Schneider-Hohendorf describe expression of adhesion molecules MCAM and PSGL-1 on human CD4+ T cells and Th17 T cells in multiple sclerosis patients under long-term natalizumab treatment. The authors identify that despite blockade of VLA-4, MCAM+ T cells can migrate through the blood–brain barrier to access the CNS through PSGL-1 and MCAM.
Abstract
The focus of this study is the characterization of human T cell blood–brain barrier migration and corresponding molecular trafficking signatures. We examined peripheral blood and cerebrospinal fluid immune cells from patients under long-term anti–very late antigen-4 (VLA-4)/natalizumab therapy (LTNT) and from CNS specimens. LTNT patients’ cerebrospinal fluid T cells exhibited healthy central-/effector-memory ratios, but lacked CD49d and showed enhanced myeloma cell adhesion molecule (MCAM) expression. LTNT led to an increase of PSGL-1 expression on peripheral T cells. Although vascular cell adhesion molecule-1 (VLA-4 receptor) was expressed at all CNS barriers, P-selectin (PSGL-1-receptor) was mainly detected at the choroid plexus. Accordingly, in vitro experiments under physiological flow conditions using primary human endothelial cells and LTNT patients’ T cells showed increased PSGL-1–mediated rolling and residual adhesion, even under VLA-4 blockade. Adhesion of MCAM+/TH17 cells was not affected by VLA-4 blocking alone, but was abrogated when both VLA-4 and MCAM were inhibited. Consistent with these data, MCAM+ cells were detected in white matter lesions, and in gray matter of multiple sclerosis patients. Our data indicate that lymphocyte trafficking into the CNS under VLA-4 blockade can occur by using the alternative adhesion molecules, PSGL-1 and MCAM, the latter representing an exclusive pathway for TH17 cells to migrate over the blood–brain barrier.
In recent years, several different migratory routes for immune cells over different cellular barriers into the CNS parenchyma have been characterized (Engelhardt and Sorokin, 2009; Wilson et al., 2010; Larochelle et al., 2011; Engelhardt and Ransohoff, 2012; Alvarez et al., 2013). However, the precise molecular mechanism responsible for homeostatic CNS immune surveillance and how inflammatory processes differ from regulatory/homeostatic processes on the immune cell side remain unclear (Flügel et al., 2011). Pressure to advance knowledge on the mechanisms of trans-endothelial diapedesis arose with the approval of therapeutic monoclonal antibodies interfering with cell trafficking. Such antibodies have been effective in several conditions, ranging from suppression of rejection of transplants in the case of Muronomab (Hooks et al., 1991) and Daclizumab (Saghafi et al., 2012) to amelioration of autoimmune disorders like psoriasis using anti-CD11a/LFA-1 treatment (Dubertret et al., 2006) or multiple sclerosis using anti–very late antigen 4 (VLA-4) treatment (Yednock et al., 1992; Polman et al., 2006). However, concerns have been raised that beneficial immune responses may be inhibited as well as detrimental ones (Stüve and Wiendl, 2009; Steinman, 2014). Although the vast majority of patients reacted very favorably to the treatments, in rare cases these concerns were found to be true, particularly in emerging cases of progressive multifocal leukoencephalopathy associated with anti–LFA-1 (integrin αLβ2 = CD11a/CD18) and anti–VLA-4 (integrin α4β1 = CD49d/CD29) treatment (Bloomgren et al., 2012; Schwab et al., 2012a,b). Our study combines analyses of biomaterials obtained from patients treated with the monoclonal antibody natalizumab (anti–VLA-4) with in vitro experiments addressing the mechanisms used by immune cells to transmigrate the blood–brain barriers (Huang et al., 2009; Schneider-Hohendorf et al., 2010). Although blockade of VLA-4 was thought to completely abrogate CNS entry of T lymphocytes, patients under natalizumab treatment have lower but still detectable numbers of immune cells in the cerebrospinal fluid (CSF; Stüve et al., 2006a; Stenner et al., 2008), suggesting that there are alternative or compensatory molecular mechanisms for some immune cell populations to enter the CNS. Characterizing such alternative pathways was the goal of this study; we were especially interested in the details of the mechanisms involved in early migration events, involving principally VLA-4 and P-selectin glycoprotein ligand-1 (PSGL-1 = CD162), which together with their receptors (vascular cell adhesion molecule-1 [VCAM-1] in the case of VLA-4 and P-selectin in the case of PSGL-1) are involved in tethering, rolling, and adhesion of T cells to endothelial barriers and are prerequisites for successful extravasation into the CNS (Engelhardt and Ransohoff, 2012).
RESULTS
CSF isolated from multiple sclerosis (MS) patients under long-term treatment with natalizumab reflects a normalization of the central nervous system immune response
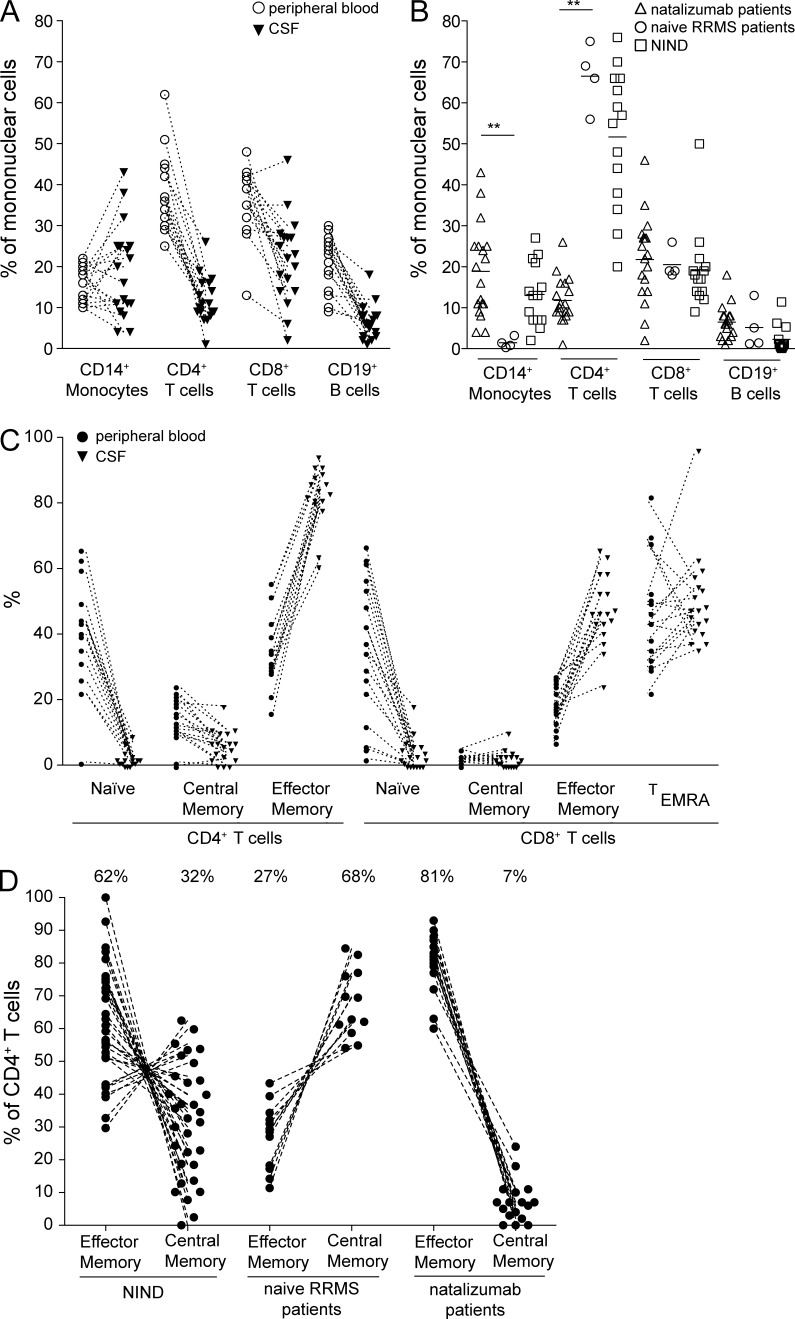
Flow cytometric analysis of PBMCs from long-term natalizumab-treated (LTNT; ≥18 mo of continuous treatment) relapsing-remitting MS (RRMS) patients revealed that the proportions of all major immune cell subsets was within normal limits (unpublished data). However, assessment of CSF immune cells of clinically stable LTNT patients (Fig. 1 A) revealed clear differences in immune cell subsets compared with treatment-naive, stable RRMS patients: the percentage of CD14+ monocytes was elevated in natalizumab-treated patients (18.9%) and, interestingly, was similar to that of control subjects without any neurological disease (13.2%), but differed significantly from the percentage in naive RRMS patients (1.4%). The percentage of CD4+ T cells was reduced (11.8 vs. 66.5%), whereas CD8+ T cells were unchanged, resulting in a reversed CD4/CD8 ratio compared with untreated MS patients (0.54 vs. 3.24; Fig. 1 B). Comparing the CD4+ and CD8+ T cell effector-memory (EM) compartments, there was an expected shift toward memory cells in the CSF compared with the peripheral EM compartments (Fig. 1 C).
Figure 1.
Changes in the cerebrospinal fluid under long-term treatment with natalizumab reflect a normalization of the central immune response in MS patients. (A) CSF composition of long-term treated natalizumab patients (filled triangles; n = 18) when compared with corresponding peripheral blood samples (open circles). (B) Comparison between CSF values from either LTNT patients (open triangles; n = 18), untreated RRMS patients (open circles; n = 4), or control patients (open squares; n = 14). **, P ≤ 0.01. (C) EM compartments of CD4+ and CD8+ T cells in the CSF of long-term treated natalizumab patients (n = 18). Naive (CD45RA+CCR7+), CM (CD45RA−CCR7+), and EM (CD45RA−CCR7−) CD4+ and CD8+ T cells are shown. Additionally, in the case of CD8+ T cells, TEMRA (CD45RA+CCR7−) are shown. (D) CSF composition concerning EM CD4+ T cells and CM CD4+ T cells in noninflammatory neurological diseases (NIND; n = 33), RRMS patients (n = 12), and MS patients under long-term natalizumab therapy (n = 18).
Importantly, the ratio between CD4 EM and central-memory (CM) cells in LTNT patients (EM/CM 81%:7%) was inverted when compared with treatment-naive, stable RRMS patients (27:68%). The difference here was even more pronounced than between noninflammatory neurological diseases (62:32%) and treatment-naive, stable RRMS patients (Fig. 1 D). These results suggest that EM cells have mechanisms that allow better access to the CNS than CM cells under continuous VLA-4 blockade.
Decrease of CD49d expression in peripheral and central T cell compartments under long-term natalizumab therapy
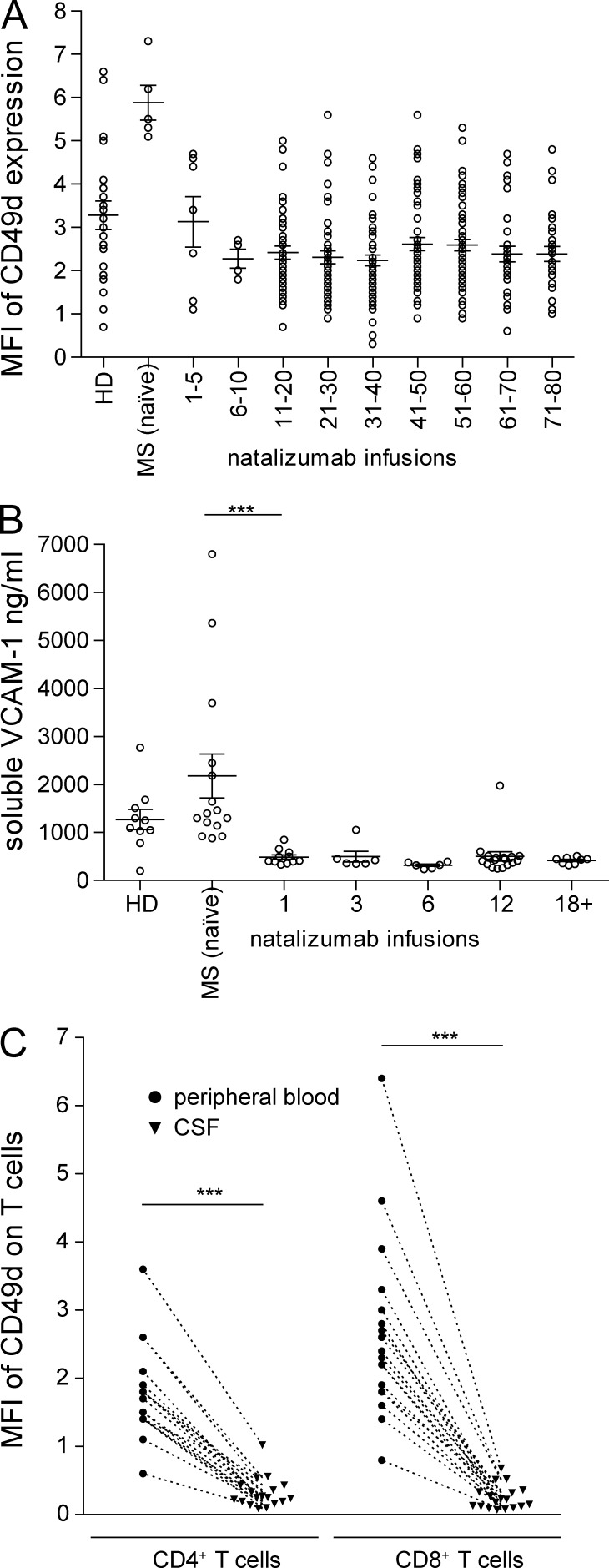
In addition to changes in immune cell populations and T cell subsets, we assessed the expression of the molecule targeted by natalizumab, CD49d, and its ligand VCAM-1. Natalizumab treatment induced a rapid decrease of CD49d surface expression on peripheral blood CD4+ T cells, which was not accompanied by a down-regulation in gene expression (Fig. 2 A and not depicted). Of note, the antibody clone used in flow cytometry analyses binds a different epitope on CD49d to natalizumab, so there is no competition/blocking (unpublished data). Also, already at the first infusion, natalizumab substantially reduced the soluble form of the CD49d ligand, VCAM-1, in the serum (Fig. 2 B). Surprisingly, CD49d expression was not detectable on CSF T cells of LTNT patients, even when compared with the down-regulated/low CD49d levels on peripheral T cells (Fig. 2 C), indicating alternative molecular patterns allowing for CNS entry under continuous VLA-4 blockade.
Figure 2.
CD49d expression in peripheral and central T cell compartments under long-term natalizumab therapy. (A) Expression of CD49d on peripheral CD4+ T cells of either healthy controls (n = 22) or MS patients before the start of natalizumab therapy (MS naive; n = 5), or during natalizumab therapy (n = 322). (B) Soluble VCAM-1 in the serum of healthy controls (n = 10), MS patients before treatment (n = 15), and during treatment with natalizumab (n = 49). (C) Comparison of the expression of CD49d on peripheral and CSF CD4+ and CD8+ T cells of long-term treated natalizumab patients (n = 18). ***, P ≤ 0.001.
Natalizumab treatment induces up-regulation of PSGL-1
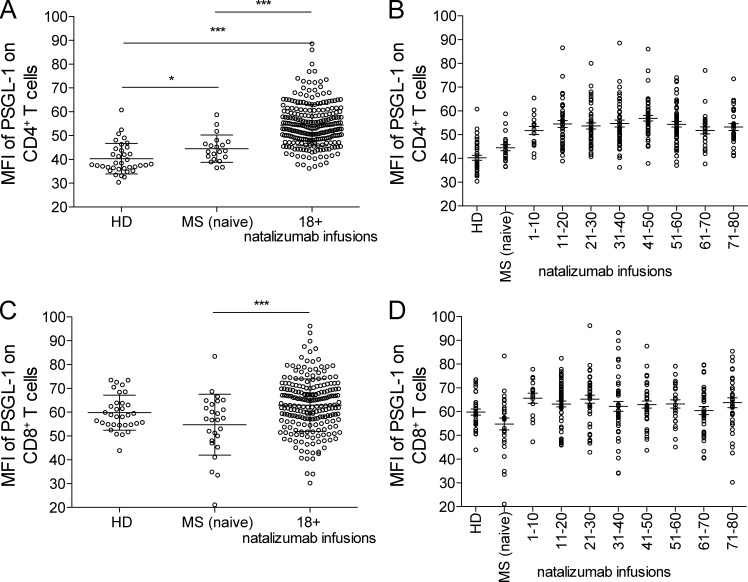
In accordance with previous observations showing sequestering of specific immune cell subsets (Kivisäkk et al., 2009), the adhesion molecule P-selectin glycoprotein ligand-1 (PSGL-1) was strongly up-regulated on CD4+ T cells in LTNT patients (CD4 MFI 54.2 ± SD 8.8) compared with patients before treatment (CD4 MFI 44.5 ± SD 5.7; Fig. 3 A). There was a clear and reproducible time kinetic in PSGL-1 up-regulation, peaking at four years of treatment (Fig. 3 B). Similar results were obtained for CD8+ T cells of MS patients with an up-regulation from MFI 54.8 ± SD 7.4 to MFI 63.0 ± SD 11.0 by natalizumab therapy (Fig. 3, C and D).
Figure 3.
Natalizumab treatment induces up-regulation of PSGL-1 on T cells. (A) Surface expression level of PSGL-1 on either healthy controls (HD, n = 36), patients before (MS [naïve], n = 20), or after long-term natalizumab therapy (18–80 natalizumab infusions; n = 252) on CD4+ T cells. (B) Time course of PSGL-1 expression during natalizumab treatment (n = 312) for CD4+ T cells when compared with healthy controls (HD, n = 36) and patients before natalizumab treatment (MS [naïve], n = 20). (C) Surface expression level of PSGL-1 on either healthy controls (HD, n = 33), patients before (MS [naïve], n = 26) or after long-term natalizumab therapy (18–80 natalizumab infusions, n = 217) on CD T cells. (D) Time course of PSGL-1 expression during natalizumab treatment (n = 292) for CD8 T cells when compared with healthy controls (HD, n = 33) and patients before natalizumab treatment (MS [naïve], n = 26). *, P ≤ 0.05; ***, P ≤ 0.001.
Expression of VCAM-1 and P-selectin at possible CNS entry sites in MS patient and control tissues
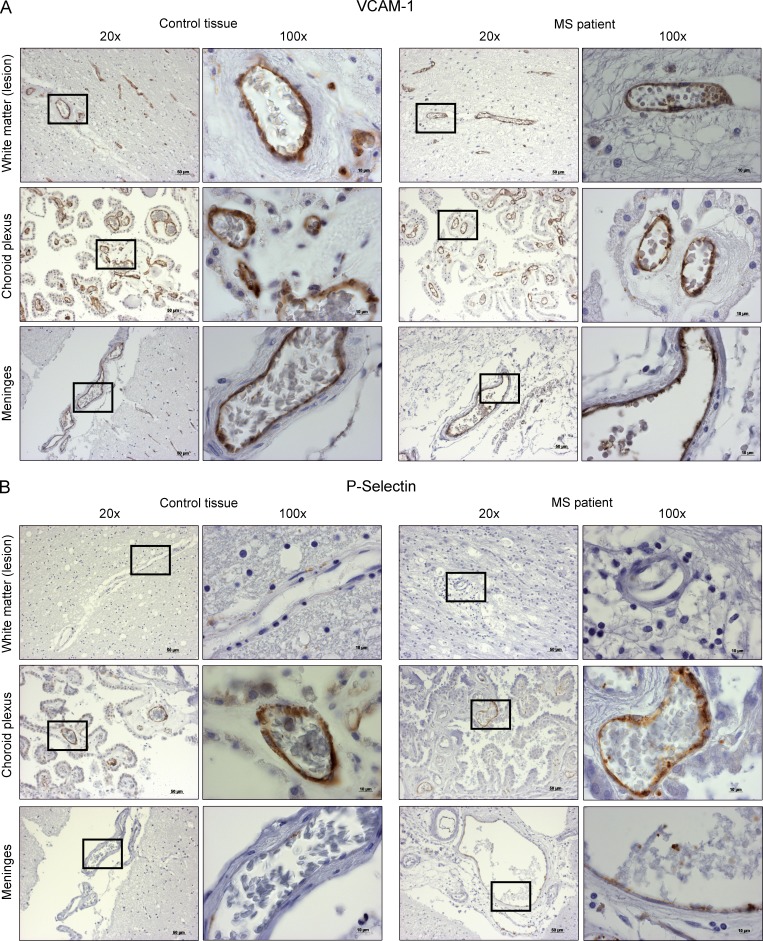
To assess the relevance of our findings for the migration of T cells over different blood–CNS barriers in vivo, we stained for the primary ligands of CD49d and PSGL-1 (i.e., VCAM-1 and P-selectin) in CNS tissue specimens from MS and control patients. The regions of interest were the meninges to assess the blood-leptomeningeal barrier, normal-appearing/lesional white matter to assess the direct migration from blood vessels to CNS tissue/traditional blood–brain barrier, and the choroid plexus to assess the blood–CSF barrier. The expression intensities are listed in Table S1. Importantly, we could show that there are varying amounts of VCAM-1 immunoreactivity on all the endothelial barriers, but the expression of P-selectin was much more restricted. There was no expression of P-selectin on the endothelium of vessels in white matter lesions, very low expression on meningeal vessels, and pronounced expression on choroid plexus vessels. Notably, the expression on the choroid plexus endothelium in the tissue of MS patients was generally more pronounced than in control tissues (Fig. 4).
Figure 4.
Expression of VCAM-1 and P-selectin on possible routes of entry in MS CNS tissue specimen and control tissue. VCAM-1 (A) and P-selectin (B) expression in the parenchymal (lesion area), choroid plexus, and meningeal blood vessels. The inset shows 100× magnification. DAB was used as chromogenic substrate and hematoxylin was used for counter stain.
Influence of natalizumab on rolling and adhesion of CD4+ T cells to CD49d and PSGL-1 ligands (i.e., VCAM-1/P-selectin)
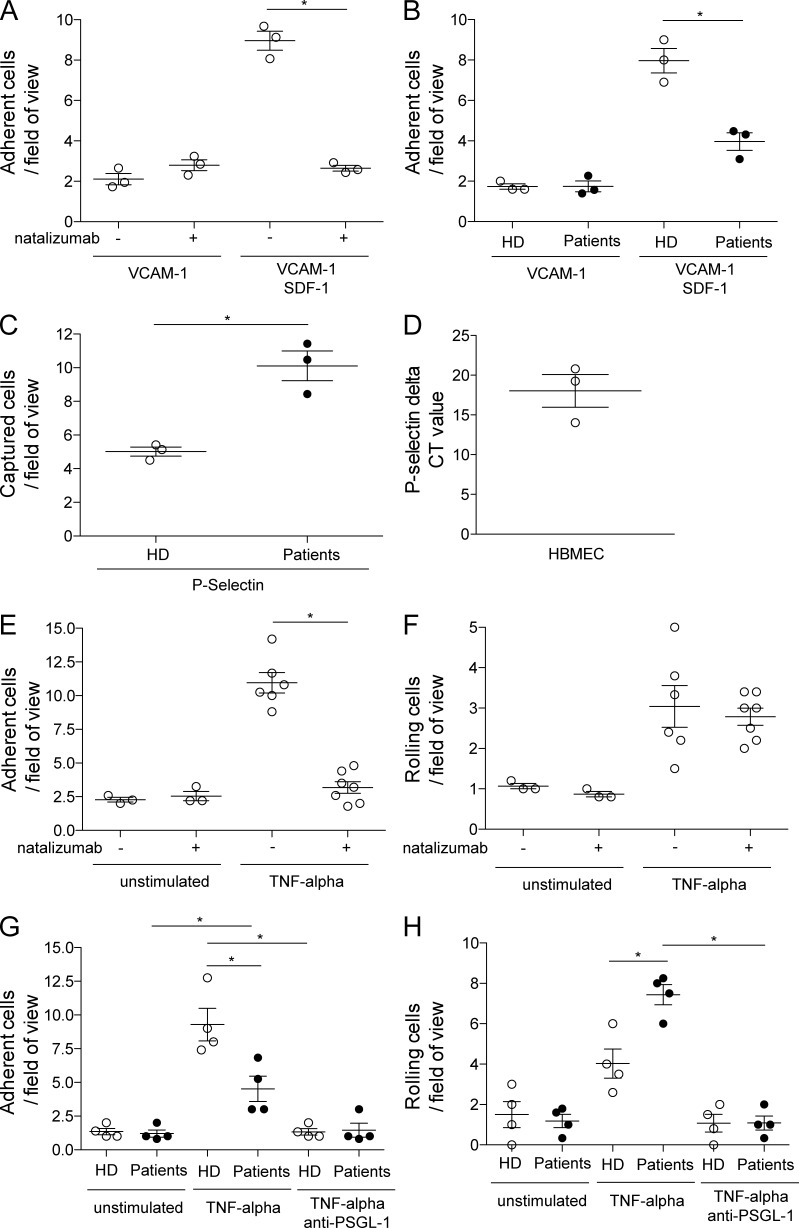
Both CD49d and PSGL-1 are molecules critically involved in the first steps of lymphocyte extravasation: tethering, rolling, and adhesion (Engelhardt and Ransohoff, 2012). We therefore assessed whether natalizumab-mediated blockade of CD49d and the subsequent up-regulation of PSGL-1 are functionally relevant under shear-flow conditions. In vitro treatment of healthy control patients’ CD4+ T cells with natalizumab abrogated the interaction of CD49d with VCAM-1 in a glass capillary-based system and, in accordance with the established view, adhesion to VCAM-1 required the conformational change of VLA-4 by a chemokine stimulus (SDF-1/CXCL12; Fig. 5 A). We subsequently showed that this reduced adhesion was also evident in freshly isolated CD4+ T cells from the blood of patients under LTNT (Fig. 5 B), demonstrating the treatment effect on immune cells in vitro. Analysis of P-selectin (PSGL-1 receptor)–coated glass capillaries revealed that the capturing capacity of patient cells was significantly elevated compared with healthy control cells (Fig. 5 C). Primary human brain–derived microvascular endothelial cells (HBMECs) grown to confluence were used in the next step to mimic the natural expression of the CD49d- and PSGL-1–binding partners. These cells express P-selectin when cultured in vitro (Fig. 5 D). We could show that CD4+ T cells of healthy controls cannot adhere to the endothelium when preincubated with natalizumab, proving the relevance of the VLA-4–VCAM-1 interaction in our system (Fig. 5 E). Interestingly, blockade of CD49d had no influence on the rolling capacity of CD4+ T cells on endothelium (Fig. 5 F). Firm adhesion to the endothelial layer was reduced, but not completely abrogated in natalizumab-treated patients and both patient and control cells showed no residual adhesion in the presence of a PSGL-1–blocking antibody (Fig. 5 G). As a final step, we could show that the rolling of CD4+ T cells over HBMECs, which was shown in the capillary system (Fig. 5 F) to be independent of CD49d, was more pronounced in LTNT patients and that this rolling could be inhibited by addition of a PSGL-1–blocking antibody (Fig. 5 H).
Figure 5.
Influence of natalizumab treatment on rolling and adhesion of CD4+ T cells to the ligands of CD49d and PSGL-1 (i.e., VCAM-1/P-selectin). (A) Adherent CD4+ T cells/field of view of a VCAM-1–coated capillary flow chamber with and without SDF-1 and with and without natalizumab (n = 4). (B) Adherent CD4+ T cells of natalizumab-treated patients and controls/field of view on a VCAM-1–coated capillary flow chamber with and without SDF-1 (n = 4). (C) Captured CD4+ T cells of healthy controls and natalizumab-treated patients/field of view on P-selectin coated capillary flow chambers (n = 4). (D) qPCR data of P-selectin expression in cultured primary human brain endothelial cells (n = 3). Adherent (E) or rolling (F) CD4+ T cells/field of view on parallel plate flow chambers coated with either noninflamed or TNF-inflamed primary endothelial cells with and without addition of natalizumab (n = 4). Adherent (G) or rolling (H) CD4+ T cells of natalizumab-treated patients and controls/field of view on parallel plate flow chambers coated with either noninflamed or TNF-inflamed primary endothelial cells with and without PSGL-1 blocking antibodies (n = 4). *, P ≤ 0.05.
TH17 cells can use myeloma cell adhesion molecule(MCAM) to adhere to endothelium independently of VCAM-1–VLA-4 interactions
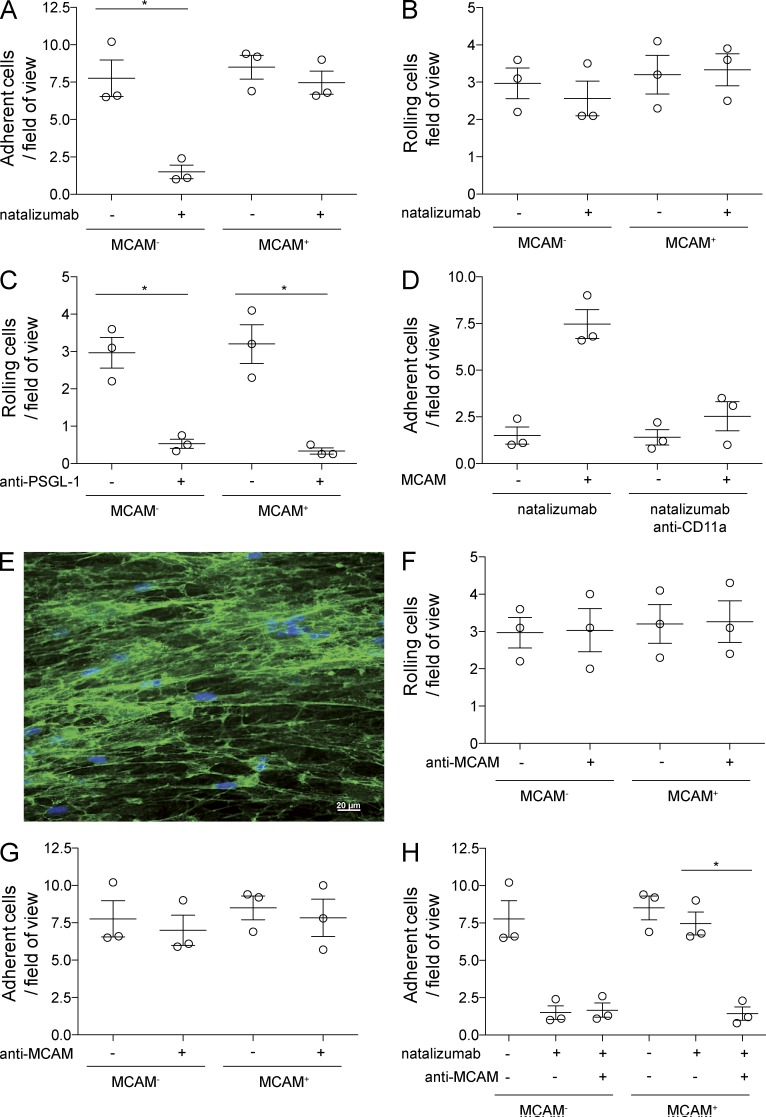
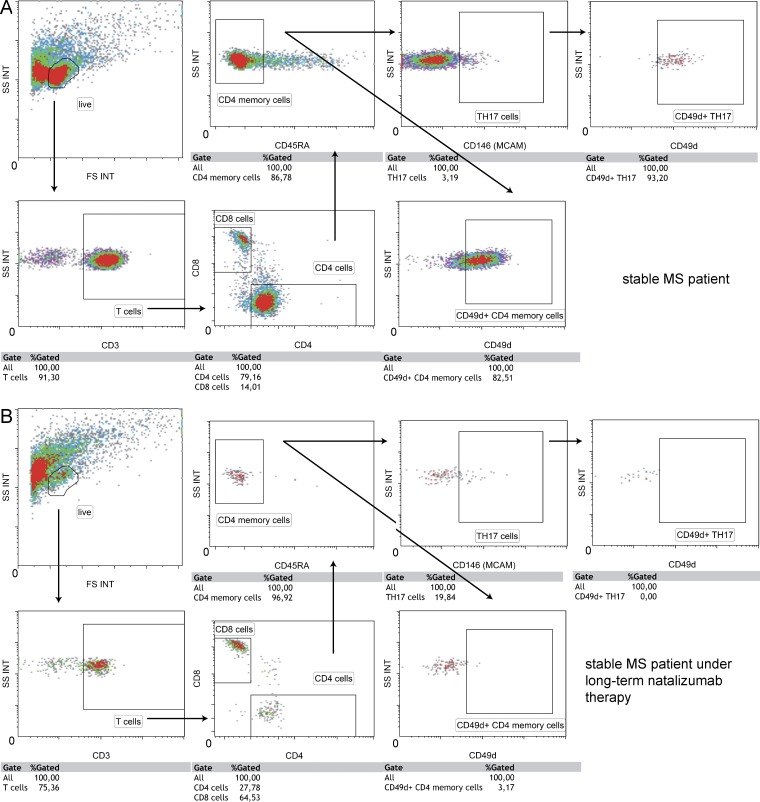
We observed residual adhesion of LTNT patients’ CD4+ T cells to HBMEC (Fig. 5 F), even though the CSF and peripheral T cells of the same patients did not express CD49d (Fig. 2, A and C). As recent studies have shown that TH17-polarized cells can induce experimental autoimmune encephalomyelitis (EAE) in CD49d-deficient mice (Rothhammer et al., 2011) and that TH17 cells use VLA-4–independent mechanisms to access the CNS during viral infection (Rothhammer et al., 2014), we next investigated whether TH17 cells could enter the CSF under LTNT conditions in the human system. The melanoma cell adhesion molecule (MCAM; CD146) was recently shown to be a highly specific extracellular marker for TH17 cells in humans (Larochelle et al., 2012; Flanagan et al., 2012), opening the possibility of functional analysis of rolling and adhesion of TH17/MCAM+ cells. By examining healthy controls’ PBMC under shear flow conditions, we could show that blockade of CD49d by natalizumab abrogated the adhesion of MCAM− memory cells (CD3+CD4+CD45RO+MCAM−) but did not affect adhesion of MCAM+ memory cells (Fig. 6 A). We had the possibility to investigate CSF-derived cells of two MS patients by flow cytometry and found that the percentage of MCAM+ cells of CD3+CD4+CD45RA− cells was much higher in a LTNT patient when compared with a stable MS patient without natalizumab treatment (19.8 vs. 3.2%), suggesting a prevalence of TH17 cells in the CSF of LTNT patients. These TH17 cells, as shown above for whole CD4+ T cells in Fig. 2 C, also did not express CD49d in LTNT patients (Fig. 7). There was no enhanced in vitro rolling of healthy controls’ MCAM+ cells and blockade of CD49d did not influence capturing (Fig. 6 B). PSGL-1 blockade abrogated rolling in both populations (Fig. 6 C). By blocking CD11a in addition to CD49d, we could reduce adhesion of TH17 cells (Fig. 6 D). Finally, we had the opportunity to block their signature molecule MCAM in vitro with an antibody developed for a potential clinical application (i.e., blocking the function of TH17 cells in vivo to interfere with autoimmune processes). Immunocytochemistry showed that laminin α4, a ligand of MCAM, was present on the cultured HBMECs (Fig. 6 E). MCAM blockade alone did not influence rolling (Fig. 6 F) or adhesion (Fig. 6 G) to HBMECs. However, the blockade of CD49d and MCAM together abrogated the adhesion of MCAM+ cells to HBMECs (Fig. 6 H).
Figure 6.
TH17 cells can use MCAM for firm adhesion to primary human brain endothelium CD4+CD45RO+MCAM+/− T cells on parallel plate flow chambers coated with TNF inflamed primary endothelial cells. (A) Adherent cells/field of view with and without addition of natalizumab (n = 3). (B) Rolling cells/field of view with and without addition of natalizumab (n = 3). (C) Rolling cells/field of view with and without blockade of PSGL-1 (n = 3). (D) Adherent cells/field of view with and without addition of natalizumab and blockade of CD11a (n = 3). (E) α4 laminin staining on cultured primary endothelium. Bar, 20 µm. (F) Rolling cells/field of view with and without blockade of MCAM (n = 3). (G) Adherent cells/field of view with and without blockade of MCAM (n = 3). (H) Adherent cells/field of view with and without addition of natalizumab and blockade of MCAM (n = 3). *, P ≤ 0.05.
Figure 7.
Long-term natalizumab-treated MS patient shows a putative enrichment of TH17 (MCAM+) cells in the CSF. Flow cytometry stainings of one stable MS patient without therapy (A) and one stable MS patient under long-term natalizumab therapy (B).
In situ detection of MCAM+ T cells in MS lesions suggests a pathogenic role of TH17 cells
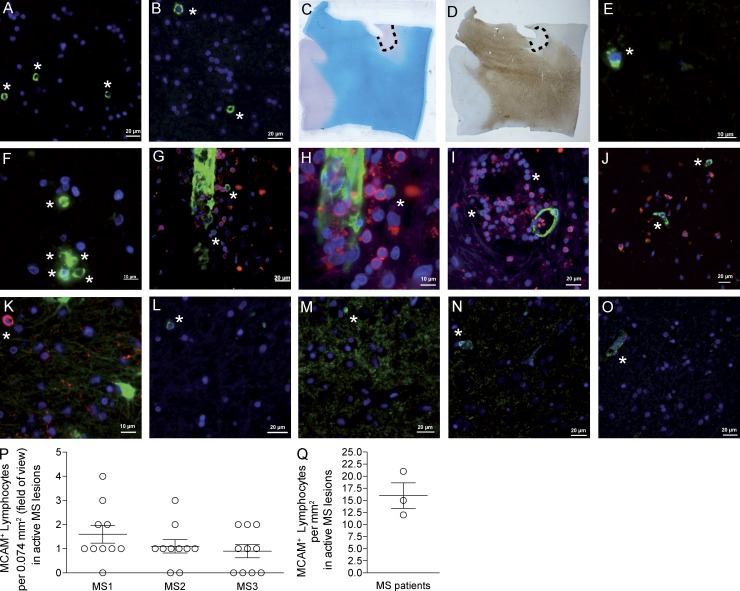
To provide support for the relevance of our observations with TH17 cells in MS pathogenesis, CNS tissue specimens of MS white matter lesions were assessed for the presence of MCAM+ lymphocytes (Fig. 8, A–F). Even though there is recent evidence for the expression of MCAM on TC17 cells (Dagur et al., 2014), the MCAM+ cells were shown to be CD8− (Fig. 8 G–I), CD68− (Fig. 8 J), and GFAP− (Fig. 8 K). MCAM+ cells could also be detected in gray matter of MS patients (Fig. 8, L–O) with isolated cells in direct apposition to blood vessel walls (Fig. 8, N and O). Quantification of MCAM+ lymphocytes resulted in an average of 16 ± 4.6 cells/mm2 in active MS white matter lesions (Fig. 8, P and Q).
Figure 8.
MCAM+ lymphocytes are found in active MS lesions. Individual examples of active white matter lesions from three MS patient autopsies. (A–F) with MCAM shown in green and DAPI shown in blue. C (LFB) and D (PLP) stainings illustrate the location of the white matter lesion shown in A+B. To exclude the possibility of MCAM+CD8+ lymphocytes, G-I show a double staining for MCAM (green) and CD8 (red) with DAPI counter staining, showing the prominent MCAM expression of endothelial cells and less intense expression on MCAM+ lymphocytes. J shows MCAM (green) and CD68 (red) with no double-positive cells, K shows MCAM (red) and GFAP (green) with no double-positive cells. L-O show examples of MCAM+ cells in gray matter from three MS patient autopsies. MCAM+ lymphocytes are marked with asterisks. P shows quantification of three MS patients’ active white matter lesion (MCAM+ lymphocytes per field of view = 0.074 mm2; n = 10 each). Q shows calculated amount of MCAM+ lymphocytes per mm2 in active white matter MS lesions (n = 3).
DISCUSSION
Our data suggest that PSGL-1 supports extravasation of CD4+ T cells into the CNS in conditions where VLA-4-mediated processes are blocked. Furthermore, we could show for the first time that TH17 cells can use MCAM together with VLA-4 to mediate adhesion to human brain endothelium. These data suggest that TH17 cells have a migratory advantage during homeostasis and early inflammation when VCAM-1 levels on endothelial cells are low, but also explain why TH17 cells can still migrate to the CNS during VLA-4 blockade.
Over time, the continuous blockade of CD49d was associated with a strong and persistent up-regulation of PSGL-1 on peripheral T cells. As there was no transcriptional connection between CD49d and PSGL-1, it is tempting to speculate that this up-regulation might be a selection process, favoring T cells with higher PSGL-1 expression over PSGL-1 low-expressers over time. The fact that both VLA-4 and PSGL-1 are important for early steps of extravasation (Kerfoot et al., 2006) might suggest the PSGL-1 up-regulation is a compensatory mechanism of T cells during the treatment with natalizumab. We used two models of cell interaction under shear-flow conditions to assess the functional relevance of our observations: coated glass capillaries were used to answer questions of specific molecular interactions (Zarbock et al., 2007), whereas primary HBMECs were used to more closely mimic the in vivo setting (Schwab et al., 2012a; adapted to shear-flow conditions). We could show that HBMECs expressed functionally relevant amounts of P-selectin in vitro, allowing us to assess both VLA-4 and PSGL-1–mediated effects in our model. The shear-flow experiments showed that a chemokine-triggered CD49d/VCAM-1 interaction is mandatory for the adhesion of CD4+ T cells in healthy controls, confirming previous studies (Campbell et al., 1998; Bauer et al., 2009). The PSGL-1 up-regulation associated with natalizumab treatment enhances the ability of patients’ CD4+ T cells to roll over P-selectin. As expected, natalizumab treatment in vitro blocks firm adhesion to inflamed endothelial cells, but did not interfere with rolling, proving that this step in the migration cascade does not involve VLA-4, which has previously only been shown in the murine system (Coisne et al., 2009). The fact that inflammation is necessary even though there is expression of VCAM-1 on resting endothelium is consistent with murine findings that the endothelium needs to secrete and present inflammation-induced chemokines (e.g., SDF-1) for successful CD49d/VCAM-1 interactions (Piccio et al., 2002). Blockade of PSGL-1 completely abrogates subsequent firm adhesion, suggesting that PSGL-1–mediated rolling is a prerequisite for any (integrin-mediated) firm adhesion in our system. Lastly, patients’ CD4+ T cells show functional enhancement of PSGL-1–mediated rolling, consistent with our hypothesis that PSGL-1 up-regulation is a compensatory mechanism, ultimately allowing for an enhanced migration of immune cells expressing alternative integrins other than CD49d to subsequently adhere firmly to the endothelium.
Although the inverted CD4/CD8 ratio in the cellular CSF compartment could be expected in our patient cohort (Stüve et al., 2006a), the surprisingly high percentage of CSF monocytes shows that these cells are potentially not dependent on VLA-4–VCAM-1 interactions to enter the CSF and might even be considered immunoregulatory, as they are also present in healthy CSF material. Accordingly, the CSF T cell repertoire in LTNT patients was shifted toward EM cells, similar to the healthy CSF composition, but showed a reversed EM/CM ratio when compared with RRMS patients without natalizumab treatment. Together with published data (Stüve et al., 2006b) and our own observations that the CSF T cell count is strongly reduced in natalizumab-treated patients, one might speculate that CSF entry of CM cells is specifically blocked by natalizumab, whereas (some) EM cells are still able to migrate into the CSF space. As both EM and CM cells express CD49d to similarly high amounts (unpublished data), this might suggest that there is at least one subset of EM cells, which does not require CD49d to cross into the CSF. Also consistent with this theory is the observation that CSF T cells completely lack CD49d expression in LTNT patients; this is a specific observation for LTNT, as CSF T cells are usually exclusively memory cells with very high expression levels of CD49d (Barrau et al., 2000; and unpublished data).
Histological stainings of autopsy tissue showed that there is ubiquitous but varying degrees of expression of VCAM-1 on endothelial barriers in vivo, whereas P-selectin is only selectively expressed. There was no P-selectin expression on vessels in the white matter, very weak expression on meningeal vessels, and strong expression on the endothelium of choroid plexus vessels. This could suggest that cells with an enhanced PSGL-1 expression would selectively migrate over the choroid plexus and not via the deep tissue postcapillary venules. Potentially, TH17 cells may also migrate over the leptomeningeal barrier or even the deep vessels in the parenchyma, where the higher PSGL-1 expression in natalizumab-treated individuals might be sufficient to support successful PSGL-1/P-selectin interactions even in the case of low P-selectin expression. This would be consistent with our hypothesis that in the human system specific immune subpopulations such as monocytes or T cells with a specific EM phenotype (e.g., TH17 cells) are able to use a CD49d-independent mechanism to migrate into the CNS, or more specifically, the CSF space. It seems reasonable that if this process is dependent on PSGL-1–mediated rolling over P-selectin expressing endothelium, cells that express more PSGL-1 have a migratory advantage in the migration over the choroid plexus into the CSF space.
It has previously been shown in animal studies that some subpopulations of T cells (i.e., TH17 cells) do not need CD49d to specifically migrate over the choroid plexus into the CSF space, but do depend on LFA-1–ICAM-1 interactions (Rothhammer et al., 2011, 2014). Our study supports the relevance of these findings in the human system as MCAM+ memory cells (TH17 cells; Flanagan et al., 2012; Larochelle et al., 2012) could still adhere to endothelium under CD49d-blocking conditions. An additional blockade of LFA-1 could abrogate this adhesion, in accordance with previously published data, suggesting that LFA-1 is important for the CNS infiltration of all T cells (Rothhammer et al., 2011). Interestingly, the blockade of both VLA-4 and MCAM impaired adhesion of MCAM+ cells, suggesting that TH17 cells use both VLA-4 and their signature molecule MCAM to mediate firm adhesion to endothelium, introducing MCAM as part of the migratory cascade into target tissue, specifically for TH17 cells. The relative timing and contribution of MCAM versus LFA-1 and α-4 integrin to the adhesion cascade has not yet been established. MCAM binds to a laminin isoform produced by endothelial cells (laminin α4), and is part of the subendothelial basement membrane. However, the binding partner on the endothelial side remains unclear, as there is no evidence yet of laminin α4 expression on the luminal surface of endothelial cells in vivo. Binding to MCAM on TH17 cells could therefore be facilitated by laminin α4, homophilic MCAM interaction (Wang and Yan, 2013), or a yet unknown binding partner. We could show a mean of 16 ± 4.6 MCAM+ lymphocytes/mm2 in active white matter MS lesions, which is in accordance with the seminal work of Lucchinetti et al. (2000), who counted between 133 and 145 T cells/mm2 in pattern II/III lesions, therefore suggesting that between 11 and 12% of infiltrating T cells in these MS lesions might be TH17/MCAM+ cells. This would also be in agreement with data from Larochelle et al. (2012) who could show that >8% of CD4+CD45RO+ cells express MCAM in CSF specimens of MS patients.
It is clear that TH17 cells have additional adhesive mechanisms that permit CNS entry even in the presence of natalizumab. LFA-1 is likely to play an important role, consistent with the findings of Rothhammer et al. (Rothhammer et al., 2011) in EAE using conditional α4 integrin knockout mice and potentially also MCAM/laminin, which is consistent with the results from Flanagan et al. (2012) and (Larochelle et al. (2012). We hypothesize that the cells that are still able to enter the CSF in natalizumab-treated patients are most likely to be TH17 cells, suggesting a role for this population not only in pathology (Kebir et al., 2007) but also in CNS immune surveillance (Axtell and Steinman, 2009). Alternatively, the presence of TH17 cells alone without the presence of TH1 cells may not be sufficient to mediate pathology in MS, unlike in EAE where TH17 cells have been shown to induce EAE symptoms (Rothhammer et al., 2011). This could be the reason why some patients exhibit strong relapses after cessation of natalizumab treatment (Melis et al., 2013), as TH1 cells can again gain entry into the CNS and the TH17 cells, which are already in situ, could exacerbate tissue damage and clinical symptoms. This dataset suggests that a blockade of VLA-4 and MCAM together might be an even stronger treatment than VLA-4 blockade alone or could be an efficient treatment alternative in cases where the blockade of TH1 migration is insufficient to alleviate clinical symptoms (e.g., cases with a strong TH17-biased presentation such as neuromyelitis optica; Varrin-Doyer et al., 2012; Kieseier et al., 2013). One more speculative point concerns progression and gray matter atrophy in MS: our data are consistent with the hypothesis that TH17 cells might mediate residual progression under natalizumab, as we also find these cells in gray matter and their entry is not blocked by the treatment. If that holds true in further studies, there is a possibility that progression in secondary progressive MS and primary progressive MS might also have a TH17 component and that a combined blockade of VLA-4 and MCAM could turn out to be a valid treatment option for these diseases. Collectively, our data concerning lymphocyte trafficking into the human CNS under VLA-4 blockade provide an explanation for “compensatory” mechanisms of specific immune cell subsets and suggest preferred trafficking routes. This could have implications for the immune surveillance under homeostatic, as well as inflammatory conditions and once again underlines the importance of the blood–CSF barrier of the choroid plexus in the context of immune surveillance and pathology.
MATERIALS AND METHODS
Patients and healthy controls.
381 patients with the diagnosis of clinically definite active RRMS according to the 2005 revised McDonald diagnostic criteria (Polman et al., 2005) were enrolled in this study. These MS patients had been treated continuously with natalizumab for up to 80 mo and were stable by assessment of clinical and MRI parameters. 21 patients in this cohort underwent analysis of CSF in parallel to assessment of peripheral blood. 39 age- and sex-matched healthy donors (HD) with no previous history of neurological or immune-mediated diseases served as controls. Furthermore, 49 natalizumab-naive MS patients, 32 patients with noninflammatory neurological diseases, and 14 patients without neurological diseases served as controls.
The study was approved by the local ethics committee (Ethik-Kommission der medizinischen Fakultät der Universität Würzburg, registration number 155/06; Ethik-Kommission der Ärztekammer Westfalen-Lippe und der Medizinischen Fakultät der Westfälischen Wilhelms-Universität, registration number: 2010–245-f-S) and informed written consent was obtained from all participants. This study was performed according to the Declaration of Helsinki.
Biomaterials and flow cytometry.
PBMCs were isolated by density gradient centrifugation using lymphocyte separation medium (PAA Laboratories) as previously described (Schwab et al., 2010). Flow cytometry analysis of CSF was performed as described previously (Schwab et al., 2012a). Ex vivo isolated or cultured cells were washed with PBS supplemented with 0.1% BSA and stained with fluorescence-labeled mAbs together with blocking mouse IgG (Sigma-Aldrich) at 4°C for 30 min. Cells then were measured on a FACSCalibur (BD) and Gallios as well as Navios Flow Cytometer (Beckman Coulter) and analyzed using FlowJo (Tree Star) and Kaluza (Beckman Coulter) software. mAbs used in the study are shown in Table S2. The flow cytometry anti-CD49d mAb (clone 9F10) enables a simultaneous labeling of CD49d and bound natalizumab (secondarily labeled with IgG, data not shown).
Immunohistochemistry.
For histochemical studies, 4-µm-thick formalin fixed paraffin embedded (FFPE) tissues sections from three MS patients and three patients without inflammatory demyelinating CNS disease were stained with luxol fast blue for myelin integrity and H&E for inflammatory infiltrates. For immunohistochemical studies, the antibodies used were anti-CD106 (VCAM1, mouse-IgG1, 1.4C3; Acris), CD62P (P-selectin, mouse IgG2a, 1E3; Santa Cruz Biotechnology, Inc.), and PLP (Proteo lipid protein, mouse-IgG2a, plpc1; AbD Serotec). The secondary antibody (EnVision+ Dual Link System-HRP; DAKO) was HRP conjugated and diaminobenzidine (DAKO) was used as a chromogenic substrate. Antigen retrieval was done using EnVision FLEX Target Retrieval Solution, Low pH (DAKO), and endogenous peroxidase activity was blocked with 3% hydrogen peroxide in methanol. For immunofluorescence studies, the primary antibodies used were CD146 (MCAM, rabbit monoclonal IgG, EPR3208; Millipore), CD8 (mouse monoclonal, IgG1, 144B; Abcam), rabbit anti-laminin α4 (Ringelmann et al., 1999), GFAP (mouse monoclonal IgG1, 131-17719; Invitrogen) and CD68 (mouse monoclonal IgG3k, PG-M1; Dako). The secondary antibodies used were goat anti–rabbit-HRP (IgG; Life Technologies), donkey anti–mouse-568 (IgG; Invitrogen), and streptavidin-488 (Invitrogen). For staining MCAM, amplification with TSA Plus Biotin kit was done according to the manufacturers instruction. In total, three MS patients and three controls were used for the staining.
Blocking antibodies for flow chamber experiments.
The blocking antibodies natalizumab (anti-CD49d, Tysabri; Biogen Idec), anti-PSGL-1 (KPL1; Santa Cruz Biotechnology, Inc.), and anti-MCAM (clone 2107; Prothena) were used at a concentration of 10 µg/ml. Lymphocytes were preincubated for 30 min in PBS before washing out of the blocking antibodies and application to the flow chamber experiments.
Capillary flow chamber.
To investigate leukocyte adhesion, we used a human flow chamber system. In brief, glass capillaries were coated with P-selectin (20 µg/ml; R&D Systems) and VCAM-1 (20 µg/ml; R&D Systems) or P-selectin (20 µg/ml; R&D Systems), VCAM-1 (20 µg/ml; R&D Systems) and SDF1 (20 µg/ml; R&D Systems) for 2 h. Chambers were blocked with 1% casein (Thermo Fisher Scientific) for 1 h and afterward perfused with isolated CD4 cells at a constant shear stress of 1 dyne/cm2. To investigate the capturing of CD4 cells, flow chambers were coated with P-selectin (20 µg/ml) alone and were perfused with isolated CD4 cells at a constant shear stress of 1 dyne/cm2 for 2 min and, subsequently, the number of rolling cells per field of view was determined. Videos were obtained with an inverted TS100 transmission light microscope (Nikon) equipped with a 10×/0.25 NA objective and a digital camera (Pixelfly; Cooke Corporation).
Quantitative real-time PCR.
PCR has been performed as described previously (Breuer et al., 2014). In short, RNA was extracted from primary human brain microvascular endothelial cells using TRIzol reagent (Invitrogen). cDNA was synthesized from 1 µg of total RNA using a standard protocol with random hexamer primers (Thermo Fisher Scientific). Real-time quantitative PCR (qPCR) was performed in a StepOnePlus cycler (Applied Biosystems) using endogen control primers for 18sRNA as well as TaqMan Gene Expression Assays specific for human P-selectin (Applied Biosystems).
Parallel plate flow chamber.
Primary human brain microvascular endothelial cells (HBMEC) were purchased from ScienCell Research Laboratories and grown to confluence on 35-mm culture dishes. If indicated, HBMECs were stimulated with TNF (10 nM) for 16 h. Human CD4+ T cells were counted and resuspended in perfusion buffer (Medium 199 supplemented with FCS and Hepes). Cells were perfused through the parallel plate flow chamber (Glykotech) at a constant shear stress of 0.25 dyne/cm2 for 8 min. Cells were observed using an inverted microscope (Nikon TS-100) equipped with a 10×/0.25 NA objective and a digital camera. Videos were recorded using CamWare software (PCO Ag) and rolling and adherent cells per field of view were analyzed.
Statistical analysis.
Statistical significance of differences between two groups was determined using unpaired Student’s t test except for comparisons between peripheral blood and CSF of the same patient, where the paired Student’s t test was used. Differences were considered statistically significant with the following p-values: *, P < 0.05; **, P < 0.01; and ***, P < 0.001. Software for statistical and correlation assessment was Prism 5 (GraphPad).
Acknowledgments
We would like to thank Theresa Henninger (Department of Neurology, Würzburg), Daniela Roosterman, Barbara Wrobel, Julia Schlingmann, and Verena Schütte (all from Department of Neurology, Münster) for excellent technical assistance. Importantly, we thank the donors for their cooperation. We thank Prothena for providing us with the anti–human MCAM antibody. We also thank the Netherlands Brain Bank for providing human MS tissue material for histology and Heike Blum for help with the illustrations.
This study was funded by the Deutsche Forschungsgesellschaft (DFG) grant CRC128/B1 to H. Wiendl and N. Schwab, CRC128/Z2 to H. Wiendl, and CRC128/Z1 to T. Kuhlmann, ZA428/6-1 to A. Zarbock, SFB 1009/A3 to H. Wiendl, SFB 1009/A5 to A. Zarbock, DFG EXC 1003 Cells in Motion - Cluster of Excellence (Project B, Cells in barriers), Münster, Germany to H. Wiendl, DFG_GR3946_1_1 to C.C. Gross, and the EU (BEST-MS, FP7, 305477) to H. Wiendl, as well as the German Ministry for Health and Education “German competence network of MS” (KKNMS), project B1 “Pharmacovigilance Study Natalizumab” to H. Wiendl.
Ken Flanagan is an employee of Prothena Corporation. The authors have no additional competing financial interests.
Footnotes
Abbreviations used:
- CM
- central-memory
- CSF
- cerebrospinal fluid
- EAE
- experimental autoimmune encephalomyelitis
- EM
- effector-memory
- FFPE
- formalin-fixed, paraffin-embedded
- HBMEC
- human brain microvascular endothelial cell
- MCAM
- myeloma cell adhesion molecule
- MFI
- mean fluorescence intensity
- MS
- multiple sclerosis
- PLP
- proteolipid protein
- RRMS
- relapsing-remitting multiple sclerosis
- VCAM-1
- vascular cell adhesion molecule-1
- VLA-4
- very late antigen 4
References
- Alvarez, J.I., Katayama T., and Prat A.. 2013. Glial influence on the blood brain barrier. Glia. 61:1939–1958 10.1002/glia.22575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Axtell, R.C., and Steinman L.. 2009. Gaining entry to an uninflamed brain. Nat. Immunol. 10:453–455 10.1038/ni0509-453 [DOI] [PubMed] [Google Scholar]
- Barrau, M.A., Montalban X., Sáez-Torres I., Brieva L., Barberà N., and Martínez-Cáceres E.M.. 2000. CD4(+)CD45RO(+)CD49d(high) cells are involved in the pathogenesis of relapsing-remitting multiple sclerosis. J. Neuroimmunol. 111:215–223 10.1016/S0165-5728(00)00357-X [DOI] [PubMed] [Google Scholar]
- Bauer, M., Brakebusch C., Coisne C., Sixt M., Wekerle H., Engelhardt B., and Fässler R.. 2009. Beta1 integrins differentially control extravasation of inflammatory cell subsets into the CNS during autoimmunity. Proc. Natl. Acad. Sci. USA. 106:1920–1925 10.1073/pnas.0808909106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloomgren, G., Richman S., Hotermans C., Subramanyam M., Goelz S., Natarajan A., Lee S., Plavina T., Scanlon J.V., Sandrock A., and Bozic C.. 2012. Risk of natalizumab-associated progressive multifocal leukoencephalopathy. N. Engl. J. Med. 366:1870–1880 10.1056/NEJMoa1107829 [DOI] [PubMed] [Google Scholar]
- Breuer, J., Schwab N., Schneider-Hohendorf T., Marziniak M., Mohan H., Bhatia U., Gross C.C., Clausen B.E., Weishaupt C., Luger T.A., et al. 2014. Ultraviolet B light attenuates the systemic immune response in central nervous system autoimmunity. Ann. Neurol. 75:739–758 10.1002/ana.24165 [DOI] [PubMed] [Google Scholar]
- Campbell, J.J., Hedrick J., Zlotnik A., Siani M.A., Thompson D.A., and Butcher E.C.. 1998. Chemokines and the arrest of lymphocytes rolling under flow conditions. Science. 279:381–384 10.1126/science.279.5349.381 [DOI] [PubMed] [Google Scholar]
- Coisne, C., Mao W., and Engelhardt B.. 2009. Cutting edge: Natalizumab blocks adhesion but not initial contact of human T cells to the blood-brain barrier in vivo in an animal model of multiple sclerosis. J. Immunol. 182:5909–5913 10.4049/jimmunol.0803418 [DOI] [PubMed] [Google Scholar]
- Dagur, P.K., Biancotto A., Stansky E., Sen H.N., Nussenblatt R.B., and McCoy J.P.. 2014. Secretion of interleukin-17 by CD8+ T cells expressing CD146 (MCAM). Clin. Immunol. 152:36–47 10.1016/j.clim.2014.01.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubertret, L., Sterry W., Bos J.D., Chimenti S., Shumack S., Larsen C.G., Shear N.H., and Papp K.A.. CLEAR Multinational Study Group. 2006. CLinical experience acquired with the efalizumab (Raptiva) (CLEAR) trial in patients with moderate-to-severe plaque psoriasis: results from a phase III international randomized, placebo-controlled trial. Br. J. Dermatol. 155:170–181 10.1111/j.1365-2133.2006.07344.x [DOI] [PubMed] [Google Scholar]
- Engelhardt, B., and Ransohoff R.M.. 2012. Capture, crawl, cross: the T cell code to breach the blood-brain barriers. Trends Immunol. 33:579–589 10.1016/j.it.2012.07.004 [DOI] [PubMed] [Google Scholar]
- Engelhardt, B., and Sorokin L.. 2009. The blood-brain and the blood-cerebrospinal fluid barriers: function and dysfunction. Semin. Immunopathol. 31:497–511 10.1007/s00281-009-0177-0 [DOI] [PubMed] [Google Scholar]
- Flanagan, K., Fitzgerald K., Baker J., Regnstrom K., Gardai S., Bard F., Mocci S., Seto P., You M., Larochelle C., et al. 2012. Laminin-411 is a vascular ligand for MCAM and facilitates TH17 cell entry into the CNS. PLoS ONE. 7:e40443 10.1371/journal.pone.0040443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flügel, A., Schläger C., Lühder F., and Odoardi F.. 2011. Autoimmune disease in the brain—how to spot the culprits and how to keep them in check. J. Neurol. Sci. 311:S3–S11 [DOI] [PubMed] [Google Scholar]
- Hooks, M.A., Wade C.S., and Millikan W.J. Jr. 1991. Muromonab CD-3: a review of its pharmacology, pharmacokinetics, and clinical use in transplantation. Pharmacotherapy. 11:26–37 [PubMed] [Google Scholar]
- Huang, Y.-H., Zozulya A.L., Weidenfeller C., Metz I., Buck D., Toyka K.V., Brück W., and Wiendl H.. 2009. Specific central nervous system recruitment of HLA-G(+) regulatory T cells in multiple sclerosis. Ann. Neurol. 66:171–183 10.1002/ana.21705 [DOI] [PubMed] [Google Scholar]
- Kebir, H., Kreymborg K., Ifergan I., Dodelet-Devillers A., Cayrol R., Bernard M., Giuliani F., Arbour N., Becher B., and Prat A.. 2007. Human TH17 lymphocytes promote blood-brain barrier disruption and central nervous system inflammation. Nat. Med. 13:1173–1175 10.1038/nm1651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerfoot, S.M., Norman M.U., Lapointe B.M., Bonder C.S., Zbytnuik L., and Kubes P.. 2006. Reevaluation of P-selectin and alpha 4 integrin as targets for the treatment of experimental autoimmune encephalomyelitis. J. Immunol. 176:6225–6234 10.4049/jimmunol.176.10.6225 [DOI] [PubMed] [Google Scholar]
- Kieseier, B.C., Stüve O., Dehmel T., Goebels N., Leussink V.I., Mausberg A.K., Ringelstein M., Turowski B., Aktas O., Antoch G., and Hartung H.-P.. 2013. Disease amelioration with tocilizumab in a treatment-resistant patient with neuromyelitis optica: implication for cellular immune responses. JAMA Neurol. 70:390–393 10.1001/jamaneurol.2013.668 [DOI] [PubMed] [Google Scholar]
- Kivisäkk, P., Healy B.C., Viglietta V., Quintana F.J., Hootstein M.A., Weiner H.L., and Khoury S.J.. 2009. Natalizumab treatment is associated with peripheral sequestration of proinflammatory T cells. Neurology. 72:1922–1930 10.1212/WNL.0b013e3181a8266f [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larochelle, C., Alvarez J.I., and Prat A.. 2011. How do immune cells overcome the blood-brain barrier in multiple sclerosis? FEBS Lett. 585:3770–3780 10.1016/j.febslet.2011.04.066 [DOI] [PubMed] [Google Scholar]
- Larochelle, C., Cayrol R., Kebir H., Alvarez J.I., Lécuyer M.-A., Ifergan I., Viel É., Bourbonnière L., Beauseigle D., Terouz S., et al. 2012. Melanoma cell adhesion molecule identifies encephalitogenic T lymphocytes and promotes their recruitment to the central nervous system. Brain. 135:2906–2924 10.1093/brain/aws212 [DOI] [PubMed] [Google Scholar]
- Lucchinetti, C., Brück W., Parisi J., Scheithauer B., Rodriguez M., and Lassmann H.. 2000. Heterogeneity of multiple sclerosis lesions: implications for the pathogenesis of demyelination. Ann. Neurol. 47:707–717 [DOI] [PubMed] [Google Scholar]
- Melis, M., Cocco E., Frau J., Lorefice L., Fenu G., Coghe G., Mura M., and Marrosu M.G.. 2013. Post-natalizumab clinical and radiological findings in a cohort of multiple sclerosis patients: 12-month follow-up. Neurol. Sci. 35:401–408 [DOI] [PubMed] [Google Scholar]
- Piccio, L., Rossi B., Scarpini E., Laudanna C., Giagulli C., Issekutz A.C., Vestweber D., Butcher E.C., and Constantin G.. 2002. Molecular mechanisms involved in lymphocyte recruitment in inflamed brain microvessels: critical roles for P-selectin glycoprotein ligand-1 and heterotrimeric G(i)-linked receptors. J. Immunol. 168:1940–1949 10.4049/jimmunol.168.4.1940 [DOI] [PubMed] [Google Scholar]
- Polman, C.H., Reingold S.C., Edan G., Filippi M., Hartung H.-P., Kappos L., Lublin F.D., Metz L.M., McFarland H.F., O’Connor P.W., et al. 2005. Diagnostic criteria for multiple sclerosis: 2005 revisions to the “McDonald Criteria”. Ann. Neurol. 58:840–846 10.1002/ana.20703 [DOI] [PubMed] [Google Scholar]
- Polman, C.H., O’Connor P.W., Havrdova E., Hutchinson M., Kappos L., Miller D.H., Phillips J.T., Lublin F.D., Giovannoni G., Wajgt A., et al. AFFIRM Investigators. 2006. A randomized, placebo-controlled trial of natalizumab for relapsing multiple sclerosis. N. Engl. J. Med. 354:899–910 10.1056/NEJMoa044397 [DOI] [PubMed] [Google Scholar]
- Ringelmann, B., Röder C., Hallmann R., Maley M., Davies M., Grounds M., and Sorokin L.. 1999. Expression of laminin alpha1, alpha2, alpha4, and alpha5 chains, fibronectin, and tenascin-C in skeletal muscle of dystrophic 129ReJ dy/dy mice. Exp. Cell Res. 246:165–182 10.1006/excr.1998.4244 [DOI] [PubMed] [Google Scholar]
- Rothhammer, V., Heink S., Petermann F., Srivastava R., Claussen M.C., Hemmer B., and Korn T.. 2011. Th17 lymphocytes traffic to the central nervous system independently of α4 integrin expression during EAE. J. Exp. Med. 208:2465–2476 10.1084/jem.20110434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothhammer, V., Muschaweckh A., Gasteiger G., Petermann F., Heink S., Busch D.H., Heikenwälder M., Hemmer B., Drexler I., and Korn T.. 2014. α4-integrins control viral meningoencephalitis through differential recruitment of T helper cell subsets. Acta Neuropathol Commun. 2:27 10.1186/2051-5960-2-27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saghafi, H., Rahbar K., Nobakht Haghighi A., Qoreishi M., and Safdari F.. 2012. Efficacy of anti-interleukin-2 receptor antibody (daclizumab) in reducing the incidence of acute rejection after renal transplantation. Nephrourol Mon. 4:475–477 10.5812/numonthly.1806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider-Hohendorf, T., Stenner M.-P., Weidenfeller C., Zozulya A.L., Simon O.J., Schwab N., and Wiendl H.. 2010. Regulatory T cells exhibit enhanced migratory characteristics, a feature impaired in patients with multiple sclerosis. Eur. J. Immunol. 40:3581–3590 10.1002/eji.201040558 [DOI] [PubMed] [Google Scholar]
- Schwab, N., Zozulya A.L., Kieseier B.C., Toyka K.V., and Wiendl H.. 2010. An imbalance of two functionally and phenotypically different subsets of plasmacytoid dendritic cells characterizes the dysfunctional immune regulation in multiple sclerosis. J. Immunol. 184:5368–5374 10.4049/jimmunol.0903662 [DOI] [PubMed] [Google Scholar]
- Schwab, N., Ulzheimer J.C., Fox R.J., Schneider-Hohendorf T., Kieseier B.C., Monoranu C.M., Staugaitis S.M., Welch W., Jilek S., Du Pasquier R.A., et al. 2012a. Fatal PML associated with efalizumab therapy: insights into integrin αLβ2 in JC virus control. Neurology. 78:458–467, discussion:465 10.1212/WNL.0b013e3182478d4b [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwab, N., Höhn K.G., Schneider-Hohendorf T., Metz I., Stenner M.-P., Jilek S., Du Pasquier R.A., Gold R., Meuth S.G., Ransohoff R.M., et al. 2012b. Immunological and clinical consequences of treating a patient with natalizumab. Mult. Scler. 18:335–344 10.1177/1352458511421919 [DOI] [PubMed] [Google Scholar]
- Steinman, L.2014. Immunology of relapse and remission in multiple sclerosis. Annu. Rev. Immunol. 32:257–281 10.1146/annurev-immunol-032713-120227 [DOI] [PubMed] [Google Scholar]
- Stenner, M.-P., Waschbisch A., Buck D., Doerck S., Einsele H., Toyka K.V., and Wiendl H.. 2008. Effects of natalizumab treatment on Foxp3+ T regulatory cells. PLoS ONE. 3:e3319 10.1371/journal.pone.0003319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stüve, O., and Wiendl H.. 2009. Iatrogenic immunosuppression with biologics in MS: expecting the unexpected? Neurology. 73:1346–1347 10.1212/WNL.0b013e3181beed38 [DOI] [PubMed] [Google Scholar]
- Stüve, O., Marra C.M., Bar-Or A., Niino M., Cravens P.D., Cepok S., Frohman E.M., Phillips J.T., Arendt G., Jerome K.R., et al. 2006a. Altered CD4+/CD8+ T-cell ratios in cerebrospinal fluid of natalizumab-treated patients with multiple sclerosis. Arch. Neurol. 63:1383–1387 10.1001/archneur.63.10.1383 [DOI] [PubMed] [Google Scholar]
- Stüve, O., Marra C.M., Jerome K.R., Cook L., Cravens P.D., Cepok S., Frohman E.M., Phillips J.T., Arendt G., Hemmer B., et al. 2006b. Immune surveillance in multiple sclerosis patients treated with natalizumab. Ann. Neurol. 59:743–747 10.1002/ana.20858 [DOI] [PubMed] [Google Scholar]
- Varrin-Doyer, M., Spencer C.M., Schulze-Topphoff U., Nelson P.A., Stroud R.M., Cree B.A.C., and Zamvil S.S.. 2012. Aquaporin 4-specific T cells in neuromyelitis optica exhibit a Th17 bias and recognize Clostridium ABC transporter. Ann. Neurol. 72:53–64 10.1002/ana.23651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, Z., and Yan X.. 2013. CD146, a multi-functional molecule beyond adhesion. Cancer Lett. 330:150–162 10.1016/j.canlet.2012.11.049 [DOI] [PubMed] [Google Scholar]
- Wilson, E.H., Weninger W., and Hunter C.A.. 2010. Trafficking of immune cells in the central nervous system. J. Clin. Invest. 120:1368–1379 10.1172/JCI41911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yednock, T.A., Cannon C., Fritz L.C., Sanchez-Madrid F., Steinman L., and Karin N.. 1992. Prevention of experimental autoimmune encephalomyelitis by antibodies against alpha 4 beta 1 integrin. Nature. 356:63–66 10.1038/356063a0 [DOI] [PubMed] [Google Scholar]
- Zarbock, A., Lowell C.A., and Ley K.. 2007. Spleen tyrosine kinase Syk is necessary for E-selectin-induced alpha(L)beta(2) integrin-mediated rolling on intercellular adhesion molecule-1. Immunity. 26:773–783 10.1016/j.immuni.2007.04.011 [DOI] [PMC free article] [PubMed] [Google Scholar]