Abstract
Background
The purpose of this study was to assess aspiration pneumonia (AsPn) rates and predictors after chemo-irradiation for head and neck cancer.
Methods
The was a prospective study of 72 patients with stage III to IV oropharyngeal cancer treated definitively with intensity-modulated radiotherapy (IMRT) concurrent with weekly carboplatin and paclitaxel. AsPn was recorded prospectively and dysphagia was evaluated longitudinally through 2 years posttherapy by observer-rated (Common Toxicity Criteria version [CTCAE]) scores, patient-reported scores, and videofluoroscopy.
Results
Sixteen patients (20%) developed AsPn. Predictive factors included T classification (p = .01), aspiration detected on videofluoroscopy (videofluoroscopy-asp; p = .0007), and patient-reported dysphagia (p = .02–.0003), but not observer-rated dysphagia (p = .4). Combining T classification, patient reported dysphagia, and videofluoroscopy-asp, provided the best predictive model.
Conclusion
AsPn continues to be an under-reported consequence of chemo-irradiation for head and neck cancer. These data support using patient-reported dysphagia to identify high-risk patients requiring videofluoroscopy evaluation for preventive measures. Reducing videofluoroscopy-asp rates, by reducing swallowing structures radiation doses and by trials reducing treatment intensity in patients predicted to do well, are likely to reduce AsPn rates.
Keywords: oropharyngeal cancer, head and neck cancer, IMRT, aspiration pneumonia, dysphagia
Introduction
Long-term dysphagia is a major sequel of chemo-irradiation of head and neck cancer according to both observer-rated1 and patient-reported2 assessments. Swallowing studies in these patients are characterized by decreased coordination between the various phases of the swallow process, reduced laryngeal elevation and glottic closure, and reduced inversion of the epiglottis during the swallow, all of which promote aspiration.3–5 In addition, reduced movement of the base of tongue and pooling of residue in the vallecula and pyriform sinuses promote aspiration after the swallow.3–5 Loss of laryngeal sensation may accompany these abnormalities causing “silent aspiration,” in which the cough reflex is absent or is not effective in expelling the aspirate.4–5
We have previously reported an association between dysphagia in patients receiving intensive chemo-irradiation for head and neck cancer and aspiration pneumonia (AsPn).3 Literature review at the time showed only anecdotal previous reports, suggesting that AsPn was an under-reported sequel of chemo-irradiation. Several subsequent reports confirmed this observation, with AsPn rates of 8% to 20%, including cases of death,6–9 and an even higher aspiration rate of 38% in patients referred for an evaluation of dysphagia after therapy.10 However, the large majority of trials of chemo-irradiation trials for head and neck cancer in the past decade have not detailed AsPn as a potential sequel of therapy, and its reporting continues to be anecdotal. For example, pooled database of late toxicities in Radiation Therapy Oncology Group studies of chemo-irradiation detailed very few cases of infections but not AsPn.1 Thus, although AsPn is the most common cause of death in patients with dysphagia because of neurologic disorders,11 its rates and consequences after chemo-irradiation for head and neck cancer are poorly documented.
The purpose of the current study was to assess the long-term rate of AsPn and its predictors in a prospective study of chemotherapy concurrent with intensity-modulated radiotherapy (IMRT) for patients with advanced oropharyngeal cancer. IMRT planning aimed specifically at reducing the doses to the swallowing-related anatomic structures. The clinical results of the study showed on average mild posttherapy dysphagia.12 The analyses presented in this article aimed to assess the risk and the potential predictors of AsPn, including prospective measures of aspiration risk detected on modified barium swallow (videofluoroscopy), clinical factors, and observer-rated and patient-reported dysphagia, collected longitudinally in the study. Our main goal was to identify which patients are at high-risk of AsPn so that preventive measures can be undertaken.
Patients and Methods
From 2003 to 2008, 72 patients with stage III to IV oropharyngeal cancer participated in a prospective study approved by the University of Michigan Review Board and all patients signed an informed consent. All received weekly chemotherapy with carboplatin (area under the curve [AUC] = 1) and paclitaxel (30 mg/m2) concurrent with radiotherapy. IMRT prescribed doses were 70 Gy to gross tumor/involved nodal metastases, 59 to 63 Gy to high-risk subclinical disease, and 56 to 59 Gy to low-risk nodal regions, all in 35 fractions over 7 weeks. Planning priorities included sparing the parts of the oral cavity, major salivary glands, pharyngeal constrictors, larynx, and esophagus, which were outside the targets, in an effort to reduce dysphagia and xerostomia, as detailed elsewhere.12
Episodes of AsPn were prospectively recorded as part of the toxicity recording for the clinical study. Patients were considered to have AsPn based on clinical symptoms with corresponding characteristic radiographic changes.11
Videofluoroscopy was performed before therapy and at 3, 12, and 24 months posttreatment, according to previously detailed methods.12 Patients with persistent lymphadenopathy or positron emission tomography-based suspected residual lymph node disease posttherapy (n = 22) underwent planned neck dissection at 3 months and additional videofluoroscopy at approximately 4 months. Videofluoroscopy-based aspiration (videofluoroscopy-asp) was defined once the bolus passed the level of the vocal folds and entered the subglottic region. For each video-fluoroscopy study, the number of aspiration events and the penetration/aspiration (P/A) score13 were recorded, with the highest (worst) score of the various boluses used for each patient's score at each time point. Silent aspiration or inefficient clearance were noted when an aspiration event produced nonproductive or no spontaneous cough. Patients demonstrating videofluoroscopy-asp were instructed in using maneuvers to reduce aspiration and swallow exercises to improve the safety and efficiency of the swallow.14 Feeding tubes were considered for symptomatic patients who did not improve following instructions. No preventive swallowing exercise therapy was used.
Patients completed 2 validated quality of life (QOL) instruments at baseline and 1, 3, 6, 12, 18, and 24 months posttreatment: the Head and Neck QOL (HN-QOL) and the University of Washington QOL (UW-QOL) questionnaires, detailed elsewhere.12 In this study, we analyzed the results of the dysphagia-related questions 8 and 9 from the HN-QOL questionnaire (“How much are you bothered by problems with swallowing liquids,” and “how much are you bothered by problems with swallowing solids?”), question 6b from the UW-QOL questionnaire (“How well do you swallow?”), and the Eating Domain Score from HN-QOL (a composite score from 6 questions about problems chewing, dryness while eating, taste, swallowing solids, and swallowing liquids). The scaling of scores for each question was modified to range from 0 to 100, with higher scores corresponding to worse condition, as detailed elsewhere.12 After scaling, each item score in the Eating Domain was added and the average sum, including all the items in the Domain, was transformed linearly to produce the summary Domain score, with 0 the best and 100 the worst score.
Observer-rated toxicities using the Common Terminology Criteria for Adverse Events (CTCAE) version 2 scale were prospectively recorded at baseline and at each follow-up (every 2 months) throughout 2 years. CTCAEdysphagia scores were used in this analysis (0 = no dysphagia; 1 = mild dysphagia without dietary modifications; 2 = moderate dysphagia requiring change in diet; 3 = supplemental enteral feeding required; and 4 = oral feeding is not feasible).
Statistical analysis
Logistic regression models were used to assess the relation between the covariates (clinical factors, observer-rated and patient-reported dysphagia scores, and videofluoroscopy scores). Models for the longitudinally measured covariates included multiple observations per subject corresponding to each time a covariate was measured with associated binary outcome defined as the presence/absence of any subsequent development of AsPn. To account for subject correlation, generalized estimating equations with a first order autoregressive correlation structure were used to fit the model.
Separate multivariate models were fit using the clinical variables, the QOL questionnaire and CTCAEdysphagia scores, and videofluoroscopy-based variables, by including all univariately significant variables. Further multivariate models were then built by combining significant factors from these multivariate models. Empirical receiver operating characteristic curves were generated from both univariate and multivariate models. The associated AUC values were calculated as an overall summary of the discriminatory ability of the model. Whether a particular variable added to the predictive ability of a model containing another variable was assessed using the multivariate p value from the model containing both variables. Leave 1 out, cross-validation was used to obtain realistic estimates of AUC from the multivariate models. The software packages R and SAS (V9.2, Cary, NC) were used for analysis.
Results
Patient characteristics are listed in Table 1. Seventy-two patients were accrued; 1 patient who declined continued participation a few months after therapy was excluded from analysis. Median follow-up for toxicity was 49 months (range, 12–97 months). Eighteen patients (25%) required feeding tubes during therapy, of whom 1 (1.4%) required a feeding tube >6 months posttherapy.
Table 1. Patient characteristics.
| Age | |
| Median 54.6 y | (range, 40–75 y) |
| Sex | |
| Male | 63 (89%) |
| Female | 8 (11%) |
| Disease site | |
| Base of tongue | 38 (54%) |
| Tonsil | 33 (46%) |
| T classification | |
| T1 | 9 (13%) |
| T2 | 28 (39%) |
| T3 | 16 (23%) |
| T4 | 18 (25%) |
| N classification | |
| N0 | 5 (7%) |
| N1 | 6 (8%) |
| N2a | 8 (11%) |
| N2b | 32 (45%) |
| N2c | 14 (20%) |
| N3 | 6 (8%) |
| AJCC stage | |
| III | 8 (11%) |
| IVA | 57 (80%) |
| IVB | 6 (8.5%) |
| BMI (pretreatment) | |
| <18.50 | 0 |
| 18.50–24.99 | 12 (17%) |
| 25.00–29.99 | 27 (38%) |
| ≥30.00 | 32 (45%) |
| Weight loss from pretreatment to 3 mo | |
| <10 lbs | 9 (13%) |
| 10–20 lbs | 16 (23%) |
| 20–30 lbs | 17 (24%) |
| 30–40 lbs | 14 (20%) |
| 40–50 lbs | 11 (15%) |
| >50 lbs | 4 (5%) |
| Smoking status* | |
| Current smoker | 16 (22%) |
| Former smoker | 29 (41%) |
| Never smoker | 26 (37%) |
| Follow-up | |
| Median 49 mo | (range, 12.2–97.2 mo) |
Abbreviations: AJCC, American Joint Committee on Cancer; BMI, body mass index.
For current and past cigarette smokers, the median number of pack-years smoked was 21 (range, 1–140 pack-years).
Sixteen patients developed AsPn with 12 requiring hospitalization and all recovered after various antibiotic regimens. The actuarial rate of AsPn was 15% at 24 months and 20% at 36 months, with median time from start of therapy to AsPn at 18 months (range, 1–72 months; Figure 1). No patient had recurrent pneumonia. Radiological findings included consolidations/opacities of bilateral lower lobes,5 right lower lobe,3 left lower lobe,5 posterior segments of left upper lobe (1 patient), and unavailable radiological findings in 2 patients diagnosed with AsPn and treated elsewhere. Bacterial diagnosis was made through bronchoscopy in only 1 patient recovering from oral flora.
Figure 1.
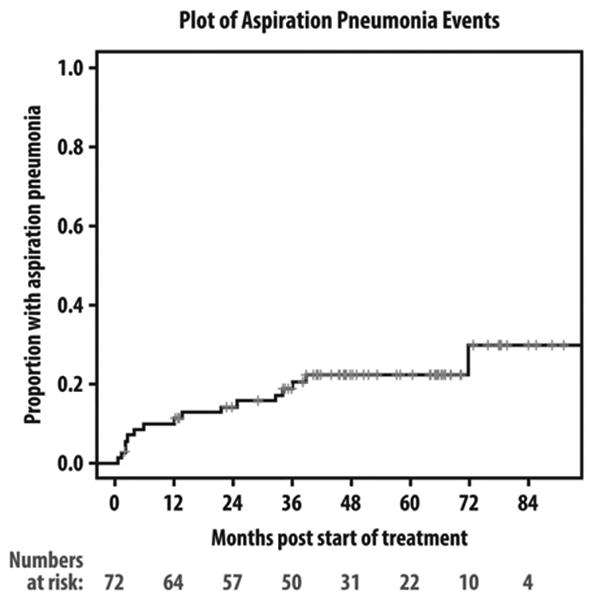
Kaplan–Meier plot of aspiration pneumonia (AsPn) events since starting therapy.
Of the clinical variables tested (Table 2), univariate analysis (UVA) showed current smoking (p = .04) and T classification (p = .005) to be significantly associated with the development of AsPn. T classification remained significant on multivariate analysis (MVA; 0 of 9, 3 of 28, 6 of 16, and 7 of 18 patients with pneumonia/total patients with T classifications 1, 2, 3, and 4, respectively; p = .01).
Table 2.
Univariate and multivariate analyses of the correlations of clinical variables and aspiration pneumonia.
| Variable | Odds ratio | 95% Confidence interval | Univariate p value | Univariate AUC | Multivariate p value | |
|---|---|---|---|---|---|---|
| Age | 1.06 | 0.99 | 1.14 | .0935 | 0.624 | |
| Smoking history | 1.94 | 0.56 | 6.79 | .2992 | 0.571 | |
| Current smoker | 3.58 | 1.08 | 11.9 | .0376 | 0.629 | .1386 |
| Baseline BMI | 0.97 | 0.85 | 1.10 | .605 | 0.54 | |
| Disease site | ||||||
| Base of tongue | 1.67 | 0.53 | 5.21 | .380 | 0.562 | |
| Tumor volume, cc | 1.01 | 1.00 | 1.01 | .0688 | 0.672 | |
| T classification | 2.54 | 1.32 | 4.87 | .0050 | 0.736 | .0123 |
| N classification | 1.24 | 0.77 | 1.99 | .3705 | 0.614 | |
| AJCC stage | 0.80 | 0.23 | 2.80 | .721 | 0.52 | |
| Sex | 0.43 | 0.09 | 2.01 | .281 | 0.549 | |
| Neck dissection | 0.704 | 0.199 | 2.49 | .589 | 0.536 | |
Abbreviations: AUC, area under the curve; BMI, body mass index; AJCC, American Joint Committee on Cancer.
The longitudinal distributions of the patient-reported and observer-rated dysphagia scores in this study were detailed elsewhere.12 The scores of the patient-reported Eating Domain and the dysphagia-related questions 8 and 9 from HN-QOL and question 6b from UW-QOL were all statistically significant predictors of AsPn on UVA, but the observer-rated dysphagia scores were not. Of the 16 episodes of pneumonia, the preceding CTCAEdysphagia scores were 0 to 1 in 11 cases and 2 to 3 in 5 cases (p = .4; Table 3). Eating Domain Score and UW-QOL question 6b remained significant on MVA (Table 3).
Table 3.
Univariate and multivariate analysis of the correlation between observer-rated or patient-reported dysphagia variables and subsequent aspiration pneumonia.
| Variable | Odds ratio* | 95% Confidence interval | Univariate p value | Univariate AUC | Multivariate p value | |
|---|---|---|---|---|---|---|
| Observer-rated dysphagia | 1.03 | 0.96 | 1.10 | .41 | 0.654 | |
| HN-QOL question 8 | 1.16 | 1.03 | 1.30 | .012 | 0.63 | .402 |
| HN-QOL question 9 | 1.06 | 1.01 | 1.10 | .013 | 0.662 | .072 |
| UW-QOL question 6b | 1.02 | 1.00 | 1.04 | .027 | 0.665 | .021 |
| Eating Domain Score | 1.12 | 1.04 | 1.21 | .0029 | 0.673 | .0003 |
Abbreviations: AUC, area under the curve; HN-QOL, Head and Neck Quality of Life questionnaire; UW-QOL, University of Washington Quality of Life questionnaire. * The reported odds ratio for the Eating Domain Score is scaled for a 10% increase in Eating Domain Score.
Details of the longitudinal results of videofluoroscopy in this study were provided elsewhere.12 All patients had a posttherapy videofluoroscopy study at a minimum of 2 time points. All had a pretreatment videofluoroscopy evaluation, with 94%, 92%, and 80% having follow-up studies at 3, 12, and 24 months, respectively. Twenty-two patients had planned neck dissection 3 months posttherapy and all had an additional videofluoroscopy study 4 months posttherapy. Videofluoroscopy-asp was noted in 35 patients at least once. Fifty-seven percent of patients with videofluoroscopy-asp aspirated at only 1 examination, whereas the rest aspirated on multiple posttherapy videofluoroscopies. Of 16 patients with AsPn, 13 showed evidence of videofluoroscopy-asp before the episode of AsPn. Posttherapy videofluoroscopy-asp (representing any aspiration found on videofluoroscopy), videofluoroscopy-based silent aspiration, high P/A score, and the number of aspiration events recorded on a single videofluoroscopy study, were all statistically significant predictors of AsPn on UVA (Table 4). Because of the strong association between all the videofluoroscopy variables (Spearman Correlation ≥ 0.8), we used videofluoroscopy-asp in further modeling because of its highest AUC (0.73). No difference was observed in the predictive value of early posttherapy (3 months) versus late (12 or 24 months) videofluoroscopy-asp in predicting AsPn (AUC 0.76 vs 0.71, respectively). In contrast to the posttherapy videofluoroscopy-asp, aspiration on pretherapy videofluoroscopy was not a significant factor in developing AsPn (p = .2).
Table 4.
Univariate analysis of videofluoroscopy variables and their correlations with subsequent aspiration pneumonia.
| Variable | Odds ratio | 95% Confidence interval | Univariate p value | Univariate AUC | |
|---|---|---|---|---|---|
| Videofluoroscopy-asp | 2.46 | 1.46 | 4.14 | .0007 | 0.731 |
| No. of aspiration events/study | 1.16 | 1.00 | 1.33 | .043 | 0.718 |
| P/A scale | 1.19 | 1.09 | 1.31 | .0003 | 0.724 |
| Silent aspiration | 1.29 | 0.95 | 1.75 | .047 | 0.652 |
Abbreviations: AUC, area under the curve; asp, aspiration; P/A, penetration/aspiration.
Combining the clinical, patient-reported, and video-fluoroscopy factors found significant on MVAs (T classification, Eating Domain Score, UW-QOL question 6b, and videofluoroscopy-asp) into a single model improved the AUC significantly (0.87) compared to either factor alone (Figure 2). Eating Domain scores had the highest AUC among the patient-reported outcomes (Table 3), therefore, they were used in the receiver operating characteristic curves.
Figure 2.
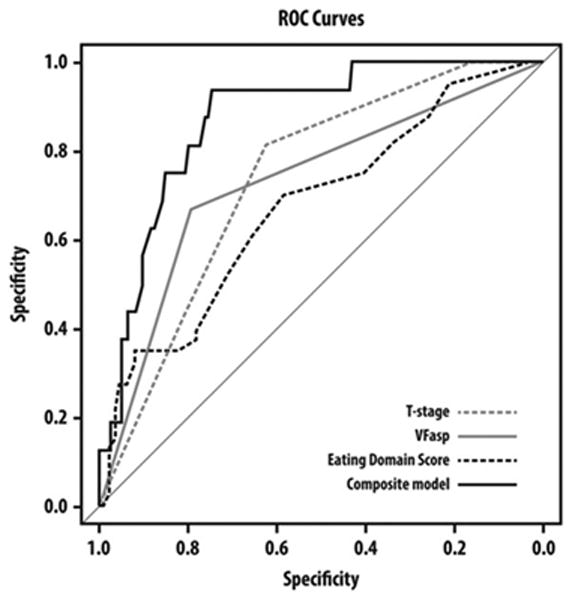
Receiver operator characteristics curves comparing the ability of T classification, videofluoroscopy-aspiration (asp), Eating Domain Score, and a composite model to predict aspiration pneumonia.
Discussion
The 20% actuarial rate of long-term posttreatment AsPn observed in this study is consistent with the rates reported in previous series, which specifically aimed to assess AsPn after chemo-irradiation of head and neck cancer.6–9 However, major clinical trials continue to under-report the incidence of AsPn, and almost all the trials detailed in a recent review of chemo-irradiation of head and neck cancer15 have not captured this information at all. We suggest that AsPn should be routinely included as an item in the reporting of toxicities after chemo-irradiation for head and neck cancer.
In patients with AsPn, the aspiration episode is generally not witnessed and the diagnosis is inferred when a patient at risk has radiographic evidence of an infiltrate in a characteristic bronchopulmonary segment that depends on patient position at the time of aspiration: basal segments of lower lobes in patients aspirating while in an upright or semirecumbent position, and posterior segments of the upper lobes and apical segments of the lower lobes in patients aspirating in the recumbent position.11 This radiological variety was observed in the patients in our series. The usual clinical course is an acute pneumonic process similar to typical community-acquired pneumonia, however, without effective treatment there is a higher incidence of cavitation and lung abscess formation.11
Rehabilitation of the patient at risk of aspiration is performed by speech-language therapists using techniques including postural changes in head position during the swallow, swallowing maneuvers designed to close the entrance to the airway early in the swallow, and diet modifications.4,5,14 In typical clinical practice, patients are referred for swallow evaluation only if they present with high-grade observer-rated dysphagia.10 However, in the current study, observer-rated dysphagia did not predict subsequent AsPn. Rather, patient-reported dysphagia, videofluoroscopy-asp, and advanced T classification, were significant predictors of AsPn. These findings suggest that referral for swallow evaluation that includes video-fluoroscopy should be considered routine practice, especially in patients with advanced T classification, and not restricted to those with overt observer-rated dysphagia. In addition, routine patient-based symptom grading should be done in addition to observer-rated grading. Dysphagia is a subjective symptom, likely to be underestimated by clinicians.16,17 We have previously found that grade 1 observer-rated dysphagia that persisted through 12 months posttherapy was associated with patient-reported dysphagia and videofluoroscopy findings similar to those found in patients with higher-grade observer-rated dysphagia.18 Similarly, the Northwestern University group found that patient self-perception of dysphagia had moderate correlation with videofluoroscopy findings in patients with head and neck cancer, supporting its complementary role in the assessment of swallowing.19
AsPn predictors were analyzed by Purkey et al10 in a retrospective review of 52 patients who presented with clinical symptoms of dysphagia after therapy. They found significant correlation between videofluoroscopy-asp and current smoking and the development of AsPn. Videofluoroscopy-asp was also a significant factor in our study, which assessed all patients regardless of symptoms, and current smoking was a significant factor on UVA.
The strength of our study was its prospective and longitudinal design. The multiple observations per patient over time allowed us to calculate the correlations between the various observations at each time point and subsequent AsPn, providing predictive estimates. To our knowledge, this is the first such study of AsPn in head and neck cancer. For example, as 57% of videofluoroscopy-asp occurred on only 1 videofluoroscopy study, it is likely that some episodes of videofluoroscopy-asp would have been missed had we not repeated videofluoroscopies at several time points in each patient. Limitations of this study include the need to validate its findings in an independent cohort and larger patient numbers.
For the radiation oncologist planning IMRT for head and neck cancer, prospective and retrospective studies in recent years have provided data about the anatomic structures whose damage causes dysphagia and aspiration, and their dose-effect relationships related to videofluoroscopy-asp.20–22 Although the current study has produced some of these dose-effect data,21 they were not yet available during the study. Using these data to guide IMRT planning to further improve the sparing of the swallowing structures outside the targets, as well as trials assessing reduced treatment intensity in patients predicted to do well, may reduce the rates of videofluoroscopy-asp. Taking into account the significant correlations demonstrated in the current study, reducing videofluoroscopy-asp rates is likely to reduce the risk of AsPn.
Acknowledgments
Contract grant sponsor: National Institutes of Health (NIH) grant PO1 CA59827 and the Newman Family Foundation.
Footnotes
This work was presented in part at the 8th International Conference on Head and Neck Cancer, Toronto, Canada, July 21–25, 2012.
References
- 1.Machtay M, Moughan J, Trotti A, et al. Factors associated with severe late toxicity after concurrent chemoradiation for locally advanced head and neck cancer: an RTOG analysis. J Clin Oncol. 2008;26:3582–3589. doi: 10.1200/JCO.2007.14.8841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Langendijk JA, Doornaert P, Verdonck–de Leeuw IM, Leemans CR, Aaronson NK, Slotman BJ. Impact of late treatment-related toxicity on quality of life among patients with head and neck cancer treated with radiotherapy. J Clin Oncol. 2008;26:3770–3776. doi: 10.1200/JCO.2007.14.6647. [DOI] [PubMed] [Google Scholar]
- 3.Eisbruch A, Lyden T, Bradford CR, et al. Objective assessment of swallowing dysfunction and aspiration after radiation concurrent with chemotherapy for head-and-neck cancer. Int J Radiat Oncol Biol Phys. 2002;53:23–28. doi: 10.1016/s0360-3016(02)02712-8. [DOI] [PubMed] [Google Scholar]
- 4.Rosenthal DI, Lewin JS, Eisbruch A. Prevention and treatment of dysphagia and aspiration after chemoradiation for head and neck cancer. J Clin Oncol. 2006;24:2636–2643. doi: 10.1200/JCO.2006.06.0079. [DOI] [PubMed] [Google Scholar]
- 5.Murphy BA, Gilbert J. Dysphagia in head and neck cancer patients treated with radiation: assessment, sequelae, and rehabilitation. Semin Radiat Oncol. 2009;19:35–42. doi: 10.1016/j.semradonc.2008.09.007. [DOI] [PubMed] [Google Scholar]
- 6.Chen SW, Yang SN, Liang JA, Lin FJ. Outcome and prognostic factors in patients with aspiration pneumonia during concurrent chemoradiotherapy for head and neck cancer. Eur J Cancer Care (Engl) 2010;19:631–635. doi: 10.1111/j.1365-2354.2009.01104.x. [DOI] [PubMed] [Google Scholar]
- 7.Francis DO, Weymuller EA, Jr, Parvathaneni U, Merati AL, Yueh B. Dysphagia, stricture, and pneumonia in head and neck cancer patients: does treatment modality matter? Ann Otol Rhinol Laryngol. 2010;119:391–397. doi: 10.1177/000348941011900605. [DOI] [PubMed] [Google Scholar]
- 8.Nguyen NP, Smith HJ, Dutta S, et al. Aspiration occurrence during chemoradiation for head and neck cancer. Anticancer Res. 2007;27:1669–1672. [PubMed] [Google Scholar]
- 9.van den Broek GB, Balm AJ, van den Brekel MW, Hauptmann M, Schornagel JH, Rasch CR. Relationship between clinical factors and the incidence of toxicity after intra-arterial chemoradiation for head and neck cancer. Radiother Oncol. 2006;81:143–150. doi: 10.1016/j.radonc.2006.09.002. [DOI] [PubMed] [Google Scholar]
- 10.Purkey MT, Levine MS, Prendes B, Norman MF, Mirza N. Predictors of aspiration pneumonia following radiotherapy for head and neck cancer. Ann Otol Rhinol Laryngol. 2009;118:811–816. [PubMed] [Google Scholar]
- 11.Marik PE. Aspiration pneumonitis and aspiration pneumonia. N Engl J Med. 2001;344:665–671. doi: 10.1056/NEJM200103013440908. [DOI] [PubMed] [Google Scholar]
- 12.Feng FY, Kim HM, Lyden TH, et al. Intensity-modulated chemoradiotherapy aiming to reduce dysphagia in patients with oropharyngeal cancer: clinical and functional results. J Clin Oncol. 2010;28:2732–2738. doi: 10.1200/JCO.2009.24.6199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rosenbek JC, Robbins JA, Roecker EV, Boyle JL, Wood JL. A penetration-aspiration scale. Dysphagia. 1996;11:93–98. doi: 10.1007/BF00417897. [DOI] [PubMed] [Google Scholar]
- 14.Logemann JA. Treatment of oral and pharyngeal dysphagia. Phys Med Rehabil Clin N Am. 2008;19:803–816. doi: 10.1016/j.pmr.2008.06.003. [DOI] [PubMed] [Google Scholar]
- 15.Bernier J. Current state-of-the-art for concurrent chemoradiation. Semin Radiat Oncol. 2009;19:3–10. doi: 10.1016/j.semradonc.2008.09.002. [DOI] [PubMed] [Google Scholar]
- 16.Basch E, Iasonos A, McDonough T, et al. Patient versus clinician symptom reporting using the National Cancer Institute Common Terminology Criteria for Adverse Events: results of a questionnaire-based study. Lancet Oncol. 2006;7:903–909. doi: 10.1016/S1470-2045(06)70910-X. [DOI] [PubMed] [Google Scholar]
- 17.Jensen K, Bonde Jensen B, Grau C. The relationship between observer-based toxicity scoring and patient assessed symptom severity after treatment for head and neck cancer. A correlative cross sectional study of the DAHANCA toxicity scoring system and the EORTC quality of life questionnaires Radiother Oncol. 2006;78:298–305. doi: 10.1016/j.radonc.2006.02.005. [DOI] [PubMed] [Google Scholar]
- 18.Gluck I, Feng FY, Lyden T, et al. Evaluating and reporting dysphagia in trials of chemoirradiation for head-and-neck cancer. Int J Radiat Oncol Biol Phys. 2010;77:727–733. doi: 10.1016/j.ijrobp.2009.05.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ding R, Logemann JA. Patient self-perceptions of swallowing difficulties as compared to expert ratings of videofluorographic studies. Folia Phoniatr Logop. 2008;60:142–150. doi: 10.1159/000120622. [DOI] [PubMed] [Google Scholar]
- 20.Rancati T, Schwartz M, Allen AM, et al. Radiation dose-volume effects in the larynx and pharynx. Int J Radiat Oncol Biol Phys. 2010;76(3 Suppl):S64–S69. doi: 10.1016/j.ijrobp.2009.03.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Eisbruch A, Kim HM, Feng FY, et al. Chemo-IMRT of oropharyngeal cancer aiming to reduce dysphagia: swallowing organs late complication probabilities and dosimetric correlates. Int J Radiat Oncol Biol Phys. 2011;81:e93–e99. doi: 10.1016/j.ijrobp.2010.12.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schwartz DL, Hutcheson K, Barringer D, et al. Candidate dosimetric predictors of long-term swallowing dysfunction after oropharyngeal intensity-modulated radiotherapy. Int J Radiat Oncol Biol Phys. 2010;78:1356–1365. doi: 10.1016/j.ijrobp.2009.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]


