Abstract
The therapeutic and psychoactive properties of cannabinoids have long been recognized. The type 2 receptor for cannabinoids (CB2) has emerged as an important therapeutic target in several pathologies, as it mediates beneficial effects of cannabinoids while having little if any psychotropic activity. Difficulties associated with the development of CB2-based therapeutic agents have been related to its intricate pharmacology, including the species specificity and functional selectivity of the CB2-initiated responses. We postulated that a plasmalemmal or subcellular location of the receptor may contribute to the differential signaling pathways initiated by its activation. To differentiate between these two, we used extracellular and intracellular administration of CB2 ligands and concurrent calcium imaging in CB2-expressing U2OS cells. We found that extracellular administration of anandamide was ineffective, whereas 2-arachidonoyl glycerol (2-AG) and WIN55,212-2 triggered delayed, CB2-dependent Ca2+ responses that were Gq protein-mediated. When microinjected, all agonists elicited fast, transient, and dose-dependent elevations in intracellular Ca2+ concentration upon activation of Gq-coupled CB2 receptors. The CB2 dependency was confirmed by the sensitivity to AM630, a selective CB2 antagonist, and by the unresponsiveness of untransfected U2OS cells to 2-AG, anandamide, or WIN55,212-2. Moreover, we provide functional and morphological evidence that CB2 receptors are localized at the endolysosomes, while their activation releases Ca2+ from inositol 1,4,5-trisphosphate-sensitive- and acidic-like Ca2+ stores. Our results support the functionality of intracellular CB2 receptors and their ability to couple to Gq and elicit Ca2+ signaling. These findings add further complexity to CB2 receptor pharmacology and argue for careful consideration of receptor localization in the development of CB2-based therapeutic agents.
Although cannabinoids are active at several G protein-coupled receptors and ion channels, only two “true” cannabinoid receptors are recognized, namely CB1 and CB2.1 Interest in the latter has sparked as it appeared as an important therapeutic target in inflammatory and painful conditions,2,3 while not being involved in the psychoactive cannabinoid effects, which are mainly CB1-mediated. As such, increasing effort is being spent in the development of CB2-based therapeutic agents.4,5 Nonetheless, controversies exist, for instance, in CB2 pharmacology and distribution.6−8 At least two CB2 receptor isoforms have been identified, with tissue- and species-specific expression patterns.8,9 It has been found that CB2 agonists may elicit distinct responses at CB2 receptors from different species.10 Moreover, functional selectivity, defined as the ability of a receptor to couple to different signaling pathways depending on the ligand that stimulates it,11 has been reported for CB2.7 Further complexity is added to the CB2 receptor pharmacology with the recent finding that their intracellular activation modulates neuronal function.12 Because CB2 receptors have been found to signal through Ca2+,12−15 we used calcium imaging and extracellular and intracellular administration of cannabinoid ligands to investigate the functionality of plasmalemmal versus intracellular CB2 receptors in U2OS cells stably expressing CB2.
Experimental Procedures
Chemicals
Anandamide, AM630, WIN55,212-2, 2-arachidonoyl glycerol (2-AG), and d-[Trp7,9,10]-substance P were obtained from Tocris Bioscience (R&D Systems, Minneapolis, MN). All other chemicals were from Sigma (St. Louis, MO).
Cell Culture
The CB2-β-arrestin2-GFP-U2OS (CB2-U2OS) cell line was kindly provided by M. Caron and L. S. Barak (Duke University, Durham, NC); the CB2 receptor sequence is the CNR2_Human sequence (GenBank accession code P34972). CB2-U2OS cells were maintained in DMEM supplemented with 10% fetal bovine serum, 100 mg/mL Zeocin, and 200 μg/mL G418 at 37 °C in a humidified incubator with 5% CO2. The serum was removed 24 h prior to experimentation. In experiments that aimed to evaluate Gq-dependent signaling, cell starvation was concomitant with incubation of d-[Trp7,9,10]-substance P (24 h).
Immunocytochemistry and Confocal Imaging Studies
U2OS cells transiently transfected with the GFP-tagged CB2 receptor (kindly provided by M. Caron and L. S. Barak) and with Rab7-RFP (Addgene, Cambridge, MA) 48 h earlier were fixed with 4% paraformaldehyde, washed in phosphate-buffered saline, and mounted with DAPI Fluoromont G (Southern Biotech, Birmingham, AL). Cells were imaged using a Carl Zeiss 710 two-photon confocal microscope with a 63× oil immersion objective, using a 1× digital zoom, with excitations set for DAPI, GFP, and DsRed at 405, 488, and 561 nm, respectively. Images were analyzed using Zen 2010 (Zeiss), as previously reported.16
Calcium Imaging
Measurements of [Ca2+]i were performed as previously described.16−19 Briefly, cells were incubated with 5 μM Fura-2 AM (Invitrogen) in HBSS at room temperature for 45 min, washed with dye-free HBSS, and incubated for an additional 45 min to allow dye de-esterification. Coverslips were mounted in an open bath chamber (RP-40LP, Warner Instruments, Hamden, CT) on the stage of an inverted microscope (Nikon Eclipse TiE, Nikon Inc., Melville, NY), equipped with a Perfect Focus System and a Photometrics CoolSnap HQ2 CCD camera (Photometrics, Tucson, AZ). During the experiments, the Perfect Focus System was activated. Fura-2 AM fluorescence (emission at 510 nm), following alternate excitation at 340 and 380 nm, was acquired at a frequency of 0.25 Hz. Images were acquired and analyzed using NIS-Elements AR version 3.1 (Nikon Inc.). After appropriate calibration with ionomycin and CaCl2, and Ca2+-free and EGTA, respectively, the ratio of the fluorescence signals (340 nm to 380 nm) was converted to Ca2+ concentration.20
Intracellular Microinjection
Injections were performed using Femtotips II, InjectMan NI2, and FemtoJet systems (Eppendorf) as previously reported.16−19 Pipettes were backfilled with an intracellular solution containing 110 mM KCl, 10 mM NaCl, and 20 mM HEPES (pH 7.2) or the compounds to be tested. The injection time was 0.4 s at 60 hectoPascal with a compensation pressure of 20 hectoPascal to ensure that the microinjected volume was <1% of the cell volume. The intracellular concentration of chemicals was determined on the basis of the concentration in the pipet and the volume of injection. The cells to be injected were Z-scanned before injection, and the cellular volume was automatically calculated by NIS-Elements AR version 3.1 (Nikon Inc.).
Statistics
Data are expressed as means and the standard error of the mean. One-way analysis of variance, followed by post hoc Bonferroni and Tukey tests, was used to assess significant differences between groups; P < 0.05 was considered statistically significant.
Results
Effects of Extracellular versus Intracellular Administration of CB2 Agonist 2-Arachidonoyl Glycerol on the Cytosolic Ca2+ Concentration of CB2-Expressing U2OS Cells
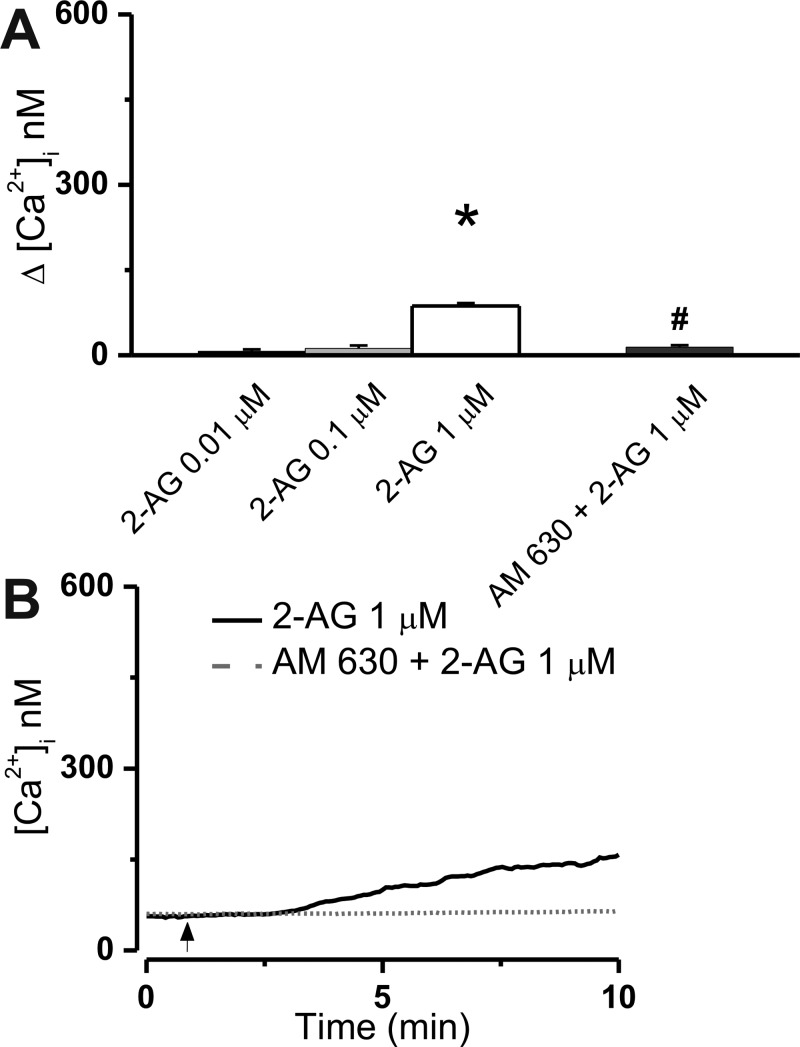
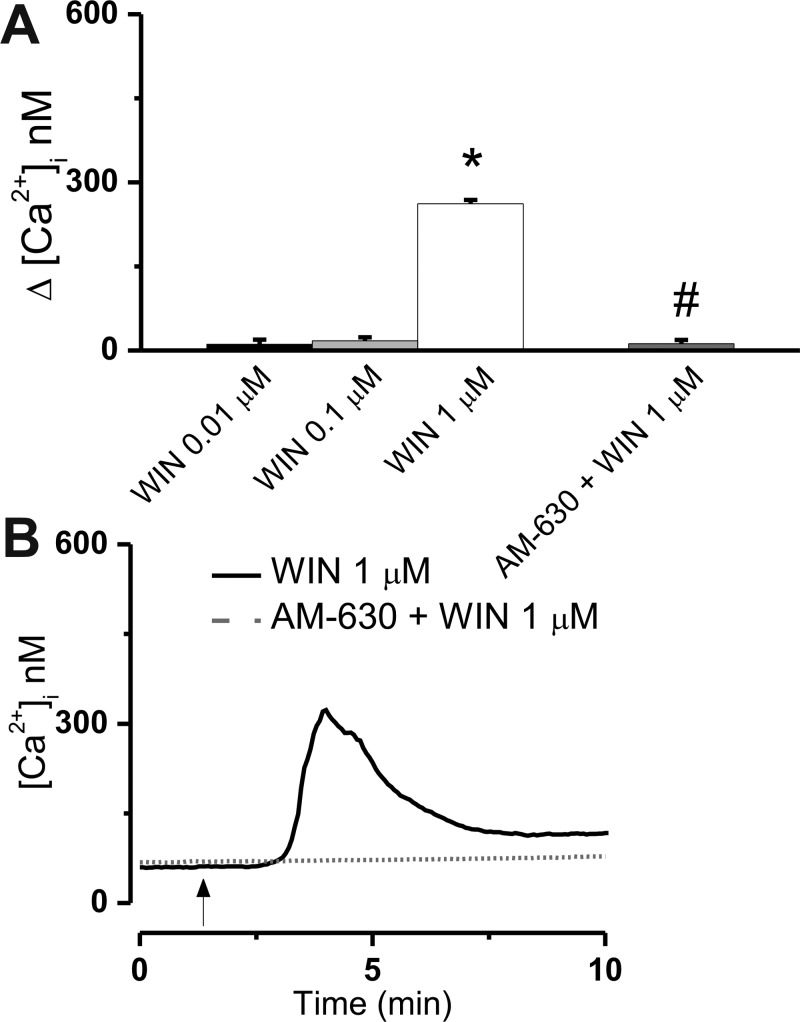
We evaluated the Ca2+ response of U2OS cells stably expressing the CB2 receptor to bath application and intracellular microinjection of the endocannabinoid 2-arachidonoyl glycerol (2-AG). Increasing concentrations of 2-AG (0.01, 0.1, and 1 μM) were applied extracellularly to CB2-U2OS cells, which elevated the intracellular Ca2+ concentration, [Ca2+]i, by 7 ± 3.4 nM (n = 31 cells), 12 ± 5.1 nM (n = 43 cells), and 87 ± 3.1 nM (n = 56 cells), respectively (Figure 1A). The effect of the latter concentration of 2-AG was statistically significant (P < 0.05) and sensitive to CB2 blockade with AM630 (1 μM, 10 min). In the presence of AM630, Δ[Ca2+]i was reduced to 14 ± 3.6 nM (n = 49) (Figure 1A,B). The effect of 2-AG on [Ca2+]i was delayed by 1–2 min and increased gradually (Figure 1B).
Figure 1.
Extracellular administration of 2-arachidonoyl glycerol (2-AG) to CB2-U2OS cells elevates [Ca2+]i. (A) Comparison of the increases in [Ca2+]i produced by extracellular administration of 2-AG (0.01–1 μM) and 1 μM 2-AG in the presence of CB2 receptor antagonist AM630 (1 μM); P < 0.05 compared with basal levels (∗) or with 1 μM 2-AG (#). (B) Representative recordings of increases in [Ca2+]i in response to 1 μM 2-AG in absence or presence of 1 μM CB2 antagonist AM630.
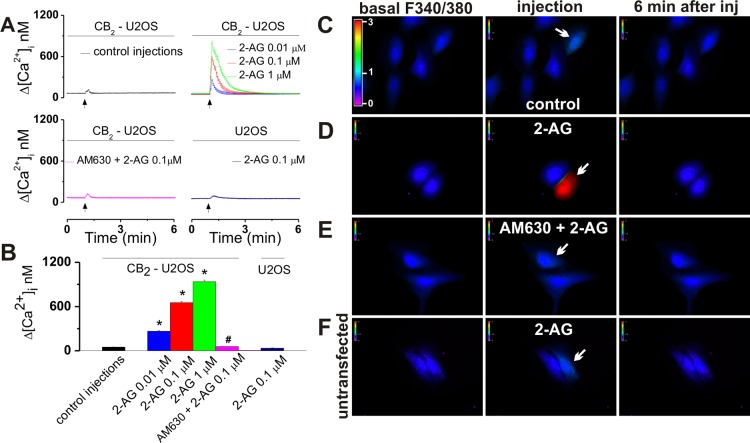
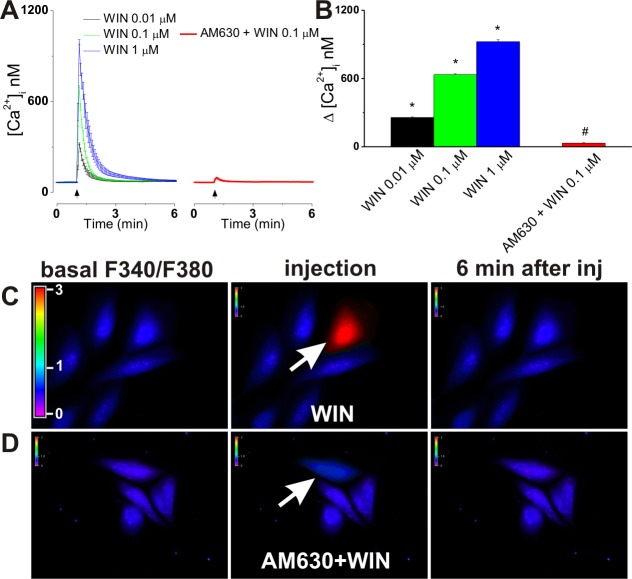
In experiments using the intracellular microinjection technique, injection of 0.01, 0.1, and 1 μM 2-AG (final concentrations inside the cell) induced robust and significant increases in [Ca2+]i of CB2-U2OS cells of 264 ± 11 nM (n = 6 cells), 653 ± 19 nM (n = 6 cells), and 938 ± 22 nM (n = 6 cells), respectively, while control buffer microinjection had an insignificant effect of 49 ± 4 nM (n = 6 cells) (Figure 2A–D). In the presence of co-injected CB2 antagonist AM630 (1 μM), 0.1 μM 2-AG elevated [Ca2+]i by 58 ± 4 nM (n = 6 cells), similar to that of control buffer microinjection; the effect of 0.1 μM 2-AG on [Ca2+]i of untransfected U2OS cells was also insignificant, measuring 34 ± 5 nM (n = 6 cells) (Figure 2A,B,E,F).
Figure 2.
Intracellular microinjection of 2-AG into CB2-U2OS cells produces CB2-dependent Ca2+ elevation. (A) Averaged increases in [Ca2+]i produced by intracellular administration of control buffer (top left) or 2-AG (0.01, 0.1, and 1 μM, final concentrations inside the cell, top right), co-injection of 0.1 μM 2-AG with CB2 antagonist AM630 (1 μM, bottom left) in CB2-U2OS cells, or microinjection of 0.1 μM 2-AG into control U2OS cells (bottom right). (B) Comparison of the Ca2+ responses elicited by the treatments of CB2-U2OS or control untransfected U2OS cells mentioned above; P < 0.05 when compared with the control (∗) or with 2-AG alone (#). (C–F) Characteristic fluorescence images of Fura-2 AM-loaded CB2-U2OS cells before (left), during (middle), and 6 min after (right) intracellular administration of control buffer (C), 0.1 μM 2-AG alone (D), or 0.1 μM 2-AG in the presence of 1 μM AM630 (E) or of U2OS cells treated with 0.1 μM 2-AG (F). Arrows denote the injected cells; the fluorescence scale (0–3) is illustrated in each panel and magnified in the left panel of part C.
In all series of experiments using intracellular injection, CB2-U2OS cells were pretreated for 1 min with 1 μM AM630 (applied extracellularly), to exclude the possibility that the injected cannabinoid ligands leaked from the pipet and triggered plasma membrane receptor-mediated effects.
Functional and Morphological Evidence of Localization of CB2 to Endolysosomes in CB2-Transfected U2OS Cells
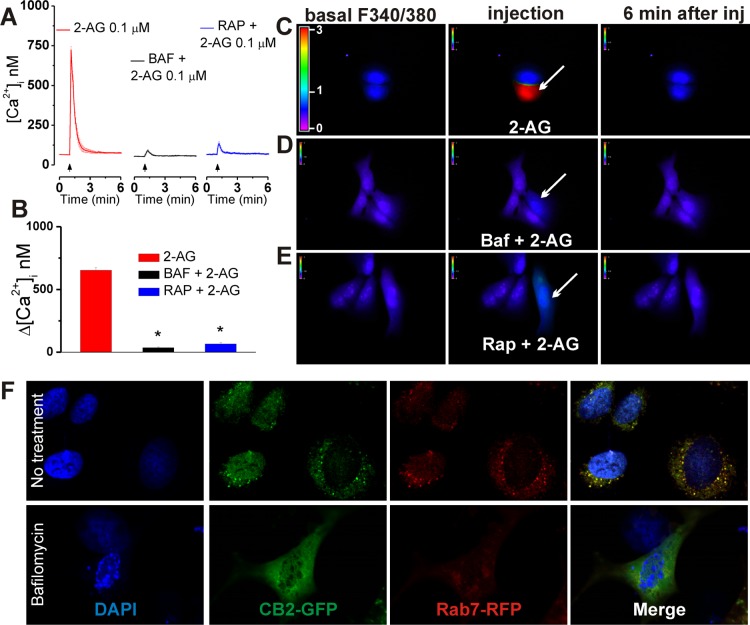
CB2-U2OS cells were incubated for 1 h with either 1 μM bafilomycin A1, a V-type ATPase that prevents lysosomal acidification,21 or 30 μM rapamycin, which blocks the last step of the engulfment of molecules by endolysosomes via microautophagy.22 Under the conditions described above, the effect of 0.1 μM 2-AG on [Ca2+]i, initially measuring 653 ± 19 nM, was drastically reduced to 36 ± 4.8 nM (n = 6 cells) and 67 ± 9.1 nM (n = 6 cells) (Figure 3A–E). In U2OS cells co-expressing GFP-tagged CB2 receptors and RFP-tagged Rab7, a small GTPase associated with both endosomes and lysosomes,23 we observed an extensive colocalization of CB2 and Rab7 (Figure 3F). Lysosomal disruption using bafilomycin A1 greatly reduced the extent of the merged signal of CB2 and Rab7 (Figure 3F).
Figure 3.
Intracellular CB2 receptors are located at endolysosomes in CB2-expressing U2OS cells. (A) Averaged Ca2+ responses of CB2-U2OS cells to intracellular administration of 0.1 μM 2-AG in the absence (left) or presence of lysosomal disruptor bafilomycin A1 (1 μM, incubation for 1 h, middle) or microautophagy inhibitor rapamycin (30 μM, incubation for 1 h, right). (B) Comparison of the increases in [Ca2+]i elicited by 2-AG under the conditions described above; P < 0.05 when compared with 2-AG microinjection (∗). (C–E) Representative fluorescence images of Fura-2 AM-loaded CB2-U2OS cells before (left), during (middle), and 6 min after (right) intracellular administration of 0.1 μM 2-AG in the absence (C) and presence of bafilomycin A1 (D) or rapamycin (E). (F) Confocal images (top row) showing the colocalization of the GFP-tagged CB2 receptor and RFP-tagged Rab7, an endolysosomal marker, in GFP-CB2- and RFP-Rab7-transfected U2OS cells; the nuclei are labeled with DAPI (blue). Lysosomal disruption (bottom row) with bafilomycin A1 markedly reduces the extent of the merged immunostaining of CB2 and Rab7.
Intracellular, but Not Extracellular, Administration of Anandamide Elevates the Cytosolic Ca2+ Concentration of CB2-Expressing U2OS Cells
CB2-U2OS cells extracellularly treated with progressive concentrations of anandamide (0.01, 0.1, and 1 μM) failed to respond with a significant increase in [Ca2+]i (Figure 1 of the Supporting Information); incubation with CB2 antagonist AM630 (1 μM) did not modify the response to 1 μM anandamide (Figure 1 of the Supporting Information).
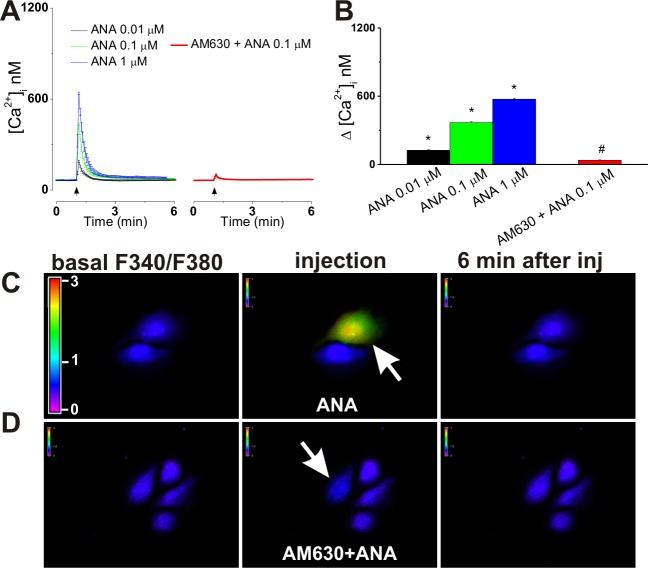
However, CB2-U2OS cells responded to intracellular administration of anandamide (0.01, 0.1, and 1 μM, final concentrations inside the cell) with significant and concentration-dependent elevations of [Ca2+]i: 124 ± 7.4, 368 ± 8.4, and 574 ± 7.2 nM, respectively [n = 6 cells for each concentration tested (Figure 4A,B)]. The anandamide-induced increase in [Ca2+]i was fast and transient (Figure 4A); blockade of intracellular CB2 receptors upon co-injection of 1 μM AM630 prevented the effect of microinjected anandamide (1 μM), reducing it to 37 ± 4.1 nM [n = 6 cells (Figure 4A–D)], a response similar to that elicited by control buffer microinjection (Figure 2). Characteristic images depicting the increases in the Fura-2 fluorescence ratio at 340 and 380 nm upon microinjection of anandamide (1 μM) in the absence or presence of AM630 (1 μM) are shown in panels C and D of Figure 4.
Figure 4.
Intracellular administration of anandamide increases [Ca2+]i in CB2-U2OS cells. (A) Averaged Ca2+ responses induced by increasing doses of microinjected anandamide (ANA, 0.01–1 μM, left) or by co-injection of 0.1 μM anandamide and 1 μM CB2 antagonist AM630 (right). (B) Comparison of the increases in [Ca2+]i produced by microinjected anandamide (0.01, 0.1, and 1 μM) and anandamide (0.1 μM) in the presence of AM630 (1 μM); P < 0.05 compared with the control (∗) (see Figure 2) or with 0.1 μM anandamide alone (#). (C and D) Typical fluorescence images of Fura-2 AM-loaded CB2-U2OS cells before (left), during (middle), and 6 min after (right) intracellular administration of 0.1 μM anandamide alone (C) or in the presence of 1 μM AM630 (D). Arrows denote the injected cells; the fluorescence scale (0–3) is illustrated in each panel and magnified in the left panel of part C.
Anandamide produced negligible effects in control untransfected U2OS cells regardless of whether it was applied extracellularly (Figure 2 of the Supporting Information) or intracellularly (Figure 3 of the Supporting Information).
WIN55,212-2 Produces CB2 Receptor-Dependent Increases in Ca2+ Concentration in CB2-U2OS Cells
Extracellular administration of WIN55,212-2 (0.01, 0.1, and 1 μM) elevated [Ca2+]i by 11 ± 8.1 nM (n = 27 cells), 17 ± 6.3 nM (n = 38 cells), and 262 ± 6.8 nM [n = 46 cells (Figure 5A,B)]; the latter effect was statistically significant (P < 0.05) and sensitive to CB2 blockade with AM630 [1 μM, incubation for 10 min, Δ[Ca2+]i reduced to 12 ± 6.8 nM (n = 36 cells)] (Figure 5A,B). The Ca2+ response elicited by WIN55,212-2 occurred with a latency of 1–2 min (Figure 5B), similar to the effect of bath-applied 2-AG (Figure 1B).
Figure 5.
Extracellular administration of WIN55,212-2 to CB2-U2OS cells elevates [Ca2+]i. (A) Comparison of the increases in [Ca2+]i produced by extracellular administration of WIN55,212-2 (WIN, 0.01–1 μM) and 1 μM WIN55,212-2 in the presence of CB2 receptor antagonist AM630 (1 μM); P < 0.05 compared with basal levels (∗) or with 1 μM WIN55,212-2 (#). (B) Representative recordings of increases in [Ca2+]i in response to 1 μM WIN in the absence or presence of 1 μM CB2 antagonist AM630.
Microinjection of WIN55,212-2 (0.01, 0.1, and 1 μM, final concentrations inside the cell) into CB2-U2OS cells triggered fast, transient, and concentration-dependent increases in [Ca2+]i of 257 ± 5.3, 635 ± 7.1, and 924 ± 17 nM (n = 6 cells for each concentration tested), whereas when 1 μM AM630 was co-injected with 1 μM WIN55,212-2, a small and insignificant increase in Ca2+ concentration, of 31 ± 4.6 nM (n = 6 cells), was apparent (Figure 6A,B). Representative examples of the increase in the F340/F380 fluorescence ratio produced by intracellular administration of WIN55,212-2 or AM630 and WIN55,212-2 are shown in panels C and D of Figure 6. The insignificant Ca2+ responses to extracellular and intracellular administration of WIN55,212-2 in control U2OS cells are illustrated in Figures 2 and 3 of the Supporting Information, respectively.
Figure 6.
Microinjection of WIN55,212-2 increases [Ca2+]i in CB2-U2OS cells. (A) Averaged [Ca2+]i elevations produced by increasing doses of microinjected WIN55,212-2 (WIN 0.01–1 μM, left) or by co-injection of 0.1 μM WIN55,212-2 and 1 μM CB2 antagonist AM630 (right). (B) Comparison of the increases in [Ca2+]i elicited by microinjected WIN55,212-2 (0.01, 0.1, and 1 μM) and WIN55,212-2 (0.1 μM) in the presence of AM630 (1 μM); P < 0.05 compared with the control (∗) (see Figure 2) or with 0.1 μM WIN55,212-2 alone (#). (C and D) Characteristic fluorescence images of Fura-2 AM-loaded CB2-U2OS cells before (left), during (middle), and 6 min after (right) intracellular administration of 0.1 μM WIN55,212-2 alone (C) or in the presence of 1 μM AM630 (D). Arrows denote the injected cells; the fluorescence scale (0–3) is illustrated in each panel and magnified in the left panel of part C.
Effects of Extracellular 2-AG and WIN55,212-2 Are Mediated by Gq Coupling of CB2 Receptors
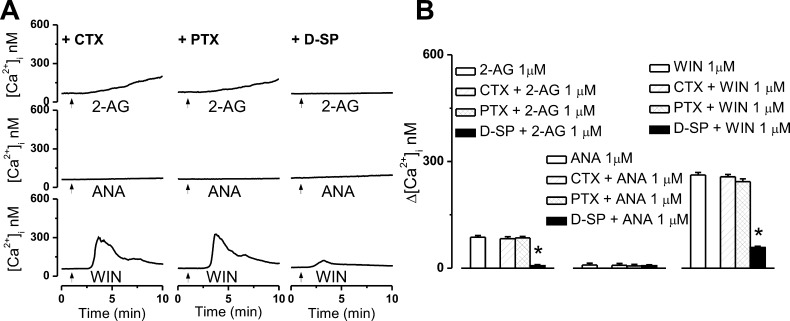
2-AG (1 μM), anandamide (1 μM), or WIN55,212-2 (1 μM) was bath-applied to CB2-U2OS cells treated overnight with chemicals interfering with G protein signaling. After cholera toxin (CTX, 100 ng/mL) pretreatment, 2-AG produced an increase in the [Ca2+]i of 83 ± 5.1 nM [n = 61 cells (Figure 7A,B)] similar to the effect of 2-AG on untreated cells. CTX irreversibly abolishes the GTPase activity of the Gs α subunit, inducing continual activation of adenylyl cyclase and increased intracellular levels of cAMP. In this manner, it hinders ligands of Gs-coupled receptors from eliciting their Gs-dependent effects. After pretreatment with the Gi/o inhibitor pertussis toxin (PTX, 100 ng/mL), the response of 2-AG was negligibly modified, measuring 85 ± 4.2 nM [n = 53 cells (Figure 7A,B)]. Conversely, pretreatment of CB2-U2OS cells with Gq blocker d-[Trp7,9,10]-substance P (D-SP, 100 ng/mL)24 greatly reduced the effect of 2-AG to 7 ± 2.7 nM [n = 67 cells (Figure 7A,B)].
Figure 7.
The Ca2+ responses produced by bath-applied 2-AG and WIN55,212-2 are prevented by a Gq protein inhibitor. (A) Representative examples of the Ca2+ responses induced by 1 μM 2-AG (top), 1 μM anandamide (ANA, middle), or 1 μM WIN55,212-2 (WIN, bottom), applied by bath to CB2-expressing U2OS cells in the presence of cholera toxin (CTX, 100 nM, top), which occludes Gs-dependent signaling, Gi/o blocker pertussis toxin (PTX, 100 nM, middle), or Gq protein inhibitor d-[Trp7,9,10]-substance P (D-SP, 100 μM, bottom); 2-AG and WIN55,212-2 increased [Ca2+]i, and the response was Gq protein-mediated. (B) Comparison of the mean amplitude of the Ca2+ responses produced by extracellular administration of 1 μM 2-AG (left), 1 μM anandamide (middle), or 1 μM WIN55,212-2 (right) in the absence and presence of the indicated G protein inhibitors; P < 0.05 compared with WIN55,212-2 alone (∗).
The Ca2+ response elicited by anandamide was largely similar and not significant regardless of whether anandamide was applied alone [Δ[Ca2+]i of 9 ± 4.7 nM (n = 33 cells)] or after stimulating Gs-dependent signaling with CTX (100 ng/mL), inhibiting Gi/o with PTX (100 ng/mL), and inhibiting Gq with D-SP (100 ng/mL). The Δ[Ca2+]i values were 8 ± 5.2 nM (n = 29 cells), 6 ± 4.8 nM (n = 41 cells), and 7 ± 2.3 nM (n = 36 cells), respectively (Figure 7A,B).
D-SP pretreatment drastically diminished the effect induced by WIN55,212-2, reducing it to 58 ± 3.9 nM, while in the presence of CTX or PTX, WIN55,212-2 increased [Ca2+]i to similar extents as in their absence: Δ[Ca2+]i values were 257 ± 6.8 nM (n = 53 cells) and 243 ± 7.9 nM (n = 47 cells) in cells pretreated with CTX and PTX, respectively, and 262 ± 6.8 nM (n = 49 cells) for WIN55,212-2 alone (Figure 7A,B).
Intracellular CB2 Receptors Couple to Gq Proteins
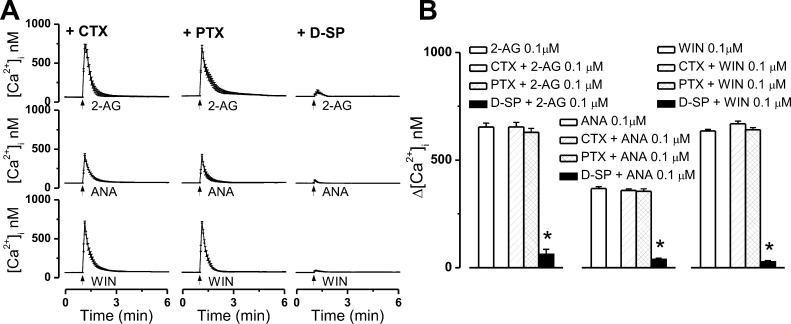
Overnight treatment of CB2-U2OS cells with CTX (100 ng/mL) or PTX (100 ng/mL) failed to significantly modify the Ca2+ response elicited by intracellular administration of 0.1 μM 2-AG (Figure 8A,B). Under the conditions described above, 2-AG increased [Ca2+]i by 654 ± 21 nM (n = 6 cells) and 629 ± 18 nM (n = 6 cells), respectively, while the response to 2-AG alone measured 652 ± 19 nM [n = 6 cells (Figure 8A,B)]. After incubation of cells with D-SP (100 ng/mL), the effect of 2-AG was greatly decreased to 62 ± 23 nM (n = 6 cells), indicating participation of a Gq-dependent pathway in the response (Figure 8A,B).
Figure 8.
Intracellular microinjection of 2-AG, anandamide, or WIN55,212-2 produces Gq-dependent Ca2+ responses in CB2-expressing U2OS cells. (A) Averaged Ca2+ responses induced by intracellular administration of 0.1 μM 2-AG (top), 0.1 μM anandamide (ANA, middle), or 0.1 μM WIN55,212-2 (WIN, bottom) in CB2-U2OS cells pretreated with cholera toxin (CTX, 100 nM, top), pertussis toxin (PTX, 100 nM, middle), or Gq protein inhibitor d-[Trp7,9,10]-substance P (D-SP, 100 μM, bottom); each ligand increased [Ca2+]i, and the response was sensitive to Gq protein blockade. (B) Comparison of the mean amplitude of the Ca2+ responses produced by intracellular administration of 0.1 μM 2-AG (left), 0.1 μM anandamide (middle), or 0.1 μM WIN55,212-2 (right) in absence and presence of the indicated G protein inhibitors; in each group of data, P < 0.05 compared with 2-AG, anandamide, or WIN55,212-2 alone (∗).
Upon pretreatment with CTX (100 ng/mL) or PTX (100 ng/mL), intracellular injection of anandamide (0.1 μM) elevated [Ca2+]i of CB2-U2OS cells by 359 ± 7.2 nM (n = 6 cells) or 355 ± 11.2 nM (n = 6 cells), respectively; these effects are similar to those triggered by microinjected anandamide alone [368 ± 8.4 nM (n = 6 cells)], indicating that Gs and Gi/o proteins are not mediating its effect. Upon D-SP (100 ng/mL) pretreatment, intracellular anandamide produced an insignificant Ca2+ response of 38 ± 6.3 nM [n = 6 cells (Figure 8A,B)].
Similarly, CTX and PTX (both at 100 ng/mL) pretreatment did not significantly modify the response of CB2-U2OS cells to microinjected WIN55,212-2 (0.1 μM). Δ[Ca2+]i values were 668 ± 13 nM (n = 6 cells) and 641 ± 9.8 nM (n = 6 cells) after CTX and after PTX, respectively, and 635 ± 7.1 nM for WIN55,212-2 alone. However, blocking Gq proteins with 100 ng/mL D-SP basically abolished the effect of intracellular WIN55,212-2. In this case, Δ[Ca2+]i decreased to 27 ± 5.4 nM [n = 6 cells (Figure 8A,B)].
Activation of Intracellular CB2 Mobilizes Ca2+ from Distinct Pools
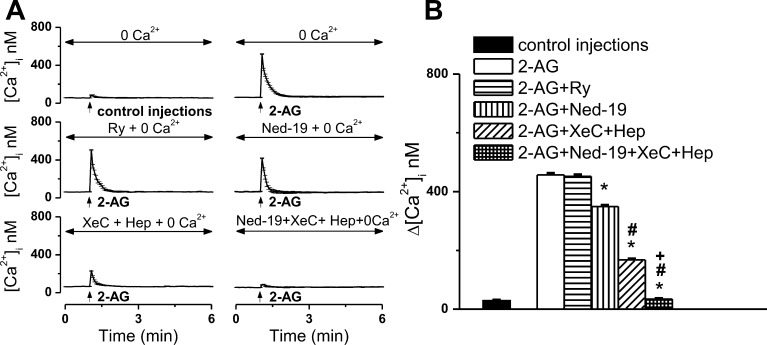
CB2-U2OS cells incubated with Ca2+-free saline responded to microinjection of 0.1 μM 2-AG with a 457 ± 5.2 nM increase in [Ca2+]i (n = 6 cells), while the effect of control buffer microinjection was negligible {Δ[Ca2+]i = 29 ± 1.7 nM [n = 6 cells (Figure 9A,B)]}. The response to 2-AG was reduced in Ca2+-free versus Ca2+-containing HBSS [Δ[Ca2+]i = 653 ± 19 nM (Figure 2)], indicating mobilization of both intracellular and extracellular Ca2+ pools. Using a pharmacological approach, we further sought to identify the intracellular sources of Ca2+ involved in the response to microinjected 2-AG. Pretreatment of cells with ryanodine receptor blocker ryanodine (10 μM, 1 h) did not significantly modify the Ca2+ response to 2-AG, which measured 452 ± 5.9 nM [n = 6 cells (Figure 9A,B)]. Conversely, pretreatment with Ned-19 (5 μM, 15 min), which blocks endolysosomal Ca2+ release via the NAADP-sensitive two-pore channels,25 decreased the extent of the Ca2+ elevation produced by 2-AG to 349 ± 4.3 nM (n = 6 cells); inositol 1,4,5-trisphosphate receptor (IP3R) inhibition induced by treating CB2-U2OS cells with heparin and xestospongin C (10 μM, 15 min) also induced a significant decrease in the response to 2-AG {Δ[Ca2+]i = 168 ± 3.6 nM [n = 6 cells (Figure 9A,B)]}. When both IP3Rs and the two-pore channels were blocked, the Ca2+ response to intracellular microinjection of 2-AG was completely abolished {Δ[Ca2+]i = 34 ± 2.4 nM [n = 6 cells (Figure 9A,B)]}.
Figure 9.
2-AG mobilizes endoplasmic reticulum and acidic-like Ca2+ stores. (A) Averaged Ca2+ responses of CB2-U2OS cells in Ca2+-free saline, microinjected with either control buffer or 0.1 μM 2-AG in the absence or presence of ryanodine receptor blocker ryanodine (Ry), two-pore channel antagonist Ned-19, IP3R inhibitors xestospongin C (XeC) and heparin (Hep), or a combination of Ned-19, XeC, and Hep. (B) Comparison of the Ca2+ increases produced by the treatments described in (A); P < 0.05 compared with 2-AG microinjection (∗), 2-AG in the presence of Ned-19 (#), or 2-AG in the presence of IP3R blockers (+).
Discussion
In addition to the effects initiated at the plasma membrane, G protein-coupled receptors (GPCRs) may also trigger signaling cascades upon their activation within the cell.26 The emerging paradigm of functional intracellular GPCRs is particularly significant in the case of lipid messengers that are generated intracellularly and may target their receptors at both sites, (reviewed in ref (27)). We and others reported the functionality of intracellularly expressed CB1 receptors,17,28 as well as their ability to use Ca2+ as a second messenger.17 To evaluate whether CB2 receptors elicit Ca2+ signaling upon plasmalemmal or intracellular activation, cannabinoid ligands were administered extracellularly or microinjected into U2OS cells stably expressing CB2.
We noticed that extracellular administration of 2-AG induced a small increase in [Ca2+]i of CB2-U2OS cells only at the highest concentration tested here (1 μM). Bath application of anandamide did not elevate [Ca2+]i of CB2-U2OS cells. While 2-AG produced a gradual increase in [Ca2+]i of CB2-U2OS cells, a robust Ca2+ response was induced by extracellular administration of WIN55,212-2, which is a cannabinoid agonist displaying high affinity and/or intrinsic activity at this receptor.1,29 The CB2 specificity was indicated by the ability of CB2 antagonist AM63029 to inhibit the effects of extracellular administration of 2-AG and WIN55,212-2 in CB2-U2OS cells and by the unresponsiveness of untransfected U2OS cells to bath application of WIN55,212-2.
Similar to CB1, CB2 receptors couple to Gi proteins and inhibit cAMP formation or activate MAPK.6,29 However, coupling to Gs or Gq of CB1 receptors has also been reported.30,31 While a cannabinoid-dependent [Ca2+]i increase may occur downstream of Gi,32,33 we noticed that the CB2-dependent effects of 2-AG or WIN55,212-2 were completely contingent on Gq in our paradigm. WIN55,212-2 also activates Gq downstream of CB1;31 moreover, it can trigger a CB2-dependent signaling pathway different from that elicited by other ligands.7 Thus, the discrepancy between the effects of extracellular administration of anandamide, 2-AG, and WIN55,212-2 in our study may be a result of the reported functional selectivity at CB2 receptors.7,34
In a recent study, we demonstrated that anandamide can trigger fast CB1-dependent Ca2+ signaling upon microinjection but is ineffective upon extracellular administration in CB1-transfected cells.17 To test the hypothesis of functional intracellular CB2 receptors in CB2-U2OS cells, intracellular injections of 2-AG, anandamide, or WIN55,212-2 were conducted. When 2-AG or anandamide was microinjected, concentration-dependent elevations of [Ca2+]i were observed, and the Ca2+ response pattern was very fast, which is in contrast with the delayed, modest response and lack of effect, respectively, observed with the extracellular administration of these two agonists. Likewise, intracellular administration of WIN55,212-2 resulted in a concentration-dependent and robust effect. WIN55,212-2 and 2-AG produced Ca2+ responses with an amplitude higher than that of anandamide, which is consistent with the full agonistic activity at CB2 receptors reported for these ligands.1,29 The sensitivity of these responses to the intracellular blockade of CB2 and the unresponsiveness of control, untransfected U2OS cells to microinjected cannabinoids were considered to be indicative of CB2 specificity. We further noticed that interfering with Gq-dependent, but not with Gs- or Gi/o-dependent, signaling prevented the effect of microinjected 2-AG, anandamide, or WIN55,212-2. Moreover, the endocannabinoids and WIN55,212-2 trigger similar signaling pathways upon intracellular administration, despite their unrelated chemical structure.
The accumulating evidence pointing to endolysosomes as intracellular locations where GPCRs initiate signaling16,18,19,35,36 prompted us to evaluate whether this may be the case for CB2. In an initial functional study, we found that the effect of intracellular 2-AG is abolished by lysosomal disruption. Because rapamycin reduced the effects of microinjected 2-AG, ligand microautophagy22 may be a necessary step in the activation of endolysosomally located CB2. The localization of CB2 to lysosomes was confirmed by an additional study, providing morphological evidence that CB2 colocalizes with the endolysosomal-associated small GTPase Rab7. Lysosomal disruption with bafilomycin A1 markedly reduced the fluorescence intensity of both CB2 and Rab7, supporting the findings of the functional study.
Next, we sought to examine the pools of Ca2+ mobilized upon activation of intracellularly located CB2. The reduction in the amplitude of the Ca2+ response of CB2-U2OS cells incubated with Ca2+-free saline to microinjected 2-AG indicated that both influx of extracellular Ca2+ and release of Ca2+ from intracellular stores occur downstream of intracellular CB2 activation. Using a pharmacological approach, we further determined that 2-AG-induced stimulation of intracellular CB2 results in Ca2+ mobilization from endolysosomes via NAADP-sensitive two-pore channels and from the endoplasmic reticulum via the IP3R. This effect correlates well with the localization of CB2 at the endolysosmes, as well as with the localization of NAADP-generating enzymes37,38 at the membrane of acidic organelles.39
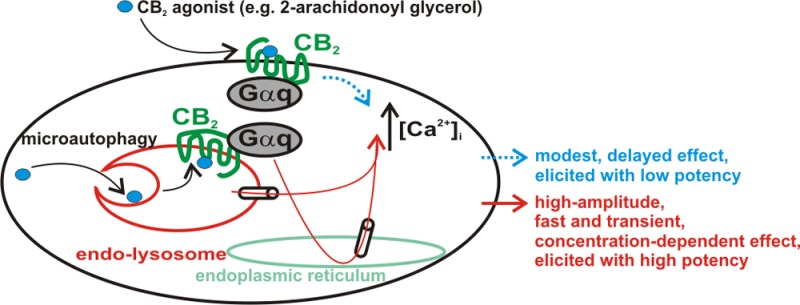
Thus, we conclude that intracellular CB2 receptors are functional, endolysosomally located, and Gq-coupled in CB2-U2OS cells, and their activation mobilizes NAADP-sensitive acidic-like Ca2+ stores and inositol 1,4,5-trisphosphate-sensitive endoplasmic reticulum pools. In addition, we note that CB2 agonists elicit discrepant and delayed effects and exhibit a considerably lower potency when administered extracellularly as opposed to intracellularly. Although these effects appear to be Gq-mediated, it may be that a considerable pool of plasmalemmal CB2 receptors do not couple with Gq/Ca2+ signaling. Conversely, Gq coupling and associated Ca2+ responses may be a characteristic of intracellularly located CB2, in which case the delay in the effects elicited by agonists upon extracellular administration may be a result of the latency necessary for membrane permeation. Nonetheless, differential activation of plasmalemmal CB2 by the three agonists (applied extracellularly) is more likely to be a result of the functional selectivity at CB2,7,34 which is supported by the previous finding that 2-AG (which promotes agonist-directed trafficking at CB234) has a low potency at eliciting CB2-mediated Ca2+ responses.34 Binding of a particular agonist to a GPCR results in the enrichment of a unique set of receptor conformations based on the microaffinity of the agonist for each conformation; because distinct conformations presumably couple receptors differently to specific G proteins and intracellular effectors, individual agonists ultimately produce distinct effects.11 As such, anandamide, 2-AG, and WIN55,212-2 may induce conformational changes in plasmalemmal CB2, resulting in distinct responses, as observed in the study presented here. However, we note that this is not the case with intracellular, endolysosomally located CB2, which appears to couple to the same Gq-mediated Ca2+ pathway in response to intracellular administration of the three agonists; moreover, the three agonists have higher potency and efficacy at eliciting intracellularly initiated CB2-dependent Ca2+ responses. Thus, functional selectivity may apply more to plasmalemmal than to intracellular CB2.
On a different note, plasma membrane-located CB2 is internalized upon agonist stimulation and slowly recycled back to the cell surface via a Rab11-dependent pathway, while rapid recycling, via Rab4, does not occur.40 Moreover, in the case of CB2, not all receptors are recycled, and chronic agonist stimulation does not promote switching from recycling to degradative pathways, supporting the hypothesis of a putative role of sequestration of the CB2 receptor to the cytoplasm following internalization.40
Our results, together with those of others,12 add further complexity to the CB2 receptor pharmacology and argue for careful consideration of receptor localization in the development of CB2-based therapeutic agents. Moreover, because 2-AG is intracellularly produced “on demand”,41 its first target may be intracellularly located CB2, which may be relevant both in physiological states and in pathologies with an increased level of production of endocannabinoids.
Acknowledgments
We thank Drs. Marc Caron and Lawrence S. Barak (Duke University) for providing the CB2-U2OS cell line and the CB2-GFP cDNA.
Glossary
Abbreviations
- 2-AG
2-arachidonoyl glycerol
- [Ca2+]i
intracellular Ca2+ concentration
- CB2-U2OS
CB2-β-arrestin 2-green fluorescent protein-U2OS
- CTX
cholera toxin
- DMEM
Dulbecco’s modified Eagle’s medium
- D-SP
d-[Trp7,9,10]-substance P
- EGTA
ethylene glycol tetraacetic acid
- GFP
green fluorescent protein
- GPCR
G protein-coupled receptor
- HBSS
Hank’s balanced salt solution
- IP3R
inositol 1,4,5-trisphosphate receptor
- MAPK
mitogen-activated protein kinase
- NAADP
nicotinic acid adenine dinucleotide phosphate
- PTX
pertussis toxin
- RFP
red fluorescent protein.
Supporting Information Available
Evidence of negligible effects of extracellular anandamide on CB2-expressing U2OS cells or untransfected U2OS cells, insignificant responses produced by microinjected anandamide in untransfected U2OS cells is provided as supplemental data, and insignificant Ca2+ responses to extracellular or intracellular administration of WIN55,212-2 in untransfected U2OS cells. This material is available free of charge via the Internet at http://pubs.acs.org.
Author Contributions
G.C.B. and E.D. contributed equally to this work.
This work was supported by National Institutes of Health Grants HL090804 (to E.B.) and DA035926 and DA023204 (to M.E.A.).
The authors declare no competing financial interests.
Funding Statement
National Institutes of Health, United States
Supplementary Material
References
- Pertwee R. G.; Howlett A. C.; Abood M. E.; Alexander S. P.; Di Marzo V.; Elphick M. R.; Greasley P. J.; Hansen H. S.; Kunos G.; Mackie K.; Mechoulam R.; Ross R. A. (2010) International Union of Basic and Clinical Pharmacology. LXXIX. Cannabinoid receptors and their ligands: Beyond CB1 and CB2. Pharmacol. Rev. 62, 588–5631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anand P.; Whiteside G.; Fowler C. J.; Hohmann A. G. (2009) Targeting CB2 receptors and the endocannabinoid system for the treatment of pain. Brain Res. Rev. 60, 255–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabral G. A.; Griffin-Thomas L. (2009) Emerging role of the cannabinoid receptor CB2 in immune regulation: Therapeutic prospects for neuroinflammation. Expert Rev. Mol. Med. 11, e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riether D. (2012) Selective cannabinoid receptor 2 modulators: A patent review 2009–present. Expert Opin. Ther. Pat. 22, 495–510. [DOI] [PubMed] [Google Scholar]
- Ruhl T.; Deuther-Conrad W.; Fischer S.; Gunther R.; Hennig L.; Krautscheid H.; Brust P. (2012) Cannabinoid receptor type 2 (CB2)-selective N-aryl-oxadiazolyl-propionamides: Synthesis, radiolabelling, molecular modelling and biological evaluation. Org. Med. Chem. Lett. 2, 32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atwood B. K.; Mackie K. (2010) CB2: A cannabinoid receptor with an identity crisis. Br. J. Pharmacol. 160, 467–479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atwood B. K.; Wager-Miller J.; Haskins C.; Straiker A.; Mackie K. (2012) Functional selectivity in CB2 cannabinoid receptor signaling and regulation: Implications for the therapeutic potential of CB2 ligands. Mol. Pharmacol. 81, 250–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onaivi E. S.; Ishiguro H.; Gu S.; Liu Q. R. (2012) CNS effects of CB2 cannabinoid receptors: Beyond neuro-immuno-cannabinoid activity. J. Psychopharmacol. 26, 92–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Q. R.; Pan C. H.; Hishimoto A.; Li C. Y.; Xi Z. X.; Llorente-Berzal A.; Viveros M. P.; Ishiguro H.; Arinami T.; Onaivi E. S.; Uhl G. R. (2009) Species differences in cannabinoid receptor 2 (CNR2 gene): Identification of novel human and rodent CB2 isoforms, differential tissue expression and regulation by cannabinoid receptor ligands. Genes Brain Behav. 8, 519–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bingham B.; Jones P. G.; Uveges A. J.; Kotnis S.; Lu P.; Smith V. A.; Sun S. C.; Resnick L.; Chlenov M.; He Y.; Strassle B. W.; Cummons T. A.; Piesla M. J.; Harrison J. E.; Whiteside G. T.; Kennedy J. D. (2007) Species-specific in vitro pharmacological effects of the cannabinoid receptor 2 (CB2) selective ligand AM1241 and its resolved enantiomers. Br. J. Pharmacol. 151, 1061–1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urban J. D.; Clarke W. P.; von Zastrow M.; Nichols D. E.; Kobilka B.; Weinstein H.; Javitch J. A.; Roth B. L.; Christopoulos A.; Sexton P. M.; Miller K. J.; Spedding M.; Mailman R. B. (2007) Functional selectivity and classical concepts of quantitative pharmacology. J. Pharmacol. Exp. Ther. 320, 1–13. [DOI] [PubMed] [Google Scholar]
- den Boon F. S.; Chameau P.; Schaafsma-Zhao Q.; van Aken W.; Bari M.; Oddi S.; Kruse C. G.; Maccarrone M.; Wadman W. J.; Werkman T. R. (2012) Excitability of prefrontal cortical pyramidal neurons is modulated by activation of intracellular type-2 cannabinoid receptors. Proc. Natl. Acad. Sci. U.S.A. 109, 3534–3539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Petrocellis L.; Marini P.; Matias I.; Moriello A. S.; Starowicz K.; Cristino L.; Nigam S.; Di Marzo V. (2007) Mechanisms for the coupling of cannabinoid receptors to intracellular calcium mobilization in rat insulinoma β-cells. Exp. Cell Res. 313, 2993–3004. [DOI] [PubMed] [Google Scholar]
- Sugiura T.; Kondo S.; Kishimoto S.; Miyashita T.; Nakane S.; Kodaka T.; Suhara Y.; Takayama H.; Waku K. (2000) Evidence that 2-arachidonoylglycerol but not N-palmitoylethanolamine or anandamide is the physiological ligand for the cannabinoid CB2 receptor. Comparison of the agonistic activities of various cannabinoid receptor ligands in HL-60 cells. J. Biol. Chem. 275, 605–612. [DOI] [PubMed] [Google Scholar]
- Zoratti C.; Kipmen-Korgun D.; Osibow K.; Malli R.; Graier W. F. (2003) Anandamide initiates Ca2+ signaling via CB2 receptor linked to phospholipase C in calf pulmonary endothelial cells. Br. J. Pharmacol. 140, 1351–1362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu J.; Deliu E.; Zhang X. Q.; Hoffman N. E.; Carter R. L.; Grisanti L. A.; Brailoiu G. C.; Madesh M.; Cheung J. Y.; Force T.; Abood M. E.; Koch W. J.; Tilley D. G.; Brailoiu E. (2013) Differential Activation of Cultured Neonatal Cardiomyocytes by Plasmalemmal Versus Intracellular G Protein-coupled Receptor 55. J. Biol. Chem. 288, 22481–22492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brailoiu G. C.; Oprea T. I.; Zhao P.; Abood M. E.; Brailoiu E. (2011) Intracellular cannabinoid type 1 (CB1) receptors are activated by anandamide. J. Biol. Chem. 286, 29166–29174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deliu E.; Brailoiu G. C.; Eguchi S.; Hoffman N. E.; Rabinowitz J. E.; Tilley D. G.; Madesh M.; Koch W. J.; Brailoiu E. (2014) Direct evidence of intracrine angiotensin II signaling in neurons. Am. J. Physiol. 306, C736–C744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deliu E.; Brailoiu G. C.; Mallilankaraman K.; Wang H.; Madesh M.; Undieh A. S.; Koch W. J.; Brailoiu E. (2012) Intracellular endothelin type B receptor-driven Ca2+ signal elicits nitric oxide production in endothelial cells. J. Biol. Chem. 287, 41023–41031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grynkiewicz G.; Poenie M.; Tsien R. Y. (1985) A new generation of Ca2+ indicators with greatly improved fluorescence properties. J. Biol. Chem. 260, 3440–3450. [PubMed] [Google Scholar]
- Bowman E. J.; Siebers A.; Altendorf K. (1988) Bafilomycins: A class of inhibitors of membrane ATPases from microorganisms, animal cells, and plant cells. Proc. Natl. Acad. Sci. U.S.A. 85, 7972–7976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunz J. B.; Schwarz H.; Mayer A. (2004) Determination of four sequential stages during microautophagy in vitro. J. Biol. Chem. 279, 9987–9996. [DOI] [PubMed] [Google Scholar]
- Wang T.; Ming Z.; Xiaochun W.; Hong W. (2011) Rab7: Role of its protein interaction cascades in endo-lysosomal traffic. Cell. Signalling 23, 516–521. [DOI] [PubMed] [Google Scholar]
- Mukai H.; Munekata E.; Higashijima T. (1992) G protein antagonists. A novel hydrophobic peptide competes with receptor for G protein binding. J. Biol. Chem. 267, 16237–16243. [PubMed] [Google Scholar]
- Naylor E.; Arredouani A.; Vasudevan S. R.; Lewis A. M.; Parkesh R.; Mizote A.; Rosen D.; Thomas J. M.; Izumi M.; Ganesan A.; Galione A.; Churchill G. C. (2009) Identification of a chemical probe for NAADP by virtual screening. Nat. Chem. Biol. 5, 220–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jalink K.; Moolenaar W. H. (2010) G protein-coupled receptors: The inside story. BioEssays 32, 13–16. [DOI] [PubMed] [Google Scholar]
- Zhu T.; Gobeil F.; Vazquez-Tello A.; Leduc M.; Rihakova L.; Bossolasco M.; Bkaily G.; Peri K.; Varma D. R.; Orvoine R.; Chemtob S. (2006) Intracrine signaling through lipid mediators and their cognate nuclear G-protein-coupled receptors: A paradigm based on PGE2, PAF, and LPA1 receptors. Can. J. Physiol. Pharmacol. 84, 377–391. [DOI] [PubMed] [Google Scholar]
- Benard G.; Massa F.; Puente N.; Lourenco J.; Bellocchio L.; Soria-Gomez E.; Matias I.; Delamarre A.; Metna-Laurent M.; Cannich A.; Hebert-Chatelain E.; Mulle C.; Ortega-Gutierrez S.; Martin-Fontecha M.; Klugmann M.; Guggenhuber S.; Lutz B.; Gertsch J.; Chaouloff F.; Lopez-Rodriguez M. L.; Grandes P.; Rossignol R.; Marsicano G. (2012) Mitochondrial CB1 receptors regulate neuronal energy metabolism. Nat. Neurosci. 15, 558–564. [DOI] [PubMed] [Google Scholar]
- Howlett A. C.; Barth F.; Bonner T. I.; Cabral G.; Casellas P.; Devane W. A.; Felder C. C.; Herkenham M.; Mackie K.; Martin B. R.; Mechoulam R.; Pertwee R. G. (2002) International Union of Pharmacology. XXVII. Classification of cannabinoid receptors. Pharmacol. Rev. 54, 161–202. [DOI] [PubMed] [Google Scholar]
- Glass M.; Felder C. C. (1997) Concurrent stimulation of cannabinoid CB1 and dopamine D2 receptors augments cAMP accumulation in striatal neurons: Evidence for a Gs linkage to the CB1 receptor. J. Neurosci. 17, 5327–5333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauckner J. E.; Hille B.; Mackie K. (2005) The cannabinoid agonist WIN55,212-2 increases intracellular calcium via CB1 receptor coupling to Gq/11 G proteins. Proc. Natl. Acad. Sci. U.S.A. 102, 19144–19149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugiura T.; Kodaka T.; Kondo S.; Nakane S.; Kondo H.; Waku K.; Ishima Y.; Watanabe K.; Yamamoto I. (1997) Is the cannabinoid CB1 receptor a 2-arachidonoylglycerol receptor? Structural requirements for triggering a Ca2+ transient in NG108-15 cells. J. Biochem. 122, 890–895. [DOI] [PubMed] [Google Scholar]
- Sugiura T.; Kodaka T.; Kondo S.; Tonegawa T.; Nakane S.; Kishimoto S.; Yamashita A.; Waku K. (1996) 2-Arachidonoylglycerol, a putative endogenous cannabinoid receptor ligand, induces rapid, transient elevation of intracellular free Ca2+ in neuroblastoma x glioma hybrid NG108-15 cells. Biochem. Biophys. Res. Commun. 229, 58–64. [DOI] [PubMed] [Google Scholar]
- Shoemaker J. L.; Ruckle M. B.; Mayeux P. R.; Prather P. L. (2005) Agonist-directed trafficking of response by endocannabinoids acting at CB2 receptors. J. Pharmacol. Exp. Ther. 315, 828–838. [DOI] [PubMed] [Google Scholar]
- Irannejad R.; Tomshine J. C.; Tomshine J. R.; Chevalier M.; Mahoney J. P.; Steyaert J.; Rasmussen S. G.; Sunahara R. K.; El-Samad H.; Huang B.; von Zastrow M. (2013) Conformational biosensors reveal GPCR signalling from endosomes. Nature 495, 534–538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deliu E.; Tica A. A.; Motoc D.; Brailoiu G. C.; Brailoiu E. (2011) Intracellular angiotensin II activates rat myometrium. Am. J. Physiol. 301, C559–C565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosker F.; Cheviron N.; Yamasaki M.; Menteyne A.; Lund F. E.; Moutin M. J.; Galione A.; Cancela J. M. (2010) The ecto-enzyme CD38 is a nicotinic acid adenine dinucleotide phosphate (NAADP) synthase that couples receptor activation to Ca2+ mobilization from lysosomes in pancreatic acinar cells. J. Biol. Chem. 285, 38251–38259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S. Y.; Cho B. H.; Kim U. H. (2010) CD38-mediated Ca2+ signaling contributes to angiotensin II-induced activation of hepatic stellate cells: Attenuation of hepatic fibrosis by CD38 ablation. J. Biol. Chem. 285, 576–582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis L. C.; Morgan A. J.; Ruas M.; Wong J. L.; Graeff R. M.; Poustka A. J.; Lee H. C.; Wessel G. M.; Parrington J.; Galione A. (2008) Ca2+ signaling occurs via second messenger release from intraorganelle synthesis sites. Curr. Biol. 18, 1612–1618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimsey N. L.; Goodfellow C. E.; Dragunow M.; Glass M. (2011) Cannabinoid receptor 2 undergoes Rab5-mediated internalization and recycles via a Rab11-dependent pathway. Biochim. Biophys. Acta 1813, 1554–1560. [DOI] [PubMed] [Google Scholar]
- Hashimotodani Y.; Ohno-Shosaku T.; Tanimura A.; Kita Y.; Sano Y.; Shimizu T.; Di Marzo V.; Kano M. (2013) Acute inhibition of diacylglycerol lipase blocks endocannabinoid-mediated retrograde signalling: Evidence for on-demand biosynthesis of 2-arachidonoylglycerol. J. Physiol. 591, 4765–4776. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.