In 1986, Higa, Jefford, and co-workers reported the isolation of a structurally novel polycyclic alkaloid, manzamine A, 1, from a sponge harvested near the coast of Okinawa.1 The unique structure of 1 consists of a β-carboline heterocycle attached to a novel pentacyclic diamine core containing both eight- and thirteen-membered rings on a pyrrolo[2,3-i]isoquinoline framework. The cytotoxic activity (IC50 = 0.07 µg/mL against P-388 mouse leukemia cells) and unique structure of 1 have stimulated considerable interest and activity directed toward the total synthesis of manzamine A which has not yet been successfully achieved to date.2 The intramolecular vinylogous amide photoaddition/fragmentation/Mannich closure sequence that we have developed has been applied to the stereoselective synthesis of complex structural types including mesembrine and the aspidosperma alkaloids from simple precursors.3 We have described the application of this methodology to the construction of the tetracyclic core of the manzamine alkaloids, in which the single stereocenter on the unsaturated eight-membered ring template 2 dictates all of the requisite stereochemical relationships embodied in 3, which represents the tetracyclic core of manzamine A.4 Outlined herein is the extension of these preliminary investigations to the first total synthesis of manzamine A.
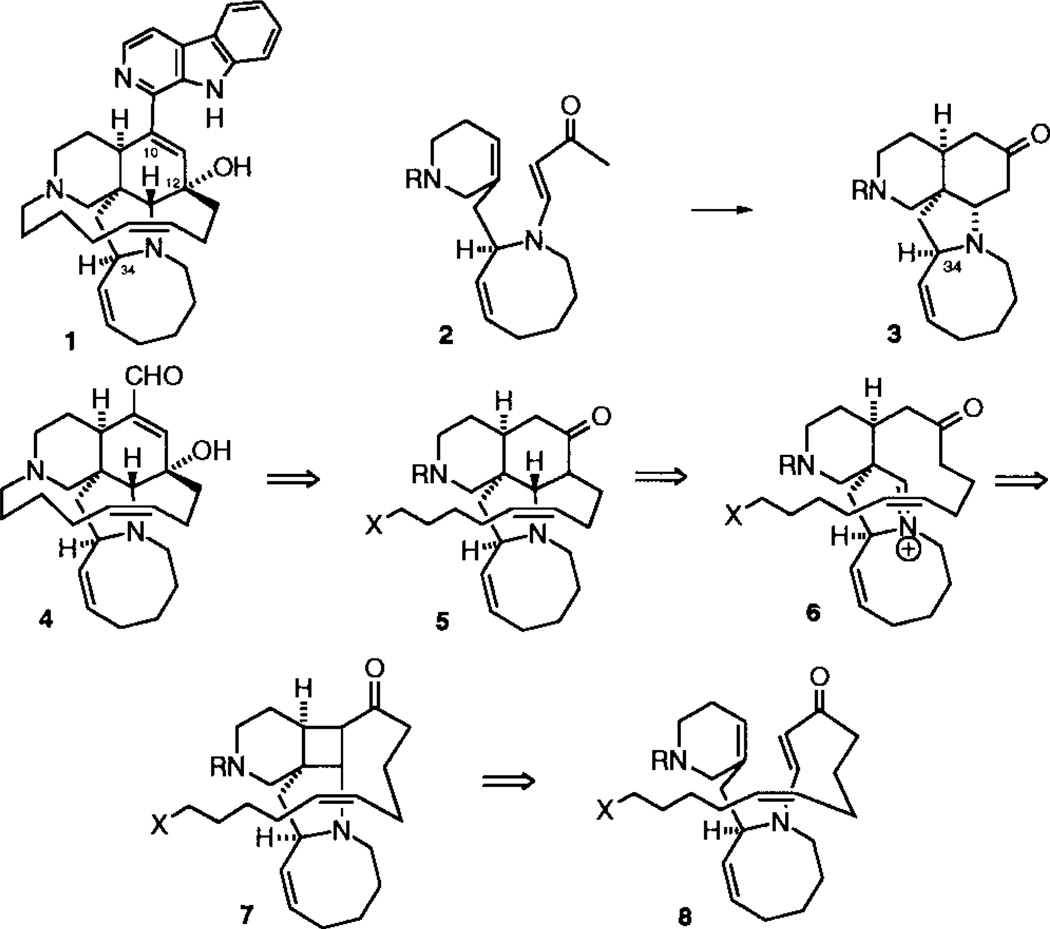
The retrosynthetic analysis for our approach to the synthesis of manzamine A is outlined in Scheme 1. Disconnection of the β-carboline from 1 leads to ircinal A, 4, a naturally occurring compound that has been converted to 1 by Pictet–Spengler cyclization followed by DDQ oxidation.5 We anticipated that ircinal A could be formed by B-ring functionalization and macrocyclization of 5. The tetracyclic ring system of 5 would result from the Mannich closure of ketoiminium 6, which is derived by retro-Mannich fragmentation of 7, the product of intramolecular cycloaddition of 8.
Scheme 1.
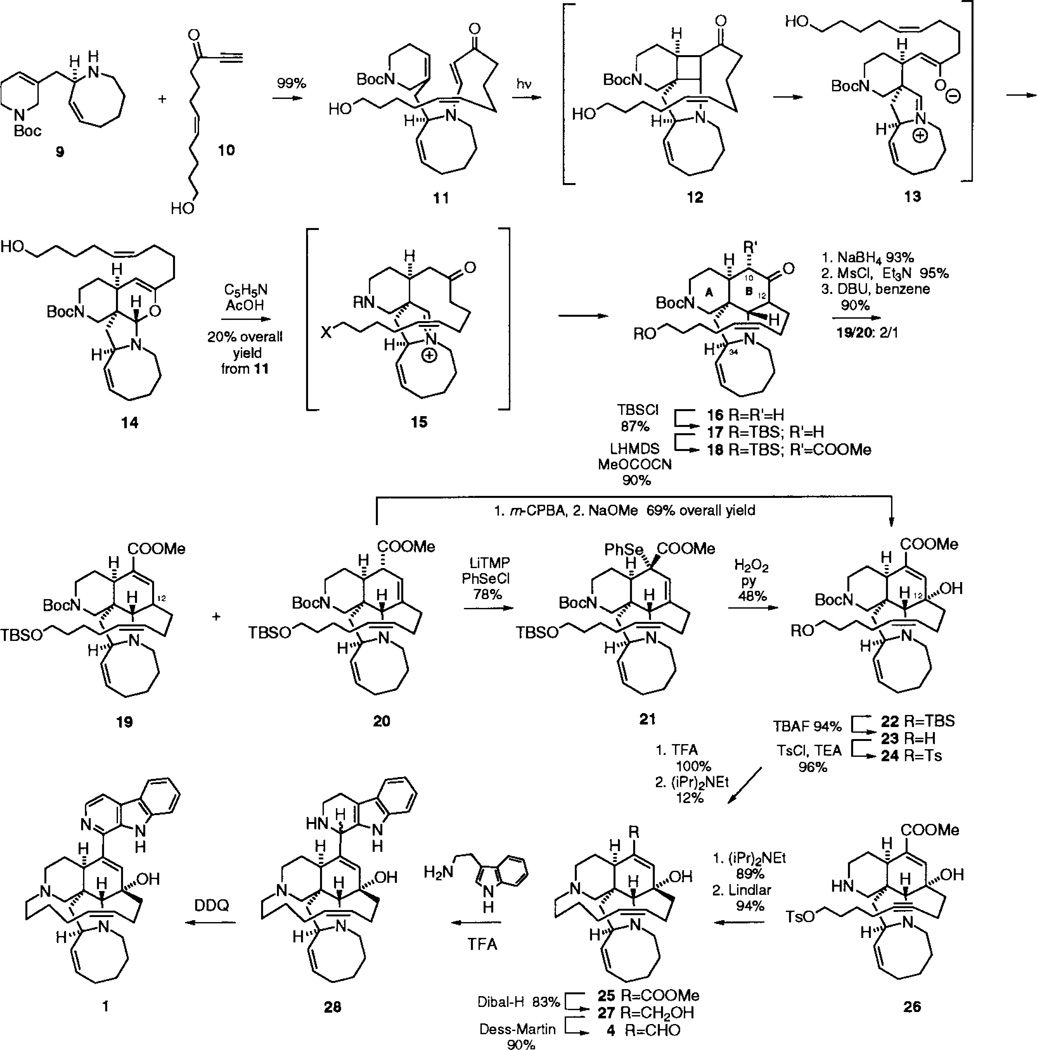
The preparation and reaction of the requisite photosubstrate is outlined in Scheme 2.6 Reaction of the previously described secondary amine 97 with acetylenic ketone 108 gave the requisite vinylogous amide photosubstrate 11 in 99% yield from 9. Photoaddition and retro-Mannich fragmentation of 11 led, via O-closure of the ketoiminium intermediate 13, to aminal 14. The isomerization of 14 to the manzamine tetracycle 16 proceeded on exposure of 14 to pyridinium acetate to give 16 as a single stereoisomer in 20% overall yield from 11 (an average of 60% yield/step for photoaddition, fragmentation, and Mannich closure). The assignment of the relative stereochemistry shown in 16 follows from our published studies on the photocycloaddition of 24 and the conversion of 16 to manzamine A, as detailed below. The unique stereochemistry of the C-12 substituent in 16, which is not critical to the subsequent stereoselective introduction of the C-12α hydroxyl moiety, was not established at this stage.
Scheme 2.
The elaboration of the B ring of 16 to include the functionality present in manzamine A was achieved as follows: Carboxylation of the kinetic enolate derived from 17, the silyl ether of 16, with Mander’s reagent gave ketoester 18, with the C-10α ester on the convex face of the AB ring system. Reduction of the C-11 ketone, followed by elimination of the derived mesylate with DBU in refluxing benzene, gave a 2:1 mixture of the α,β- and β,γ-unsaturated esters 19 and 20, respectively. Equilibration of 19 to a 2:1 mixture of 19 and 20 could be achieved in quantitative yield by reexposure of 19 to DBU in refluxing benzene.
Selenation of the conjugate base of 19 (LiTMP) led to the formation of the α-selenated product 21 in ca. 40% yield, while selenation of the deconjugated ester 20 led to the formation of the same product in 78% yield. We attribute this difference in reactivity to the relative difficulty of deprotonation of the C-12 hydrogen in 19. Oxidation of selenide 21 resulted in the formation of the desired C-12α alcohol 22, the stereochemical assignment of which was supported by the H bonding observed between the hydroxyl hydrogen and the azocine nitrogen by 1H NMR (br s, δ 6.5, exchanges with D2O) and subsequently confirmed by the conversion of 22 to manzamine A. The same product 22 could be obtained more efficiently via epoxidation of the β,γ –unsaturated ester 20 and treatment of the derived epoxide with sodium methoxide (69% overall yield of 22 from 20). The closure of the macrocyclic 13-membered ring to complete the synthesis of the pentacyclic ring system of manzamine A proved challenging. Deprotection of silyl ether 22, followed by tosylation of the derived alcohol 23, gave 24. Removal of the Boc protecting group and exposure of the secondary amine to Hünig’s base under high dilution conditions (1 mM) led to the formation of methyl ircinate 25 in a disappointing 12% yield. We were delighted to find that cyclization of the acetylenic substrate 269 under the same reaction conditions led to the formation of the desired macrocyclic product in 89% yield, which on Lindlar reduction gave 25 in 94% yield.
Reaction of the unsaturated ester 25 with DIBAL-H resulted in the first total synthesis of ircinol A, 27, [α]D = −18° (c = 0.30, MeOH), in 83% yield, the isolation of which was recently reported by Kobayashi and co-workers.10 Oxidation of 27 with the Dess–Martin reagent gave a 90% yield of ircinal A, 4, ([α]D = +46° (c = 0.23, CHCl3); lit.5 [α]D = +48° (c) = 2.9, CHCl3)), the transformation of which to manzamine A has been reported by Kobayashi.5 Following that procedure, reaction of 4 with tryptamine in the presence of trifluoroacetic acid gave manzamine D, 28, in 58% yield, which on oxidation with DDQ provided manzamine A, 1 (50% yield), which was identical in all respects with an authentic sample kindly provided to us by Professor Kobayashi.
The completion of the first total synthesis of manzamine A in 17 steps from the readily available bicyclic precursor 9 (which was prepared in 14 steps from pyridine-3-methanol)4 underscores the utility of the vinylogous amide photoaddition/ fragmentation/Mannich closure sequence that we have developed for the synthesis of complex structures from simple precursors. The establishment of all of the stereochemical relationships in 1 from the single stereogenic center in 9 further attests to the remarkable levels of stereochemical control that are possible using this photochemical cascade in organic synthesis.
Supplementary Material
Acknowledgment
J.M.A. is the recipient of a Division of Organic Chemistry Graduate Fellowship, sponsored by Abbott Laboratories and administered by the American Chemical Society. We thank Professor J. Kobayashi (Hokkaido University) for helpful discussions and a generous sample of manzamine A. We also warmly acknowledge the invaluable contributions of Dr. Miles G. Siegel and Dr. John E. Stelmach to the early stages of this work and Dr. Abdelhakim Hammach for his assistance in the preparation of 9. The generous financial support of the National Institutes of Health (Grant CA40250), SmithKline Beecham, Wyeth-Ayerst, and Pfizer is gratefully acknowledged.
Footnotes
Supporting Information Available: Preparation pocedures for 1, 11, 14, and 16–28 with spectral data (21 pages, print/PDF). See any current masthead page for ordering and Web access instructions.
References
- 1.(a) Sakai R, Higa T, Jefford CW, Bernardinelli G. J. Am. Chem. Soc. 1986;108:6404. [Google Scholar]; (b) Nakamura H, Deng S, Kobayashi J, Ohizumi Y, Tomotake Y, Matsuzaki T, Hirata Y. Tetrahedron Lett. 1987;28:621. [Google Scholar]
- 2.For an excellent review of synthetic efforts in this area, see: Matzanke N, Gregg RJ, Weinreb SM. Org. Prep. Proc. Int. 1998;30:1. and references therein. For synthetic approaches disclosed since 1996, see: Brands KMJ, DiMichele LM. Tetrahedron Lett. 1998;39:1677. Li S, Yamamura S. Tetrahedron Lett. 1998;39:2597. Li S, Yamamura S, Hosomi H, Ohba S. Tetrahedron Lett. 1998;39:2601. Baldwin JE, Bischoff L, Claridge TDW, Heupel FA, Spring DR, Whitehead RC. Tetrahedron. 1997;53:2271. Li S, Ohba S, Kosemura S, Yamamura S. Tetrahedron Lett. 1996;37:7365. Baldwin JE, Claridge TDW, Culshaw AJ, Heupel FA, Smrckova S, Whitehead RC. Tetrahedron Lett. 1996;37:6919. Torisawa Y, Hosaka T, Tanabe K, Suzuki N, Motohashi Y, Hino T, Nakagawa M. Tetrahedron. 1996;52:10597. Martin SF, Chen H-J, Courtney AK, Liao Y, Pätzel M, Ramser MN, Wagman AS. Tetrahedron. 1996;52:7251. Pandit UK, Borer B, Bieraugel H. Pure Appl. Chem. 1996;68:659.
- 3. Winkler JD, Mazur Bowen C, Liotta F. Chem. Rev. 1995;95:2003. For application to the manzamines, see: Winkler JD, Siegel MG, Stelmach JE. Tetrahedron Lett. 1993;34:6509. Winkler JD, Stelmach J, Siegel MG, Haddad N, Axten JM, Dailey WP., III Isr. J. Chem. 1997;37:47.
- 4.Winkler JD, Axten JM, Hammach A, Kwak Y-S, Lucero M, Houk KN. Tetrahedron. in press (honoring Professor M. Joullié). [Google Scholar]
- 5.Kondo K, Shigemori H, Kikuchi Y, Ishibashi M, Sasaki T, Kobayashi J. J. Org. Chem. 1992;57:2480. [Google Scholar]
- 6.All compounds were fully purified (>95%) and characterized by 1H and 13C NMR, IR, HRMS, and specific rotation. See the Supporting Information for experimental procedures, tabulated data, and copies of spectra.
- 7.The eight-membered ring of 9 was prepared by intramolecular alkylation of the corresponding N-Alloc O-tosylate using NaH (82%), followed by nitrogen deprotection (Pd0, 90%) as described in ref 4.
- 8.The acetylenic ketone 10 was prepared in three steps from the known methyl 10-hydroxy-5-decynoate ( Nowak W, Gerlach H. Liebigs Ann. Chem. 1993:153.) by the following sequence: (1) formation of the Weinreb amide (Me3Al, MeNHOMe-HCl, 94%); (2) semi-hydrogenation (Lindlar, 99%); (3) reaction with ethynylmagnesium bromide (79%).
- 9.The acetylenic substrate 26 was prepared from 9 and the diynone corresponding to 10 (which was available by the route outlined in ref 8, albeit without semi-hydrogenation of the intermediate alkyne) by the same reaction sequence employed for the preparation of 24 from 9 and 10.
- 10.The levorotatory rotation that we observe for ircinol A, which differs in sign from that of the previously published report ( Tsuda M, Kawasaki N, Kobayashi J. Tetrahedron. 1994;50:7957.), is consistent with data recently obtained by Professor Kobayashi (personal communication).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.