Fluc channels protect bacteria from accumulating F− in acidic environments.
Abstract
Fluoride ion (F−) is a ubiquitous environmental threat to microorganisms, which have evolved a family of highly selective “Fluc” F− channels that export this inhibitory anion from their cytoplasm. It is unclear, however, how a thermodynamically passive mechanism like an ion channel can protect against high concentrations of external F−. We monitored external F− concentrations in Escherichia coli suspensions and showed that, in bacteria lacking Fluc, F− accumulates when the external medium is acidified, as a predicted function of the transmembrane pH gradient. This weak acid accumulation effect, which results from the high pKa (3.4) and membrane permeability of HF, is abolished by Fluc channels. We also found that, although bacterial growth is inhibited by high concentrations of F−, bacteria can withstand cytoplasmic F− at levels a hundred times higher than those that inhibit proliferation, resuming growth when the F− challenge is removed.
INTRODUCTION
Aqueous F−, ubiquitous in the environment at 10–100-µM levels, is potentially toxic to cells because at these concentrations it inhibits phosphoryl-transfer enzymes essential for energy production and nucleic acid synthesis (Adamek et al., 2005; Breaker, 2012). F− is a threat to unicellular organisms in particular because they are directly exposed to it, in contrast to animals, whose excretory epithelia do not transport this halide into the bloodstream. Accordingly, plasma membranes of many bacteria, yeasts, and protozoa carry F− exporter proteins to maintain cytoplasmic F− below inhibitory concentrations (Breaker, 2012; Stockbridge et al., 2012, 2013; Li et al., 2013). In the absence of such proteins, these microorganisms become hypersensitive to F−, an effect exacerbated by low extracellular pH. Two phylogenetically unrelated classes of F− exporters were recently identified: an exclusively prokaryotic clade of F−/H+ antiporters of the CLC anion-transporter superfamily and the “Fluc” family of F−-specific ion channels, also found in eukaryotes. These F− exporters can be considered analogous to the widespread multidrug resistance transporters that protect all organisms from natural organic xenobiotics and man-made drugs. F−/H+ antiporters, like multidrug resistance pumps, use energy sources (proton gradients or ATP hydrolysis) to drive thermodynamically uphill efflux of unwanted substrates. But how do Fluc ion channels, which can mediate only passive, downhill F− movement, export the anion to resist environmental stress?
At the present, early stage of F− membrane biology research, this question has been answered only with a hypothesis: Fluc F− channels are used to undermine the weak acid accumulation, or “ion trapping,” effect that would otherwise concentrate F− in the cytoplasm far above its extracellular concentration (Marquis et al., 2003; Breaker, 2012; Li et al., 2013; Stockbridge et al., 2013). This effect arises because HF is both a weak acid (pKa = 3.4) and a membrane-permeant molecule (Fig. 1). Without any F− anion exit pathway in the plasma membrane, a microorganism that finds itself in an acidic niche will accumulate intracellular F− according to the transmembrane proton gradient:
| (1) |
Figure 1.
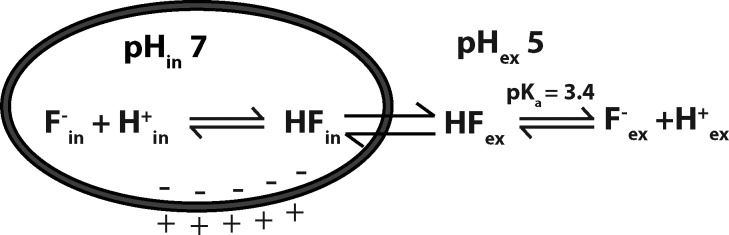
Weak acid accumulation effect for F−. A cell devoid of any membrane pathway for F− anion is depicted in a medium of pH 5.0. If cytoplasmic pH is regulated near neutrality, under such conditions F− will necessarily accumulate in the cell because of the high pKa and membrane permeability of HF.
According to this mechanism, in a hypoxic milieu of pH 5.5, for example, a bacterium lacking F− exporters, when experiencing mild acid stress, with pH 7.0 cytoplasm (Wilks and Slonczewski, 2007), would accumulate the anion ∼30-fold into the millimolar range, where enzyme inhibition would be severe. Fluc channels would prevent this concentrative uptake from occurring by permitting the anion to exit the cell down its electrochemical gradient. Although this idea rests on a rigorous thermodynamic argument, it calls out for experimental tests along the lines of those recently shown qualitatively in Fluc-knockout yeast (Li et al., 2013). By measuring F− uptake into Fluc-knockout Escherichia coli under a variety of pH conditions, we now show that these bacteria display pH-dependent hypersensitivity to F−, that the weak acid accumulation effect operates quantitatively as expected, and that Fluc channels completely abolish F− accumulation, permitting growth in the presence of F−. We also show that although F− accumulation stops growth of Fluc-knockout cells, it does not kill them; instead, it puts them into a quiescent condition from which they rapidly recover when F− is subsequently removed.
MATERIALS AND METHODS
Fluoride sensitivity growth assays for wild-type (strain BW25113) and Fluc-deleted (ΔFluc; kanr) E. coli strains have been described previously (Breaker, 2012; Stockbridge et al., 2012). E. coli strains do not possess CLCF F−/H+ antiporters. For F− uptake experiments, bacteria were grown to stationary phase in LB, pelleted, and resuspended in recording medium (150 mM NaCl, 0.1–1.3 mM NaF, 10 mM KPi, and 10 mM MES, pH 7.0). F− uptake was continuously monitored in a 2-ml stirred cell as a loss of F− from the extracellular recording medium, using an F−-specific electrode, as previously described for liposome suspensions (Stockbridge et al., 2013). We found empirically that 20–30 min was sufficient for the F− response to level off to a steady state. Intracellular F− concentration was estimated from the loss of extracellular anion followed electrochemically after an acid pulse, equivalent to the F− taken into the cytoplasmic volume, 0.8–1.0 fl/cell water, given the cell density (cells/ml). Cytoplasmic water content was determined as described previously (Stock et al., 1977). Cells in recording medium supplemented with 0.1% glucose were treated with [14C]inulin or [14C]sucrose, pelleted by centrifugation, weighed, and thoroughly dried by lyopholization. Water content was corrected for extracellular and periplasmic spaces by monitoring the distributions of [14C]inulin, which cannot cross the outer membrane, and [14C]sucrose, which crosses the outer membrane but is not transported into the cytoplasm. This cytoplasmic volume value agrees well with previously published measurements (Kubitschek and Friske, 1986; Volkmer and Heinemann, 2011). The ΔFluc knockout strain of E. coli was provided by R.R. Breaker (Yale University, New Haven, CT).
RESULTS
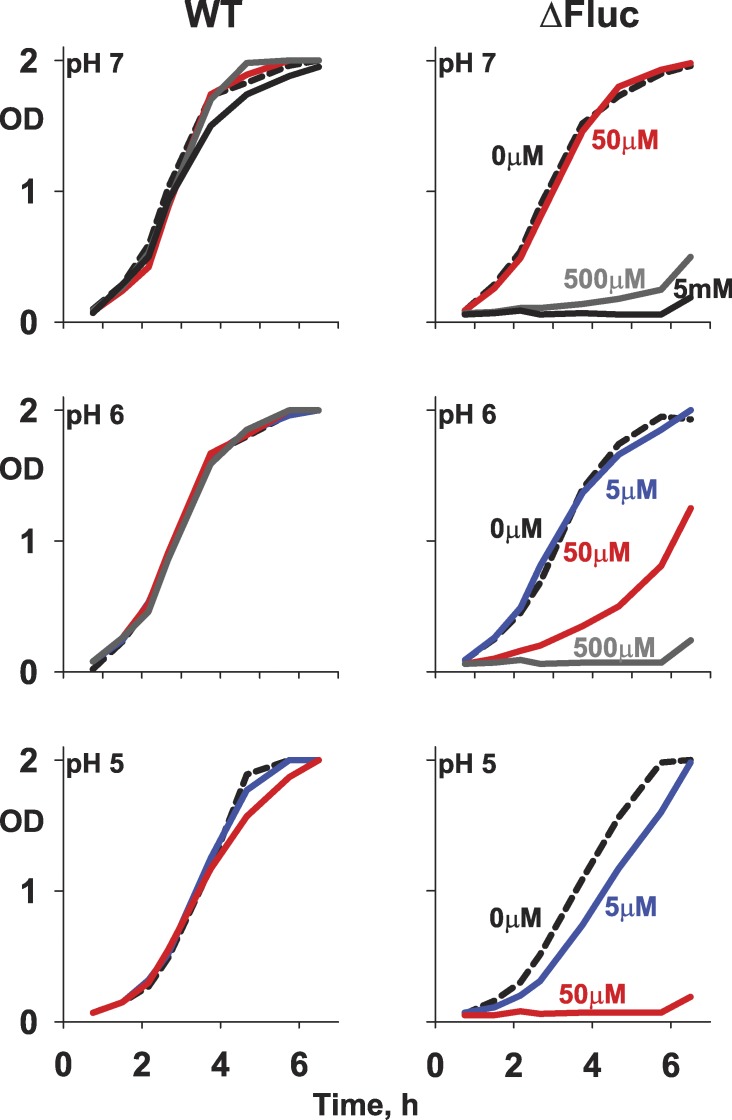
In previous work, a Fluc-knockout (ΔFluc) strain of E. coli was shown to be hypersensitive to F−, as assessed by growth in pH-neutral media, and this effect could be reversed by expressing F− exporters in the bacteria with a rescue plasmid (Breaker, 2012; Stockbridge et al., 2012). The ion-trapping effect predicts that the F− sensitivity of such bacteria should intensify as the growth medium is acidified, i.e., as the HF concentration in the medium increases at a given concentration of total fluoride. This prediction is confirmed in F− sensitivity experiments run at varying pH (Fig. 2). Although wild-type E. coli withstands F− in all conditions tested, growth inhibition of ΔFluc bacteria appears at progressively lower F− concentrations as pH is lowered from 7.0 to 5.0. However, this acid-enhanced sensitivity to F− might merely reflect a greater cellular susceptibility to two separate, independent stresses rather than to the intimate linkage between F− and pH that defines the weak acid accumulation effect.
Figure 2.
F− inhibition of E. coli growth at varying external pH. Growth curves are shown for wild-type and ΔFluc E. coli in media adjusted to the indicated pH and NaF concentrations.
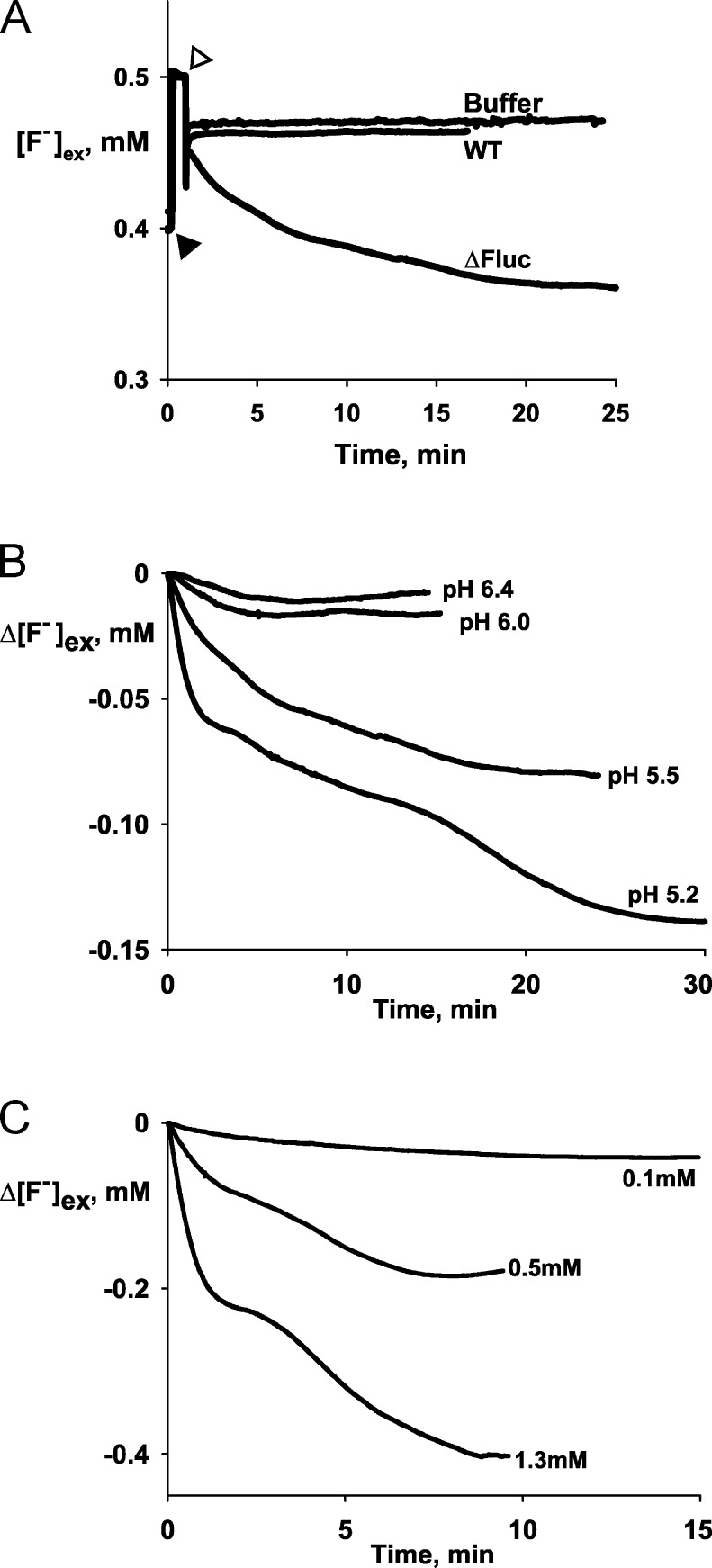
The central prediction of this effect is that F− should become concentrated in the cytoplasm as extracellular pH is lowered according to Eq. 1. This is readily tested by equilibrating the bacteria with F− at pH 7.0 and then acidifying the medium; under such conditions, F− transport into the bacteria should ensue, an expectation verified in Fig. 3 A. Bacteria were suspended in pH 7.0 media containing 0.5 mM F−, and extracellular F− concentration was continuously monitored electrochemically. Upon acidification to pH 5.5, extracellular F− immediately begins to drop in ΔFluc suspensions, indicating uptake of the halide into these bacteria over and above the initial equilibrated concentration. No such F− uptake is observed with wild-type bacteria. Moreover, F− uptake into ΔFluc bacteria increases as extracellular pH is lowered (Fig. 3 B), again as expected. A further prediction is that the amount of F− transported in response to acidification should increase with extracellular F− concentration, as is also observed (Fig. 3 C).
Figure 3.
F− uptake by E. coli. (A) Raw data traces of extracellular F− concentration in suspensions of wild-type and ΔFluc E. coli in response to acid exposure or control buffer without cells. Calibration pulse of 0.1 mM NaF (closed triangle) and addition of HCl to drop the pH from 7.5 to 5.5 (open triangle) are indicated. (B) Representative F− electrode traces of F− uptake by ΔFluc E. coli cells in 0.5 mM F− medium, with changing external pH to the indicated values at t = 0. (C) F− uptake at pH 5.3 in the indicated concentrations of external F−.
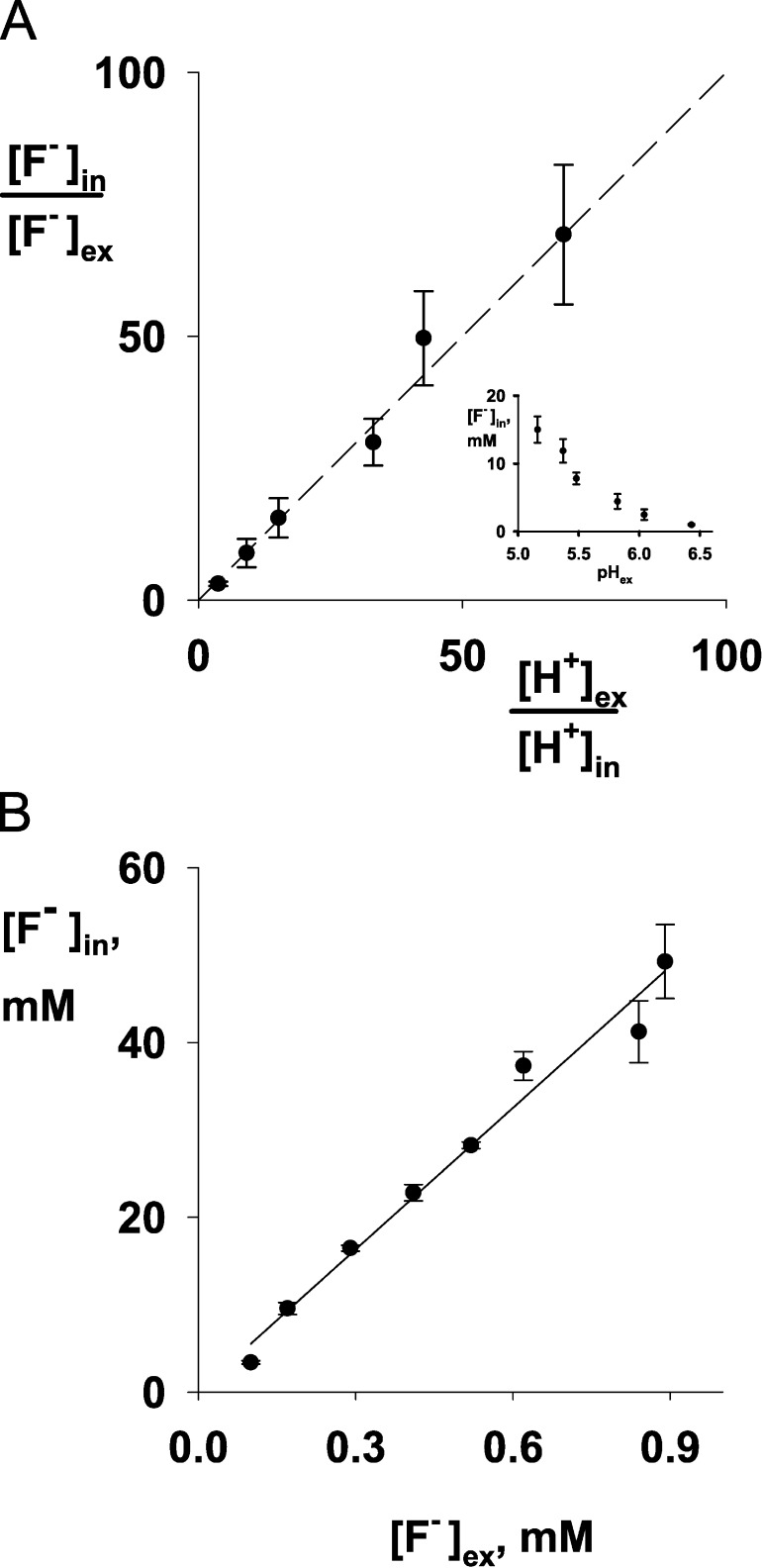
The F− uptake results above are qualitative. To test the ion-trapping mechanism quantitatively, the acid-induced loss of extracellular F− is used to estimate the absolute concentration in the bacterial cytoplasm. In these experiments (Fig. 4), ΔFluc bacteria exposed to a pH drop are allowed to take up F− for 20–30 min to reach steady-state, while extracellular F− concentration is monitored. The cells are then harvested and cytoplasmic F− concentration is estimated. The results agree precisely with the ion-trapping mechanism. The ratio of cytoplasmic to extracellular F− is numerically equal to the extracellular to cytoplasmic proton activity ratio over two orders of magnitude (Fig. 4 A), assuming that cytoplasmic pH is regulated near neutrality under these conditions, as has been long known to be the case (Slonczewski et al., 1981; Zilberstein et al., 1984; Wilks and Slonczewski, 2007). Furthermore, at a fixed pH gradient, cytoplasmic F− concentration varies linearly with extracellular F−, also as predicted (Fig. 4 B). The slope of the linear fit is equivalent to a pH gradient of 1.7 units, which is in good agreement with the value (1.8 units) under the assumption that cytoplasmic pH remains constant at 7.0 under these conditions.
Figure 4.
F− uptake by ΔFluc E. coli cells as a function of pH or F− concentration. (A) Ratio of internal and external [F−] with varying pH. The dashed line has a slope of 1, as predicted by Eq. 1. Inset plots data directly against medium pH. (B) Ratio of internal and external F−. The solid line is a fit to the data with slope of 54, corresponding to a transmembrane pH differential of 1.7 units. F− uptake was determined as in Fig 3. Each point shows mean (±SEM) of three independent measurements. Internal pH was assumed to remain constant at 7.0.
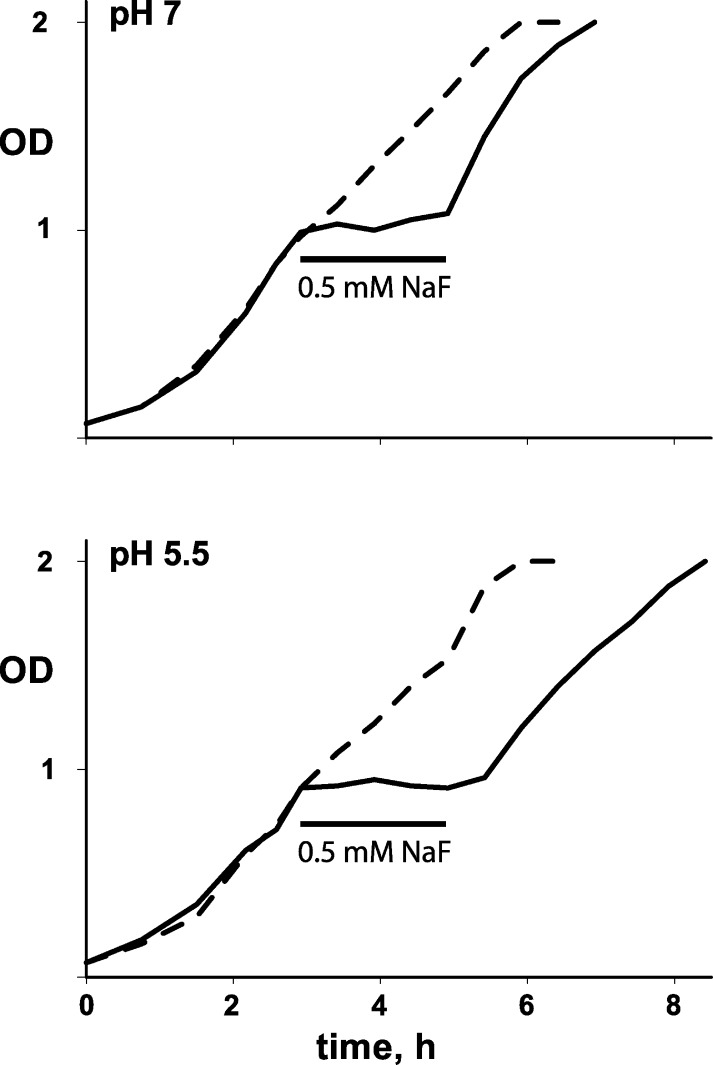
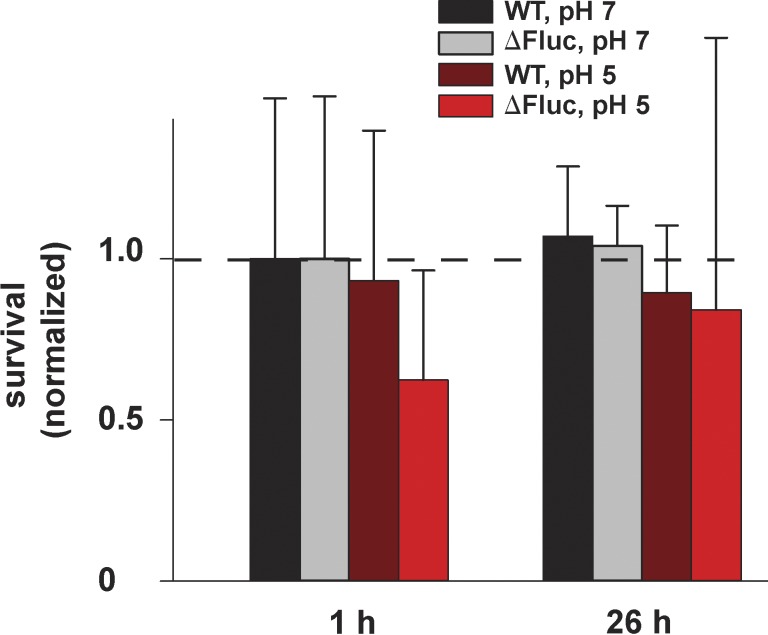
The aforementioned results verify the idea that HF acts as a membrane-permeant weak acid to deliver F− anion to the bacterial cytoplasm and trap it there at high concentrations, thus preventing growth. However, they do not address the question of whether bacteria are killed by such F− exposure. The experiments of Fig. 5 show that the bacteria are not killed, but merely stalled. Here, ΔFluc cells are grown at pH 7.0 for 3 h, at which time the cells are pelleted, washed, and resuspended at pH 7.0 or 5.5 with 0.5 mM F− added to the medium, an insult which stops growth immediately in both pH conditions. After 2 h, the cells are washed free of F−, and growth resumes after a pH-dependent lag. At pH 7.0, where cytoplasmic F− is low (∼0.5 mM), no lag is observed, but at pH 5.5, where intracellular F− has accumulated to ∼15 mM, a lag of roughly 30 min is necessary for full recovery of growth rate. The ability of the ΔFluc cells to hunker down and wait out a long-term F− challenge is further illustrated in Fig. 6. Here, stationary-state cells are exposed to 0.5 mM F− at pH 7.0 or 5.5 for 2–26 h, after which time survivors are counted by growth of colonies on F−-free plates. Survival of all cells, wild-type and ΔFluc, is close to 100% for both pH conditions. These results with E. coli are in harmony with a recent report (Li et al., 2013) that long-term exposure of several yeast species to F− prevents growth at 10 mM but does not kill the cells until concentrations as high as 0.1–0.2 M are used.
Figure 5.
F− exposure during bacterial growth. ΔFluc E. coli in log-phase growth was treated with 0.5 mM NaF at the indicated pH. After 2 h, the F− was washed away, the pH was returned to 7.0, and optical density of the culture was monitored. Dashed curve is control without F− exposure.
Figure 6.
Bacterial survival after F− exposure. Wild-type or ΔFluc E. coli culture was incubated with 0.5 mM NaF at the indicated pH for the indicated time. Afterward, cells were plated on neutral-pH LB plates, and colonies were counted after 16 h. The number of colonies was normalized against a wild-type culture that was not treated with F− (dashed line indicates 1.4 × 109 CFU/ml). Points represent means ± SEM of three independent measurements.
DISCUSSION
The experiments here are aimed at a counterintuitive solution to the problem of environmental F− toxicity that many microorganisms have reached: the use of an ion channel, instead of an energy-consuming pump, as an exporter for cytoplasmic F−. Though thermodynamically passive, Fluc-type F− channels act to keep cytoplasmic F− below extracellular concentrations and below levels that would otherwise inhibit metabolism. At first glance, we might expect that an ion channel would work against this purpose by ushering extracellular F− into the cell rather than out of it. But two physiological circumstances common to most microorganisms operate so that these channels provide a net export pathway for the anion. First, nearly all cells maintain negative membrane potentials, and these can be quite large for bacteria and yeast under energy-using conditions. For a bacterial cell with a −100-mV potential, the equilibrium level of cytoplasmic F− would be ∼50-fold lower than the extracellular concentration, i.e., <1 µM (far below inhibitory levels; Adamek et al., 2005). Second, an ion channel would be of particular utility for a microorganism haunting diverse niches through its life cycle and encountering a weakly acidic environment. In such a case, any organism without an F− permeability pathway would necessarily concentrate the halide in its cytoplasm far above the extracellular concentration via the weak acid accumulation effect. An F− channel present in the cell membrane would abolish this effect by collapsing the F− gradient, exporting the anion to its low equilibrium level.
It is now firmly established that Fluc proteins are F−-specific ion channels that protect E. coli and yeasts from F− toxicity (Baker et al., 2012; Stockbridge et al., 2012, 2013; Li et al., 2013). But the relevance of the weak acid accumulation mechanism to F− toxicity and Fluc function has not been previously examined. These experiments demonstrate quantitatively that Fluc channels are required to counteract cytoplasmic F− accumulation driven by pH gradients across the bacterial inner membrane set up by mild acidification of the growth medium, a condition which mimics environments that microorganisms sporadically encounter.
These experiments also bear upon the question of Fluc channels as novel targets for antibiotics (Li et al., 2013; Nelson et al., 2014). A Fluc-directed antibiotic would not by itself be bacteriocidal. Instead, by shutting down Fluc channels of a pathogenic bacterium or fungus, it could retard growth, but eliminating the stalled organism would require cooperation of the host’s immune system. Moreover, for such a drug to be effective, co-administration of F− would be necessary, perhaps along with a lowered pH of the infected region. Such maneuvers are straightforward in the laboratory, but would call for clever bioengineering in clinical applications.
Acknowledgments
The authors declare no competing financial interests.
Michael Pusch served as guest editor.
References
- Adamek, E., Pawłowska-Góral K., and Bober K.. 2005. In vitro and in vivo effects of fluoride ions on enzyme activity. Ann. Acad. Med. Stetin. 51:69–85 [PubMed] [Google Scholar]
- Baker, J.L., Sudarsan N., Weinberg Z., Roth A., Stockbridge R.B., and Breaker R.R.. 2012. Widespread genetic switches and toxicity resistance proteins for fluoride. Science. 335:233–235 10.1126/science.1215063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breaker, R.R.2012. New insight on the response of bacteria to fluoride. Caries Res. 46:78–81 10.1159/000336397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubitschek, H.E., and Friske J.A.. 1986. Determination of bacterial cell volume with the Coulter Counter. J. Bacteriol. 168:1466–1467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, S., Smith K.D., Davis J.H., Gordon P.B., Breaker R.R., and Strobel S.A.. 2013. Eukaryotic resistance to fluoride toxicity mediated by a widespread family of fluoride export proteins. Proc. Natl. Acad. Sci. USA. 110:19018–19023 10.1073/pnas.1310439110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marquis, R.E., Clock S.A., and Mota-Meira M.. 2003. Fluoride and organic weak acids as modulators of microbial physiology. FEMS Microbiol. Rev. 26:493–510 10.1111/j.1574-6976.2003.tb00627.x [DOI] [PubMed] [Google Scholar]
- Nelson, J.W., Zhou Z., and Breaker R.R.. 2014. Gramicidin D enhances the antibacterial activity of fluoride. Bioorg. Med. Chem. Lett. 24:2969–2971 10.1016/j.bmcl.2014.03.061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slonczewski, J.L., Rosen B.P., Alger J.R., and Macnab R.M.. 1981. pH homeostasis in Escherichia coli: measurement by 31P nuclear magnetic resonance of methylphosphonate and phosphate. Proc. Natl. Acad. Sci. USA. 78:6271–6275 10.1073/pnas.78.10.6271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stock, J.B., Rauch B., and Roseman S.. 1977. Periplasmic space in Salmonella typhimurium and Escherichia coli. J. Biol. Chem. 252:7850–7861 [PubMed] [Google Scholar]
- Stockbridge, R.B., Lim H.H., Otten R., Williams C., Shane T., Weinberg Z., and Miller C.. 2012. Fluoride resistance and transport by riboswitch-controlled CLC antiporters. Proc. Natl. Acad. Sci. USA. 109:15289–15294 10.1073/pnas.1210896109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stockbridge, R.B., Robertson J.L., Kolmakova-Partensky L., and Miller C.. 2013. A family of fluoride-specific ion channels with dual-topology architecture. Elife (Cambridge). 2:e01084 10.7554/eLife.01084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkmer, B., and Heinemann M.. 2011. Condition-dependent cell volume and concentration of Escherichia coli to facilitate data conversion for systems biology modeling. PLoS ONE. 6:e23126 10.1371/journal.pone.0023126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilks, J.C., and Slonczewski J.L.. 2007. pH of the cytoplasm and periplasm of Escherichia coli: rapid measurement by green fluorescent protein fluorimetry. J. Bacteriol. 189:5601–5607 10.1128/JB.00615-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zilberstein, D., Agmon V., Schuldiner S., and Padan E.. 1984. Escherichia coli intracellular pH, membrane potential, and cell growth. J. Bacteriol. 158:246–252 [DOI] [PMC free article] [PubMed] [Google Scholar]