Abstract
In 1998, the landmark paper describing the isolation and culture of human embryonic stem cells (ESCs) was published. Since that time, the main goal of many diabetes researchers has been to derive β cells from ESCs as a renewable cell-based therapy for the treatment of patients with diabetes. In working toward this goal, numerous protocols that attempt to recapitulate normal pancreatic development have been published that result in the formation of pancreatic cell types from human pluripotent cells. This review examines stem cell differentiation methods and places them within the context of pancreatic development. We additionally compare strategies that are currently being used to generate pancreatic cell types and contrast them with approaches that have been used to generate functional cell types in different lineages. In doing this, we aim to identify how new approaches might be used to improve yield and functionality of in vitro–derived pancreatic β cells as an eventual cell-based therapy for type 1 diabetes.
Keywords: pluripotent stem cell, β cell, differentiation, endoderm, pancreas, diabetes
Introduction
Isolated from the inner cell mass of the preimplantation embryo, embryonic stem cells (ESCs) are immortal, pluripotent cells that retain the capacity to differentiate into cell types of all three germ layers. The isolation of human embryonic stem cells (hESCs)1 and their subsequently demonstrated ability to be differentiated toward specific cell fates2 raised the possibility that hESC-derived cells could form a basis for future cell-based therapies. In 2007, the generation of human-induced pluripotent stem cells (PSCs)3—an alternate source of PSCs—opened up the possibility of isogenic cell-based therapies. These advances have generated an intense interest in identifying methods to direct PSC differentiation in vitro toward therapeutically relevant cell types, such as pancreatic β cells, which could potentially replace the use of cadaveric islets in the treatment of type 1 diabetes. This review examines progress that has been made toward the differentiation of PSCs toward pancreatic β cells. We discuss how this progress was only possible because of our knowledge of pancreas development and how additional knowledge in this area may yield the key for generating fully functional β cells from PSCs in vitro.
Developmental pathway of β cells
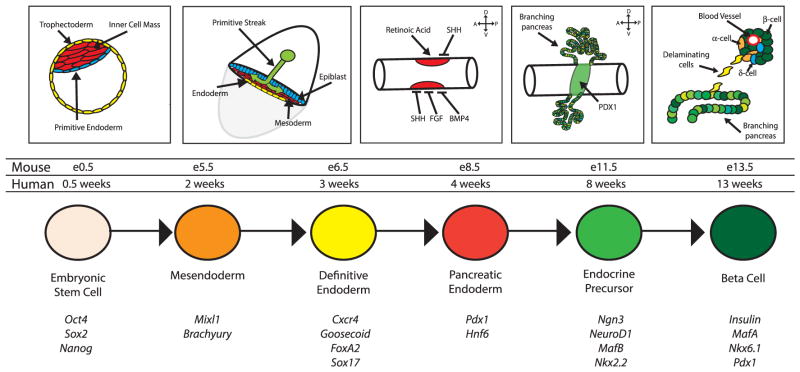
Successful protocols that promote differentiation of PSCs toward a β cell fate have attempted to recapitulate the signaling processes that function during embryonic pancreas development (Fig. 1). This is achieved by the addition of growth factors and/or inhibitors at specific times during PSC differentiation that are meant to approximate in vivo signaling events that guide β cell development. A summation of the critical stages of pancreas development and the growth factors/inhibitors that have been used in attempts to direct differentiation of hPSCs toward β cells is shown in Figure 2.
Figure 1.
Schematic depicting key developmental stages and corresponding morphogenetic processes occurring in the embryo during pancreas formation. The lower rows show the relative mouse and human developmental timelines, as well as some of the pivotal genes used to identify these stages.
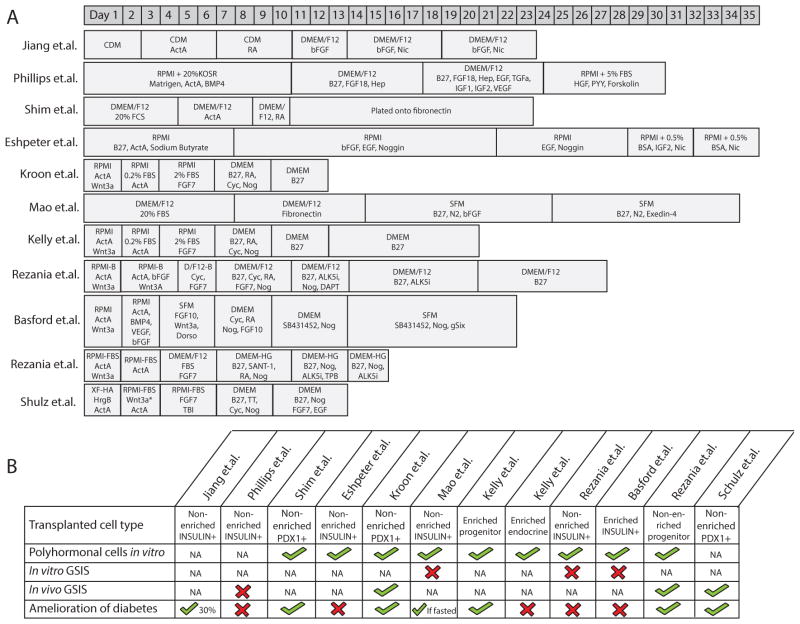
Figure 2.
(A) Timelines of published multistep procedures that have been used to induce differentiation of hESCs toward insulin-secreting cells before cell transplantation. (B) Characteristics of hESC-derived pancreatic cells that have been differentiated toward insulin-secreting cells. ActA, activin A; ALK5i, ALK5 inhibitor; BSA, bovine serum albumin; CDM, chemically defined media; Cyc, cyclopamine; D/F12-B, DMEM/F12 with BSA; EGF, epidermal growth factor; FBS, fetal bovine serum; FCS, fetal calf serum; Hep, heparin; HrgB, heregulin 1b; Nic, nicotinamide; Nog, Noggin; RA, retinoic acid; RPMI-B, RPMI1640 with BSA; SFM, serum-free medium; TBI, TGF-βR1 kinase inhibitor; TT, TTNPB.
Formation of definitive endoderm
The first step in differentiating hPSCs toward pancreatic β cells is the formation of definitive endoderm. There is some evidence, both in vitro4 and in vivo,5,6 that definitive endoderm derives from a bipotential mesendoderm progenitor that is marked by the expression of the transcription factors Brachyury and Mixl1.7–9 Specification of the definitive endoderm lineage is accompanied by the upregulation of a number of transcription factors, most importantly, Goosecoid, Sox17, and FoxA2.10–13 This same progression of gene expression occurs during both mouse and human PSC differentiation into definitive endoderm.4,14,15
In the majority of human PSC differentiation protocols, definitive endoderm formation is induced by treating cells with a high concentration of activin A—a transforming growth factor β (TGF-β) family member used to mimic the activity of Nodal. Nodal proteins play an evolutionarily conserved role in initiating gastrulation and in driving endoderm formation in all studied vertebrate species.16 In fish, frogs, and mammals, Nodal proteins act in a concentration-dependent fashion to promote formation of the mesoderm lineage at low concentrations and endoderm at high concentrations. The levels of Nodal signaling have also been linked to embryonic and germ layer patterning along the anterior–posterior axis such that high levels of Nodal promote anterior mesoderm and endoderm.17 Consistent with these studies in the embryo, the nodal mimetic activin A has distinct concentration-dependent effects. It was seen by Kubo et al. that different concentrations induce different developmental outcomes: high concentrations of activin A favored dorsal mesoderm and endoderm fates, whereas low concentrations of activin A favored more ventral mesoderm fates.18
Subsequent studies utilizing hESCs have demonstrated that PI3K signaling must be suppressed for cells to optimally respond to activin/Nodal.19 Compounds such as wortmannin, which inhibits PI3K signaling, have been found to promote definitive endoderm formation20 and have been used in combination with activin A to induce definitive endoderm from hESCs. To increase the robustness of differentiation and reduce costs, researchers have sought to discover small molecule alternatives that have the ability to direct hPSC differentiation into definitive endoderm. Two such molecules, IDE1 and IDE2, have been identified and have been shown to be able to induce definitive endoderm formation (in the presence of serum) with similar efficiencies to activin A.21 The specific target molecule for these compounds has not been identified, though experiments indicate that activation of TGF-β signaling may be involved.21
Other signaling pathways appear to modify the activity of activin A during the definitive endoderm induction step. In the embryo, these signaling pathways function during gastrulation and act downstream of Nodal. One example is the TGF-β superfamily molecule, BMP4, which is expressed in the posterior primitive streak.22 Mouse embryos lacking BMP4 fail to express genes associated with mesoderm formation, such as Brachyury, and die during gastrulation (at approximately embryonic day (E) 6.5). A number of studies have reported that the inclusion of low concentrations of BMP4 along with activin A on the first day of differentiation improves the efficiency of definitive endoderm formation.23–26 Other signaling pathways that are essential for gastrulation have been shown to play a role in activin-induced definitive endoderm formation. For example, Wnt signaling is essential for vertebrate gastrulation and mouse embryos lacking Wnt3 fail to gastrulate.27 If canonical Wnt signaling is blocked during ESC differentiation, mesendoderm fails to form,28,29 whereas activation of canonical Wnt signaling enhances differentiation of hESCs toward definitive endoderm.30
Formation of posterior foregut and pancreatic endoderm from definitive endoderm
In the vertebrate embryo, a number of molecular and morphogenic changes take place between the initial formation of definitive endoderm and emergence of Pdx1-expressing pancreatic endoderm. The definitive endoderm transitions from a two-dimensional sheet into a primitive gut tube that becomes patterned along the anterior–posterior and dorsal–ventral axes. During gut tube morphogenesis, the presumptive pancreatic endoderm comes in contact with mesodermal cell types including the septum transversum mesenchyme (ventral) and notochord (dorsal).31
These mesodermal cell types are the source of several signaling molecules that regulate the formation of Pdx1-expressing endoderm, including fibroblast growth factor (FGF), retinoic acid (RA), and hedgehog. FGF signaling is involved in many stages of pancreas development, including global patterning of the endoderm along the anterior–posterior axis as well as posterior foregut patterning. In chick and mouse, low levels of FGF signaling promote posterior foregut fate and expression of Pdx1, whereas higher levels promote more posterior endoderm fates.32–34
The notochord, which is located adjacent to the dorsal endoderm until around E8.0, is an important source of several of these signaling molecules and has been shown to play an essential role in dorsal pancreatic development. Removal of the notochord in chick embryos before pancreatic bud formation resulted in failure to initiate dorsal pancreas development, though ventral pancreas development was unaffected.35 Further studies found that signaling molecules from notochord, including activin βB and FGF2, were able to repress the expression of sonic hedgehog (Shh) in the adjacent prepancreatic endoderm,36 though this was inhibited in the presence of exogenous Shh, suggesting that Shh acts as an antipancreatic factor.36
RA signaling plays an essential role in the morphogenesis and organogenesis of numerous foregut organs, including the pancreas.37 The distribution of RA in the embryo is tightly controlled by synthesis from circulating retinol in a two-step reaction involving specific retinol and retinaldehyde dehydrogenases (RALDHs),38 as well as by degradation by specific cytochrome P450s (CYP26s).37 A variety of gain and loss studies indicate that retinoid signaling is required for pancreatic specification in a number of species.38–41 For example, in zebrafish, it has been shown that retinoid signaling is essential for both pancreas and liver specification, and that exogenous RA treatment induces ectopic expression of pancreatic and liver markers.39 In both Xenopus41 and quail,40 it has been shown that retinoid signaling is essential for the formation of the dorsal pancreas, though it has no effect on ventral pancreas genesis. Further studies in Xenopus have demonstrated that while RA was sufficient to induce pancreas-specific genes in the dorsal pancreas, it was unable to induce these same genes in the ventral pancreas.42 Furthermore, in mice, loss of RALDH activity results in broad foregut organ abnormalities, including dorsal pancreas agenesis. Moreover, it was shown that RA signaling was sufficient to induce Pdx1 expression in the anterior endoderm.38,43
Following from these developmental studies, nearly every published ESC differentiation protocol requires the addition of exogenous RA and/or FGF to promote the transition of definitive endoderm to Pdx1+ endoderm in mouse44–46 and human (Fig. 2).23,25,47–53 Although most protocols use FGF and/or RA, the interpretation of how these pathways promote expression of Pdx1 expression remains poorly understood.
Pancreatic endoderm to endocrine precursor cells
The commitment of pancreatic endoderm to endocrine precursor cells is an obligate step during the formation of β cells, though most differentiation protocols do not incorporate factors specifically designed to promote or enhance this process. This is due in part to a paucity of information about the signaling pathways that initiate endocrine cell formation and guide subsequent differentiation into the β cell lineage. There is a significant amount of information about the transcription factors that regulate endocrine and β cell fate in vivo,54 and these factors serve as useful markers to identify cell types in ESC-derived pancreatic cultures. For example, the transcription factor neurogenin 3 (Neurog3) has been an important marker because it is expressed early in the specification of the pancreatic endocrine lineage.55 However, as it is only expressed transiently and marks all endocrine cells, this makes it problematic to use as a marker to identify cells committing to a β cell fate.
In the mouse, Neurog3 has a bimodal expression pattern, which is thought to mark the first and second waves of endocrine cell formation. The first wave of Neurog3 expression is between E8.5 and E11.5, and the second wave of expression is initiated at E12, with peak expression observed at E15.5.56,57 Studies in both chick and mouse have shown that Neurog3 is involved in the epithelial–mesenchymal transition58 that is associated with endocrine cell development (Fig. 1), during which delaminating endocrine cells are marked by the expression of EphB3.59 It is thought that only endocrine cells specified during the secondary transition (E12 onwards) form definitive single-hormone cells in the adult pancreas,60 while endocrine cells formed during the primary transition (E8.5 through E11.5) do not contribute to the adult pancreas, instead playing a role during embryonic pancreatic function.61 This paradigm, however, is not supported by all studies of pancreatic cell development, some of which have demonstrated that a small number of endocrine progenitor cells formed before the primary transition are able to form hormone cells during the secondary transition, and hence, can contribute to the adult pancreas.56
Perhaps the best-studied developmental signaling pathway involved in controlling endocrine precursor formation is Notch signaling. In a number of reports, it has been found that impaired Notch receptor activation or signaling resulted in upregulation of Neurog3 expression, leading to premature endocrine cell differentiation at the expense of both pancreatic progenitor cell expansion and exocrine cell differentiation.62–68 In contrast, active Notch signaling appears to maintain cells as undifferentiated progenitors that are able to contribute to both proliferation and later differentiation. In this sense, the function of Notch signaling during endocrine precursor specification is analogous to its function during early mammalian neurogenesis.69,70 Despite a clear role for Notch signaling in repressing endocrine cell fate in vivo, relatively few protocols utilize Notch inhibitors such as DAPT during ESC differentiation (Fig. 2). This suggests that the endogenous Notch signaling levels in these cultures is not inhibitory to endocrine differentiation.
FGF10 produced by the surrounding pancreatic mesenchyme is also involved in pancreatic progenitor cell proliferation and acts upstream of Notch signaling. The role of FGF10 was initially identified in mouse knockout studies, where FGF10−/−mice exhibit severe pancreatic hypoplasia, owing to a profound reduction in the proliferation of pancreatic epithelial progenitor cells.71 By contrast, in transgenic mice that overexpress FGF10, pancreatic hyperplasia is seen, which results from the decreased differentiation of endocrine progenitor cells and concurrent expansion of progenitor cell numbers.72,73 This proliferative effect of FGF10 has also been observed in vitro in both isolated mouse74 and rat75 pancreatic epithelia. A study using isolated E10.5 mouse pancreatic epithelia found that the exogenous FGF10 increased the proliferation of pancreatic progenitor cells and promoted the expression of Hes1, a downstream target of Notch signaling. Moreover, in FGF10-treated epithelium, inhibition of Notch signaling caused the loss of Hes1 expression and a decrease in pancreatic progenitor cell proliferation, suggesting that Notch is downstream of FGF10.74
Other signaling ligands that are secreted by the pancreatic mesenchyme also play a role in endocrine cell development. For example, mesenchyme-derived TGF-β signals were shown to be involved in regulating cell fate choice between endocrine and exocrine lineages.76–78 There was, however, some degree of uncertainty as to which lineages TGF-β signaling was affecting: endocrine, exocrine, or both lineages via a common progenitor cell. Recently, the TGF-β superfamily members, TGF-β2 and TGF-β3, have been identified to play key roles in controlling the balance between endocrine precursor proliferation and differentiation in hESC cultures. In their report, Guo et al. identified a set of signaling factors, including TGF-β ligands that are expressed by the pancreatic mesenchyme in the embryo and were able to promote the expansion of hESC-derived pancreatic progenitors through a mechanism that is dependent on the duration of ligand signaling.79 It was seen that while a short burst of TGF-β signaling promoted upregulation of both Neurog3 expression and endocrine differentiation, prolonged TGF-β signaling lead to expansion of the progenitor pool and attenuation of endocrine development.79
During the formation of the pancreas in human embryogenesis, NEUROG3 expression is observed at week (W) 8 in development, where it is coexpressed with other pancreatic transcription factors such as PDX1, as well as hormones such as insulin and glucagon. As in the mouse, NEUROG3 expression levels diminish over time, though it was still observable at W21.80 In contrast to Neurog3 expression patterns reported in the mouse, NEUROG3 expression has not been seen to be biphasic during human development.81 This may indicate that distinct primary and secondary transition events do not occur during human pancreatic development. There is, in fact, some debate about the functional necessity of NEUROG3 in human endocrine pancreas development. Several published reports describe patients with homozygous mutations in NEUROG382,83 who were born with varying levels of circulating C-peptide, although one patient described had severely reduced C-peptide levels. Despite all patients’ eventually developing diabetes, the fact that these patients often have detectable C-peptide suggests that human NEUROG3 mutations may not result in complete loss of function, or that NEUROG3 is not required for embryonic specification of all β cells in humans.
Commitment of endocrine progenitor cells to β cells
The signals that control the transition from endocrine precursors into functional β cells are not yet defined. It is, therefore, not surprising that differentiation of PSC-derived pancreatic progenitors into mature, functional β cells in vitro has not yet been achieved. Historically, studies have used insulin expression to identify β cells; however, it is now clear from mouse experiments that early insulin-expressing cells do not go on to become definitive β cells. This was initially demonstrated in mice, using a Cre/loxP tagging strategy, in which the early endocrine cells were irreversibly tagged using the activity of Cre recombinase, allowing for cell-lineage tracing. This work showed that the definitive β cells did not arise from early endocrine cells that were specified during the primary transition, but instead arose from a Pdx1+ progenitor cell that had not previously expressed insulin.61 This work suggests that it may be prudent to use other markers in addition to insulin in order to identify PSC-derived β cells.
There have been multiple studies that describe the emergence of hormone-expressing cells during human pancreas development. One report found that insulin-expressing cells first appear at W8 before other hormone-expressing cell types, such as glucagon or somatostatin,84 which were detected at W8.5, followed by pancreatic polypeptide (PP)-expressing cells at W10.85 At these early stages, insulin-expressing cells coexpress glucagon at frequencies ranging from 10% to 92%84–86 and coexpress somatostatin at frequencies ranging from 10% to 97%.84,86 The existence of tri-hormonal (insulin/glucagon/somatostain) cells has also been reported. In contrast, PP-expressing cells have only been observed as monohormonal cells during human fetal development.86 Polyhormonal cells were seen to decrease in frequency after W984 and were not detected in pancreata at W22.87
The use of hormone (markers) as a readout of PSC-derived β cells has misdirected many researchers into focusing on primary transition insulin+ cells. As is observed during embryonic development, human PSC-derived insulin-expressing cells are generally polyhormonal, expressing glucagon and/or somatostatin. These in vitro–derived polyhormonal cells are functionally immature and lack the capacity for glucose-stimulated insulin secretion (GSIS),24,47 which is the key hallmark of a functional β cell. As yet, however, there have been no reports suggesting that polyhormonal endocrine cells give rise to functional monohormonal endocrine cells, which is consistent with lineage-tracing experiments previously performed in mice.
To better identify cell types that can give rise to mature β cells, the field has focused on transcription factors, including Nkx6.1, MafB, and MafA, which mark progenitors and maturing β cells in vivo. For example, Nkx6.1 is initially broadly expressed in the mouse pancreas at E10.588 but becomes restricted to the insulin-expressing cells and scattered ductal cells between E13 and E15.5. In the adult mouse, Nkx6.1 is exclusively seen in the β cells.89 A similar pattern of NKX6.1 expression has been observed during human development, where NKX6.1 is broadly expressed in the pancreatic epithelium from W9, with subsequent downregulation of NKX6.1 expression in noninsulin-expressing cells by W13. Similar to the mouse, NKX6.1 expression is restricted to β cells in the adult human pancreas.90 Loss-of-function studies in the mouse showed that Nkx6.1 is required for β cell specification89 and in vitro overexpression studies suggest that it may improve β cell function,91 although this was not true in vivo.92
There are now several reports examining the expression of NKX6.1 in differentiating hPSC cultures. Two early studies identified expression of NKX6.1 RNA48 and protein47 following the expression of earlier transcription factors such as PDX1; however, neither report demonstrated coexpression of NKX6.1 with other markers of β cell differentiation, such as insulin. More recently, NKX6.1 was observed to be coexpressed with C-peptide,20 PDX1,20,52,93,94 or insulin.25 However, these cells either demonstrated limited functionality20 or functionality was not examined.25,93,94 It is therefore unclear if coexpression of NKX6.1 with either PDX1 or insulin will be a useful identifier of the functional β cell lineage. A recent study sorted differentiated hESCs on the basis of high (~80%) or low (~25%) NKX6.1 expression, then transplanted these different populations into diabetic mice. It was seen that while diabetes was reversed within 3 months in NKX6.1-high recipient mice, NKX6.1-low recipient mice remained hyperglycemic.95 This would suggest that high NKX6.1 expression may be an indicator of the definitive β cell lineage.
MafA and MafB are transcription factors that are expressed in both the mouse and human pancreas in a temporospatially regulated fashion. In the mouse, MafB is first observed at E10.5 during the primary transition96 in Neurog3+ progenitors and in insulin+ and glucagon+ cells. MafB expression then becomes restricted to glucagon+ cells (β cells) in the adult.96,97 In contrast, MafA expression is not observed until later in development—at E13.5 in the insulin+ cells specified during the secondary transition.98 In addition to their different temporal and spatial distributions, MafB and MafA play different functional roles during β cell differentiation. Surprisingly, only MafB is required for β cell development in the mouse—β cell numbers are not affected in mice lacking pancreatic Mafa. However, it is possible that MafB is able to compensate for the loss of MafA in this context.97 In contrast, MafA has been shown to control the glucose-responsive transcription of insulin and other associated genes in the definitive β cells.99,100 Mafa−/− mice have impaired glucose tolerance and defects in insulin secretion, emphasizing the importance of MafA in the maintenance of an appropriate GSIS response.101
Interestingly, the expression pattern of MAFA and MAFB differ in the human, both spatially and temporally, as compared to the mouse. MAFA has been seen to be expressed throughout the developing pancreatic epithelium, including in the nascent endocrine cells from W9.90 In insulin+ cells, a strong nuclear expression of MAFA was seen, in contrast to weaker expression throughout the remaining epithelium. From W13 onwards, MAFA expression was downregulated, with a low level of expression restricted to the insulin+ cells, which was followed by complete loss of expression by W21. However in the adult, nuclear-localized MAFA expression was observed in β cells, similar to that seen in mice,90 suggesting possible biphasic expression of MAFA in humans. A second study found that MAFA transcripts increased from W9 through to W23, albeit at levels lower than those observed in the adult.102 The expression of MAFB initially resembled that of MAFA, in that at W9, expression is seen in both the pancreatic epithelium and in insulin+ cells. However, by W14, expression of MAFB is seen to be restricted to both insulin+ and glucagon+ cells. This spatial patterning is maintained throughout development, with expression of MAFB in both the β and β cells of the adult.90 This differs from the mouse, where MafB expression has not been observed in the β cells of the adult islet.96
Other tissues important for β cell development
Many tissues are known to play a role in controlling β cell development (Fig. 3). Earlier, we have discussed the role of both notochord-and mesenchyme-derived signals. The signaling molecules from these tissues have been at least partially identified. In contrast, it has been observed that both neural crest cells and endothelial cells play important roles in β cell development, though the signaling molecules derived from these tissues have not been well characterized.
Figure 3.

Schematic depicting the influence of surrounding tissues on β cell development. (A) At E8.0, the notochord plays an important role in the suppression of Shh in the pancreatic endoderm. (B) At E10.5, the mesenchyme surrounding the pancreas produces FGF10 that signals to the pancreatic epithelium and plays an important role in endocrine progenitor cell proliferation, replication, and differentiation. (C) At E13.5, endocrine cells produce proangiogenic factors, such as angiopoeitin and VEGF-A, in order to attract the developing endothelium. Ang, angiopoeitin; DS, dorsal somite; Ec, ectoderm; En, endoderm; IM, intermediate mesoderm; LPM, lateral plate mesoderm; No, notochord; NT, neural tube; Shh, sonic hedgehog; VS, ventral somite.
Neural crest
Neural crest cells derive from the dorsal neural tube, where they migrate ventrally and infiltrate the foregut mesenchyme at ~E10.0. Neural crest cells play an important role in control of progenitor cell replication and β cell maturation.103 A study utilizing mice where FoxD3 was specifically deleted in the neural crest cells resulted in a loss of neural crest cells and, interestingly, increased insulin+ cell proliferation but decreased β cell maturity.104 This correlated with in vitro studies that identified a role for neural crest cells in β cell replication. A study in which isolated mouse islets were cocultured with neural crest stem cells found up to a 10-fold increase in β cell replication, as compared to β cells found in isolated islets.105 To date, no study has reported the coculture of neural crest cells with human PSC-derived pancreatic endoderm or endocrine cells. Given the role that neural crest plays in β cell maturation, this may be an approach that is able to influence maturity of human PSC-derived pancreatic cells in vitro.
Endothelium
The endothelium or vasculature plays multiple roles in controlling pancreatic and β cell development. Early in development, at around E8, fusion of the dorsal aortae displaces the notochord and the dorsal foregut. These aortic endothelial cells have been shown to induce pancreatic-bud structures as well as Pdx1 expression in the adjacent endoderm.106 In Xenopus that were engineered to lack a dorsal aorta, there was a loss of pancreatic marker expression.106 Interestingly, it was seen that recombination of prepancreatic endoderm with other tissues (such as mesenchyme or umbilical artery) that contained endothelial cells also allowed for the induction of pancreatic differentiation (as marked by the expression of insulin).106 Further studies found that while the endothelial cells of the dorsal aorta were not required for the initial induction of Pdx1, they were, however, required for the maintenance of Pdx1 expression, as well as inducing expression of the pancreatic transcription factor Ptf1a in the dorsal pancreas. In contrast, ventrally located endothelial tissues are unnecessary for Pdx1 and Ptf1a expression in the ventral pancreas.107
Work in the chicken has also suggested a role for endothelial signals early in pancreatic development. Before formation of the blood vessels, it was seen that CXCR4-expressing angioblasts migrated to the prepancreatic endodermal region because of recruitment by the CXCL12-expressing endoderm. Following migration, the angioblasts were able to induce Pdx1 expression in the prepancreatic domain.108,109 Studies have demonstrated that over-expression of the CXCL12 ligand resulted in ectopic recruitment of angioblasts and a concurrent expansion of the pancreatic domain, while blockade of the CXCR4 receptor resulted in the absence of dorsal aorta formation, and consequently a decrease in Pdx1 and insulin expression.108
The endothelium is also known to play a role later in pancreatic development, in addition to its earlier role in dorsal bud induction. At ~E13.5, endocrine cells express the angiogenic factors angiopoeitin and vascular endothelial growth factor A (VEGF-A) and are located in close proximity to the newly forming capillaries.110 As the developing endocrine cells are unable to synthesize their own basement membrane, they rely on the adjacent endothelial cells for the formation of their basement membrane.111 In addition, VEGF-A expression by β cells is required for establishing the capillary network that is necessary for islet function in the control of glucose homeostasis. In mice that have a β cell–specific inactivation of VEGF-A, impaired GSIS was observed, despite normal β cell mass and insulin contents in the islet. It is thought that this defect in GSIS is due to a decreased intraislet capillary network leading to a reduction in insulin transport via the endothelial fenestrae.110
Functionality of β cells generated from PSCs
Although there have been no reports describing the generation of a fully functional β cell in vitro, ESC cultures routinely give rise to polyhormonal insulin-expressing cells that might be useful as insulin delivery vehicles. However, transplantation of these cells has not ameliorated diabetes in mice under nonfasting conditions50,51,112 and in some cases transplanted cells were unable to maintain an insulin+ phenotype, instead giving rise to grafts that consisted solely of glucagon-expressing cells.24,113 In lieu of attempting to generate functional β cells in vitro, Kroon et al. used a different approach by engrafting pancreatic progenitors (PDX1+ endoderm) into mice and allowing them to mature in vivo. Cells matured in vivo became insulin-expressing, glucose-responsive cells capable of regulating blood sugar levels in these animals. It was necessary to allow these grafts to develop for substantial periods of time (90–140 day posttransplant) before secretion of human insulin was seen. Following selective ablation of mouse β cells with streptozotocin (STZ), the engrafted cells were able to maintain normoglycemia until surgical graft removal (up to 88 days post-STZ treatment).114
This same approach has now been used by a number of groups49,52,53,115,116 and was most recently used to demonstrate that hESC-derived pancreatic cells were able ameliorate diabetes in both mice and rats.53 This is an important proof of principle in efforts to translate this approach into a clinical setting, as it is the first time that PSC-derived pancreatic cells have been shown to function in a nonmouse model. A study performed by Kelly et al. highlighted the difference in transplanting progenitor cells as compared to polyhormonal cells. Following transplantation, progenitor cells consistently gave rise to insulin+ cells that ameliorated diabetes, whereas polyhormonal cells gave rise to grafts containing glucagon+ and somatostatin+ cells that were therapeutically ineffective.52
Form to function: role of morphogenesis in PSC differentiation
Perhaps one reason why, as yet, there has been failure to create wholly in vitro PSC-derived β cells is a lack of supporting tissues being present—such as the mesenchyme and endothelium, whose essential roles in β cell development were earlier discussed. Another deficiency is the two-dimensional nature of many culture systems, which do not allow for the morphogenetic processes that occur during organogenesis in vivo. Several recent reports have incorporated morphogenetic processes into differentiation protocols to generate three-dimensional intestinal, retinal, and pituitary gland tissues.
One key morphogenetic event in endoderm organogenesis is the transition of definitive endoderm into a primitive gut tube, from which all endoderm organs are derived. The primitive gut tube is a simple cuboidal epithelium surrounded by a primitive mesenchyme. It was recently shown that manipulation of Wnt and FGF signaling was sufficient to induce human PSC-derived definitive endoderm to undergo gut tube–like morphogenesis. The resulting three-dimensional structures were a simple cuboidal epithelium surrounded by a primitive mesenchyme. These gut tube–like structures were competent to form complex intestinal organoids in vitro. Developing intestinal organoids underwent similar developmental processes as the embryonic intestine and gave rise to a complex array of functional intestinal cell types117 (Fig. 4A).
Figure 4.
Schematic depicting self-organization of PSCs in order to form differentiated tissues. (A) hPSCs can be differentiated into intestinal cell types. As differentiation occurs, spheroids bud from a posterior endoderm monolayer and go on to form lumen-containing organoids with both epithelial and mesenchymal tissue that contain the major cell types of the intestine. (B) mESC cell aggregates self-organize to form spherical vesicles on the edge of the aggregates, which then evaginate to form optic cup–like structures. (C) mESC cell aggregates form ectoderm on the surface that can self-organize to form anterior pituitary–like structures.
The importance of morphogenesis was also demonstrated in approaches to generate retinal cell types. Using aggregates of mESCs, Ikeda et al. were able to induce the formation of Pax6-expressing retinal cells; however, no clear formation of retinal epithelial structures was seen.118 Subsequent modifications to their protocol, most importantly, the addition of either matrigel or the combination of purified laminin, enactin, and nodal, stimulated optic cup morphogenesis and resulted in the formation of three-dimensional optic cup–like structures. Optic cup structures matured into tissues that contained all of the major cell types of the retina119 (Fig. 4B). A similar approach was used to generate three-dimensional neural tissues resembling the anterior pituitary gland structure Rathke’s pouch. In this case, mESC aggregates that were cultured in the presence of a Smoothened agonist (thus activating Hedgehog signaling) and BMP4, followed by the inhibition of Notch signaling, were able to form a structure in vitro that morphologically resembles Rathke’s pouch and contained multiple endocrine cell types that were able to secrete adrenocorticotropic hormone (ACTH)120 (Fig. 4C).
Another approach to generate three-dimentional tissues from PSCs is through the incorporation of other supporting cell types such as stromal and vascular cells. For example, addition of vascular endothelial cells and mesenchymal stem cells into PSC-derived liver cultures resulted in the formation of liver bud–like structures.121 These liver bud structures formed highly differentiated hepatocytes and contained well-formed capillaries that were capable of forming a patent, functional vascular plexus when engrafted into mice. Given the importance of vascular cells for endocrine pancreas development, incorporation of vascularity into human PSC-derived pancreatic cultures is a promising approach to promote endocrine development.
Summary
Since the first publication reporting the generation of PSC-derived pancreatic cells in 2006, a vast number of protocols have been reported. Many of these protocols utilize the same ontogenic approach to PSC differentiation, and the most success in generating functional β cells has come from transplanting PDX1+ progenitor cells, to allow for in vivo cell maturation into glucose-responsive β cells. The use of progenitor cells as a treatment for type 1 diabetes has a number of advantages as compared to the use of more differentiated insulin+ cells. Importantly, progenitors give rise to therapeutically effective insulin-expressing cells in vivo and are ready for use in clinical trials. There is no need to understand or identify the signals required for β cell maturation in order to achieve a therapeutic end point. However, pancreatic progenitor cell therapy is somewhat of a black box approach since they also give rise to the other pancreatic endocrine cell types that could be therapeutically counterproductive. When engrafting progenitors, one can only hope that they give rise to the desired number of β cells following in vivo maturation. For this reason, it is still critical to develop methods to generate fully functional β cells in vitro. These cells could be highly characterized and quality controlled, and transplanted into patients with a more predictable therapeutic outcome.
Given the recent advances in generating more functional, complex three-dimensional tissues, it should be possible to generate more functional pancreatic tissues for transplantation. This approach would allow for the incorporation of other important tissues, such as mesenchyme and endothelium, and may provide the correct signaling molecules and three-dimensional architecture to allow for the formation of mature, functional β cells.
Footnotes
Conflicts of interest
The authors declare no conflicts of interest.
References
- 1.Thomson JA, et al. Embryonic stem cell lines derived from human blastocysts. Science. 1998;282:1145–1147. doi: 10.1126/science.282.5391.1145. [DOI] [PubMed] [Google Scholar]
- 2.Reubinoff BE, et al. Embryonic stem cell lines from human blastocysts: somatic differentiation in vitro. Nat Biotechnol. 2000;18:399–404. doi: 10.1038/74447. [DOI] [PubMed] [Google Scholar]
- 3.Takahashi K, et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861–872. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- 4.Tada S, et al. Characterization of mesendoderm: a diverging point of the definitive endoderm and mesoderm in embryonic stem cell differentiation culture. Development. 2005;132:4363–4374. doi: 10.1242/dev.02005. [DOI] [PubMed] [Google Scholar]
- 5.Lawson KA, Pedersen RA. Cell fate, morphogenetic movement and population kinetics of embryonic endoderm at the time of germ layer formation in the mouse. Development. 1987;101:627–652. doi: 10.1242/dev.101.3.627. [DOI] [PubMed] [Google Scholar]
- 6.Lawson KA, Meneses JJ, Pedersen RA. Clonal analysis of epiblast fate during germ layer formation in the mouse embryo. Development. 1991;113:891–911. doi: 10.1242/dev.113.3.891. [DOI] [PubMed] [Google Scholar]
- 7.Wilkinson DG, Bhatt S, Herrmann BG. Expression pattern of the mouse T gene and its role in mesoderm formation. Nature. 1990;343:657–659. doi: 10.1038/343657a0. [DOI] [PubMed] [Google Scholar]
- 8.Pearce JJ, Evans MJ. Mml, a mouse Mix-like gene expressed in the primitive streak. Mech Dev. 1999;87:189–192. doi: 10.1016/s0925-4773(99)00135-5. [DOI] [PubMed] [Google Scholar]
- 9.Robb L, et al. Cloning, expression analysis, and chromosomal localization of murine and human homologues of a Xenopus mix gene. Dev Dyn. 2000;219:497–504. doi: 10.1002/1097-0177(2000)9999:9999<::AID-DVDY1070>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 10.Blum M, et al. Gastrulation in the mouse: the role of the homeobox gene goosecoid. Cell. 1992;69:1097–1106. doi: 10.1016/0092-8674(92)90632-m. [DOI] [PubMed] [Google Scholar]
- 11.Ang SL, et al. The formation and maintenance of the definitive endoderm lineage in the mouse: involvement of HNF3/forkhead proteins. Development. 1993;119:1301–1315. doi: 10.1242/dev.119.4.1301. [DOI] [PubMed] [Google Scholar]
- 12.Sasaki H, Hogan BL. Differential expression of multiple fork head related genes during gastrulation and axial pattern formation in the mouse embryo. Development. 1993;118:47–59. doi: 10.1242/dev.118.1.47. [DOI] [PubMed] [Google Scholar]
- 13.Hudson C, et al. Xsox17alpha and -beta mediate endoderm formation in Xenopus. Cell. 1997;91:397–405. doi: 10.1016/s0092-8674(00)80423-7. [DOI] [PubMed] [Google Scholar]
- 14.Yasunaga M, et al. Induction and monitoring of definitive and visceral endoderm differentiation of mouse ES cells. Nat Biotechnol. 2005;23:1542–1550. doi: 10.1038/nbt1167. [DOI] [PubMed] [Google Scholar]
- 15.Green MD, et al. Generation of anterior foregut endoderm from human embryonic and induced pluripotent stem cells. Nat Biotechnol. 2011;29:267–272. doi: 10.1038/nbt.1788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zorn AM, Wells JM. Molecular basis of vertebrate endoderm development. Int Rev Cytol. 2007;259:49–111. doi: 10.1016/S0074-7696(06)59002-3. [DOI] [PubMed] [Google Scholar]
- 17.Robertson EJ, et al. Control of early anterior-posterior patterning in the mouse embryo by TGF-beta signalling. Philos Trans R Soc Lond B Biol Sci. 2003;358:1351–1357. doi: 10.1098/rstb.2003.1332. discussion 1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kubo A, et al. Development of definitive endoderm from embryonic stem cells in culture. Development. 2004;131:1651–1662. doi: 10.1242/dev.01044. [DOI] [PubMed] [Google Scholar]
- 19.McLean AB, et al. Activin a efficiently specifies definitive endoderm from human embryonic stem cells only when phosphatidylinositol 3-kinase signaling is suppressed. Stem Cells. 2007;25:29–38. doi: 10.1634/stemcells.2006-0219. [DOI] [PubMed] [Google Scholar]
- 20.Zhang D, et al. Highly efficient differentiation of human ES cells and iPS cells into mature pancreatic insulin-producing cells. Cell Res. 2009;19:429–438. doi: 10.1038/cr.2009.28. [DOI] [PubMed] [Google Scholar]
- 21.Borowiak M, et al. Small molecules efficiently direct endodermal differentiation of mouse and human embryonic stem cells. Cell Stem Cell. 2009;4:348–358. doi: 10.1016/j.stem.2009.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Winnier G, et al. Bone morphogenetic protein-4 is required for mesoderm formation and patterning in the mouse. Genes Dev. 1995;9:2105–2116. doi: 10.1101/gad.9.17.2105. [DOI] [PubMed] [Google Scholar]
- 23.Nostro MC, et al. Stage-specific signaling through TGFbeta family members and WNT regulates patterning and pancreatic specification of human pluripotent stem cells. Development. 2011;138:861–871. doi: 10.1242/dev.055236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Basford CL, et al. The functional and molecular characterisation of human embryonic stem cell-derived insulin-positive cells compared with adult pancreatic beta cells. Diabetologia. 2012;55:358–371. doi: 10.1007/s00125-011-2335-x. [DOI] [PubMed] [Google Scholar]
- 25.Micallef SJ, et al. INS (GFP/w derived insulin-producing cells. Diabetologia. 2012;55:694–706. doi: 10.1007/s00125-011-2379-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Teo AK, et al. Activin and BMP4 synergistically promote formation of definitive endoderm in human embryonic stem cells. Stem Cells. 2012;30:631–642. doi: 10.1002/stem.1022. [DOI] [PubMed] [Google Scholar]
- 27.Liu P, et al. Requirement for Wnt3 in vertebrate axis formation. Nat Genet. 1999;22:361–365. doi: 10.1038/11932. [DOI] [PubMed] [Google Scholar]
- 28.Gadue P, et al. Wnt and TGF-beta signaling are required for the induction of an in vitro model of primitive streak formation using embryonic stem cells. Proc Natl Acad Sci U S A. 2006;103:16806–16811. doi: 10.1073/pnas.0603916103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jackson SA, et al. Differentiating embryonic stem cells pass through ‘temporal windows’ that mark responsiveness to exogenous and paracrine mesendoderm inducing signals. PLoS One. 2010;5:e10706. doi: 10.1371/journal.pone.0010706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bone HK, et al. A novel chemically directed route for the generation of definitive endoderm from human embryonic stem cells based on inhibition of GSK-3. J Cell Sci. 2011;124:1992–2000. doi: 10.1242/jcs.081679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McCracken KW, Wells JM. Molecular pathways controlling pancreas induction. Semin Cell Dev Biol. 2012;23:656–662. doi: 10.1016/j.semcdb.2012.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wells JM, Melton DA. Early mouse endoderm is patterned by soluble factors from adjacent germ layers. Development. 2000;127:1563–1572. doi: 10.1242/dev.127.8.1563. [DOI] [PubMed] [Google Scholar]
- 33.Dessimoz J, et al. FGF signaling is necessary for establishing gut tube domains along the anterior-posterior axis in vivo. Mech Dev. 2006;123:42–55. doi: 10.1016/j.mod.2005.10.001. [DOI] [PubMed] [Google Scholar]
- 34.Deutsch G, et al. A bipotential precursor population for pancreas and liver within the embryonic endoderm. Development. 2001;128:871–881. doi: 10.1242/dev.128.6.871. [DOI] [PubMed] [Google Scholar]
- 35.Kim SK, Hebrok M, Melton DA. Notochord to endoderm signaling is required for pancreas development. Development. 1997;124:4243–4252. doi: 10.1242/dev.124.21.4243. [DOI] [PubMed] [Google Scholar]
- 36.Hebrok M, Kim SK, Melton DA. Notochord repression of endodermal Sonic hedgehog permits pancreas development. Genes Dev. 1998;12:1705–1713. doi: 10.1101/gad.12.11.1705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rhinn M, Dolle P. Retinoic acid signalling during development. Development. 2012;139:843–858. doi: 10.1242/dev.065938. [DOI] [PubMed] [Google Scholar]
- 38.Martin M, et al. Dorsal pancreas agenesis in retinoic acid-deficient Raldh2 mutant mice. Dev Biol. 2005;284:399–411. doi: 10.1016/j.ydbio.2005.05.035. [DOI] [PubMed] [Google Scholar]
- 39.Stafford D, Prince VE. Retinoic acid signaling is required for a critical early step in zebrafish pancreatic development. Curr Biol. 2002;12:1215–1220. doi: 10.1016/s0960-9822(02)00929-6. [DOI] [PubMed] [Google Scholar]
- 40.Stafford D, et al. A conserved role for retinoid signaling in vertebrate pancreas development. Dev Genes Evol. 2004;214:432–441. doi: 10.1007/s00427-004-0420-6. [DOI] [PubMed] [Google Scholar]
- 41.Chen Y, et al. Retinoic acid signaling is essential for pancreas development and promotes endocrine at the expense of exocrine cell differentiation in Xenopus. Dev Biol. 2004;271:144–160. doi: 10.1016/j.ydbio.2004.03.030. [DOI] [PubMed] [Google Scholar]
- 42.Pan FC, et al. Retinoic acid-mediated patterning of the pre-pancreatic endoderm in Xenopus operates via direct and indirect mechanisms. Mech Dev. 2007;124:518–531. doi: 10.1016/j.mod.2007.06.003. [DOI] [PubMed] [Google Scholar]
- 43.Molotkov A, Molotkova N, Duester G. Retinoic acid generated by Raldh2 in mesoderm is required for mouse dorsal endodermal pancreas development. Dev Dyn. 2005;232:950–957. doi: 10.1002/dvdy.20256. [DOI] [PubMed] [Google Scholar]
- 44.Micallef SJ, et al. Retinoic acid induces Pdx1-positive endoderm in differentiating mouse embryonic stem cells. Diabetes. 2005;54:301–305. doi: 10.2337/diabetes.54.2.301. [DOI] [PubMed] [Google Scholar]
- 45.Shi Y, et al. Inducing embryonic stem cells to differentiate into pancreatic beta cells by a novel three-step approach with activin A and all-trans retinoic acid. Stem Cells. 2005;23:656–662. doi: 10.1634/stemcells.2004-0241. [DOI] [PubMed] [Google Scholar]
- 46.Schroeder IS, et al. Differentiation of mouse embryonic stem cells to insulin-producing cells. Nat Protoc. 2006;1:495–507. doi: 10.1038/nprot.2006.71. [DOI] [PubMed] [Google Scholar]
- 47.D’Amour KA, et al. Production of pancreatic hormone-expressing endocrine cells from human embryonic stem cells. Nat Biotechnol. 2006;24:1392–1401. doi: 10.1038/nbt1259. [DOI] [PubMed] [Google Scholar]
- 48.Jiang J, et al. Generation of insulin-producing islet-like clusters from human embryonic stem cells. Stem Cells. 2007;25:1940–1953. doi: 10.1634/stemcells.2006-0761. [DOI] [PubMed] [Google Scholar]
- 49.Shim JH, et al. Directed differentiation of human embryonic stem cells towards a pancreatic cell fate. Diabetologia. 2007;50:1228–1238. doi: 10.1007/s00125-007-0634-z. [DOI] [PubMed] [Google Scholar]
- 50.Eshpeter A, et al. In vivo characterization of transplanted human embryonic stem cell-derived pancreatic endocrine islet cells. Cell Proliferation. 2008;41:843–858. doi: 10.1111/j.1365-2184.2008.00564.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mao GH, et al. The reversal of hyperglycaemia in diabetic mice using PLGA scaffolds seeded with islet-like cells derived from human embryonic stem cells. Biomaterials. 2009;30:1706–1714. doi: 10.1016/j.biomaterials.2008.12.030. [DOI] [PubMed] [Google Scholar]
- 52.Kelly OG, et al. Cell-surface markers for the isolation of pancreatic cell types derived from human embryonic stem cells. Nat Biotechnol. 2011;29:750–756. doi: 10.1038/nbt.1931. [DOI] [PubMed] [Google Scholar]
- 53.Rezania A, et al. Maturation of human embryonic stem cell-derived pancreatic progenitors into functional islets capable of treating pre-existing diabetes in mice. Diabetes. 2012;61:2016–2029. doi: 10.2337/db11-1711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pan FC, Wright C. Pancreas organogenesis: from bud to plexus to gland. Dev Dyn. 2011;240:530–565. doi: 10.1002/dvdy.22584. [DOI] [PubMed] [Google Scholar]
- 55.Gradwohl G, et al. Neurogenin3 is required for the development of the four endocrine cell lineages of the pancreas. Proc Natl Acad Sci U S A. 2000;97:1607–1611. doi: 10.1073/pnas.97.4.1607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gu G, Dubauskaite J, Melton DA. Direct evidence for the pancreatic lineage: NGN3 +cells are islet progenitors and are distinct from duct progenitors. Development. 2002;129:2447–2457. doi: 10.1242/dev.129.10.2447. [DOI] [PubMed] [Google Scholar]
- 57.Villasenor A, Chong DC, Cleaver O. Biphasic Ngn3 expression in the developing pancreas. Dev Dyn. 2008;237:3270–3279. doi: 10.1002/dvdy.21740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gouzi M, et al. Neurogenin3 initiates stepwise de-lamination of differentiating endocrine cells during pancreas development. Dev Dyn. 2011;240:589–604. doi: 10.1002/dvdy.22544. [DOI] [PubMed] [Google Scholar]
- 59.Villasenor A, et al. EphB3 marks delaminating endocrine progenitor cells in the developing pancreas. Dev Dyn. 2012;241:1008–1019. doi: 10.1002/dvdy.23781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Pictet RL, et al. An ultrastructural analysis of the developing embryonic pancreas. Dev Biol. 1972;29:436–467. doi: 10.1016/0012-1606(72)90083-8. [DOI] [PubMed] [Google Scholar]
- 61.Herrera PL. Adult insulin- and glucagon-producing cells differentiate from two independent cell lineages. Development. 2000;127:2317–2322. doi: 10.1242/dev.127.11.2317. [DOI] [PubMed] [Google Scholar]
- 62.Apelqvist A, et al. Notch signalling controls pancreatic cell differentiation. Nature. 1999;400:877–881. doi: 10.1038/23716. [DOI] [PubMed] [Google Scholar]
- 63.Jacquemin P, et al. Transcription factor hepatocyte nuclear factor 6 regulates pancreatic endocrine cell differentiation and controls expression of the proendocrine gene ngn3. Mol Cell Biol. 2000;20:4445–4454. doi: 10.1128/mcb.20.12.4445-4454.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Jensen J, et al. Independent development of pancreatic alpha- and beta-cells from neurogenin3-expressing precursors: a role for the notch pathway in repression of premature differentiation. Diabetes. 2000;49:163–176. doi: 10.2337/diabetes.49.2.163. [DOI] [PubMed] [Google Scholar]
- 65.Murtaugh LC, et al. Notch signaling controls multiple steps of pancreatic differentiation. Proc Natl Acad Sci U S A. 2003;100:14920–14925. doi: 10.1073/pnas.2436557100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Esni F, et al. Notch inhibits Ptf1 function and acinar cell differentiation in developing mouse and zebrafish pancreas. Development. 2004;131:4213–4224. doi: 10.1242/dev.01280. [DOI] [PubMed] [Google Scholar]
- 67.Sumazaki R, et al. Conversion of biliary system to pancreatic tissue in Hes1-deficient mice. Nat Genet. 2004;36:83–87. doi: 10.1038/ng1273. [DOI] [PubMed] [Google Scholar]
- 68.Fukuda A, et al. Ectopic pancreas formation in Hes1-knockout mice reveals plasticity of endodermal progenitors of the gut, bile duct, and pancreas. J Clin Invest. 2006;116:1484–1493. doi: 10.1172/JCI27704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lewis J. Neurogenic genes and vertebrate neurogenesis. Curr Opin Neurobiol. 1996;6:3–10. doi: 10.1016/s0959-4388(96)80002-x. [DOI] [PubMed] [Google Scholar]
- 70.Beatus P, Lendahl U. Notch and neurogenesis. J Neurosci Res. 1998;54:125–136. doi: 10.1002/(SICI)1097-4547(19981015)54:2<125::AID-JNR1>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 71.Bhushan A, et al. FGF10 is essential for maintaining the proliferative capacity of epithelial progenitor cells during early pancreatic organogenesis. Development. 2001;128:5109–5117. doi: 10.1242/dev.128.24.5109. [DOI] [PubMed] [Google Scholar]
- 72.Hart A, Papadopoulou S, Edlund H. FGF10 maintains notch activation, stimulates proliferation, and blocks differentiation of pancreatic epithelial cells. Dev Dyn. 2003;228:185–193. doi: 10.1002/dvdy.10368. [DOI] [PubMed] [Google Scholar]
- 73.Norgaard GA, Jensen JN, Jensen J. FGF10 signaling maintains the pancreatic progenitor cell state revealing a novel role of Notch in organ development. Dev Biol. 2003;264:323–338. doi: 10.1016/j.ydbio.2003.08.013. [DOI] [PubMed] [Google Scholar]
- 74.Miralles F, et al. Interplay between FGF10 and Notch signalling is required for the self-renewal of pancreatic progenitors. Int J Dev Biol. 2006;50:17–26. doi: 10.1387/ijdb.052080fm. [DOI] [PubMed] [Google Scholar]
- 75.Miralles F, et al. Signaling through fibroblast growth factor receptor 2b plays a key role in the development of the exocrine pancreas. Proc Natl Acad Sci U S A. 1999;96:6267–6272. doi: 10.1073/pnas.96.11.6267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Sanvito F, et al. TGF-beta 1 influences the relative development of the exocrine and endocrine pancreas in vitro. Development. 1994;120:3451–3462. doi: 10.1242/dev.120.12.3451. [DOI] [PubMed] [Google Scholar]
- 77.Miralles F, Czernichow P, Scharfmann R. Follistatin regulates the relative proportions of endocrine versus exocrine tissue during pancreatic development. Development. 1998;125:1017–1024. doi: 10.1242/dev.125.6.1017. [DOI] [PubMed] [Google Scholar]
- 78.Tulachan SS, et al. TGF-beta isoform signaling regulates secondary transition and mesenchymal-induced endocrine development in the embryonic mouse pancreas. Dev Biol. 2007;305:508–521. doi: 10.1016/j.ydbio.2007.02.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Guo T, et al. Factors expressed by murine embryonic pancreatic mesenchyme enhance generation of insulin-producing cells from hESCs. Diabetes. 2013;62:1581–1592. doi: 10.2337/db12-0167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Lyttle BM, et al. Transcription factor expression in the developing human fetal endocrine pancreas. Diabetologia. 2008;51:1169–1180. doi: 10.1007/s00125-008-1006-z. [DOI] [PubMed] [Google Scholar]
- 81.Jennings RE, et al. Development of the human pancreas from foregut to endocrine commitment. Diabetes. 2013;62:3514–3522. doi: 10.2337/db12-1479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Wang J, et al. Mutant neurogenin-3 in congenital malabsorptive diarrhea. N Engl J Med. 2006;355:270–280. doi: 10.1056/NEJMoa054288. [DOI] [PubMed] [Google Scholar]
- 83.Rubio-Cabezas O, et al. Permanent neonatal diabetes and enteric anendocrinosis associated with biallelic mutations in NEUROG3. Diabetes. 2011;60:1349–1353. doi: 10.2337/db10-1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Polak M, et al. Early pattern of differentiation in the human pancreas. Diabetes. 2000;49:225–232. doi: 10.2337/diabetes.49.2.225. [DOI] [PubMed] [Google Scholar]
- 85.Jeon J, et al. Endocrine cell clustering during human pancreas development. J Histochem Cytochem. 2009;57:811–824. doi: 10.1369/jhc.2009.953307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Piper K, et al. Beta cell differentiation during early human pancreas development. J Endocrinol. 2004;181:11–23. doi: 10.1677/joe.0.1810011. [DOI] [PubMed] [Google Scholar]
- 87.De Krijger RR, et al. The midgestational human fetal pancreas contains cells coexpressing islet hormones. Dev Biol. 1992;153:368–375. doi: 10.1016/0012-1606(92)90121-v. [DOI] [PubMed] [Google Scholar]
- 88.Rudnick A, et al. Pancreatic beta cells express a diverse set of homeobox genes. Proc Natl Acad Sci U S A. 1994;91:12203–12207. doi: 10.1073/pnas.91.25.12203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Sander M, et al. Homeobox gene Nkx6.1 lies downstream of Nkx2.2 in the major pathway of beta-cell formation in the pancreas. Development. 2000;127:5533–5540. doi: 10.1242/dev.127.24.5533. [DOI] [PubMed] [Google Scholar]
- 90.Riedel MJ, et al. Immunohistochemical characterisation of cells co-producing insulin and glucagon in the developing human pancreas. Diabetologia. 2012;55:372–381. doi: 10.1007/s00125-011-2344-9. [DOI] [PubMed] [Google Scholar]
- 91.Schisler JC, et al. Stimulation of human and rat islet beta-cell proliferation with retention of function by the homeodomain transcription factor Nkx6.1. Mol Cell Biol. 2008;28:3465–3476. doi: 10.1128/MCB.01791-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Schaffer AE, et al. Transgenic overexpression of the transcription factor Nkx6.1 in beta-cells of mice does not increase beta-cell proliferation, beta-cell mass, or improve glucose clearance. Mol Endocrinol. 2011;25:1904–1914. doi: 10.1210/me.2011-1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Van Hoof D, et al. Differentiation of human embryonic stem cells into pancreatic endoderm in patterned size-controlled clusters. Stem Cell Res. 2011;6:276–285. doi: 10.1016/j.scr.2011.02.004. [DOI] [PubMed] [Google Scholar]
- 94.Xu X, Browning VL, Odorico JS. Activin, BMP and FGF pathways cooperate to promote endoderm and pancreatic lineage cell differentiation from human embryonic stem cells. Mech Dev. 2011;128:412–427. doi: 10.1016/j.mod.2011.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Rezania A, et al. Enrichment of human embryonic stem cell-derived NKX6.1-expressing pancreatic progenitor cells accelerates the maturation of insulin-secreting cells in vivo. Stem Cells. 2013;31:2432–2442. doi: 10.1002/stem.1489. [DOI] [PubMed] [Google Scholar]
- 96.Artner I, et al. MafB: an activator of the glucagon gene expressed in developing islet alpha- and beta-cells. Diabetes. 2006;55:297–304. doi: 10.2337/diabetes.55.02.06.db05-0946. [DOI] [PubMed] [Google Scholar]
- 97.Artner I, et al. MafA and MafB regulate genes critical to beta-cells in a unique temporal manner. Diabetes. 2010;59:2530–2539. doi: 10.2337/db10-0190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Matsuoka TA, et al. The MafA transcription factor appears to be responsible for tissue-specific expression of insulin. Proc Natl Acad Sci U S A. 2004;101:2930–2933. doi: 10.1073/pnas.0306233101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Matsuoka TA, et al. Members of the large Maf transcription family regulate insulin gene transcription in islet beta cells. Mol Cell Biol. 2003;23:6049–6062. doi: 10.1128/MCB.23.17.6049-6062.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Wang H, et al. MAFA controls genes implicated in insulin biosynthesis and secretion. Diabetologia. 2007;50:348–358. doi: 10.1007/s00125-006-0490-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Zhang C, et al. MafA is a key regulator of glucose-stimulated insulin secretion. Mol Cell Biol. 2005;25:4969–4976. doi: 10.1128/MCB.25.12.4969-4976.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Sarkar SA, et al. Global gene expression profiling and histochemical analysis of the developing human fetal pancreas. Diabetologia. 2008;51:285–297. doi: 10.1007/s00125-007-0880-0. [DOI] [PubMed] [Google Scholar]
- 103.Nekrep N, et al. Signals from the neural crest regulate beta-cell mass in the pancreas. Development. 2008;135:2151–2160. doi: 10.1242/dev.015859. [DOI] [PubMed] [Google Scholar]
- 104.Plank JL, et al. Influence and timing of arrival of murine neural crest on pancreatic beta cell development and maturation. Dev Biol. 2011;349:321–330. doi: 10.1016/j.ydbio.2010.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Grouwels G, et al. Differentiating neural crest stem cells induce proliferation of cultured rodent islet beta cells. Diabetologia. 2012;55:2016–2025. doi: 10.1007/s00125-012-2542-0. [DOI] [PubMed] [Google Scholar]
- 106.Lammert E, Cleaver O, Melton D. Induction of pancreatic differentiation by signals from blood vessels. Science. 2001;294:564–567. doi: 10.1126/science.1064344. [DOI] [PubMed] [Google Scholar]
- 107.Yoshitomi H, Zaret KS. Endothelial cell interactions initiate dorsal pancreas development by selectively inducing the transcription factor Ptf1a. Development. 2004;131:807–817. doi: 10.1242/dev.00960. [DOI] [PubMed] [Google Scholar]
- 108.Katsumoto K, Kume S. Endoderm and mesoderm reciprocal signaling mediated by CXCL12 and CXCR4 regulates the migration of angioblasts and establishes the pancreatic fate. Development. 2011;138:1947–1955. doi: 10.1242/dev.058719. [DOI] [PubMed] [Google Scholar]
- 109.Katsumoto K, Kume S. The role of CXCL12-CXCR4 signaling pathway in pancreatic development. Theranostics. 2013;3:11–17. doi: 10.7150/thno.4806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Brissova M, et al. Pancreatic islet production of vascular endothelial growth factor–a is essential for islet vascularization, revascularization, and function. Diabetes. 2006;55:2974–2985. doi: 10.2337/db06-0690. [DOI] [PubMed] [Google Scholar]
- 111.Nikolova G, et al. The vascular basement membrane: a niche for insulin gene expression and Beta cell proliferation. Dev Cell. 2006;10:397–405. doi: 10.1016/j.devcel.2006.01.015. [DOI] [PubMed] [Google Scholar]
- 112.Phillips BW, et al. Directed differentiation of human embryonic stem cells into the pancreatic endocrine lineage. Stem Cells Dev. 2007;16:561–578. doi: 10.1089/scd.2007.0029. [DOI] [PubMed] [Google Scholar]
- 113.Rezania A, et al. Production of functional glucagon-secreting alpha-cells from human embryonic stem cells. Diabetes. 2011;60:239–247. doi: 10.2337/db10-0573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Kroon E, et al. Pancreatic endoderm derived from human embryonic stem cells generates glucose-responsive insulin-secreting cells in vivo. Nat Biotechnol. 2008;26:443–452. doi: 10.1038/nbt1393. [DOI] [PubMed] [Google Scholar]
- 115.Jiang W, et al. In vitro derivation of functional insulin-producing cells from human embryonic stem cells. Cell Res. 2007;17:333–344. doi: 10.1038/cr.2007.28. [DOI] [PubMed] [Google Scholar]
- 116.Schulz TC, et al. A scalable system for production of functional pancreatic progenitors from human embryonic stem cells. PLoS One. 2012;7:e37004. doi: 10.1371/journal.pone.0037004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Spence JR, et al. Directed differentiation of human pluripotent stem cells into intestinal tissue in vitro. Nature. 2011;470:105–109. doi: 10.1038/nature09691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Ikeda H, et al. Generation of Rx+/Pax6+ neural retinal precursors from embryonic stem cells. Proc Natl Acad Sci U S A. 2005;102:11331–11336. doi: 10.1073/pnas.0500010102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Eiraku M, et al. Self-organizing optic-cup morphogenesis in three-dimensional culture. Nature. 2011;472:51–56. doi: 10.1038/nature09941. [DOI] [PubMed] [Google Scholar]
- 120.Suga H, et al. Self-formation of functional adenohy-pophysis in three-dimensional culture. Nature. 2011;480:57–62. doi: 10.1038/nature10637. [DOI] [PubMed] [Google Scholar]
- 121.Takebe T, et al. Vascularized and functional human liver from an iPSC-derived organ bud transplant. Nature. 2013;499:481–484. doi: 10.1038/nature12271. [DOI] [PubMed] [Google Scholar]