Abstract
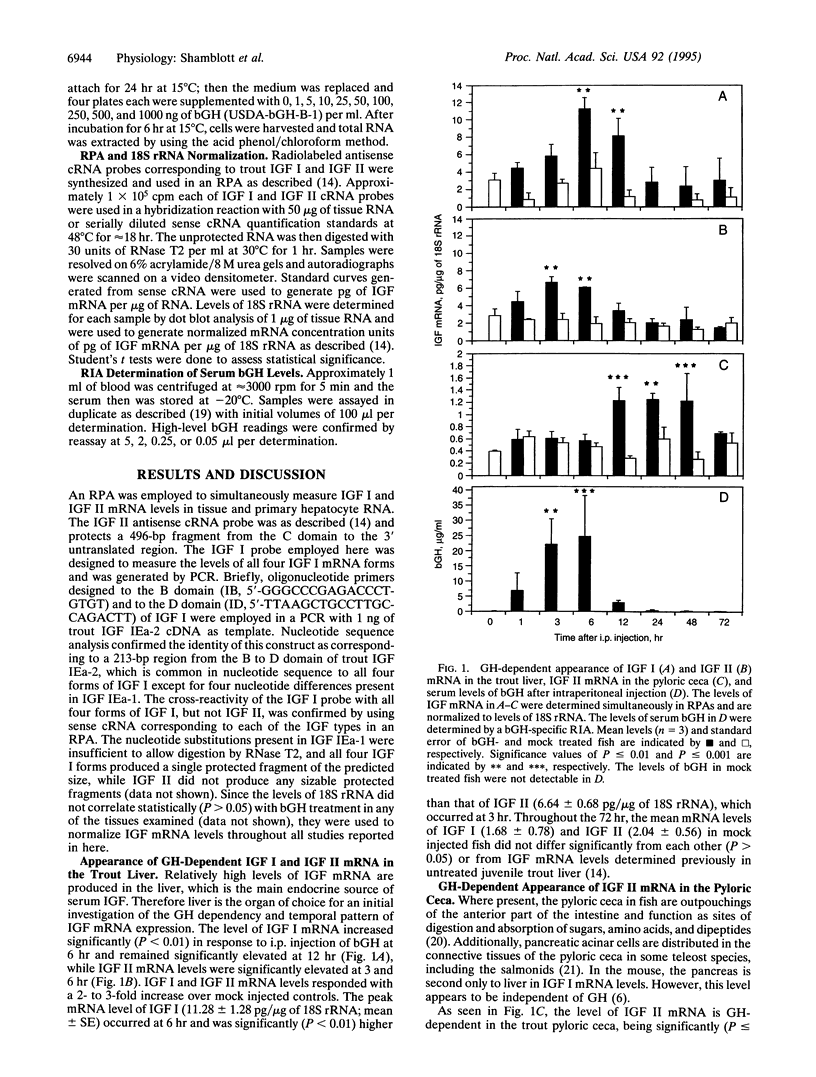
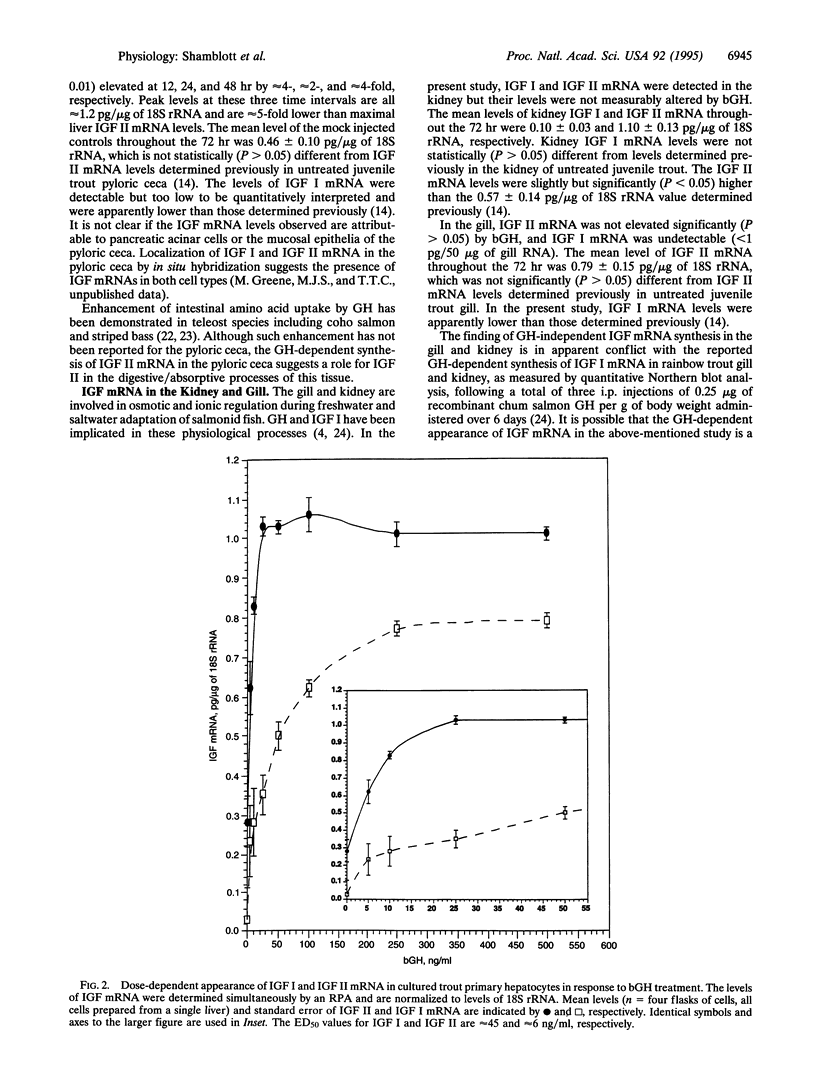
Augmentation of vertebrate growth by growth hormone (GH) is primarily due to its regulation of insulin-like growth factor I (IGF I) and IGF II levels. To characterize the effect of GH on the levels of IGF I and IGF II mRNA in a teleost, 10 micrograms of bovine GH (bGH) per g of body weight was administered to juvenile rainbow trout (Oncorhynchus mykiss) through i.p. injection. The levels of IGF I and IGF II mRNA were determined simultaneously, by using RNase protection assays, in the liver, pyloric ceca, kidney, and gill at 0, 1, 3, 6, 12, 24, 48, and 72 hr after injection. In the liver, IGF I mRNA levels were significantly elevated at 6 and 12 hr (approximately 2- to 3-fold, P < or = 0.01), while IGF II mRNA levels were significantly elevated at 3 and 6 hr (approximately 3-fold, P < or = 0.01). In the pyloric ceca, IGF II mRNA levels were significantly elevated at 12, 24, and 48 hr (approximately 3-fold, P < or = 0.01), while IGF I mRNA was below the limits of assay accuracy. GH-dependent IGF mRNA appearance was not detected in the gill and kidney. Serum bGH levels, determined by using a radioimmunoassay, were significantly elevated at 3 and 6 hr (P < 0.005). In primary hepatocyte culture, IGF I and IGF II mRNA levels increased in a bGH dose-dependent fashion, with ED50 values of approximately 45 and approximately 6 ng of bGH per ml, respectively. The GH-dependent appearance of IGF II mRNA in the liver and pyloric ceca suggests important roles for this peptide hormone exclusive of IGF I.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beorlegui C., Martínez A., Sesma P. Some peptide-like colocalizations in endocrine cells of the pyloric caeca and the intestine of Oncorhynchus mykiss (Teleostei). Cell Tissue Res. 1992 Aug;269(2):353–357. doi: 10.1007/BF00319628. [DOI] [PubMed] [Google Scholar]
- Buddington R. K., Diamond J. M. Aristotle revisited: the function of pyloric caeca in fish. Proc Natl Acad Sci U S A. 1986 Oct;83(20):8012–8014. doi: 10.1073/pnas.83.20.8012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao Q. P., Duguay S. J., Plisetskaya E., Steiner D. F., Chan S. J. Nucleotide sequence and growth hormone-regulated expression of salmon insulin-like growth factor I mRNA. Mol Endocrinol. 1989 Dec;3(12):2005–2010. doi: 10.1210/mend-3-12-2005. [DOI] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Collie N. L., Stevens J. J. Hormonal effects on L-proline transport in coho salmon (Oncorhynchus kisutch) intestine. Gen Comp Endocrinol. 1985 Sep;59(3):399–409. doi: 10.1016/0016-6480(85)90397-1. [DOI] [PubMed] [Google Scholar]
- Cynober L., Aussel C., Chatelain P., Vaubourdolle M., Agneray J., Ekindjian O. G. Insulin-like growth factor I/somatomedin C action on 2-deoxyglucose and alpha-amino isobutyrate uptake in chick embryo fibroblasts. Biochimie. 1985 Oct-Nov;67(10-11):1185–1190. doi: 10.1016/s0300-9084(85)80118-8. [DOI] [PubMed] [Google Scholar]
- Daughaday W. H., Rotwein P. Insulin-like growth factors I and II. Peptide, messenger ribonucleic acid and gene structures, serum, and tissue concentrations. Endocr Rev. 1989 Feb;10(1):68–91. doi: 10.1210/edrv-10-1-68. [DOI] [PubMed] [Google Scholar]
- Elsasser T. H., Rumsey T. S., Hammond A. C. Influence of diet on basal and growth hormone-stimulated plasma concentrations of IGF-I in beef cattle. J Anim Sci. 1989 Jan;67(1):128–141. doi: 10.2527/jas1989.671128x. [DOI] [PubMed] [Google Scholar]
- Froger-Gaillard B., Hossenlopp P., Adolphe M., Binoux M. Production of insulin-like growth factors and their binding proteins by rabbit articular chondrocytes: relationships with cell multiplication. Endocrinology. 1989 May;124(5):2365–2372. doi: 10.1210/endo-124-5-2365. [DOI] [PubMed] [Google Scholar]
- Gray A., Tam A. W., Dull T. J., Hayflick J., Pintar J., Cavenee W. K., Koufos A., Ullrich A. Tissue-specific and developmentally regulated transcription of the insulin-like growth factor 2 gene. DNA. 1987 Aug;6(4):283–295. doi: 10.1089/dna.1987.6.283. [DOI] [PubMed] [Google Scholar]
- Humbel R. E. Insulin-like growth factors I and II. Eur J Biochem. 1990 Jul 5;190(3):445–462. doi: 10.1111/j.1432-1033.1990.tb15595.x. [DOI] [PubMed] [Google Scholar]
- Hynes M. A., Brooks P. J., Van Wyk J. J., Lund P. K. Insulin-like growth factor II messenger ribonucleic acids are synthesized in the choroid plexus of the rat brain. Mol Endocrinol. 1988 Jan;2(1):47–54. doi: 10.1210/mend-2-1-47. [DOI] [PubMed] [Google Scholar]
- Mathews L. S., Norstedt G., Palmiter R. D. Regulation of insulin-like growth factor I gene expression by growth hormone. Proc Natl Acad Sci U S A. 1986 Dec;83(24):9343–9347. doi: 10.1073/pnas.83.24.9343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormick S. D., Sakamoto T., Hasegawa S., Hirano T. Osmoregulatory actions of insulin-like growth factor-I in rainbow trout (Oncorhynchus mykiss). J Endocrinol. 1991 Jul;130(1):87–92. doi: 10.1677/joe.0.1300087. [DOI] [PubMed] [Google Scholar]
- Puissant C., Houdebine L. M. An improvement of the single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Biotechniques. 1990 Feb;8(2):148–149. [PubMed] [Google Scholar]
- Sakamoto T., Hirano T. Expression of insulin-like growth factor I gene in osmoregulatory organs during seawater adaptation of the salmonid fish: possible mode of osmoregulatory action of growth hormone. Proc Natl Acad Sci U S A. 1993 Mar 1;90(5):1912–1916. doi: 10.1073/pnas.90.5.1912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shamblott M. J., Chen T. T. Age-related and tissue-specific levels of five forms of insulin-like growth factor mRNA in a teleost. Mol Mar Biol Biotechnol. 1993 Dec;2(6):351–361. [PubMed] [Google Scholar]
- Shamblott M. J., Chen T. T. Identification of a second insulin-like growth factor in a fish species. Proc Natl Acad Sci U S A. 1992 Oct 1;89(19):8913–8917. doi: 10.1073/pnas.89.19.8913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stiles C. D., Capone G. T., Scher C. D., Antoniades H. N., Van Wyk J. J., Pledger W. J. Dual control of cell growth by somatomedins and platelet-derived growth factor. Proc Natl Acad Sci U S A. 1979 Mar;76(3):1279–1283. doi: 10.1073/pnas.76.3.1279. [DOI] [PMC free article] [PubMed] [Google Scholar]



