Abstract
We recently developed a new, rapid, and specific bioassay system that employs a fluorescent probe fabricated from our discovered CXCR4-specific ligand DV1. This new probe sensitively and selectively blocks the binding of native and synthetic ligands to CXCR4 at nanomolar levels, with a capability comparable to that seen with a conventional CXCR4 antibody. This nonradioactive, direct, and CXCR4-specific high-affinity screening system provides a new platform for CXCR4-targeted drug screening, as well as for the development of new probes for other GPCRs.
CXCR4 is a member of the G protein-coupled receptor (GPCR) family, which has a seven-transmembrane domain.1,2 Upon ligand binding, CXCR4 transfers a signal into the cell and triggers a series of corresponding signaling cascades.3−5 Like other GPCRs, CXCR4 consists of an amino (N) terminus, three extracellular loops, three intracellular loops, seven transmembrane (TM) helices, and a carboxyl (C) terminus.2 The multiple extracellular and TM domains of CXCR4 are required for chemokine interactions and receptor signaling.1 The structures of several chemokines, including stromal-cell-derived factor (SDF-1α)6,7 and viral macrophage inflammatory protein-II (vMIP-II),8,9 binding to CXCR4 have been determined by either nuclear magnetic resonance (NMR) or X-ray techniques. Unlike other chemokine receptors that have a number of distinct ligands, CXCR4 has only two endogenous natural ligands identified to date, known as SDF-1α (CXCL12) and ubiquitin.10−12 CXCR4 can also be recognized by an antagonistic ligand, namely vMIP-II, which is encoded by the Kaposi’s sarcoma-associated herpes virus.13 The CXCR4–SDF-1α interaction has essential physiological and pathological functions in hematopoiesis, immunomodulation, vascularization, cerebellar neuron migration, cancer metastasis, and human immunodeficiency virus (HIV) infection.14−17
As we all know, the binding assays for CXCR4 with [125I]SDF-1α are very arduous and not at all user-friendly, especially with respect to dealing with harmful radioactive materials. In the past few years, several groups, including ours, have used CXCR4-specific 12G5 antibodies in place of [125I]SDF-1α for the CXCR4 ligand competitive binding assay, because 12G5 antibodies can strongly and selectively interact with the domains of extracellular loops 1 and 2 (ECL1 and ECL2, respectively) of CXCR4.18,19 However, the 12G5 antibody-based binding assay is time-consuming and expensive because it requires both primary antibodies and fluorescently labeled secondary antibodies. The instability of these antibodies further worsens the situation by reducing the effectiveness of the assay.
DV1 is a synthetic peptide composed entirely of d-amino acids derived from the modification of a 21-residue peptide from the N-terminus of vMIP-II.20−22 DV1 has been shown to compete more efficiently with [125I]SDF-1α in the CXCR4 binding assay with an IC50 of 13 nM than the nonmodified V1 peptide (IC50 = 218 nM). Similarly, the CXCR4 binding affinity was much higher for DV1 (IC50 = 32 nM) than for the V1 peptide (IC50 = 456 nM) in the CXCR4-specific mAb 12G5 competing binding assay.22,23 In contrast to its strong interaction with CXCR4, the DV1 peptide showed no detectable CCR5 binding, even at concentrations as high as 100 μM, when competing with [125I]MIP-1β.22 These results suggest that binding of the DV1 peptide to CXCR4 is receptor-selective.
Here, we report a novel high-affinity and receptor subresidue-selective fluorescent CXCR4-specific probe, FITC-DV1. This novel probe was synthesized by adding an aminocaproic acid and a lysine to the C-terminus of DV1 and conjugating a fluorescein isothiocyanate (FITC) group onto the ε-amino moiety of the added lysine (the mass spectrometry data for the identification of DV1 and FITC-DV1 are shown in Figures S1 and S2 of the Supporting Information).
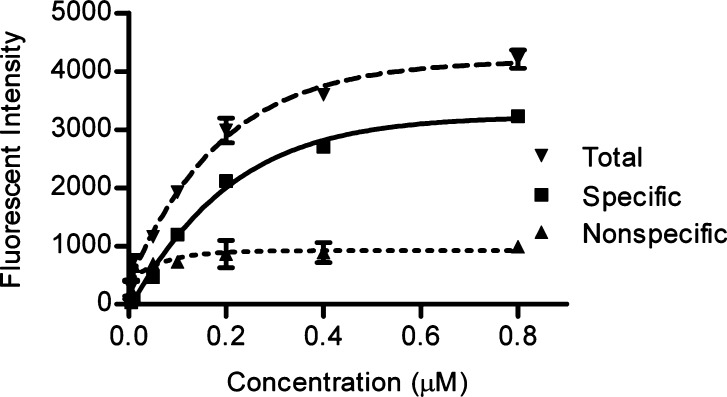
Prior to studying the binding affinity of FITC-DV1 for the CXCR4 receptor, we determined the saturation concentration for the binding of FITC-DV1 to CXCR4 in CXCR4-overexpressing cells (CHO-CXCR4 cells) (Figure 1). The specific signals for binding of FITC-DV1 to CXCR4 reached a plateau at a concentration of 800 nM.
Figure 1.
Saturation curve for binding of FITC-DV1 to CXCR4. Specific binding (■) was obtained by subtracting nonspecific binding (▲) (obtained from the binding of FITC-DV1 to wild-type CHO cells) from total binding (▼). Means ± the standard deviation; n = 3 independent experiments.
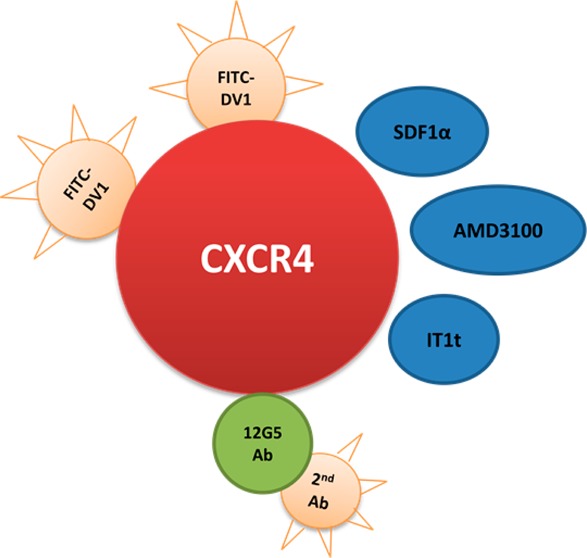
Therefore, we employed a saturation concentration of 800 nM to conduct and validate the FITC-DV1-based competitive binding assay. Our subsequent binding experiments revealed that DV1 and SDF-1α (Figure 2A and Table S1 of the Supporting Information), as well as the well-known small molecule ligands of CXCR4, AMD3100 and IT1t (Figure 2B and Table S1 of the Supporting Information), all inhibited the binding of FITC-DV1 to CXCR4 in our assay (IC50 values listed in Table S2 of the Supporting Information), suggesting that the site of binding of FITC-DV1 to CXCR4 overlaps with those of SDF-1α, 12G5 Ab, AMD3100, and IT1t. The competition curves for FITC-DV1-based and 12G5 Ab-based competitive binding were almost superimposable for all these ligands (P > 0.05). These results strongly support the idea that FITC-DV1 is a good chemical probe for ligand–CXCR4 interactions and its efficacy is comparable to that of the specific antibody 12G5.
Figure 2.
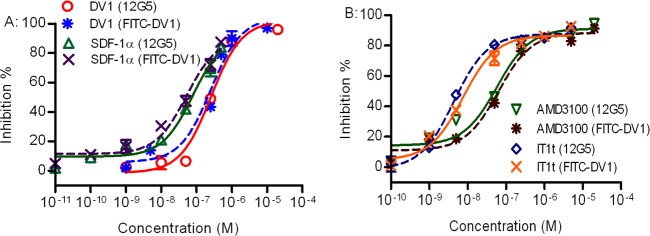
Competitive CXCR4 binding activity of DV1, SDF-1α, AMD3100, and IT1t in FITC-DV1- and 12G5 Ab-based competitive binding assays. (A) The competitive capacities of DV1 (blue asterisks) and SDF-1α (maroon times signs) in the FITC-DV1-based assay were comparable to those of DV1 (red circles) and SDF-1α (black triangles) in the 12G5 Ab-based assay, for binding CXCR4. (B) The competitive capacities of AMD3100 (black asterisks) and IT1t (red times signs) in the FITC-DV1-based assay were comparable to those of AMD3100 (black triangles) and IT1t (black diamonds) in the 12G5 Ab-based assay, for binding CXCR4. Means ± the standard deviation; n = 3 independent experiments.
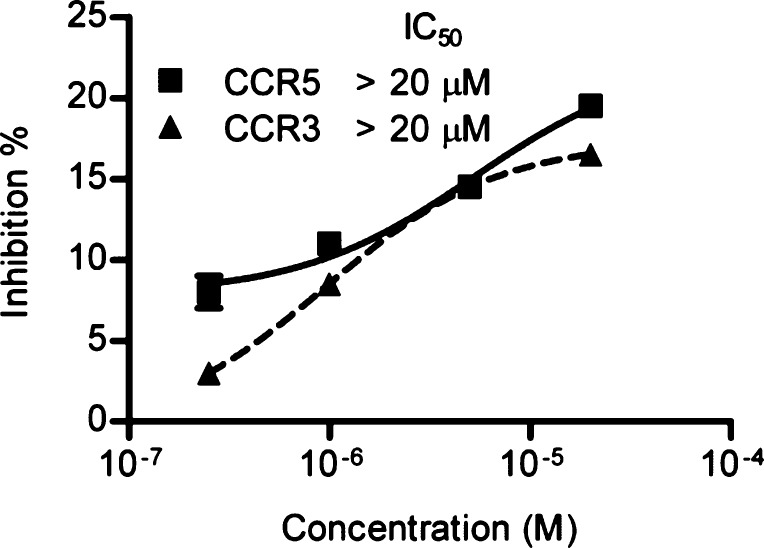
CXCR4, CCR5, and CCR3 are all chemokine receptors that have highly homologous protein sequences and three-dimensional structures. To determine whether FITC-DV1 has any cross-reactivity with CCR5 and CCR3, we performed further competitive assays. The binding activities of FITC-DV1 were very low (IC50 > 20 μM) for both CCR5 and CCR3 (Figure 3).
Figure 3.
Binding activity of FITC-DV1 with CCR5 and CCR3. The IC50 values of FITC-DV1 against CCR5 and CCR3 antibodies were both greater than 20 μM. Means ± the standard deviation; n = 3 independent experiments.
Neither ubiquitin, a ligand of CXCR4, nor RANTES (a common ligand of CCR5 and CCR3) inhibited the binding of FITC-DV1 in the corresponding CXCR4-, CCR5-, and CCR3-expressing cell lines (data not shown). Additionally, FITC-DV1 did not show any inhibitory activity against CXCR7 Ab binding with CXCR7, even at a high concentration of 5 μM. Therefore, the relatively high binding affinity, along with the receptor selectivity of FITC-DV1 toward CXCR4, makes it an excellent probe that can be used in CXCR4 binding assays.
In conclusion, conventional radioactive and antibody binding assays are very costly and time-consuming. We have developed a new fluorescent chemical ligand probe, FITC-DV1, which binds to CXCR4 with high affinity and specificity. This FITC-DV1-based CXCR4 binding assay is superior to radioactive [125I]SDF-1α- and 12G5 antibody-based CXCR4 binding assays, as it is simple, sensitive, specific, time-saving, and cost-effective. The convenience of this FITC-DV1-based assay may help to pave the new way toward a better understanding of differential ligand–CXCR4 interactions and to identify specific modulators and new candidates for CXCR4. In addition, this novel approach for developing a simple, stable, CXCR4-specific fluorescent ligand probe could be widely applied to other GPCRs of interest.
Acknowledgments
We thank the National Institutes of Health AIDS Reagent Program for providing the CCR5 and CCR3 cell lines and anti-CCR3 and CCR5 antibodies.
Supporting Information Available
Experimental methods, Figures S1 and S2, and Tables S1 and S2. This material is available free of charge via the Internet at http://pubs.acs.org.
Author Contributions
Y.Y. synthesized the peptides. Y.Y., Q.Z., and M.G. performed biological experiments. Y.Y. and Q.Z. wrote the paper. X.Y., Z.H., and J.A. supervised the work.
This work was supported by grants from the National Institutes of Health and the Carol M. Baldwin Breast Cancer Research Fund. Y.Y. was supported in part by the Chinese Scholarship Council.
The authors declare no competing financial interest.
Funding Statement
National Institutes of Health, United States
Supplementary Material
References
- Zlotnik A.; Yoshie O. (2000) Immunity 12, 121–127. [DOI] [PubMed] [Google Scholar]
- Wu B.; Chien E. Y.; Mol C. D.; Fenalti G.; Liu W.; Katritch V.; Abagyan R.; Brooun A.; Wells P.; Bi F. C.; Hamel D. J.; Kuhn P.; Handel T. M.; Cherezov V.; Stevens R. C. (2010) Science 330, 1066–1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grunbeck A.; Huber T.; Sachdev P.; Sakmar T. P. (2011) Biochemistry 50, 3411–3413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piehler J. (2011) Nat. Chem. Biol. 7, 578–579. [DOI] [PubMed] [Google Scholar]
- Congreve M.; Langmead C. J.; Mason J. S.; Marshall F. H. (2011) J. Med. Chem. 54, 4283–4311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crump M. P.; Gong J. H.; Loetscher P.; Rajarathnam K.; Amara A.; Arenzana-Seisdedos F.; Virelizier J. L.; Baggiolini M.; Sykes B. D.; Clark-Lewis I. (1997) EMBO J. 16, 6996–7007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohnishi Y.; Senda T.; Nandhagopal N.; Sugimoto K.; Shioda T.; Nagal Y.; Mitsui Y. (2000) J. Interferon Cytokine Res. 20, 691–700. [DOI] [PubMed] [Google Scholar]
- Li Y.; Liu D.; Cao R.; Kumar S.; Dong C.; An J.; Wilson S. R.; Gao Y. G.; Huang Z. (2007) Proteins 67, 243–246. [DOI] [PubMed] [Google Scholar]
- Liwang A. C.; Wang Z. X.; Sun Y.; Peiper S. C.; Liwang P. J. (1999) Protein Sci. 8, 2270–2280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teicher B. A.; Fricker S. P. (2010) Clin. Cancer Res. 16, 2927–2931. [DOI] [PubMed] [Google Scholar]
- Heveker N.; Montes M.; Germeroth L.; Amara A.; Trautmann A.; Alizon M.; Schneider-Mergener J. (1998) Curr. Biol. 8, 369–376. [DOI] [PubMed] [Google Scholar]
- Saini V.; Staren D. M.; Ziarek J. J.; Nashaat Z. N.; Campbell E. M.; Volkman B. F.; Marchese A.; Majetschak M. (2011) J. Biol. Chem. 286, 33466–33477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kledal T. N.; Rosenkilde M. M.; Coulin F.; Simmons G.; Johnsen A. H.; Alouani S.; Power C. A.; Luttichau H. R.; Gerstoft J.; Clapham P. R.; Clark-Lewis I.; Wells T. N.; Schwartz T. W. (1997) Science 277, 1656–1659. [DOI] [PubMed] [Google Scholar]
- Busillo J. M.; Benovic J. L. (2007) Biochim. Biophys. Acta 1768, 952–963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller A.; Homey B.; Soto H.; Ge N.; Catron D.; Buchanan M. E.; McClanahan T.; Murphy E.; Yuan W.; Wagner S. N.; Barrera J. L.; Mohar A.; Verastegui E.; Zlotnik A. (2001) Nature 410, 50–56. [DOI] [PubMed] [Google Scholar]
- Michael N. L.; Nelson J. A.; KewalRamani V. N.; Chang G.; O’Brien S. J.; Mascola J. R.; Volsky B.; Louder M.; White G. C. II; Littman D. R.; Swanstrom R.; O’Brien T. R. (1998) J. Virol. 72, 6040–6047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi W. T.; Duggineni S.; Xu Y.; Huang Z.; An J. (2012) J. Med. Chem. 55, 977–994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endres M. J.; Clapham P. R.; Marsh M.; Ahuja M.; Turner J. D.; McKnight A.; Thomas J. F.; Stoebenau-Haggarty B.; Choe S.; Vance P. J.; Wells T. N.; Power C. A.; Sutterwala S. S.; Doms R. W.; Landau N. R.; Hoxie J. A. (1996) Cell 87, 745–756. [DOI] [PubMed] [Google Scholar]
- Brelot A.; Heveker N.; Pleskoff O.; Sol N.; Alizon M. (1997) J. Virol. 71, 4744–4751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boshoff C.; Endo Y.; Collins P. D.; Takeuchi Y.; Reeves J. D.; Schweickart V. L.; Siani M. A.; Sasaki T.; Williams T. J.; Gray P. W.; Moore P. S.; Chang Y.; Weiss R. A. (1997) Science 278, 290–294. [DOI] [PubMed] [Google Scholar]
- Luo Z.; Fan X.; Zhou N.; Hiraoka M.; Luo J.; Kaji H.; Huang Z. (2000) Biochemistry 39, 13545–13550. [DOI] [PubMed] [Google Scholar]
- Zhou N.; Luo Z.; Luo J.; Fan X.; Cayabyab M.; Hiraoka M.; Liu D.; Han X.; Pesavento J.; Dong C. Z.; Wang Y.; An J.; Kaji H.; Sodroski J. G.; Huang Z. (2002) J. Biol. Chem. 277, 17476–17485. [DOI] [PubMed] [Google Scholar]
- Choi W. T.; Kumar S.; Madani N.; Han X.; Tian S.; Dong C. Z.; Liu D.; Duggineni S.; Yuan J.; Sodroski J. G.; Huang Z.; An J. (2012) Biochemistry 51, 7078–7086. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.