Abstract
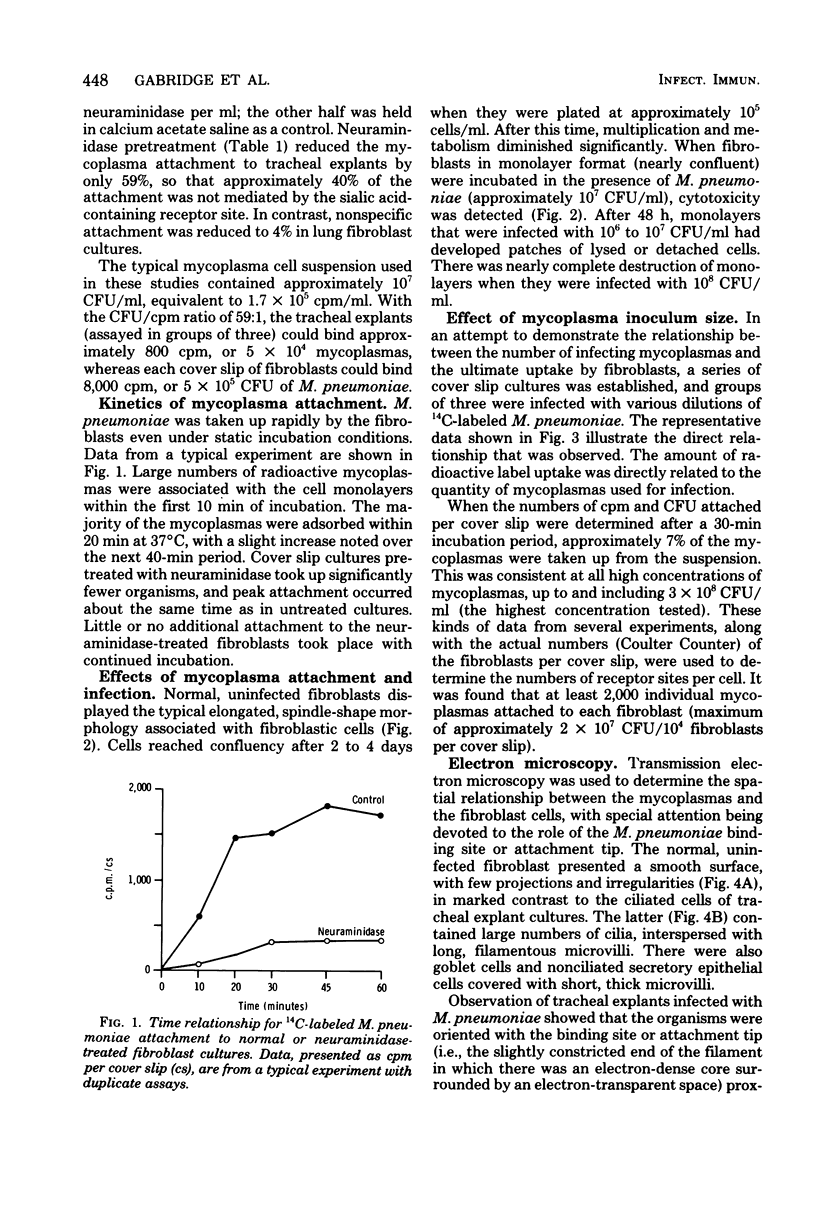
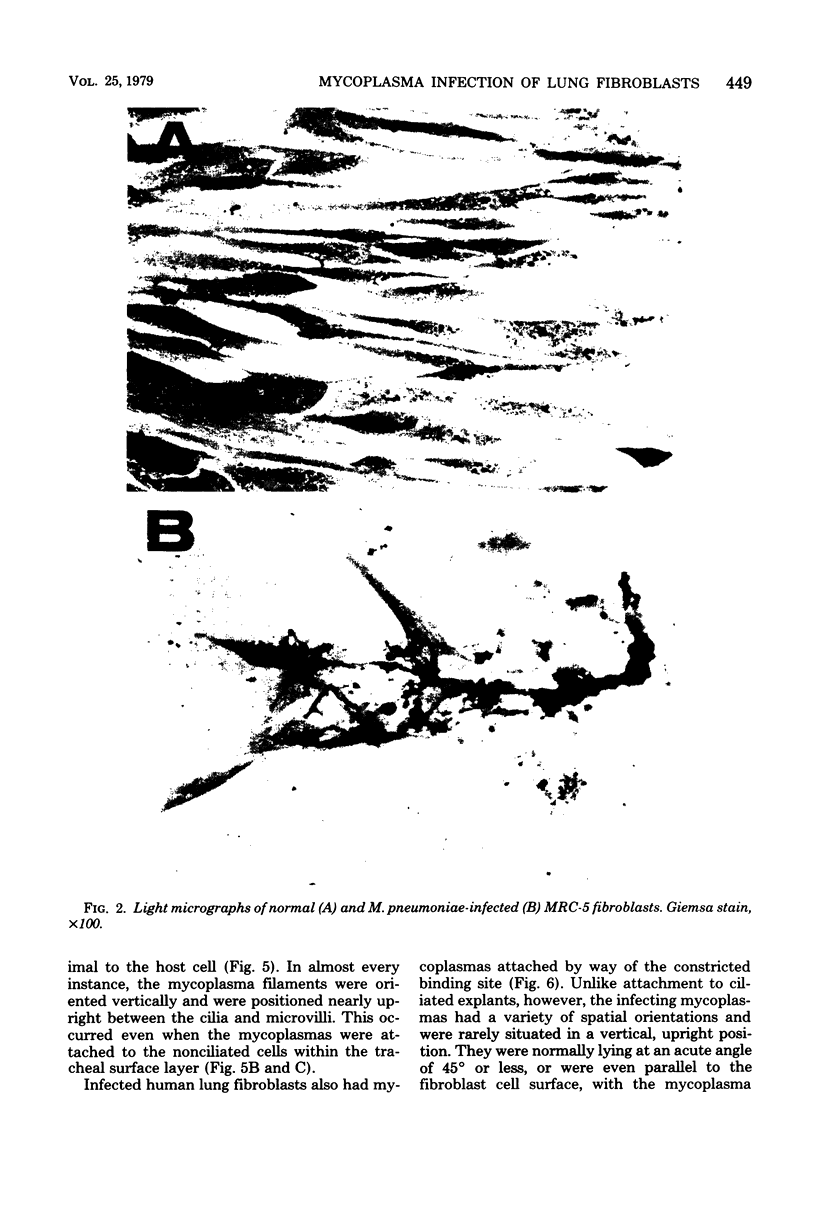
The interaction of pathogenic Mycoplasma pneumoniae and host cells was studied in cell cultures of MRC-5 human lung fibroblasts. A comparison of results obtained with fibroblasts in a monolayer format and with hamster tracheal explant cultures indicated that the former can bind significantly larger numbers of mycoplasmas. In addition, the attachment was 96% specific, that is, mediated through a neuraminidase-sensitive receptor on the host cell. Uptake of mycoplasmas was directly related to the number of mycoplasma cells present in the inoculum, and attachment was virtually complete within a 30-min period at 37 degrees C. High doses of M. pneumoniae induced a marked cytopathic effect, whereas doses of less than or equal to 10(6) colony-forming units per ml produced grossly observable cell damage that was moderate and variable. Transmission electron microscopy studies indicated that attachment of M. pneumoniae to the surface of lung fibroblasts occurred with the specialized terminal structure or binding site oriented closest to the epithelial cell surface. The filamentous mycoplasma cells were spatially arranged in several configurations and were not limited to a vertical orientation. The advantages and disadvantages of human lung fibroblast monolayer cultures, in reference to other in vitro models are discussed. A new mycoplasma agar medium (G-200 agar) with a defined tissue culture base and 10% horse serum is also described.
Full text
PDF

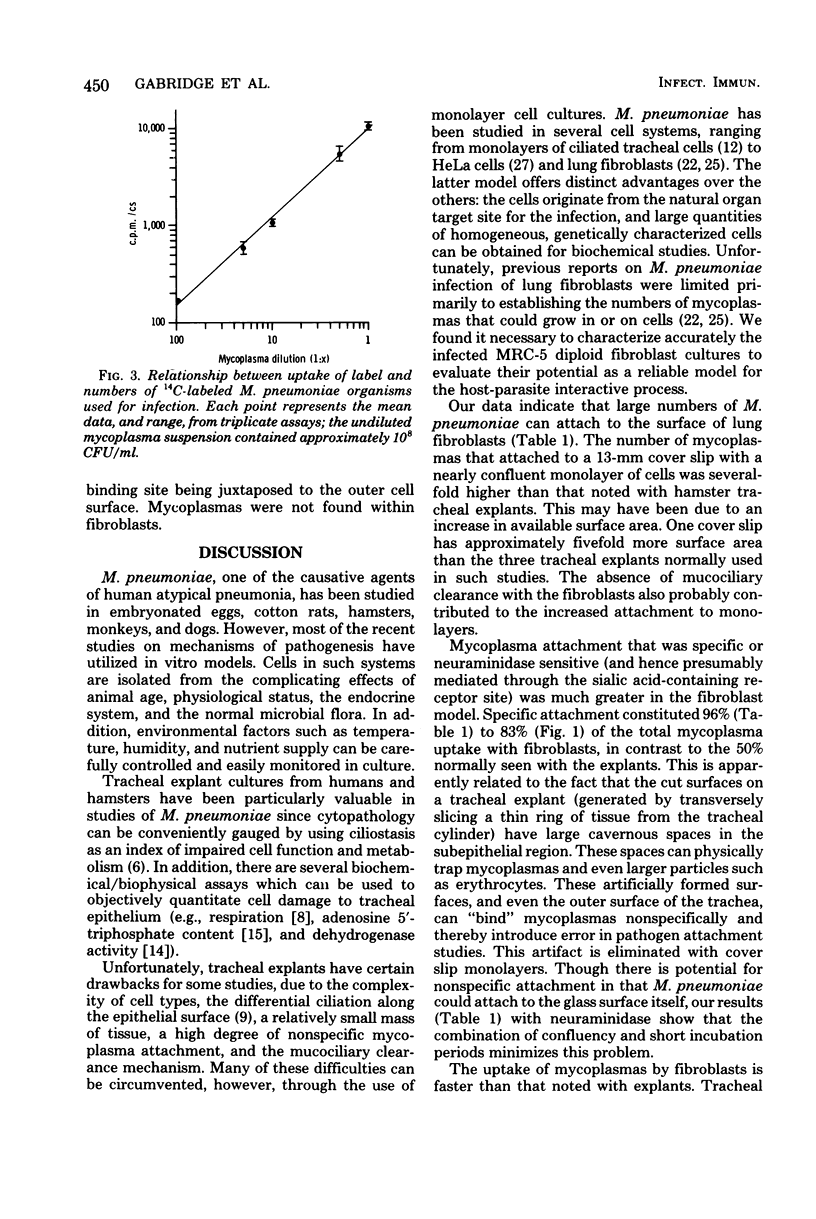
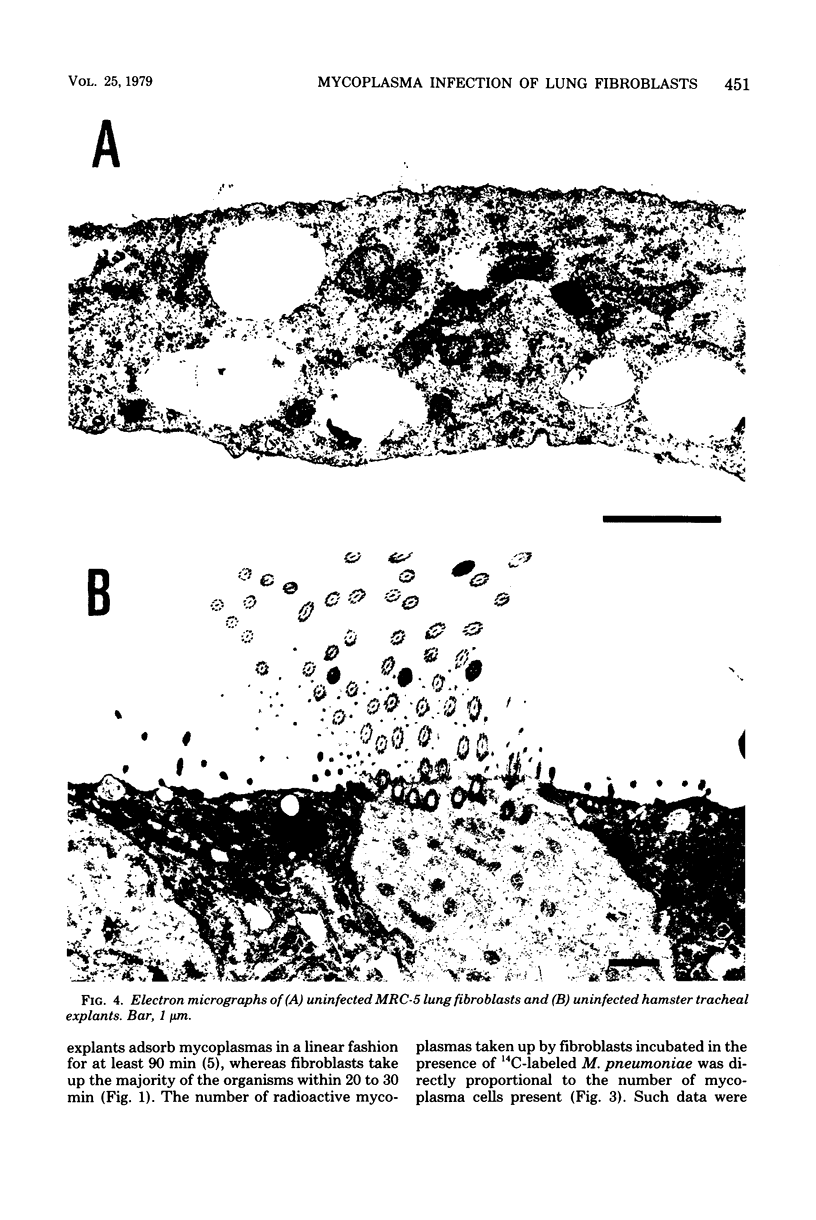
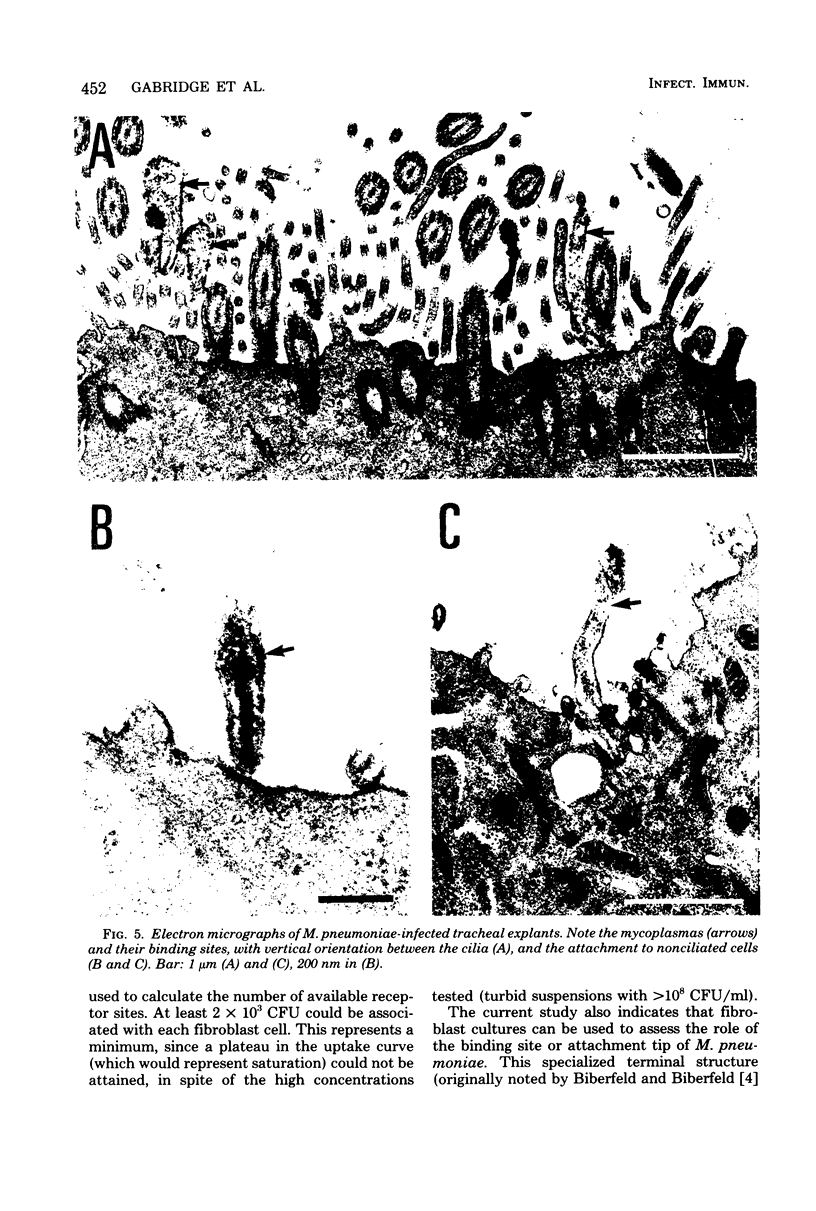


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ADA G. L., STONE J. D. Electrophoretic studies of virus-red cell interaction: mobility gradient of cells treated with viruses of the influenza group and the receptor-destroying enzyme of V. cholerae. Br J Exp Pathol. 1950 Jun;31(3):263–274. [PMC free article] [PubMed] [Google Scholar]
- Aldridge K. E., Cole B. C. Immunofluorescence and electron microscopy of the attachment of Mycoplasma synoviae to chicken embryo fibroblasts. Infect Immun. 1978 Jul;21(1):328–332. doi: 10.1128/iai.21.1.328-332.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aldridge K. E. Growth and cytopathology of Mycoplasma synoviae in chicken embryo cell cultures. Infect Immun. 1975 Jul;12(1):198–204. doi: 10.1128/iai.12.1.198-204.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biberfeld G., Biberfeld P. Ultrastructural features of Mycoplasma pneumoniae. J Bacteriol. 1970 Jun;102(3):855–861. doi: 10.1128/jb.102.3.855-861.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boatman E., Cartwright F., Kenny G. Morphology, morphometry and electron microscopy of HeLa cells infected with bovine Mycoplasma. Cell Tissue Res. 1976 Jul 20;170(1):1–16. doi: 10.1007/BF00220107. [DOI] [PubMed] [Google Scholar]
- Gabridge M. G., Agee C. C., Cameron A. M. Differential distribution of ciliated epithelial cells in the trachea of hamsters: implications for studies of pathogenesis. J Infect Dis. 1977 Jan;135(1):9–19. doi: 10.1093/infdis/135.1.9. [DOI] [PubMed] [Google Scholar]
- Gabridge M. G., Barden-Stahl Y. D., Polisky R. B., Engelhardt J. A. Differences in the attachment of Mycoplasma pneumoniae cells and membranes to tracheal epithelium. Infect Immun. 1977 Jun;16(3):766–772. doi: 10.1128/iai.16.3.766-772.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabridge M. G., Gunderson H., Schaeffer S. L., Barden-Stahl Y. D. Ciliated respiratory epithelial monolayers: new model for Mycoplasma pneumoniae infection. Infect Immun. 1978 Jul;21(1):333–336. doi: 10.1128/iai.21.1.333-336.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabridge M. G., Johnson C. K., Cameron A. M. Cytotoxicity of Mycoplasma pneumoniae Membranes. Infect Immun. 1974 Nov;10(5):1127–1134. doi: 10.1128/iai.10.5.1127-1134.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabridge M. G. Microrespirometer chamber for determinations of viability in cell and organ cultures. J Clin Microbiol. 1976 Jun;3(6):560–565. doi: 10.1128/jcm.3.6.560-565.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabridge M. G., Polisky R. B. Intracellular levels of adenosine triphosphate in hamster trachea organ cultures exposed to Mycoplasma pneumoniae cells or membranes. In Vitro. 1977 Aug;13(8):510–516. doi: 10.1007/BF02615144. [DOI] [PubMed] [Google Scholar]
- Gabridge M. G., Polisky R. B. Quantitative reduction of 2,3,4-triphenyl tetrazolium chloride by hamster trachea organ cultures: effects of Mycoplasma pneumoniae cells and membranes. Infect Immun. 1976 Jan;13(1):84–91. doi: 10.1128/iai.13.1.84-91.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabridge M. G., Singer S. E., Esposito R. A. Cultivation of mycoplasmas in a modified tissue culture medium. Appl Environ Microbiol. 1976 Jun;31(6):986–989. doi: 10.1128/aem.31.6.986-989.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabridge M. G., Singer S. E., Esposito R. A. Gradient, polyacrylamide gel electrophoresis of proteins from cytotoxic mycoplasma membranes. Biochem Biophys Res Commun. 1976 May 3;70(1):271–279. doi: 10.1016/0006-291x(76)91138-4. [DOI] [PubMed] [Google Scholar]
- Gabridge M. G., Stahl Y. D. Role of adenine in pathogenesis of Mycoplasma pneumoniae infections of tracheal epithelium. Med Microbiol Immunol. 1978 May 26;165(1):43–55. doi: 10.1007/BF02121231. [DOI] [PubMed] [Google Scholar]
- Hirsch J. G., Fedorko M. E. Ultrastructure of human leukocytes after simultaneous fixation with glutaraldehyde and osmium tetroxide and "postfixation" in uranyl acetate. J Cell Biol. 1968 Sep;38(3):615–627. doi: 10.1083/jcb.38.3.615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs J. P., Jones C. M., Baille J. P. Characteristics of a human diploid cell designated MRC-5. Nature. 1970 Jul 11;227(5254):168–170. doi: 10.1038/227168a0. [DOI] [PubMed] [Google Scholar]
- Kim C. K., Pfister R. M., Somerson N. L. Electron microscopy of Mycoplasma pneumoniae microcolonies grown on solid surfaces. Appl Environ Microbiol. 1977 Nov;34(5):591–594. doi: 10.1128/aem.34.5.591-594.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larin N. M., Saxby N. V., Buggey D. Quantitative aspects of Mycoplasma pneumoniae-cell relationships in cultures of lung diploid fibroblasts. J Hyg (Lond) 1969 Sep;67(3):375–385. doi: 10.1017/s0022172400041796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radestock U., Bredt W. Motility of Mycoplasma pneumoniae. J Bacteriol. 1977 Mar;129(3):1495–1501. doi: 10.1128/jb.129.3.1495-1501.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Razin S. The mycoplasmas. Microbiol Rev. 1978 Jun;42(2):414–470. doi: 10.1128/mr.42.2.414-470.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokhey J., Solovieva A. I., Vasilieva V. I. Induction of interferon in mice and cell cultures by Mycoplasma pneumoniae. Acta Virol. 1977 Nov;21(6):485–489. [PubMed] [Google Scholar]
- Wilson M. H., Collier A. M. Ultrastructural study of Mycoplasma pneumoniae in organ culture. J Bacteriol. 1976 Jan;125(1):332–339. doi: 10.1128/jb.125.1.332-339.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zucker-Franklin D., Davidson M., Thomas L. The interaction of mycoplasmas with mammalian cells. I. HeLa cells, neutrophils, and eosinophils. J Exp Med. 1966 Sep 1;124(3):521–532. doi: 10.1084/jem.124.3.521. [DOI] [PMC free article] [PubMed] [Google Scholar]