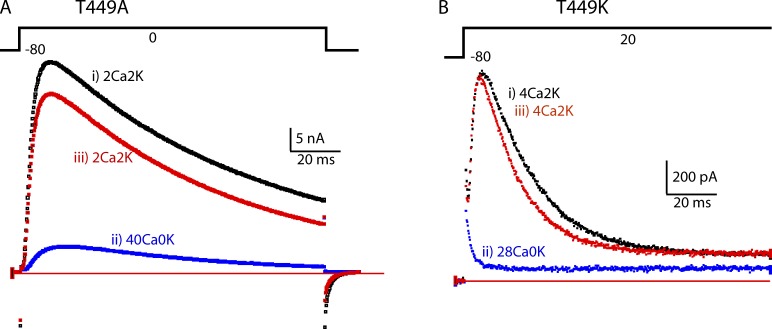
Potassium current (IK) recorded from a cell expressing the rapidly inactivating mutant T449A (López-Barneo et al., 1993) is shown in Fig. 1 A. In base extracellular medium containing 2 mM Ca2+ and 2 mM K+ (2Ca2K; see Materials and methods and Table 1 for detailed solution composition), IK during a 110-ms step to 0 mV decayed with a time constant of ∼90 ms (trace i). 2 min after resting in an extracellular solution containing 40 mM Ca2+ and 0 mM K+ (40Ca0K), the much smaller IK trace ii was recorded. The reduced IK was similar in time course to the larger IK trace, but inactivated with a slightly accelerated time constant of ∼55 ms. The effect of 40Ca0K was largely reversed on return to 2Ca2K (iii).
Figure 1.
In two mutants that have C-type inactivation, high extracellular Ca2+ depresses IK. (A) The traces show IK from a cell expressing T449A during a depolarization lasting 110 ms from −80 to 0 mV. The traces were taken in the order i–iii, with a rest of ∼2 min between them to allow full recovery from C-type inactivation. IK is strongly depressed by 40Ca0K. Note, however, that the time constant of inactivation of the current remaining in 40Ca0K is similar to that in 2Ca2K. (B) IK traces from a T449K cell. The extracellular solution was changed from 4Ca2K (i) to 28Ca0K (ii) and then back to 4Ca2K (iii).
IK from another mutant, T449K (López-Barneo et al., 1993), which inactivates even more rapidly than T449A (inactivation time constant ∼19 ms), is shown in Fig. 1 B. When the extracellular medium was changed from 4Ca2K to 28Ca0K (28 mM Ca2+), IK was reduced to a rapidly decaying component that finished within milliseconds of depolarization onset. Similar results were previously observed with the rapidly inactivating mutant E418Q (Ortega-Sáenz et al., 2000).
High Ca2+ acting on a mutant that lacks C-type inactivation
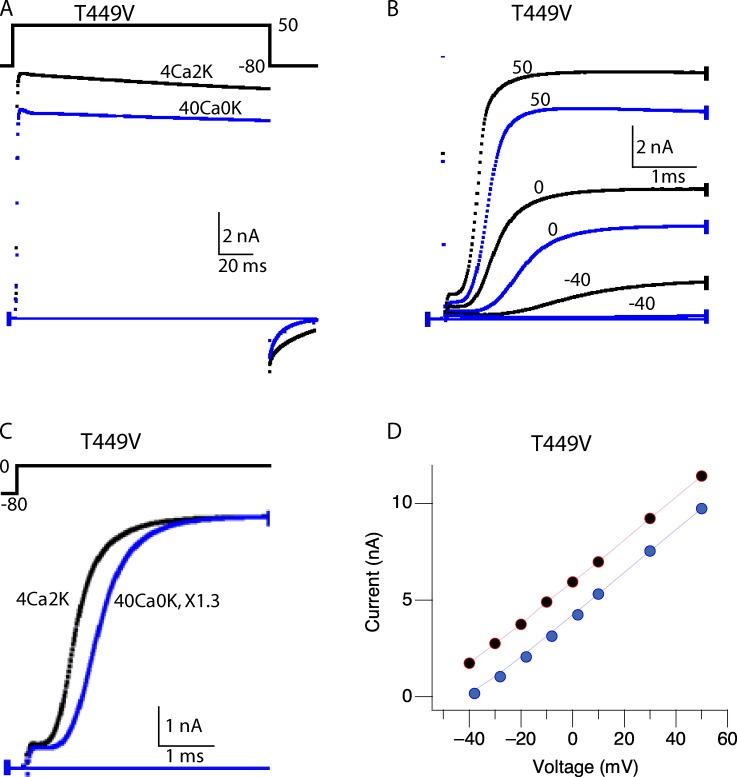
In a cell expressing T449V, which does not C-type inactivate (López-Barneo et al., 1993), changing the external solution from 4Ca2K to 40Ca0K had very little effect on IK amplitude (Fig. 2 A), in striking contrast with the current-depressing effect seen with the fast-inactivating mutants T449A and T449K (Fig. 1). Close inspection of the activation kinetics at expanded time scale (Fig. 2, B and C) shows that elevated Ca2+ slows activation of IK and that the initial lag before IK rises is longer. Fig. 2 D plots the maximum value of IK as a function of the voltage during the depolarizing step. The curve in 40Ca0K is shifted to the right by slightly less than 20 mV. These familiar effects of increased Ca2+, slower activation of IK after a longer lag and a right shift of the I-V curve, are not related to C-type inactivation, which does not occur in T449V channels (López-Barneo et al., 1993). The increased lag and slower rise of IK, sometimes called surface charge or surface potential effects (Frankenhaeuser and Hodgkin, 1957; Campbell and Hille, 1976), have more recently been attributed to di- or trivalent cation binding to negative charges on the migration path of the S4 through the membrane as the channels activate (Hoshi and Armstrong, 2012).
Figure 2.
Ca2+ does not appreciably inhibit currents through T449V channels. (A) Whole-cell currents elicited by pulses from −80 to 50 mV in 4Ca2K and 40Ca0K. (B) Slower activation time course of T449V in 40Ca0K (blue) compared with those in 4Ca2K (black). (C) The currents for pulses from −80 to 0 mV are scaled by 1.3 times to account for the familiar right shift of the I-V curve caused by high Ca2+, which is shown in D. (Gilly and Armstrong, 1982; Hille, 2001). All results shown are from the same cell.
C-type inactivation examined with short pulses
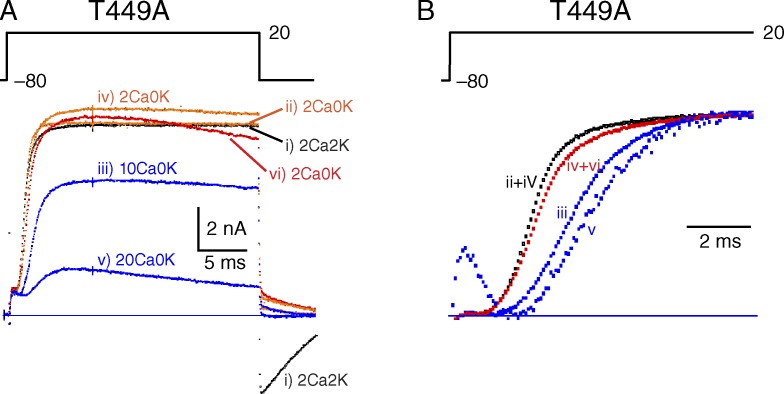
The current-depressing effect of Ca2+ on T449A seen in Fig. 1 A was also assessed by repetitively applying 25-ms depolarizing pulses to 20 mV at 0.1 Hz (Fig. 3 A). The experiment began with a large current recorded in 2Ca2K (trace i, black). After changing to 2Ca0K, IK of the 11th pulse at 0.1 Hz (trace ii, orange) was essentially unchanged except for elimination of the inward tail of current at pulse end seen in 2Ca2K. (Note: Perfusion time was not measured in this experiment.) Changing to 10Ca0K reduced IK (11th pulse at 0.1 Hz) by about a third (trace iii, blue), with complete recovery on return to 2Ca0K (11th pulse, trace iv, orange). Changing to 20Ca0K reduced IK to about a third (11th pulse, trace v, blue) and caused a slow decline of IK during the pulse, probably as the result of somewhat accelerated C-type inactivation. Again, recovery was complete on return to 2Ca0K (11th pulse, trace vi, red), but a hint of C-type inactivation remained.
Figure 3.
Ca2+ action on C-type inactivation examined with short pulses. (A) A cell expressing T449A was stimulated at 0.1 Hz in several solutions, beginning with 2Ca2K. In each solution, maximum IK was in a steady-state after ∼10 pulses or less, and a current record for the 11th pulse in each solution is displayed. The currents were elicited by 25-ms pulses from −80 to 20 mV and then back to −80 mV. (B) Slowing of activation kinetics by 10 and 20 Ca2+. The currents from A are scaled vertically for comparison and displayed on a fast time scale. ii+iv, iv+vi: mean of ii and iv or iv and vi, respectively. A gating current transient is visible in trace v, the most amplified.
The slowing of K+ channel activation seen in T449V (Fig. 2 B) is also found in the T449A mutant, as illustrated in Fig. 3 B in which the traces from Fig. 3 A have been scaled to the same amplitude to facilitate temporal comparison and shown at an expanded time scale. At elevated Ca2+, IK develops more slowly and the initial lag in its development is longer.
Another experiment using short repetitive pulses with several solution changes is summarized in Fig. 4 A. A cell expressing T449A was held at −80 mV and pulsed to 20 mV at 0.1 Hz. The extracellular Ca2+ and K+ concentrations are shown above, and peak IK amplitude recorded for each pulse is plotted below. The experiment begins in 2Ca2K, with a change to 40Ca2K at (1). This increase in [Ca2+] inhibited peak IK from ∼20 nA to ∼13 nA. Pulsing was suspended for 120 s (2), and on resumption of pulsing IK remained partially inhibited (∼13 nA); there was no recovery of IK amplitude during the suspension of pulsing. The solution was then changed back to 2Ca2K (3), and IK fully recovered to ∼20 nA (4). A suspension of pulsing caused no change in IK (4 to 5). At 440 s (5), the solution was changed to 40Ca0K, increasing [Ca2+] concurrent with removal of K+. IK progressively decreased to ∼4 nA, and suspension of pulsing did not alter IK. Decreasing extracellular [Ca2+] to 2Ca0K (6) restored IK to ∼15 nA. Full restoration to the original current size of ∼20 nA was seen when K+ was added back to the extracellular medium, making the final solution of 2Ca2K (7).
Figure 4.
Enhancement of C-type inactivation by high extracellular Ca2+ is antagonized by extracellular K+. (A) Whole-cell currents from a T449A cell were elicited by 25-ms voltage pulses from −80 to 20 mV applied at 0.1 Hz. Peak outward current in nanoamperes is plotted as a function of time as Ca2+ and K+ concentrations in the extracellular solutions were changed as indicated at the top. Solution changes were completed over the course of several pulses. The numbers (1–7) are explained in the section C-type inactivation examined with short pulses. (B) Whole-cell currents from a cell expressing T449A channels during exposure to 40Ca0K or 40Ca2K. The currents were elicited by pulses from −80 to 50 mV after a rest of 2 min.
Is the depression of IK by high Ca2+ in fact the result of enhanced C-type inactivation, as strongly suggested by its occurrence only in mutants that C-type inactivate? To further support this idea, we compared (a) the recovery time of IK after exposure to elevated Ca2+ (n = 4) with (b) the recovery time of IK after depolarization-induced C-type inactivation (n = 4). Recovery was monitored in 2 mM Ca2+ by applying short pulses at 0.1 Hz. The recovery time constant was ∼20 s for both experiments, supporting the idea that high Ca2+ depresses IK by enhancing C-type inactivation.
The results in Fig. 4 A clearly show that extracellular Ca2+ causes substantial inactivation in 40Ca2K, but much more inactivation when extracellular K+ is zero (40Ca0K; compare 3 with 6 in Fig. 4 A). Thus, a small extracellular K+ concentration largely reverses the effects of 40 mM Ca2+ on C-type inactivation. The antagonism between extracellular Ca2+ and K+ is also seen in Fig. 4 B, using currents elicited by longer depolarizing pulses to 50 mV. Each trace (in the order i–iv) was recorded after a 2-min rest in the designated solution to allow full equilibration. In traces i and iii, recorded in 40Ca0K, C-type inactivation was nearly complete, resulting in a small IK. Note that C-type inactivation had occurred at −80 mV, during a 2-min period with no pulsing. After a return to 40Ca2K with a 2-min rest, IK was much larger and C-type inactivated in the usual way (traces ii and iv). The addition of only 2 mM K+ thus was sufficient to substantially antagonize the Ca2+ effect on C-type inactivation. Judging from the results shown in Fig. 4 A, IK in Fig. 4 B would have been still larger had extracellular Ca2+ been reduced from 40 mM as K+ was added. The results thus reiterate the strong and reversible effect of elevated extracellular Ca2+ on C-type inactivation and show that even a low concentration of extracellular K+ antagonizes the current-depressing effect of Ca2+. In the Discussion, we argue that Ca2+ and K+ complete for occupancy of a site at the outer mouth of the K+ channel. Additionally, the results show that with high concentrations of Ca2+, C-type inactivation develops at the resting potential even without pulsing.
La3+ also enhances C-type inactivation
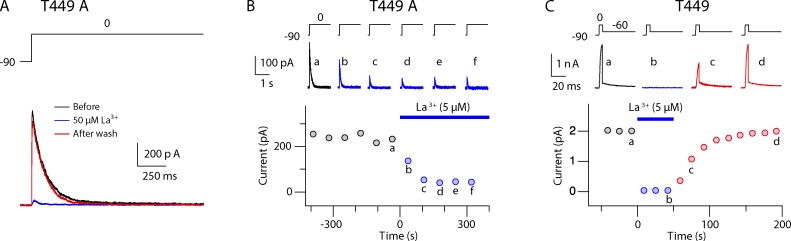
La3+ has almost the same ionic radius (1.17 Å) as Ca2+ (1.14 Å; Shannon, 1976) and has a Ca2+-like effect on C-type inactivation of T449A channels but at much lower concentrations. On depolarization to 0 mV (Fig. 5 A), IK activated quickly in 4Ca2K and then inactivated with a time constant of ∼106 ms (mean before and after). Addition of 50 µM La3+ to the external solution markedly depressed IK. After washing away the La3+ with 4Ca2K, IK recovered completely. A lower concentration of La3+ (5 µM) was also effective in progressively depressing currents through T449A K+ channels (Fig. 5 B). In our outside-out patch experiments, solution change typically required 2–5 s, which is much faster than the time course of the fall in IK in this experiment. It can be seen in Fig. 5 B that the time course of inactivation was not markedly changed by La3+, as is also true for elevated Ca2+ (Fig. 1 A).
Figure 5.
Extracellular La3+ enhances C-type inactivation in T449A and T449T (i.e., wild type) channels. (A) The black and the red trace are IK from an outside-out patch before and after exposure to 50 µM La3+, which virtually eliminated IK (blue trace). IK was elicited by 1.5-s pulses from –90 to 0 mV every 62 s. (B) Peak IK through T449A channels before and during exposure to 5 µM La3+ in the extracellular solution. Similar results were obtained in four patches. (C) Peak IK through T449T (wild type) channels before, during, and after washout of 5 µM La3+ in the extracellular solution. The patch was held at −90 mV except to measure the currents as illustrated in the insets. Similar results were obtained in four patches. The extracellular solution contained (mM) 130 NaCl, 4 KCl, 2 CaCl2, 2 MgCl2, 10 glucose, and 10 HEPES, pH 7.4, plus 5 µM La3+ in blue traces. The internal solution contained (mM) 140 KCl, 2 MgCl2, and 10 HEPES, pH 7.2.
In Fig. 5 C, La3+ was applied to wild-type ShBΔN channels with a threonine at position 449. These channels normally inactivate with a time constant of ∼1 s (Hoshi et al., 1991). At “a” the patch was depolarized to 0 mV for 3 ms, too short a time to cause appreciable C-type inactivation. That little inactivation occurred is shown by the tail of outward IK seen on repolarization to −60 mV. Immediately after the pulse, 5 µM La3+ was added externally. In the next pulse, IK was completely suppressed. We interpret this to mean that La3+ within seconds caused complete C-type inactivation at the holding potential of −90 mV, with no pulsing. IK recovered completely when La3+ was removed.
La3+ action on T449V channels that do not C-type inactivate
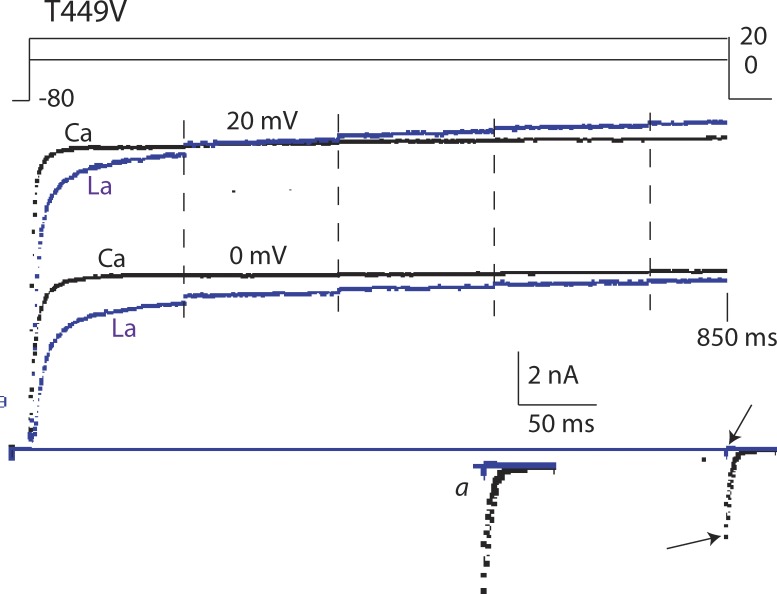
In contrast to its strong effect on T449T and rapidly inactivating T449A (Fig. 5), La3+, like Ca2+, has only mild effects on IK of T449V, as illustrated in Fig. 6. These effects, slow activation and a positive shift of the I-V curve, are similar to the effects of much higher concentrations of Ca2+ (Fig. 2) and are not related to C-type inactivation. Note that the inward tail of IK seen in 10Ca2K is suppressed by La3+, showing that at negative voltages K+ channels are blocked by this trivalent ion. A similar block at negative voltage by high external Ca2+ has been previously reported (Tytgat and Daenens, 1997; Gomez-Lagunas et al., 2003).
Figure 6.
Extracellular La3+ (10 µM) slows activation of T449V channels but causes no inactivation. IK is compared in 10KCa2K (black traces) and in 10Ca2K + La3+ (10 µM La3+; blue traces). Sampling was interrupted at each of the vertical dashed lines for 100 ms and then resumed. Total sweep duration was 850 ms. On return to −80 mV, there was a clear inward IK tail without La3+(bottom arrow), but none in the presence of La3+, which blocks K+ channels at negative voltage (top arrow). The tail is shown at slightly higher gain in inset a.
DISCUSSION
This paper presents what we think are novel aspects of the interaction of two extracellularly applied multivalent cations, Ca2+ and La3+, with voltage-gated K+ channels. These effects are used here as tools to refine ideas regarding the mechanism of C-type inactivation. Ca2+/La3+ effects on this inactivation (Hoshi et al., 1991; Kurata and Fedida, 2006) are quite distinct from the effects of multivalent cations on the voltage-controlled activation gate (Figs. 2 and 6). We think that the changes of channel properties caused by these ions are related to C-type inactivation. The main reason is that the deep depression of conductance caused by Ca2+/La3+ is seen only in channels that have what is classically defined as C-type inactivation, a relatively slow current decay that follows activation of a K+ channel’s V-gate (Hoshi et al., 1991; López-Barneo et al., 1993). Additionally, the recovery from classical, depolarization-induced C-type inactivation is the same within the accuracy of the measurement as recovery for Ca2+/La3+-induced depression of IK. The C-type inactivated state can also be accessed without experimental depolarization by the mutation W434F (Perozo et al., 1993; Yang et al., 1997) and rescued by the mutation T449V (Yang, Y., Y. Yan, and F.J. Sigworth. 2002. Biophysical Society Annual Meeting. 1138-Pos Board #B190). We think Ca2+/La3+, similar to W434F, facilitates entry of the channels into the C-type inactivated state, without depolarization.
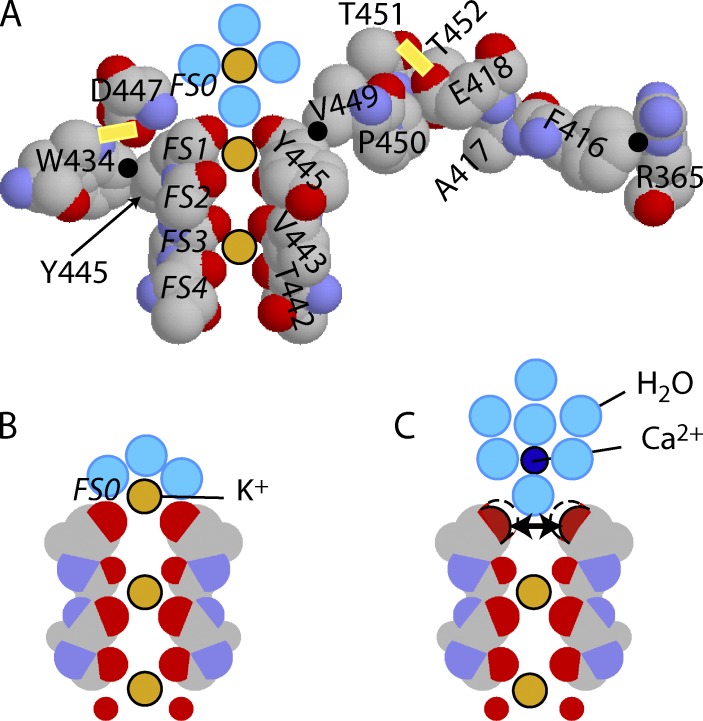
The exact nature of C-type inactivation continues to be debated and has been attributed to either pore constriction (Liu et al., 1996; Cuello et al., 2010) or dilation of the outer pore (Hoshi and Armstrong, 2013). Fig. 7 (abstracted from the chimera analyzed by Long et al. [2007]) shows a slice through the selectivity filter and some surrounding residues known to be important in C-type inactivation. For convenience, the residue numbering of the chimera has been converted to that appropriate for ShB simply by adding 72 (see the alignment of chimera and Shaker in Long et al. [2007]); in ShB, residues 451 and 452 are valine and glycine. All others are the same as in the chimera.
Figure 7.
K+ occupancy of FS1 is affected by voltage and extracellular Ca2+/La3+. (A) A slice through the selectivity filter and some surrounding residues. Points of significant close contact between residues are shown by black dots. Two significant H-bonds are indicated by the yellow bars. The spheres on the pore axis are dehydrated K+ ions in filter sites FS1 and FS3 and a hydrated K+ in FS0, just outside the filter (water molecules are light blue). (B) The filter only, in occupancy state FS2–FS4. When conducting, occupancy alternates between FS1–FS3 and FS2–FS4. At rest, when the channel is closed and Vm is negative, FS2–FS4 is the preferred state. In this state, the outermost carbonyls are stabilized by attraction to a partially dehydrated K+ ion in FS0. (C) FS0 is occupied by a Ca2+ ion. Its hydration shell is too firmly attached to allow close approach to the outer carbonyls of FS1. As a result, they repel, as indicated by the two-headed arrow, leading to dilation and C-type inactivation. Residue numbering is appropriate to ShBΔN.
Within the selectivity filter, there are four ion-binding sites (FS1 through FS4), of which only two are occupied by dehydrated K+ ions at any instant. When conducting, occupancy oscillates between FS1 and FS3 (Fig. 7 A) and FS2 and FS4 (Fig. 7 B). External to FS1 is a hydration/dehydration site (FS0); a fully hydrated K+ ion is shown just above this site in Fig. 7 A and partially hydrated in Fig. 7 B. (A partially dehydrated K+ ion in FS0 can be seen in Zhou et al. [2001b].) Precise positioning of the carbonyls is required for effective permeation: a rough electrostatic calculation shows that moving a single carbonyl 1 Å away from the K+ ion, in, e.g., FS1, decreases the binding affinity by a factor of roughly 50,000 according to Hille [2001] (Figs. S1 and S2), greatly raising the energy barrier to ion permeation.
The stability of the filter depends on the K+ ions bound within it. Each ion is bound with an energy approximating the hydration energy of a K+ ion in water (∼77 kcal/mol or 323 kJ/mol [Burgess, 1978]), making the energy barrier for entering the filter from aqueous solution low. Removing a K+ ion from a site would lead to a large mutual repulsive force among the carbonyls, tending to dilate the site. In fact, prolonged removal of all K+ ions from both sides of the membrane is known to completely destroy conduction through K+ channels (Gomez-Lagunas, 1997). In Fig. 7 A, all of the carbonyl rings except the innermost are bound electrostatically to K+. When the internal voltage is negative, the ions in the filter are drawn by the electric field to positions FS2 and FS4 (Fig. 7 B). In this case, every carbonyl is bound to an ion in the filter, except for the outermost ring of FS1, which is contributed by Y445 residues. When voltage is negative, this outer ring depends for its stability on electrostatic binding to a partially dehydrated ion in FS0 (Fig. 7 B).
The selectivity filter is surrounded by residues that support the precise positioning required for selective K+ permeation. To the left of the filter is W434, which has a close contact (Fig. 7 A, black dot) with the ring of Y445. The mutation W434F causes permanent C-type inactivation of the channel: only gating currents can be recorded (Perozo et al., 1993; Yang et al., 1997). Undoubtedly important in the positioning of W434 is a hydrogen bond from the carboxyl of D447 to the ring nitrogen of W434. The mutation D447E alters this bonding and speeds C-type inactivation (Molina et al., 1997).
To the right of the filter in the figure is V449 (T449 in wild-type ShB), which has close contact with Y445, on the side of the ring opposite to the W434 contact. Residue 449 is perhaps the most studied of the residues that affect C-type inactivation (López-Barneo et al., 1993). A449, K449, and E449 make C-type inactivation much faster, whereas V449 prevents it (summarized in Kurata and Fedida [2006]). To the right of residue 449 is a chain of contacting residues that stretches all the way to R365, which is in the voltage-sensing S4 helix (Tao et al., 2010). In the diagram, residue 449 connects to residues 450–452, which are in the loop joining the filter to S6. Both threonines in the crystal structure (ShB positions 451 and 452) form H-bonds to E418 at the top of S5 (one H-bond is shown with a yellow bar in Fig. 7 A). E418 connects to A417 and F416; the latter is in close contact (Fig. 7 A, black dot) with R365 in S4. This chain provides a likely basis for the observed coupling of C-type inactivation to S4 motion (Hoshi and Armstrong, 2013). In the resting state, the contact between F416 and R365 would not exist: R365 would be displaced inward by ∼14 Å (Jensen et al., 2012).
We postulate that S4 voltage-sensor activation perturbs the amino acid residues surrounding the ion conduction pore allosterically, destabilizing its outer mouth and allowing it to dilate, thus facilitating C-type inactivation. In normal function, we postulate, outward movement of S4 brings R365 in contact with F416 (Fig. 7 A, black dot), perturbing it and starting a wave of conformational changes. The conformational wave spreads to E418, which is hydrogen-bonded to V451 ShB (Fig. 7 A, yellow bar at right). The mutations E418C and E418Q remove this hydrogen bond and greatly accelerate inactivation (Larsson and Elinder, 2000). The wave continues from V451to D447. All of the residues in the 450–447 segment change conformation upon C-type inactivation (Liu et al., 1996; Molina et al., 1998). Based on study of the crystal structure, homology modeling, and mutation experiments, we propose that the propagated changes cause the side chain of D447 to move outward, breaking a hydrogen bond (Fig. 7 A, yellow bar at left) to the ring nitrogen of W434. Deprived of this stabilizing H-bond, W434 rotates and its ring pushes on the ring of Y445 (Fig. 7 A, black dot), tending to cause rotation of Y445 and movement of its carbonyl away from the pore axis (all seen in homology modeling [Hoshi and Armstrong, 2013]). If the residue at position 449 is a valine, close contact (Fig. 7 A, black dot) between one of the two γ-carbons of the valine and the Y445 tyrosine ring prevents rotation of the ring. This firmly stabilizes the outer carbonyl ring, explaining why T449V does not undergo C-type inactivation and why Ca2+/La3+ affects only S4 motion in this mutant. If the residue at position 449 is alanine, lysine, or one of numerous other residues, rotation is possible. If Y445 rotates, its carbonyl recedes ∼1 Å from the pore axis (seen in homology modeling [Hoshi and Armstrong, 2013]), producing a high-energy barrier for K+ entry into FS1. This greatly decreases the probability of FS1 occupancy, resulting in increased repulsion between the three other carbonyls of the outer ring, further dilating FS1 and completing C-type inactivation.
Although C-type inactivation is normally started by R365–F416 contact (we postulate), appropriate mutation of almost any of the residues in the chain from R365 to D447 can increase its likelihood (Hoshi and Armstrong, 2013). The residues in this postulated chain seem closely linked in a favored conformation, activated or inactivated: modification of one residue can affect the entire chain and, indirectly, the conformation of FS1. Furthermore, the stability of the outer carbonyl ring depends, in some cases crucially, on the presence of a K+ ion in FS0 or FS1; removing external K+ may affect the entire chain, all the way to F416 in the voltage-sensor domain.
The slowing of C-type inactivation by extracellular K+ or TEA+ is an almost defining characteristic (Choi et al., 1991; López-Barneo et al., 1993). The importance of internal K+ in slowing C-type inactivation is shown by the finding that C-type inactivation is much faster in channels that are N-type inactivated, i.e., when the N terminus of any one of the four peptides forming a Shaker K+ channel enters the inner end of an open channel and blocks IK (Hoshi et al., 1990,1991). The importance of intracellular K+ was discovered and neatly explained by Baukrowitz and Yellen (1995): “N-type inactivation…inhibits outward K+ flux, which {i.e. the flux} normally fills an external ion site and thus prevents C-type inactivation.” The “external ion site,” in our opinion, is FS1 in the selectivity filter.
As noted above, negative internal voltage draws the two ions in the filter into FS2 and FS4, leaving the outer carbonyl ring, contributed by Y445, dependent for stabilization on a partially hydrated K+ ion in FS0 (Fig. 7 B). At negative voltage, K+ efflux is vanishingly small. With no K+ ions in the external solution, K+ occupancy of FS0 is thus unlikely at negative voltage, and the carbonyl groups of FS1 thus repel each other, making dilation of the outer carbonyl ring and C-type inactivation more probable as observed experimentally (Choi et al., 1991; López-Barneo et al., 1993; Kurata and Fedida, 2006).
When both K+ and Ca2+/La3+ are present externally, we propose that they compete with K+ for occupancy of FS0 (Fig. 7 C), particularly at negative voltage. Evidence for this competition is provided by the block of inward IK observed in high Ca2+ for voltage negative to approximately −60 mV (Gomez-Lagunas et al., 2003) and by the block of inward IK by La3+ seen in Fig. 6 (also see Tytgat and Daenens [1997]). In K+/Ca2+ competition, K+ is strongly preferred over Ca2+: the enhancement of C-type inactivation caused by 40 mM Ca2+ is largely overcome by only 2 mM K+ (Fig. 4). The relatively low affinity of Ca2+ for FS0 likely arises from its strong hydration (hydration enthalpy for Ca2+ is approximately −380 kcal/mol vs. approximately −77 kcal/mol for K+ [Burgess, 1978]). A firmly fixed hydration shell, we postulate, prevents close approach of Ca2+ to the outer carbonyls of FS1 (Fig. 7 C). Failure of Ca2+ to closely approach the carbonyls makes it ineffective in overcoming their mutual repulsion (Fig. 7 C, two-headed arrow): the force vector of carbonyl-Ca2+ attraction, rather than effectively pulling the carbonyls together, is predominantly outward in direction.
Once the outer carbonyls are dilated, it may be that FS1 is occupied by a partially hydrated Na+ or Ca2+ ion. It is important in this argument that FS2 and FS4 remain K+ selective and are occupied by K+ even though the outermost carbonyl ring is dilated. Thus, for example, an Na+ ion occupying the distorted FS1 cannot move inward to replace the securely complexed K+ ion in FS2. Similarly, a K+ ion in FS2 cannot move outward because of the energy barrier constituted by the dilated outer carbonyls.
Despite its higher hydration enthalpy, La3+ has higher apparent affinity for FS0 than does Ca2+ because of its triple charge. The probable mean hydration number is not very different for Ca2+, ∼4–12, and La3+, ∼6–8 (Burgess, 1978); so each is separated from the outer carbonyls of the channel by, we postulate, a single water molecule in the firmly attached inner shell surrounding the ion (Fig. 7 C for Ca2+). If both Ca2+ and La3+ have only one firmly attached hydration shell and are thus nearly equidistant from the outer carbonyls, the important factor determining relative Ca2+/La3+ affinity for FS0 will be the higher charge on La3+. Based on these ideas, we predict that any trivalent cation with a crystal ionic radius near 1.1 Å (e.g., Gd3+ and Tl3+) will enhance C-type inactivation.
The effects of Ca2+ on the rate of C-type inactivation, large at negative voltage, are small at positive voltages where there is a strong efflux of K+ through FS1 (Figs. 1 A and 3 B). It is known that C-type inactivation can occur at a near normal rate when the extracellular solution contains no external divalent cations (López-Barneo et al., 1993), consistent with the finding that the effects of Ca2+ are mainly at negative voltage. Some indications exist in the literature that strong K+ efflux at very positive voltage decreases C-type inactivation. This may be related to a phenomenon observed in some K+ channels called U-type inactivation (Klemic et al., 2001; Jamieson and Jones, 2014). The rather modest effect of TEA+ in slowing C-type inactivation during a depolarizing pulse remains to be explained (Choi et al., 1991). We postulate that TEA+ binds near T449 in ShB, trapping K+ in FS0 or FS1 (Fig. 7 B) where it prevents dilation of the outermost carbonyls, a modified “foot-in-the-door” hypothesis (Armstrong, 1971; López-Barneo et al., 1993).
In summary, we believe that the requirements and conditions for a cation promoter of C-type inactivation are (a) an extensively hydrated cation (therefore di- or trivalent) of the appropriate size (∼1.1-Å ionic radius), too big to permeate the K+ channel when hydrated, but (b) a good fit for FS0, where (c) it binds electrostatically (especially if trivalent) to the outer carbonyl ring of FS1, if (d) there is no K+ efflux through the channel (closed V-gate or N-type inactivated) and (e) if FS1 is not K+ occupied, as would be the case at sufficiently negative voltage.
Finally, are the observations in this article more compatible with pore constriction (Yellen et al., 1994; Cuello et al., 2010) or pore dilation (Hoshi and Armstrong, 2013) as the mechanism of C-type inactivation? Before the crystal structure was available, Yellen et al. (1994) originally proposed a constriction at the level of residue 449, which is outside the filter. In the crystal structure (2R9R; Long et al., 2007), the structural components postulated to be involved in the constriction are too widely separated for the required constriction to be a reasonable possibility (Hoshi and Armstrong, 2013). A subsequent proposal (for the bacterial KcsA channel [Cuello et al., 2010]) moved the constriction into the filter itself, at the position equivalent to G444 in ShB. We think the idea of constriction at G444 is unreasonable. Clearly constriction cannot occur if either FS1 or FS2 is K+ occupied: G444 contributes a carbonyl to K+ binding in both sites, and there is simply no room for constriction when K+ (or a water molecule or a hydronium ion) is present. Removing a K+ from FS1 or FS2 would require ∼77 kcal/mol. The carbonyls of a K+-occupied site are stabilized by this binding energy, which draws them close to the K+. In the absence of a K+, the electronegative carbonyls would repel, as shown in Fig. S2: decreasing the diameter of the carbonyl ring by 1 Å is less probable than increasing it by 1 Å, by 49 kJ/mol, a factor of ∼3 × 108. In short, our evidence is compatible with dilation for the reasons cited above, and the idea of constriction is physically implausible.
Supplementary Material
Acknowledgments
We gratefully acknowledge the help of Wikipedia in many of the literature searches.
T. Hoshi was supported in part through the National Institutes of Health (grant R01GM057654).
The authors declare no competing financial interests.
Kenton J. Swartz served as editor.
References
- Åqvist, J., and Luzhkov V.. 2000. Ion permeation mechanism of the potassium channel. Nature. 404:881–884 10.1038/35009114 [DOI] [PubMed] [Google Scholar]
- Armstrong, C.M.1971. Interaction of tetraethylammonium ion derivatives with the potassium channels of giant axons. J. Gen. Physiol. 58:413–437 10.1085/jgp.58.4.413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baukrowitz, T., and Yellen G.. 1995. Modulation of K+ current by frequency and external [K+]: a tale of two inactivation mechanisms. Neuron. 15:951–960 10.1016/0896-6273(95)90185-X [DOI] [PubMed] [Google Scholar]
- Burgess, J.1978. Metal Ions in Solution. Ellis Horwood, Chichester, NY: 481 pp [Google Scholar]
- Campbell, D.T., and Hille B.. 1976. Kinetic and pharmacological properties of the sodium channel of frog skeletal muscle. J. Gen. Physiol. 67:309–323 10.1085/jgp.67.3.309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi, K.L., Aldrich R.W., and Yellen G.. 1991. Tetraethylammonium blockade distinguishes two inactivation mechanisms in voltage-activated K+ channels. Proc. Natl. Acad. Sci. USA. 88:5092–5095 10.1073/pnas.88.12.5092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuello, L.G., Jogini V., Cortes D.M., and Perozo E.. 2010. Structural mechanism of C-type inactivation in K+ channels. Nature. 466:203–208 10.1038/nature09153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- del Camino, D., and Yellen G.. 2001. Tight steric closure at the intracellular activation gate of a voltage-gated K+ channel. Neuron. 32:649–656 10.1016/S0896-6273(01)00487-1 [DOI] [PubMed] [Google Scholar]
- Demo, S.D., and Yellen G.. 1991. The inactivation gate of the Shaker K+ channel behaves like an open-channel blocker. Neuron. 7:743–753 10.1016/0896-6273(91)90277-7 [DOI] [PubMed] [Google Scholar]
- Frankenhaeuser, B., and Hodgkin A.L.. 1957. The action of calcium on the electrical properties of squid axons. J. Physiol. 137:218–244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilly, W.F., and Armstrong C.M.. 1982. Divalent cations and the activation kinetics of potassium channels in squid giant axons. J. Gen. Physiol. 79:965–996 10.1085/jgp.79.6.965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gómez-Lagunas, F.1997. Shaker B K+ conductance in Na+ solutions lacking K+ ions: a remarkably stable non-conducting state produced by membrane depolarizations. J. Physiol. 499:3–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez-Lagunas, F., Melishchuk A., and Armstrong C.M.. 2003. Block of Shaker potassium channels by external calcium ions. Proc. Natl. Acad. Sci. USA. 100:347–351 10.1073/pnas.0237122100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hille, B.2001. Ion Channels of Excitable Membranes. Third edition Sinauer, Sunderland, MA: 814 pp [Google Scholar]
- Hoshi, T., and Armstrong C.M.. 2012. Initial steps in the opening of a Shaker potassium channel. Proc. Natl. Acad. Sci. USA. 109:12800–12804 10.1073/pnas.1209665109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoshi, T., and Armstrong C.M.. 2013. C-type inactivation of voltage-gated K+ channels: pore constriction or dilation? J. Gen. Physiol. 141:151–160 10.1085/jgp.201210888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoshi, T., Zagotta W.N., and Aldrich R.W.. 1990. Biophysical and molecular mechanisms of Shaker potassium channel inactivation. Science. 250:533–538 10.1126/science.2122519 [DOI] [PubMed] [Google Scholar]
- Hoshi, T., Zagotta W.N., and Aldrich R.W.. 1991. Two types of inactivation in Shaker K+ channels: effects of alterations in the carboxy-terminal region. Neuron. 7:547–556 10.1016/0896-6273(91)90367-9 [DOI] [PubMed] [Google Scholar]
- Hoshi, T., Wissuwa B., Tian Y., Tajima N., Xu R., Bauer M., Heinemann S.H., and Hou S.. 2013. Omega-3 fatty acids lower blood pressure by directly activating large-conductance Ca2+-dependent K+ channels. Proc. Natl. Acad. Sci. USA. 110:4816–4821 10.1073/pnas.1221997110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamieson, Q., and Jones S.W.. 2014. Shaker IR T449 mutants separate C- from U-type inactivation. J. Membr. Biol. 247:319–330 10.1007/s00232-014-9634-3 [DOI] [PubMed] [Google Scholar]
- Jensen, M.Ø., Jogini V., Borhani D.W., Leffler A.E., Dror R.O., and Shaw D.E.. 2012. Mechanism of voltage gating in potassium channels. Science. 336:229–233 10.1126/science.1216533 [DOI] [PubMed] [Google Scholar]
- Klemic, K.G., Kirsch G.E., and Jones S.W.. 2001. U-type inactivation of Kv3.1 and Shaker potassium channels. Biophys. J. 81:814–826 10.1016/S0006-3495(01)75743-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurata, H.T., and Fedida D.. 2006. A structural interpretation of voltage-gated potassium channel inactivation. Prog. Biophys. Mol. Biol. 92:185–208 10.1016/j.pbiomolbio.2005.10.001 [DOI] [PubMed] [Google Scholar]
- Larsson, H.P., and Elinder F.. 2000. A conserved glutamate is important for slow inactivation in K+ channels. Neuron. 27:573–583 10.1016/S0896-6273(00)00067-2 [DOI] [PubMed] [Google Scholar]
- Liu, Y., Jurman M.E., and Yellen G.. 1996. Dynamic rearrangement of the outer mouth of a K+ channel during gating. Neuron. 16:859–867 10.1016/S0896-6273(00)80106-3 [DOI] [PubMed] [Google Scholar]
- Loboda, A., Melishchuk A., and Armstrong C.. 2001. Dilated and defunct K channels in the absence of K+. Biophys. J. 80:2704–2714 10.1016/S0006-3495(01)76239-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long, S.B., Tao X., Campbell E.B., and MacKinnon R.. 2007. Atomic structure of a voltage-dependent K+ channel in a lipid membrane-like environment. Nature. 450:376–382 10.1038/nature06265 [DOI] [PubMed] [Google Scholar]
- López-Barneo, J., Hoshi T., Heinemann S.H., and Aldrich R.W.. 1993. Effects of external cations and mutations in the pore region on C-type inactivation of Shaker potassium channels. Receptors Channels. 1:61–71 [PubMed] [Google Scholar]
- Molina, A., Castellano A.G., and López-Barneo J.. 1997. Pore mutations in Shaker K+ channels distinguish between the sites of tetraethylammonium blockade and C-type inactivation. J. Physiol. 499:361–367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molina, A., Ortega-Sáenz P., and Lopez-Barneo J.. 1998. Pore mutations alter closing and opening kinetics in Shaker K+ channels. J. Physiol. 509:327–337 10.1111/j.1469-7793.1998.327bn.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morais-Cabral, J.H., Zhou Y., and MacKinnon R.. 2001. Energetic optimization of ion conduction rate by the K+ selectivity filter. Nature. 414:37–42 10.1038/35102000 [DOI] [PubMed] [Google Scholar]
- Ortega-Sáenz, P., Pardal R., Castellano A., and López-Barneo J.. 2000. Collapse of conductance is prevented by a glutamate residue conserved in voltage-dependent K+ channels. J. Gen. Physiol. 116:181–190 10.1085/jgp.116.2.181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perozo, E., MacKinnon R., Bezanilla F., and Stefani E.. 1993. Gating currents from a nonconducting mutant reveal open-closed conformations in Shaker K+ channels. Neuron. 11:353–358 10.1016/0896-6273(93)90190-3 [DOI] [PubMed] [Google Scholar]
- Shannon, R.D.1976. Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides. Acta Crystallogr. A. 32:751–767 10.1107/S0567739476001551 [DOI] [Google Scholar]
- Tao, X., Lee A., Limapichat W., Dougherty D.A., and MacKinnon R.. 2010. A gating charge transfer center in voltage sensors. Science. 328:67–73 10.1126/science.1185954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timpe, L.C., Jan Y.N., and Jan L.Y.. 1988. Four cDNA clones from the Shaker locus of Drosophila induce kinetically distinct A-type potassium currents in Xenopus oocytes. Neuron. 1:659–667 10.1016/0896-6273(88)90165-1 [DOI] [PubMed] [Google Scholar]
- Tytgat, J., and Daenens P.. 1997. Effect of lanthanum on voltage-dependent gating of a cloned mammalian neuronal potassium channel. Brain Res. 749:232–237 10.1016/S0006-8993(96)01171-7 [DOI] [PubMed] [Google Scholar]
- Yang, Y., Yan Y., and Sigworth F.J.. 1997. How does the W434F mutation block current in Shaker potassium channels? J. Gen. Physiol. 109:779–789 10.1085/jgp.109.6.779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yellen, G., Sodickson D., Chen T.Y., and Jurman M.E.. 1994. An engineered cysteine in the external mouth of a K+ channel allows inactivation to be modulated by metal binding. Biophys. J. 66:1068–1075 10.1016/S0006-3495(94)80888-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zagotta, W.N., Hoshi T., and Aldrich R.W.. 1990. Restoration of inactivation in mutants of Shaker potassium channels by a peptide derived from ShB. Science. 250:568–571 10.1126/science.2122520 [DOI] [PubMed] [Google Scholar]
- Zhou, M., Morais-Cabral J.H., Mann S., and MacKinnon R.. 2001a. Potassium channel receptor site for the inactivation gate and quaternary amine inhibitors. Nature. 411:657–661 10.1038/35079500 [DOI] [PubMed] [Google Scholar]
- Zhou, Y., Morais-Cabral J.H., Kaufman A., and MacKinnon R.. 2001b. Chemistry of ion coordination and hydration revealed by a K+ channel-Fab complex at 2.0 Å resolution. Nature. 414:43–48 10.1038/35102009 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.