A novel site-targeted murine complement inhibitor, CR2-CD59, specifically inhibits the terminal membrane attack complex. This inhibitor dissects the complement pathway to protect against liver injury while promoting regeneration in mouse models of liver resection and acute liver failure.
Abstract
Liver resection is commonly performed under ischemic conditions, resulting in two types of insult to the remnant liver: ischemia reperfusion injury (IRI) and loss of liver mass. Complement inhibition is recognized as a potential therapeutic modality for IRI, but early complement activation products are also essential for liver regeneration. We describe a novel site-targeted murine complement inhibitor, CR2-CD59, which specifically inhibits the terminal membrane attack complex (MAC), and we use this protein to investigate the complement-dependent balance between liver injury and regeneration in a clinical setting of pharmacological inhibition. CR2-CD59 did not impact in vivo generation of C3 and C5 activation products but was as effective as the C3 activation inhibitor CR2-Crry at ameliorating hepatic IRI, indicating that the MAC is the principle mediator of hepatic IRI. Furthermore, unlike C3 or C5 inhibition, CR2-CD59 was not only protective but significantly enhanced hepatocyte proliferation after partial hepatectomy, including when combined with ischemia and reperfusion. Remarkably, CR2-CD59 also enhanced regeneration after 90% hepatectomy and improved long-term survival from 0 to 70%. CR2-CD59 functioned by increasing hepatic TNF and IL-6 levels with associated STAT3 and Akt activation, and by preventing mitochondrial depolarization and allowing recovery of ATP stores.
Liver resection is an essential component of treatment for many patients with primary or secondary liver malignancies, but there is a finite amount of liver that can be removed (∼70%) to avoid deficient regeneration and liver dysfunction (Helling, 2006; Breitenstein et al., 2009; Garcea and Maddern, 2009). Liver regeneration is also important for both donors and recipients of small-for-size liver grafts, a type of surgery which could significantly increase the donor pool but which is not widely performed primarily due to concerns of morbidity and mortality in donors (Clavien et al., 2007). Other than utilization of liver support systems, there is currently no therapy for patients with a failing remnant or small-for-size liver, and there is a significant need for strategies that can enhance the regenerative capacity of livers and increase the amount of liver that can be safely resected.
Impaired liver regeneration is associated with the extent of ischemia reperfusion injury (IRI), an unavoidable component of transplantation surgery and a component of most liver resection surgeries. Thus, ameliorating postsurgical hepatic IRI may enhance the regenerative capacity of the liver. Although currently there is no approved treatment for IRI, complement inhibition is recognized as a potential therapeutic strategy for reducing IRI (Diepenhorst et al., 2009) because complement plays a key role in post-ischemic inflammation and injury. However, complement activation products also play a critical role in liver regeneration (Mastellos et al., 2001; Strey et al., 2003; Markiewski et al., 2009), and complement inhibition would therefore appear to be contraindicative for surgery where liver regeneration is a component of recovery (He et al., 2009).
Activation of complement leads to the sequential production of the effector molecules C3a, C5a, and the membrane attack complex (MAC). C3a and C5a are soluble bioactive peptides that are cleaved from their parent proteins by enzymatic convertases, and the MAC is a terminal cytolytic protein complex assembled in cell membranes after cleavage of C5. The complement activation products C3a and/or C5a are essential for liver regeneration via their effect on cell signaling processes involved in hepatocyte proliferation (Strey et al., 2003; Markiewski et al., 2009), but a role for the MAC in liver regeneration has not been previously investigated. The precise role of complement in hepatic IRI is also not clear, with both C5a and the MAC being implicated in causing injury; deficiency of CD59 (MAC inhibitor) in mice exacerbates IRI (Zhang et al., 2011), and deficiency of C6 (MAC protein) in rats ameliorates IRI (Fondevila et al., 2008), whereas C5a receptor antagonism has also been shown to protect against hepatic IRI in rats (Arumugam et al., 2004).
Here, we describe the construction and characterization of a fusion protein, CR2-CD59, which specifically inhibits MAC assembly in mice. The complement inhibitor CD59 binds to C8 and C9 proteins in the assembling MAC to prevent it from effectively inserting into cell membranes, and because CD59 functions in a species-selective manner, it is necessary and appropriate to use a murine composition in a mouse model. The CR2 moiety of the fusion protein binds to deposited C3 cleavage products and targets the construct to sites of complement activation (Atkinson et al., 2005). The benefits of CR2-mediated targeted complement inhibition versus systemic complement inhibition have been shown previously for inhibitors of C3 activation (Crry and factor H), and include improved bioavailability, significantly improved efficacy, and maintenance of host immunity to infection (Atkinson et al., 2005; Sekine et al., 2011). Of additional importance here, unlike C3 inhibitors, soluble (untargeted) CD59 is a very poor complement inhibitor, and data indicate that it must be positioned in close proximity to the site of complement activation and MAC formation to function effectively (Zhang et al., 1999), highlighting the need for a site-targeting approach for this inhibitor. In this study, we use CR2-CD59 to dissect the complement pathway and investigate how the MAC is involved in liver injury and regeneration. The data further indicate that unlike other currently available complement inhibitors, CR2-CD59 represents a promising nontoxic therapeutic for protecting against liver injury and promoting regeneration in a variety of clinical settings, including acute liver failure after massive liver resection.
RESULTS
In vitro characterization of CR2-CD59
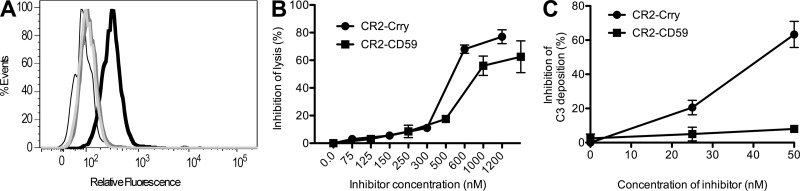
A CR2-CD59 fusion protein was designed for specific inhibition of the mouse terminal complement pathway and MAC formation at sites of complement activation (see Materials and methods). The CR2-CD59 construct targeted C3 activation fragments as demonstrated by its binding to C3 opsonized CHO cells, but not unopsonized cells (Fig. 1 A). Complement inhibitory activity of the construct was demonstrated by showing dose-dependent inhibition of serum-mediated lysis of antibody sensitized chicken erythrocytes (Fig. 1 B). CR2-CD59 was not as effective as CR2-Crry at inhibiting cell lysis on a molar basis, likely a consequence CR2-Crry acting upstream in the complement pathway and the one hit dynamics of erythrocyte lysis. However, unlike CR2-Crry, CR2-CD59 did not inhibit C3 activation (Fig. 1 C). Thus, CR2-CD59 targets to C3 opsonized cells and specifically inhibits MAC formation without affecting upstream C3 activation.
Figure 1.
In vitro characterization of CR2-CD59. (A) Flow cytometric analysis of CR2-CD59 binding to C3-opsonized CHO cells. Antibody-sensitized CHO cells were incubated with C6−/− mouse serum followed by incubation with CR2-CD59 (thick black trace) or PBS (dark gray trace). CR2-CD59 was also incubated with unopsonized cells, either without antibody (light gray trace) or without serum (thin black trace). Shown is a representative of 3 separate experiments. (B) CR2-CD59 inhibition of complement-mediated RBC lysis. Antibody-sensitized chicken RBCs were incubated with either CR2-Crry or CR2-CD59 in the presence of mouse serum. (C) Effect of CR2-CD59 on C3 activation. Activated zymosan particles were incubated with mouse serum and increasing doses of CR2-Crry or CR2-CD59, and C3 deposition on particles detected by flow cytometry. Data are presented as mean ± SEM (n = 2–3) and are representative of 2–4 independent experiments.
Hepatic IRI
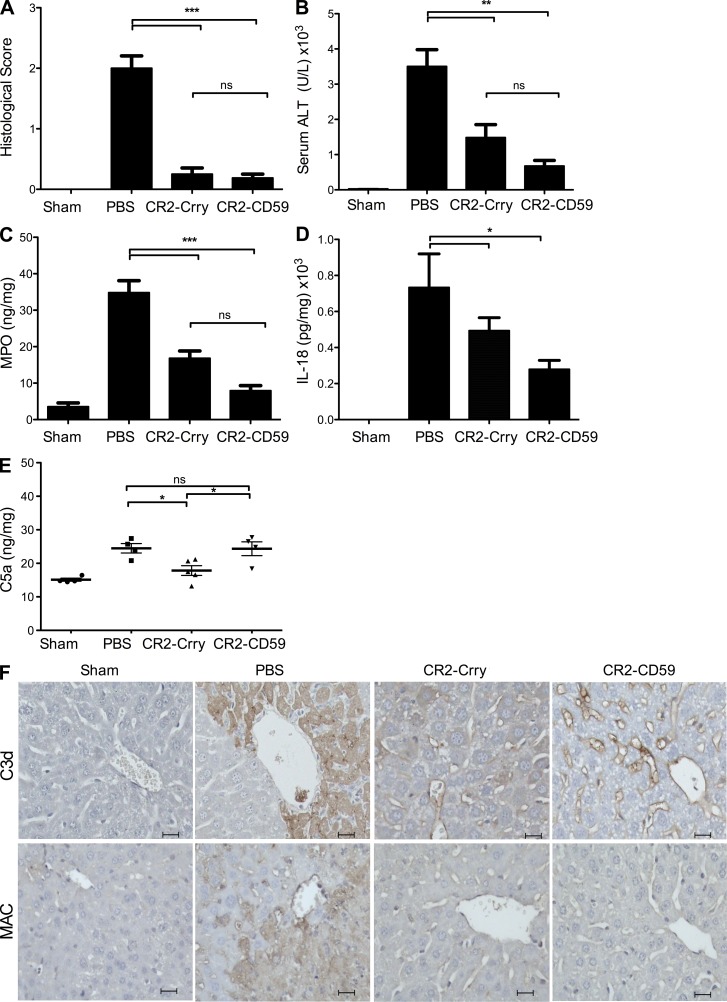
To investigate the role of the MAC in hepatic IRI in a clinically relevant setting of complement inhibition rather than deficiency, and to directly compare early pathway inhibition to terminal pathway inhibition, mice were treated with CR2-CD59, CR2-Crry, or PBS vehicle after total hepatic warm ischemia. Histological evidence of IRI and serum levels of alanine aminotransferase (ALT), a measure of liver function, were significantly and similarly reduced in CR2-Crry– and CR2-CD59–treated mice compared with PBS-treated controls (Fig. 2, A and B). The level of protection afforded by CR2-Crry was similar to that previously reported by us for mice deficient in C3 or similarly inhibited at C3 activation (He et al., 2009).
Figure 2.
Effect of CR2-CD59 and CR2-Crry on outcomes after hepatic IRI. Mice were subjected to 30 min of total warm ischemia and treated with PBS, CR2-Crry, or CR2-CD59 upon reperfusion. (A) Histological quantification of hepatic necrosis and injury in H&E-stained sections, scored on a scale of 0–3. Samples obtained 6 h after reperfusion. (B) Serum ALT levels in samples obtained 6 h after reperfusion. (C) Hepatic tissue levels of MPO measured 24 h after reperfusion by ELISA. (D) Hepatic tissue levels of IL-18 measured 24 h after reperfusion by ELISA. (E) Hepatic C5a levels 24 h after reperfusion. All ELISA measurements were normalized to total protein content. For A–E, results are expressed as mean ± SEM (n = 4–9) and are representative of 2–3 independent experiments; ***, P < 0.001; **, P < 0.01; *, P < 0.05; ns = not significant, assessed by ANOVA. (F) C3d and MAC deposition 24 h after reperfusion. Shown are representative images of immunostained liver sections, with 4 animals analyzed per group. Bars, 20 µM.
To assess whether the terminal pathway plays a role in neutrophil recruitment within the liver after IRI, myeloperoxidase (MPO) levels in liver homogenates were measured. Neutrophil infiltration is strongly associated with hepatic IRI, and MPO levels were significantly elevated in liver samples from PBS-treated control animals at 24 h after reperfusion (Fig. 2 C). However, complement inhibition with either CR2-Crry or CR2-CD59 resulted in significantly reduced levels of MPO, with no significant difference between the inhibitor-treated groups. Although C3a and C5a are known chemoattractants, these data indicate that the MAC also plays a role in hepatic neutrophil recruitment after IR. It was recently shown that the MAC can trigger NLRP3 inflammasome activation and IL-1β and IL-18 release (Triantafilou et al., 2013), so one possibility for the reduced neutrophil infiltration observed after MAC inhibition may be due to inhibition of inflammasome activation. To investigate this possibility, we assayed IL-18 in liver homogenates from the various treated groups after IR. Treatment with either CR2-CD59 or CR2-Crry, both of which inhibit MAC formation, resulted in significantly reduced levels of IL-18, the mature inflammatory form of pro–IL-18 that is cleaved by the NLRP3 inflammasome (Fig. 2 D). Collectively with previous data, this result is consistent with the MAC promoting neutrophil infiltration via inflammasome activation.
To demonstrate that CR2-CD59 is specific for the terminal pathway in vivo, the effect of CR2-CD59 on MAC deposition and generation of the upstream complement activation products C5a and C3d was assessed after hepatic IR. Although CR2-Crry and CR2-CD59 provided equivalent levels of protection, only CR2-Crry decreased C5a levels compared with PBS-treated controls (Fig. 2 E). Additionally, there were much higher levels of C3d deposited in livers from CR2-CD59–treated vs. CR2-Crry–treated animals, whereas each inhibitor similarly reduced MAC deposition (Fig. 2 F). Not surprisingly, there was extensive C3d and MAC deposition in livers from PBS-treated controls, with complement deposition in these unprotected mice focused in necrotic areas. In CR2-Crry– and CR2-CD59–treated mice, there was little to no necrosis and C3d staining was seen in a sinusoidal pattern, showing limited complement activation along the vascular endothelium, which is consistent with the amount of injury seen in these animals (Fig. 2 F). These data demonstrate that CR2-CD59 does not modulate upstream complement activation in vivo. Importantly, CR2-Crry treatment conferred no additional therapeutic benefit compared with CR2-CD59, indicating that the MAC is the critical determinant in causing hepatic IRI.
In vivo kinetics, biodistribution, and localization of CR2-CD59
Based on the protective effect of CR2-CD59 against hepatic IRI, we characterized additional aspects of the molecule that would be relevant for potential therapeutic development. After intravenous injection of radiolabeled CR2-CD59, the protein had a two-phase elimination profile: an initial rapid phase with a t1/2 of 16 min, and a second prolonged phase with a t1/2 of 6.5 h. This is somewhat shorter than that of CR2-Crry (8.7 h; Atkinson et al., 2005) and CR2-fH (8.8 h; Huang et al., 2008), but a short circulatory half-life is considered a beneficial characteristic for a site-targeted complement inhibitor because systemic effects would be minimized.
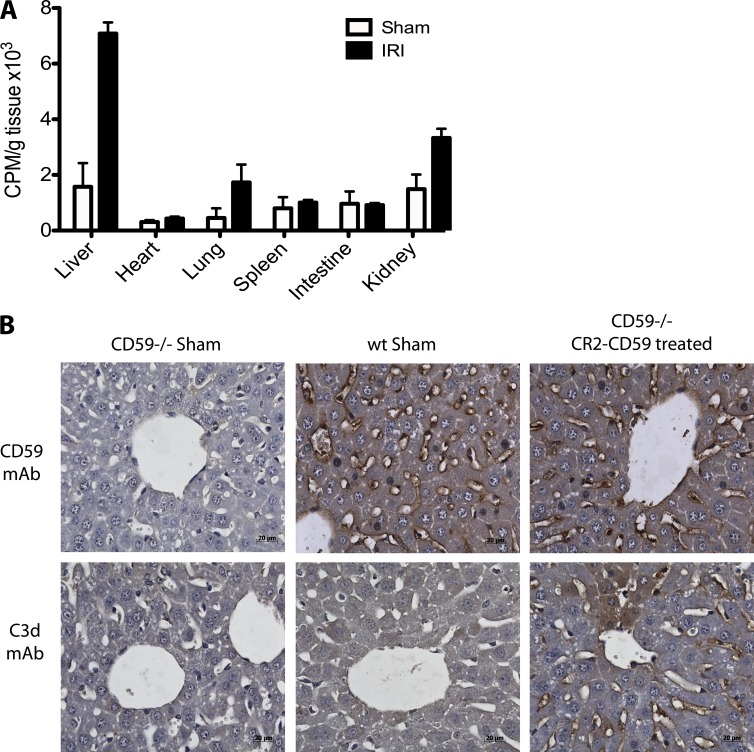
We previously demonstrated that CR2-Crry preferentially targets to the intestine in a model of intestinal IRI (Atkinson et al., 2005), and to confirm that CR2-CD59 similarly targets to sites of post-ischemic injury, we performed a biodistribution study after hepatic IR. After hepatic IRI, CR2-CD59 targeted predominantly to the liver (Fig. 3 A). Some specific targeting to the kidney and lung was also observed, but because the kidney and lung are sites of secondary injury and complement activation (Topp et al., 2004; Bilzer et al., 2006), this is not surprising and the data demonstrate injury-specific targeting. For additional and more direct evidence of CR2-CD59 targeting, we analyzed CR2-CD59 binding in the liver after IR by immunohistochemistry. For these studies, we used CD59−/− mice because the absence of endogenous CD59 allows for CR2-CD59 localization via the CD59 portion of the molecule. Mice were subjected to IRI and treated with CR2-CD59 in the therapeutic protocol described above, with analysis at 6 h after reperfusion. Analysis of liver serial sections revealed CR2-CD59 localized to the sinusoidal endothelium and areas of frank necrosis. Furthermore, analysis of C3d deposition, the target ligand for CR2, revealed a similar endothelial staining pattern (Fig. 3 B), and the pattern of C3d staining was also consistent with that seen in WT mice undergoing IRI (as shown above, Fig. 2). Compared with CR2-CD59 staining, we did observe some additional staining for C3d in the parenchyma, and it is possible that this is a reflection of the short circulatory half-life of CR2-CD59 relative to the evolution of injury and access of C3. Immunostaining for CD59 in livers from WT mice demonstrated the presence of CD59 on the central vein and sinusoidal endothelium, with no CD59 staining seen in livers from CD59−/−-deficient sham controls, confirming the specificity of the antibody. Together, these data demonstrate that CR2-CD59 colocalizes within areas of complement activation in the post-ischemic liver.
Figure 3.
Biodistribution and localization of CR2-CD59 after hepatic IRI. (A) Biodistribution of CR2-CD59 after IRI. 125I-labeled CR2-CD59 was injected into mice immediately after IR or after a sham operation, and tissue distribution of radiolabel was assessed after 2 h reperfusion. Shown are the combined results from 3 independent experiments of 1 mouse per group (mean ± SEM); *, P < 0.05 versus all other samples, as assessed by ANOVA. (B) Hepatic localization of C3d and CR2-CD59 after IRI. CR2-CD59 was injected into WT or CD59−/− mice immediately after IR or after a sham operation, and livers were isolated after 2 h reperfusion. Serial sections were prepared and analyzed by immunohistochemistry for C3d and CR2-CD59 binding. Representative images, n = 3. Bars, 20 µM.
Liver regeneration after partial hepatectomy (PHx)
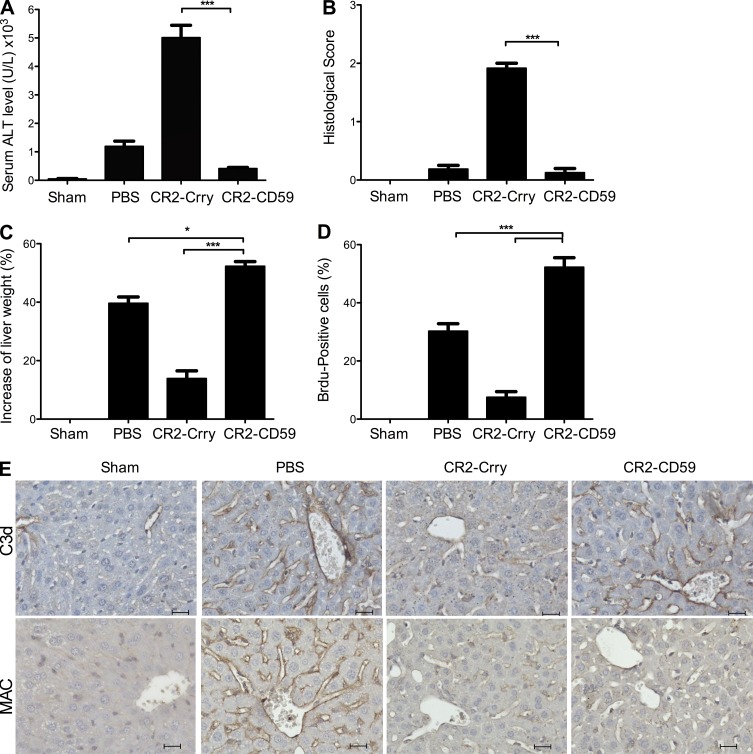
The complement activation products C3a and/or C5a are key mediators of hepatocyte proliferation and liver regeneration, and it has been shown that inhibitors of C3 and C5 activation (as well as C3 or C5 deficiency) impair the regenerative response and cause increased liver injury after toxic insult or 70% PHx (Mastellos et al., 2001; Strey et al., 2003; He et al., 2009; Markiewski et al., 2009; Singhal et al., 2012). We have previously investigated the effect of CR2-Crry on liver regeneration after 70% PHx, and although a low dose of CR2-Crry is not injurious, a dose that is optimal for protecting against IRI (the dose used in the above IRI experiments) causes liver injury and impaired regeneration, similar to other C3/C5 activation inhibitors and C3 deficiency (He et al., 2009). We show here that in marked contrast to CR2-Crry, treatment of mice with CR2-CD59 after 70% PHx significantly reduced liver injury as measured by serum ALT levels, with no increase in evidence of histological injury compared with PBS-treated controls (Fig. 4, A and B). Furthermore, CR2-CD59 treatment resulted in a significantly improved regenerative response, as measured by increased liver weight restitution and increased BrdU incorporation (Fig. 4, C and D). Complement activation in remnant livers of PBS-treated mice is shown by sinusoidal C3d deposition, which was reduced in mice treated with CR2-Crry at a dose previously shown to be optimal for reducing IRI (Fig. 4 E; He et al., 2009). In contrast, CR2-CD59 treatment did not affect C3d deposition after 70% PHx, whereas both inhibitors similarly reduced MAC deposition. These data indicate that MAC-induced injury impairs the regenerative response and that MAC inhibition enhances regeneration after PHx. As further confirmation of this conclusion, we also demonstrated increased injury, impaired regeneration, and decreased survival in CD59-deficient mice compared with PBS-treated WT controls after 70% PHx, with reversal of these effects in CD59-deficient mice treated with CR2-CD59 (unpublished data).
Figure 4.
Effect of complement inhibition on outcomes after 70% PHx. Mice were treated with PBS, CR2-Crry, or CR2-CD59 immediately after surgery, and the following determinations were made 48 h after surgery. (A) Serum ALT. (B) Histological quantification of hepatic necrosis and injury in H&E-stained sections, scored on a scale of 0–3. (C) Liver weight restitution (D) Assessment of liver regeneration by BrdU incorporation, detected by immunohistochemistry and expressed as % positive cells in 10 high-power fields. Results are expressed as mean ± SEM (n = 4–6) and are representative of 3 independent experiments; ***, P < 0.001; *, P < 0.05, assessed by ANOVA. (E) Effect of CR2-Crry and CR2-CD59 on complement activation. C3d and MAC deposition was analyzed in sections prepared from livers isolated 48 h after surgery. Shown are representative images of immunostained liver sections, with 4 animals analyzed per group. Bars, 20 µM.
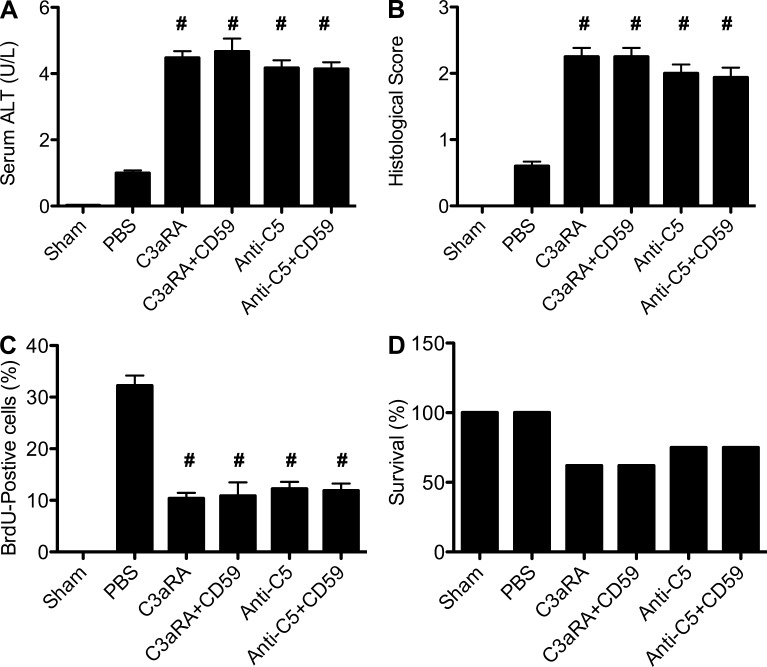
In addition to MAC inhibition, CD59 is known to have signaling and cell activation functions (Kimberley et al., 2007). Although these alternative functions are linked to its glycophosphatidylinositol anchor, we nevertheless performed an additional experiment to investigate whether CR2-CD59 may be protective after 70% PHx via a mechanism other than specific MAC inhibition that allows for the generation of C3a/C5a. It has been shown previously that C3a and C5a receptor-deficient mice, as well as WT mice treated with anti-C5 mAb, have increased hepatic injury and impaired regeneration after PHx (Strey et al., 2003; Markiewski et al., 2009). Here, we demonstrate that treatment of mice with either C3a receptor antagonist or with anti-C5 mAb increases injury and impairs regeneration after 70% PHx, and that co-treatment with CR2-CD59 does not affect any outcome measure (Fig. 5). Together with the above data and cited previous data, this result indicates that CR2-CD59 is functioning to promote liver regeneration by allowing the generation of C3a and C5a, rather than by any alternative direct protective effect.
Figure 5.
Effect of C3aRA, anti-C5 mAb, and CR2-CD59 treatment on outcome after 70 PHx. Mice were treated with C3aRA or anti-C5 mAb, with or without CR2-CD59 co-treatment, and the following determinations were made 48 h after surgery. (A) Serum ALT. (B) Histological quantification of hepatic necrosis and injury in H&E-stained sections (C) Assessment of liver regeneration by BrdU incorporation, detected by immunohistochemistry and expressed as % positive cells in 10 high-power fields. (D) Survival. Data are combined from 3 independent experiments (n = 8). Results for A–C are expressed as mean ± SEM. #, P > 0.05: no significant difference between any complement inhibitor–treated group, assessed by ANOVA.
Combined model of IRI and PHx
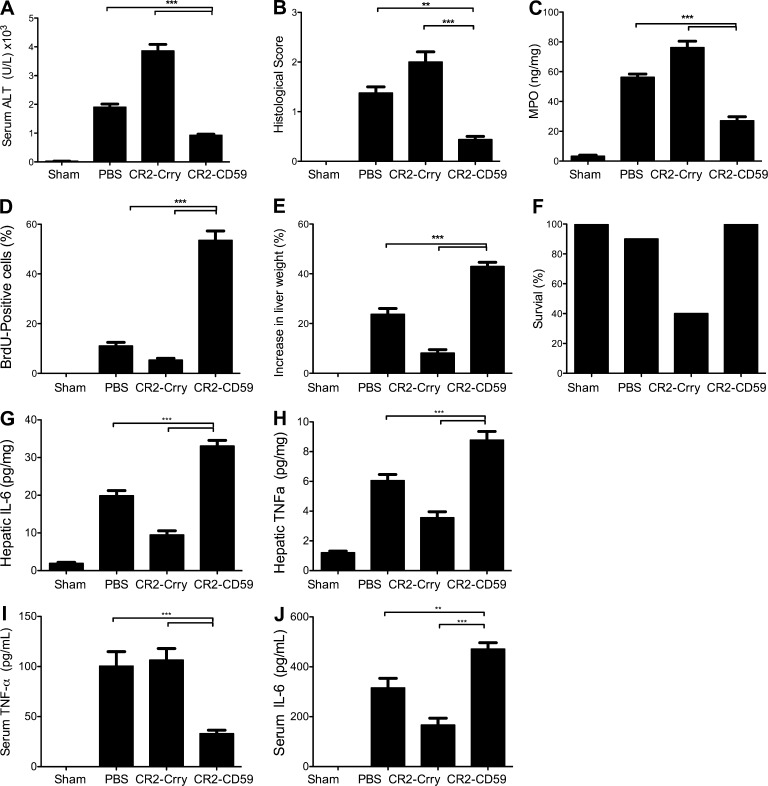
Clinically, liver resection is usually performed under the Pringle maneuver, which involves total vascular occlusion to facilitate surgery and minimize blood loss (Dixon et al., 2005). We therefore investigated the effect of MAC inhibition in a combined model of total IRI and 70% PHx. Our previous data using a similar model showed that animals treated with CR2-Crry at a dose optimal for reducing IRI fared far worse than PBS-treated animals in terms of injury, neutrophil infiltration, regeneration, and survival, with only ∼40% survival rate at day 7 after surgery (He et al., 2009). In contrast, 100% of CR2-CD59–treated mice survived, with significant improvements in all measured outcomes compared with PBS-treated controls (Fig. 6, A–F).
Figure 6.
Effect of complement inhibition on outcomes in a model incorporating both IRI and 70% PHx. Mice were treated with PBS, CR2-Crry, or CR2-CD59 immediately after combined surgery. (A) Serum ALT levels. (B) Histological quantification of hepatic necrosis and injury in H&E-stained sections. (C) Hepatic tissue levels of MPO. (D) Assessment of liver regeneration by BrdU incorporation. (E) Liver weight restitution. (F) Mouse survival. All determinations were made 48 h after surgery. (G–J) Hepatic and serum cytokines were measured by ELISA. (G) Hepatic IL-6 levels 3 h after surgery. (H) Hepatic TNF levels 3 h after surgery. (I) Serum IL-6 levels 6 h after surgery. (J) Serum TNF levels 6 h after surgery. Results are expressed as mean ± SEM (n = 4–6) and are representative of 3 independent experiments; ***, P < 0.001; **, P < 0.01, assessed by ANOVA.
To investigate the role of the MAC and potential protective mechanisms of CR2-CD59 in the combined model, we determined the effect of terminal pathway complement inhibition on TNF and IL-6 levels, two key mediators of hepatic injury and regeneration that are essential for the priming phase of the regenerative response, and expression of which can be modulated by C3a and/or C5a (Cressman et al., 1996; Yamada et al., 1997; Markiewski et al., 2009). At 3 h after surgery, which is close to the time when levels of these cytokines peak after 70% PHx, hepatic levels of both IL-6 and TNF were significantly increased in CR2-CD59–treated mice compared with PBS-treated mice, which correlated with liver regeneration profiles (Fig. 6, G and H). Serum levels of the cytokines were also measured 6 h after surgery as an indicator of systemic inflammation, and although serum IL-6 levels were also increased in CR2-CD59–treated mice compared with PBS-treated mice, systemic levels of TNF were significantly decreased (Fig. 6, I and J). Thus, CR2-CD59 increased the local production of TNF and IL-6, while also diminishing a subsequent systemic inflammatory response as indicated by decreased serum TNF.
Massive liver resection and acute liver failure
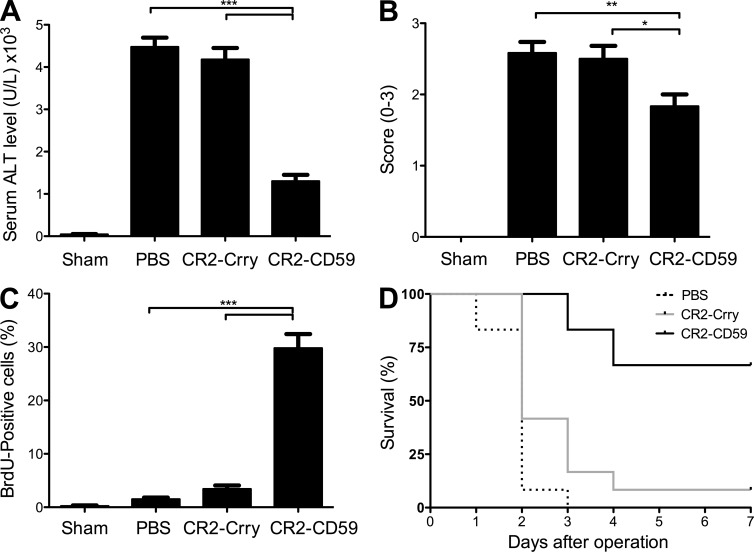
The significant improvements seen in injury and regeneration with CR2-CD59 treatment in the above models prompted us to investigate how MAC inhibition would impact outcome after 90% PHx, a model of acute hepatic failure induced by massive liver resection (Tuñón et al., 2009). Hepatic injury after 90% PHx is much more severe than the injury that occurs after 70% PHx, whereas 70% PHx in WT mice induces a proliferative response with 100% survival rate, and the remnant liver after 90% PHx fails to regenerate, with subsequent severe liver injury and a 100% mortality rate. We directly compared the effects or CR2-Crry and CR2-CD59 on outcomes after 90% PHx. We used a dose of CR2-Crry (0.08 mg) that is suboptimal for protection against IRI, but that we have previously shown is optimal for regeneration after 70% PHx (He et al., 2009). Although titration of complement inhibition would not be feasible in a clinical setting (thus precluding the clinical use of a C3 inhibitor at any dose), the lower CR2-Crry dose allows us to assess CR2-CD59 efficacy in comparison with CR2-Crry when given at an optimal dose for regeneration. In PBS-treated controls, 90% PHx resulted in liver injury and dysfunction, as demonstrated by high serum ALT levels and extensive necrosis of hepatic parenchyma with severe microvesicular steatosis. There was also little induction of a proliferative response in PBS-treated animals as indicated by the minimal amount of hepatocyte BrdU incorporation, and all mice died within 3 d of surgery (Fig. 7). Mice treated with CR2-Crry fared similarly to the PBS-treated animals in all assessed parameters. Remarkably, however, CR2-CD59 treatment resulted in significantly reduced hepatic injury and a marked hepatocyte proliferative response, with an improvement in long-term survival from 0% (in PBS-treated controls) to 70%.
Figure 7.
CR2-CD59 protects against acute hepatic failure after 90% PHx. Mice were treated with PBS, CR2-Crry, or CR2-CD59 immediately after 90% PHx. (A) Serum ALT levels 6 h after surgery. (B) Histological quantification of hepatic necrosis and injury in H&E-stained sections, scored on a scale of 0–3. (C) Assessment of liver regeneration by BrdU incorporation, detected by immunohistochemistry and expressed as % positive cells counted in 10 high-power fields, determined 24 h after surgery. Results are expressed as mean ± SEM (n = 6) and are representative of 3 independent experiments; ***, P < 0.00; **, P < 0.01; *, P < 0.05, as assessed by ANOVA. (D) Survival analysis over a 7-d period after surgery. Difference between the CR2-CD59–treated group and CR2-Crry– or PBS-treated groups was statistically significant as determined by the Kaplan-Meier test (P < 0.05). Combined data from 2 independent experiments (n = 12).
Cytokine and signaling pathways after 90% PHx
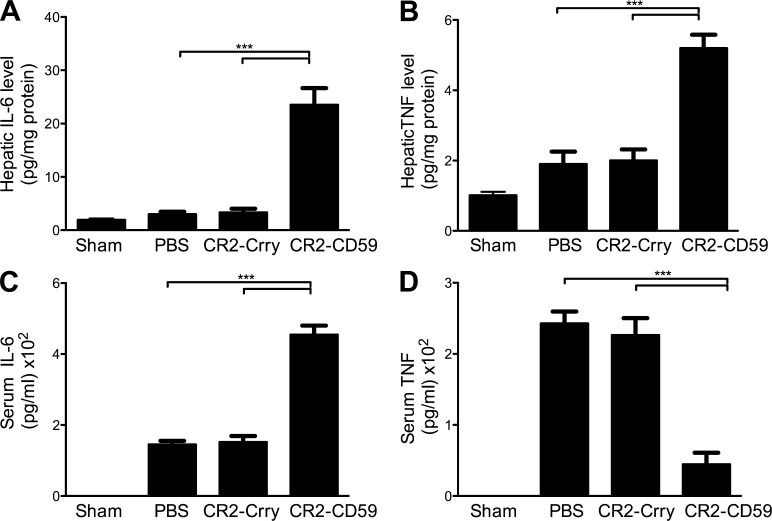
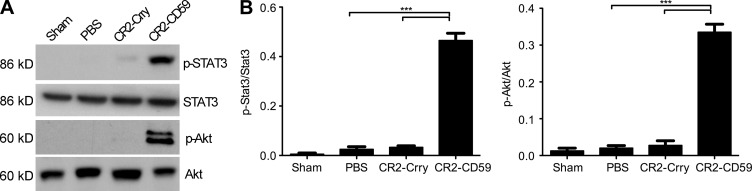
To investigate potential protective mechanisms of CR2-CD59 on the regeneration process after massive liver resection, we determined the effect of complement inhibition on TNF and IL-6 levels, two key mediators of hepatic injury and regeneration. CR2-CD59 treatment significantly increased local hepatic production of TNF and IL-6 compared with either PBS or CR2-Crry treatment (Fig. 8, A and B), similar to that seen after IRI + 70% PHx (above). There was also a significant decrease in serum TNF, although not IL-6 in CR2-CD59–treated mice, indicating diminished post-resection systemic inflammation (Fig. 8, C and D). To provide further insight into the mechanisms of CR2-CD59–mediated protection after 90% PHx, we investigated the effect of complement inhibition on STAT3 and Akt activation. Both transcription factors play an important role in the proliferative and prosurvival signaling pathways after hepatectomy, and IL-6, which was increased in remnant livers from mice treated with CR2-CD59, regulates activation of both STAT3 and Akt (Taub, 2004). At 3 h after resection, there was a significant increase in the phosphorylation of both STAT3 and Akt in remnant livers from CR2-CD59–treated mice compared with either PBS or CR2-Crry–treated mice (Fig. 9), indicating that blockade of MAC formation preserves the normal signaling pathways important for cell survival and proliferation.
Figure 8.
Systemic and local cytokine production after 90% PHx. Mice were treated with PBS, CR2-Crry, or CR2-CD59 immediately after 90% PHx. Hepatic and serum cytokines were measured by ELISA, normalized to total protein content. (A) Hepatic IL-6 levels 3 h after surgery. (B) Hepatic TNF levels 3 h after surgery. (C) Serum IL-6 levels 6 h after surgery. (D) Serum TNF levels 6 h after surgery. Results are expressed as mean ± SEM (n = 4–6) and are representative of 3 independent experiments; ***, P < 0.00, as assessed by ANOVA.
Figure 9.
Stat3 and Akt activation occurs in CR2-CD59–treated mice but not CR2-Crry–treated mice after 90% PHx. Mice were treated with PBS, CR2-Crry, or CR2-CD59 immediately after 90% PHx. (A) Representative Western blot of STAT3, p-STAT3, Akt, and p-Akt in liver homogenate at 3 h after surgery. (B) Densitometry. Results are expressed as mean ± SEM (n = 4–6) and are representative of 3 independent experiments; ***, P < 0.001, as assessed by ANOVA.
Mitochondrial dysfunction and ATP stores after 90% PHx
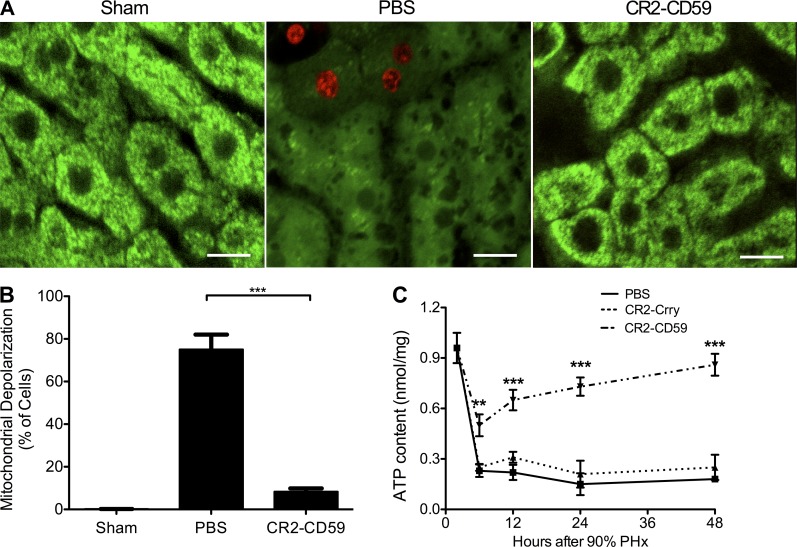
After massive liver resection, there is an increased metabolic burden put on the remnant liver to maintain cellular processes and to provide energy for regeneration. Mitochondrial synthesis of ATP is crucial to prevent liver failure and ATP depletion-dependent necrosis after massive resection and partial liver transplantation (Zhong et al., 2006; Rehman et al., 2011). To determine whether CR2-CD59 functions to prevent mitochondrial depolarization after 90% PHx, intravital confocal microscopy was performed. Live animals were injected with propidium iodide live/dead stain (red) and rhodamine (Rh)123 dye (green), the latter of which localizes to mitochondria in living cells. In sham operated animals, no dead cells were seen and all hepatocytes had punctate Rh123 fluorescence staining, indicating normal polarization of mitochondria (Fig. 10, A and B). However, PBS-treated control animals that underwent 90% PHx had diffuse Rh123 staining in a large majority of hepatocytes (75%), indicating mitochondrial depolarization. In contrast, in CR2-CD59–treated mice, only 8% of hepatocytes had depolarized mitochondria and there was little evidence of cell death as indicated by lack of PI staining.
Figure 10.
CR2-CD59 treatment prevents mitochondrial depolarization and promotes recovery of ATP stores after 90% PHx. Mice underwent 90% PHx or sham operation, and resected mice were treated with either PBS or CR2-CD59 immediately after surgery. For intravital microscopy study, live animals were infused intravascularly with Rh123 fluorescence and PI (live/dead) stain into the carotid artery 2 h after resection. (A) Representative confocal image of hepatic Rh123 (green) and PI (red). Bars, 10 µM. (B) Percentage of hepatocytes with depolarized mitochondria in 10 high-power fields. Shown are the combined results from 4 independent experiments of 1 mouse per group (mean ± SEM). (C) ATP content in liver tissue taken at different time points after 90% PHx. Results are expressed as mean ± SEM (n = 4–6) and are representative of 3 independent experiments; ***, P < 0.001; **, P < 0.01, as assessed by ANOVA.
Mitochondrial depolarization leads to swelling and rupture of the mitochondrial membrane, resulting in loss of the electrochemical gradient necessary for ATP production. To demonstrate a link between CR2-CD59–mediated protection, mitochondrial dysfunction and ATP stores, we measured ATP levels after 90% PHx. There was a marked reduction in hepatic ATP in all groups at 6 h after PHx (Fig. 10 C). However, in CR2-CD59–treated mice, ATP stores recovered to near preoperation levels by 48 h after PHx, whereas ATP levels remained low in PBS and CR2-Crry–treated mice. These data indicate that after 90% PHx, MAC formation causes mitochondrial depolarization and depletion of ATP stores, resulting in acute liver failure that can be prevented by MAC inhibition with CR2-CD59.
DISCUSSION
Complement has dual and balancing roles in hepatic injury and regeneration that are not fully understood. It is clear that the early complement activation products C3a and/or C5a play a crucial role in the hepatocyte proliferative response, but whether these proinflammatory peptides play a role in IRI is less clear. There is some evidence implicating the terminal pathway in IRI, but the MAC has not been studied in terms of liver regeneration, although there is evidence that sublytic concentrations of MAC can cause cell activation (Rus et al., 2001). Developing an effective inhibitor of MAC formation for use in experimental models has been a limiting factor in studying the role of the MAC in clinically relevant paradigms. Previous studies have used complement-deficient mice, but such animals can have inherent complications, making it sometimes difficult to reconcile data obtained using complement-deficient mice with data obtained using complement-inhibited mice.
For this study, we developed a murine complement inhibitor that targets to sites of complement activation and specifically inhibits the MAC. Although the targeting of a complement inhibitor to sites of complement activation improves bioavailability and reduces any adverse effects due to systemic inhibition, membrane localization is actually a requirement for effective CD59 function. Soluble CD59 does not effectively inhibit MAC formation, and its activity is greatly increased when it is positioned in close proximity to the site of complement activation on the membrane (Zhang et al., 1999), as is achieved by the CR2 targeting vehicle. Using CR2-CD59, we show that the MAC is the mediator of complement-dependent hepatic IRI. We show that after partial hepatectomy, with or without accompanying ischemia, specific inhibition of the MAC allows generation of early complement activation products that drive production of cytokines necessary for activation of transcription factors important for hepatocyte proliferation. We further determined that the MAC causes mitochondrial depolarization and disrupts energy supply in regenerating livers, whereas MAC blockade protects remnant livers by preserving mitochondrial function and allowing restoration of ATP stores necessary to serve the large metabolic burden in a regenerating liver.
It is interesting that CR2-CD59 reduced the level of hepatic neutrophil infiltration, even though CR2-CD59 did not affect generation of C5a, a powerful chemoattractant which has been implicated in neutrophil recruitment and IRI of various tissues and organs (Amsterdam et al., 1995; Arumugam et al., 2003, 2004; Kim et al., 2008; Lewis et al., 2008; Zheng et al., 2008). Our results indicate that after hepatic IR, neutrophil recruitment is not C5a dependent and that C5a alone is not sufficient for neutrophil recruitment when MAC formation is inhibited. It is possible that the MAC may be involved directly in neutrophil recruitment via its ability to up-regulate neutrophil adhesion markers and increase IL-8 production when deposited at sublytic concentrations (Hattori et al., 1989; Kilgore et al., 1997, 1998; Tramontini et al., 2002). Another possibility, consistent with our finding that complement inhibition decreases post-IR levels of hepatic IL-18, is that CR2-CD59 (and CR2-Crry) inhibits MAC-dependent inflammasome activation. In this regard, the MAC has been shown to trigger NLRP3 inflammasome activation and IL-1β and IL-18 release (Triantafilou et al., 2013), and the NLRP3 inflammasome has been shown to be involved in hepatic IRI and neutrophil recruitment (Huang et al., 2013).
The fact that CR2-CD59 did not impact upstream complement activation and did not impair liver regeneration after either 70% PHx or combined IRI + 70% PHx signifies the important role of early activation products in the regenerative process. Surprisingly, however, MAC inhibition actually enhanced liver regeneration compared with control treated mice. A contributing factor to enhanced regeneration in CR2-CD59–treated mice, at least in the combined model, is likely related to its role in protecting against IRI, the extent of which is thought to impact regeneration. However, CR2-CD59 also significantly increased the local production of key cytokines that are known to be important for priming the regenerative response. Interestingly, it has been shown that local and systemic IL-6 has differential effects on the production TNF. Specifically, systemic IL-6 inhibits TNF production but has little effect on tissue TNF levels (Di Santo et al., 1997). This is consistent with our data showing that CR2-CD59 treatment results in a systemic antiinflammatory profile of increased levels of serum IL-6 and decreased levels of TNF, while increasing hepatic levels of both cytokines.
Based on the significant improvements seen in CR2-CD59–treated mice after 70%, we investigated the effect of CR2-CD59 after 90% PHx. Remarkably, CR2-CD59 treatment resulted in a 70% survival rate after 90% PHx, and survival was associated with reduced hepatic injury and a dramatic increase in hepatocyte proliferation. Interestingly, although a low dose of CR2-Crry was previously shown to be protective after 70% PHx (He et al., 2009), it did not provide any protection after 90% PHx, a reflection of the unique pathophysiology of massive liver resection and acute hepatic failure. Liver regeneration after 90% PHx in CR2-CD59–treated animals was associated with a marked increase in hepatic levels of TNF and IL-6, together with STAT3 and Akt activation. IL-6 expression is dependent on TNF-induced NF-κB activation, and IL-6–induced activation of STAT3 plays a role in cell cycle progression (Cressman et al., 1996; Yamada et al., 1997; Li et al., 2002; Blindenbacher et al., 2003). IL-6 also activates the PI3K–Akt pathway, which regulates progression of the G1 phase during regeneration, as well as cell survival through up-regulation of anti-apoptotic proteins (Taub, 2004). Thus, one mechanism by which CR2-CD59 protects against acute liver failure and improves regeneration after 90% PHx is the induction of IL-6 expression and STAT3 and Akt activation, which is likely linked to the fact that CR2-CD59 does not inhibit generation of C3a/C5a, which have been shown to induce TNF and IL-6 expression after hepatectomy (Markiewski et al., 2009). Similar signaling pathways are also involved in the production of various growth factors that are essential for the regenerative process. Of note, the MAC has been shown to activate cells via the Akt pathway, although we show here that MAC inhibition by CR2-CD59 promotes Akt activation. A possible explanation for this apparent contradiction is that while CR2-CD59 effectively blocks lytic MAC formation, it may permit the limited formation of nonlethal sublytic levels of MAC that can cause cell activation (Tegla et al., 2011).
After resection, there is an excessive metabolic burden put on the remnant liver due to the large requirement of ATP needed to keep up with increased metabolic demand and biogenesis. It has been shown that accumulation of the MAC on a cell surface in vitro leads to cytosolic influx of Ca2+, which causes mitochondrial depolarization, reduction in ATP levels, and cell death (Papadimitriou et al., 1991). However, limiting cellular deposition of the MAC in vitro results in reversible cell injury, with only a transient increase in intracellular Ca2+ and a transient reduction in ATP (Scolding et al., 1989). Consistent with this earlier in vitro data, inhibiting MAC formation after 90% PHx resulted in a transient reduction of hepatic ATP with full recovery of ATP stores by 48 h. The most likely mechanism for the restoration of ATP stores in CR2-CD59–treated mice is the prevention of mitochondrial depolarization, and this was demonstrated by intravital confocal microscopy. Interestingly, a recent report presented data indicating that increased intracellular Ca2+ resulting from MAC attack causes mitochondrial depolarization leading to NLRP3 inflammasome activation (Triantafilou et al., 2013). Thus, because we found that CR2-CD59 reduces hepatic levels of the inflammasome product IL-18 after hepatic IR, it is possible that the inflammasome may provide an additional mechanistic link between the MAC, mitochondrial dysfunction, and liver injury and regeneration.
Many biological systems are poised between the effects of injury and protection, and such a balance is demonstrated here for the complement cascade in terms of liver injury and regeneration. Complement inhibitors are regarded as strong therapeutic candidates for a variety of inflammatory conditions, but the current data highlight the need to design specific inhibition strategies that can be applied to relevant clinical scenarios. We have described a targeted inhibitor, CR2-CD59, that is specific for the terminal complement activation product. We demonstrate that this inhibitor, unlike other available inhibitors that act earlier in the complement pathway, has the potential to provide a nontoxic protective strategy for both donors and recipients involved in small for size liver transplantation, as well as to extend the limits of tolerable liver resection.
MATERIALS AND METHODS
Construction, expression, and purification of complement inhibitors.
Recombinant fusion protein cDNA constructs were prepared by joining the CR2 sequence encoding the four N-terminal SCR units (residues 1–250 of mature protein, Swissprot accession no. P20023) to sequences encoding extracellular regions of mouse CD59a or Crry. The complement inhibitor sequences used encode residues 1–319 of mature Crry protein sequence (GenBank accession no. NM013499) and residues 1–73 of mature CD59a protein sequence (GenBank accession no. NM_001111060.1). Additional construction details, and expression and purification of CR2-Crry were previously described (Atkinson et al., 2005). The CR2-CD59 gene construct was prepared by standard PCR methods (Song et al., 2003). All cloning steps were performed in the pEE14.1 vector (Lonza) that was also used for protein expression in Chinese Hamster Ovary (CHO) cells transfected with FuGene-HD transfection reagent (Roche). Stably transfected clones were selected by limiting dilution, and CR2-CD59 expression by clones was quantitated by dot blot. For protein purification, CHO cells were cultured in 500 ml HYPERFlasks (Corning) and supernatant was harvested at confluence. CR2-CD59 was purified from filtered supernatants by anti–mouse CR2 (mAb 7G6) affinity chromatography as described previously for CR2-Crry (Huang et al., 2008).
In vitro characterization of CR2-CD59.
Binding of CR2-CD59 to C3-opsonized CHO cells was determined by flow cytometry as previously described (Song et al., 2003), with the exception that C6−/− mouse serum was used as the source of complement to prevent MAC-mediated cell lysis. In brief, CHO cells were sensitized with antibody, washed with PBS, and opsonized with C3 by incubating them with 10% C6−/− mouse serum for 45 min at 37°C. Opsonized cells were washed and incubated with 1 µM CR2-CD59 for 30 min at 4°C, and bound CR2-CD59 was detected using anti-CD59 mAb (BioLegend) and flow cytometry. For controls, serum, antibody or CR2-CD59 were omitted from incubations. Inhibition of complement-mediated lysis was determined as previously described (Wang et al., 1999). In brief, antibody-sensitized chicken erythrocytes (Innovative Research) were incubated for 60 min at 37°C in GVB++ buffer (CompTech) containing 20% mouse serum and increasing doses (0–2,000 nM) of either CR2-CD59 or CR2-Crry. Lysis was determined by measuring hemoglobin in the supernatant by reading absorbance at 413 nm. The effect of complement inhibitors on C3 activation was determined by assaying C3 deposition on zymosan particles, as previously described (Huang et al., 2008).
Animal care and surgeries.
C57BL/6 mice were purchased from The Jackson Laboratory. CD59a−/− mice are on a B57BL/6 background and are bred and housed at the Medical University of South Carolina, provided by P. Morgan (University of Wales, Cardiff, UK; Holt et al., 2001). C3aR/C5aR double-deficient mice on a C57BL/6 background were provided by P. Heeger (Mount Sinai Hospital, NY, NY; Kwan et al., 2013). Mice were kept on a 12 h light/dark cycle, fed a pellet diet, and given water ad libitum. For surgical procedures, mice were anesthetized with a ketamine cocktail (13 mg/ml ketamine, 2.6 mg/ml xylazine, and 0.15 mg/ml acepromazine) injected i.p. at 5 ml/kg. All procedures were approved by the Institutional Animal Care and Use Committee at the Medical University of South Carolina, in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
For hepatic ischemia and reperfusion (IR), mice were subjected to a total warm hepatic IR as previously described (Evans et al., 2008). In brief, mice were anesthetized and a small midline vertical incision was made in the abdominal wall allowing exposure of the liver and portal triad. Hepatic artery and portal vein were occluded with a microaneurysm bulldog clamp for 30 min, followed by a 6 or 24 h period of reperfusion before sacrifice. To maintain a body temperature of 37°C, animals were placed on a warming pad during and after surgery until anesthesia wore off. At sacrifice, tissues and serum were harvested and either snap frozen in liquid nitrogen for storage at −80°C, or fixed in 10% formalin for further processing.
For 70% PHx, surgery was performed as previously described (Greene and Puder, 2003). In brief, animals were anesthetized and a midline vertical incision was made to expose the liver. Resection of the median and left lateral liver lobes was done using a separate 4.0 silk suture for each lobe. Mice were sacrificed at various time points after resection, and tissue and serum was collected.
For combined ischemia and reperfusion with 70% partial hepatectomy (IR + PHx), during a 30 min period of total hepatic ischemia, as described above, 70% of the liver was resected, as described above. Mice were sacrificed after various time points of reperfusion, and tissue and serum was collected.
For 90% PHx, surgery was performed as previously described (Rehman et al., 2011). In brief, mice were anesthetized and the left lateral and median lobes were removed separately with two 4.0 silk sutures. The caudate lobes and lower right lobes were then removed separately with 6.0 silk sutures, leaving only the upper right lobe (∼10% of the liver).
For all sham operations, mice were anesthetized and a midline vertical incision was made, liver was exposed, and then the animal’s skin was sutured closed. For IR sham animals, the incisions were left open but covered with saline-soaked gauze for 30 min before the incision was closed.
Animal treatments.
Complement inhibitors were given as a single i.p. dose immediately after surgery, just before the skin being sutured closed. Dosing for CR2-Crry (0.25 mg/animal) was based on a previously established effective dose for maximum complement inhibition (He et al., 2009). For CR2-CD59 treatment, a molar equivalent dose was given (0.15 mg). An exception was for CR2-Crry treatment in the 90% PHx model, in which a lower dose of 0.08 mg/animal was given (for rationale, see Results). To investigate the possibility that the effect of CR2-CD59 on liver regeneration was due to a direct effect rather than noninterference with C3a and/or C5a generation, C57BL/6 mice were treated with either C3aRA (EMD Millipore) or anti-C5 mAb (J. Lambris, University of Pennsylvania, Philadelphia, PA), with or without CR2-CD59 treatment in the above 70% PHx protocol. Mice were treated with 1.2 mg C3aRA by i.p. injection 30 min before surgery, and again 6 h after surgery. Anti-C5 mAb was administered as a single 1 mg dose 30 min before surgery.
Liver function and injury.
Serum alanine aminotransferase (ALT) levels, a measure of liver function, were determined using an enzymatic reaction-based reagent (Pointe Scientific Inc.), according to the manufacturer’s instructions. Histological injury was assessed on 4-µm paraffin liver sections fixed in 10% formalin and stained with H&E. Histological score was determined in a blinded fashion on a semiquantitative scale ranging from 0 (no damage) to 3 (total necrotic destruction of liver). Necrotic damage was characterized by increased eosinophilia, karyolysis, vacuolization, and loss of normal hepatic architecture (Sigala et al., 2004).
Hepatic complement activation and neutrophil infiltration.
Complement activation was determined by analysis of the complement activation products C3d, C5a, and MAC. To determine C3d deposition, immunohistochemistry staining was performed on paraffin-embedded tissue sections as previously described (Moseley et al., 2010). In brief, proteinase K enzyme antigen retrieval (Dako) was performed for 5 min at room temperature followed by peroxidase and serum blocking steps. Goat anti-C3d antibody (R&D Systems) was diluted 1:40 in PBS and incubated on slides for 2 h at room temperature, followed by antibody detection with anti–goat ImmPRESS kit (Vector Laboratories). MAC deposition was visualized by IHC on paraffin-embedded tissue using rabbit anti–rat C5b-9 antibody (1:1,000, courtesy of P. Morgan), followed by anti–rabbit ImmPRESS kit (Vector Laboratories). For C5a determinations, liver homogenates were prepared from frozen liver samples homogenized in cell lysis buffer (Sigma-Aldrich) containing a protease inhibitor cocktail (Thermo Fisher Scientific). Homogenates were centrifuged at 10,000 g for 10 min at 4°C, and C5a levels in supernatant determined using an ELISA kit according to the manufacturer’s instructions (R&D Systems, BD). Infiltrating neutrophils were quantified by MPO content of liver samples using a MPO ELISA kit (Hycult Biotechnology) according to the manufacturer’s instructions.
Assessment of liver regeneration.
Mice were injected with 50 mg/kg BrdU i.p. 2 h before sacrifice, and incorporation of BrdU was visualized by immunohistochemistry using an anti-BrdU antibody (Abcam) as previously described (He et al., 2009). In brief, positive and negative cells were counted in 10 randomly selected high-power fields (∼1,000 cells total), and vessels and cell hypertrophy was controlled for by tallying the total number of cells in each field and reporting data as percentage of positive cells in each field. Restitution of liver weight is expressed as percentage of regenerated liver mass relative to total liver weight. Residual liver weight is calculated with the following equation: ([B − (C − A)]/B)*100, where A is the weight of the resected liver (∼30% of total liver), B is the estimated total liver weight based on the weight of the resected liver (B = A/0.7), and C is the final liver weight at the time of sacrifice.
In vivo kinetics, biodistribution, and localization of CR2-CD59.
The rate of CR2-CD59 clearance from the circulation of C57BL/6 mice was determined after tail vein injection of radiolabeled protein as previously described (Sharkey et al., 1991). To determine CR2-CD59 biodistribution after hepatic IRI, radiolabeled protein was injected via the tail vein upon reperfusion, and at 2 h after reperfusion mice were sacrificed and organs removed, weighed, and counted as described previously for CR2-Crry (Atkinson et al., 2005). For in vivo imaging of CR2-CD59, CD59−/− mice were subjected to IRI and treated with CR2-CD59 as described above. 6 h after reperfusion whole livers were removed for analysis. Complement activation and CR2-CD59 binding were localized on frozen serial liver sections with antibodies against C3d (R&D Systems) and CD59 (Hycult Biotech), using immunohistochemistry techniques. In brief, sections were fixed in cold acetone and endogenous peroxidase and biotin blocked to reduce nonspecific binding (Vector Laboratories). Mouse monoclonal anti-CD59 binding was detected with mouse on mouse detection system and C3d antibody binding was detected with ImmPRESS polymer reagents in accordance with the manufacturer’s instructions (Vector Laboratories). For control purposes, CD59−/− and C3−/− mouse livers were used as negative controls to test the specificity of anti-CD59 and anti-C3d antibodies. Sections immunostained with C3d and CD59 mAb were analyzed using a microscope (OX40) for the presence or absence of specific staining.
Cytokine assays.
After either combined IRI + 70% PHx or 90% PHx, TNF and IL-6 levels were measured using an ELISA kit (eBioscience) in both serum (at 3 h after surgery) and liver homogenates (at 6 h after surgery). For IRI, IL-18 levels were measured in liver homogenates prepared 24 h after surgery using an ELISA kit (R&D Systems). Liver homogenates were prepared from frozen liver samples as described above, and cytokine levels in supernatant determined by ELISA according to the manufacturer’s instructions (eBioscience). Cytokine levels were normalized to total protein content, as determined by BCA protein assay (Thermo Fisher Scientific), and results are expressed as pg/mg of protein.
Stat3 and Akt Western blotting.
Supernatants from liver homogenates (see above) were assayed for total protein content using a BCA assay (Thermo Fisher Scientific), and equal amounts of protein were added to Laemmli sample buffer (Bio-Rad Laboratories) before separation in a 4–15% Tris-HCL polyacrylamide gel. After transfer of separated proteins to a PVDF membrane (Bio-Rad Laboratories), STAT3 and Akt activation was determined by antibody detection as previously described (He et al., 2009).
ATP measurement and mitochondrial depolarization.
ATP content in liver tissue samples was measured as previously described (He et al., 2009). Mitochondrial polarization was measured by intravital confocal microscopy as previously described (Zhong et al., 2008). In brief, animals were anesthetized with 50–80 mg/kg pentobarbital i.p. 2 h after 90% PHx. The animal’s abdomen was opened and 0.4 ml rhodamine 123 and propidium iodide slowly infused into the carotid artery over a period of ∼10 min. The mice were kept alive by being connected to a small animal respirator. For live imaging, the liver was gently externalized from the body cavity and placed on a glass coverslip on the stage of a laser-scanning confocal microscope (LSM 510 NLO; Carl Zeiss) with a 25× water immersion objective lens (0.8 NA, zooming at 4×) using excitation wavelengths of 488 and 543 nm and band pass emission filters of 500–550 nm for green and 575–625 nm for red, respectively. 10 or more images were collected randomly from each mouse. Viable hepatocytes (without nuclear propidium iodide red staining) in these fields (∼220 cells) were scored in a blinded fashion for bright punctate Rh123 fluorescence (green) representing cells with polarized mitochondria versus a diffuse, dimmer cytosolic fluorescence representing cells with depolarized mitochondria.
Statistical analysis.
Statistical analysis was performed using Prism (version 5.0; GraphPad Software). Statistical significance between multiple groups was assessed using ANOVA, with a Tukey’s post test for comparison of groups. For survival analysis, a Kaplan-Meier log-rank analysis was performed. Significance was determined to be P < 0.05. All data are presented as means ± SEM, except where noted.
Acknowledgments
We thank Fei Qiao, Xiaofeng Yang, Emily Pauling, and Lixia Zhang for technical help and assistance with animal procedures.
This work was supported by grants from the National Institutes of Health (R56 AI095657, R01 HL86576, and R01 HL082485), the National Natural Science Foundation of China (81160066 and 31370917), Guangxi Natural Science Foundation (2013GXNSFCA019012), the Science & Technology Planning Project of Guangxi Province (1140003-79), and the Science &Technology Planning Project of Guilin City (20110119-1-8).
The authors declare no competing financial interests.
Footnotes
Abbreviations used:
- IRI
- ischemia reperfusion injury
- MAC
- membrane attack complex
- MPO
- myeloperoxidase
- PHx
- partial hepatectomy
References
- Amsterdam, E.A., Stahl G.L., Pan H.L., Rendig S.V., Fletcher M.P., and Longhurst J.C.. 1995. Limitation of reperfusion injury by a monoclonal antibody to C5a during myocardial infarction in pigs. Am. J. Physiol. 268:H448–H457. [DOI] [PubMed] [Google Scholar]
- Arumugam, T.V., Shiels I.A., Strachan A.J., Abbenante G., Fairlie D.P., and Taylor S.M.. 2003. A small molecule C5a receptor antagonist protects kidneys from ischemia/reperfusion injury in rats. Kidney Int. 63:134–142. 10.1046/j.1523-1755.2003.00737.x [DOI] [PubMed] [Google Scholar]
- Arumugam, T.V., Woodruff T.M., Stocks S.Z., Proctor L.M., Pollitt S., Shiels I.A., Reid R.C., Fairlie D.P., and Taylor S.M.. 2004. Protective effect of a human C5a receptor antagonist against hepatic ischaemia-reperfusion injury in rats. J. Hepatol. 40:934–941. 10.1016/j.jhep.2004.02.017 [DOI] [PubMed] [Google Scholar]
- Atkinson, C., Song H., Lu B., Qiao F., Burns T.A., Holers V.M., Tsokos G.C., and Tomlinson S.. 2005. Targeted complement inhibition by C3d recognition ameliorates tissue injury without apparent increase in susceptibility to infection. J. Clin. Invest. 115:2444–2453. 10.1172/JCI25208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilzer, M., Roggel F., and Gerbes A.L.. 2006. Role of Kupffer cells in host defense and liver disease. Liver Int. 26:1175–1186. 10.1111/j.1478-3231.2006.01342.x [DOI] [PubMed] [Google Scholar]
- Blindenbacher, A., Wang X., Langer I., Savino R., Terracciano L., and Heim M.H.. 2003. Interleukin 6 is important for survival after partial hepatectomy in mice. Hepatology. 38:674–682. 10.1053/jhep.2003.50378 [DOI] [PubMed] [Google Scholar]
- Breitenstein, S., Apestegui C., Petrowsky H., and Clavien P.A.. 2009. “State of the art” in liver resection and living donor liver transplantation: a worldwide survey of 100 liver centers. World J. Surg. 33:797–803. 10.1007/s00268-008-9878-0 [DOI] [PubMed] [Google Scholar]
- Clavien, P.A., Petrowsky H., DeOliveira M.L., and Graf R.. 2007. Strategies for safer liver surgery and partial liver transplantation. N. Engl. J. Med. 356:1545–1559. 10.1056/NEJMra065156 [DOI] [PubMed] [Google Scholar]
- Cressman, D.E., Greenbaum L.E., DeAngelis R.A., Ciliberto G., Furth E.E., Poli V., and Taub R.. 1996. Liver failure and defective hepatocyte regeneration in interleukin-6-deficient mice. Science. 274:1379–1383. 10.1126/science.274.5291.1379 [DOI] [PubMed] [Google Scholar]
- Di Santo, E., Alonzi T., Poli V., Fattori E., Toniatti C., Sironi M., Ricciardi-Castagnoli P., and Ghezzi P.. 1997. Differential effects of IL-6 on systemic and central production of TNF: a study with IL-6-deficient mice. Cytokine. 9:300–306. 10.1006/cyto.1996.0169 [DOI] [PubMed] [Google Scholar]
- Diepenhorst, G.M., van Gulik T.M., and Hack C.E.. 2009. Complement-mediated ischemia-reperfusion injury: lessons learned from animal and clinical studies. Ann. Surg. 249:889–899. 10.1097/SLA.0b013e3181a38f45 [DOI] [PubMed] [Google Scholar]
- Dixon, E., Vollmer C.M. Jr, Bathe O.F., and Sutherland F.. 2005. Vascular occlusion to decrease blood loss during hepatic resection. Am. J. Surg. 190:75–86. 10.1016/j.amjsurg.2004.10.007 [DOI] [PubMed] [Google Scholar]
- Evans, Z.P., Ellett J.D., Schmidt M.G., Schnellmann R.G., and Chavin K.D.. 2008. Mitochondrial uncoupling protein-2 mediates steatotic liver injury following ischemia/reperfusion. J. Biol. Chem. 283:8573–8579. 10.1074/jbc.M706784200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fondevila, C., Shen X.D., Tsuchihashi S., Uchida Y., Freitas M.C., Ke B., Busuttil R.W., and Kupiec-Weglinski J.W.. 2008. The membrane attack complex (C5b-9) in liver cold ischemia and reperfusion injury. Liver Transpl. 14:1133–1141. 10.1002/lt.21496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcea, G., and Maddern G.J.. 2009. Liver failure after major hepatic resection. J. Hepatobiliary Pancreat. Surg. 16:145–155. 10.1007/s00534-008-0017-y [DOI] [PubMed] [Google Scholar]
- Greene, A.K., and Puder M.. 2003. Partial hepatectomy in the mouse: technique and perioperative management. J. Invest. Surg. 16:99–102. 10.1080/08941930390194424 [DOI] [PubMed] [Google Scholar]
- Hattori, R., Hamilton K.K., McEver R.P., and Sims P.J.. 1989. Complement proteins C5b-9 induce secretion of high molecular weight multimers of endothelial von Willebrand factor and translocation of granule membrane protein GMP-140 to the cell surface. J. Biol. Chem. 264:9053–9060. [PubMed] [Google Scholar]
- He, S., Atkinson C., Qiao F., Cianflone K., Chen X., and Tomlinson S.. 2009. A complement-dependent balance between hepatic ischemia/reperfusion injury and liver regeneration in mice. J. Clin. Invest. 119:2304–2316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helling, T.S. 2006. Liver failure following partial hepatectomy. HPB (Oxford). 8:165–174. 10.1080/13651820510035712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holt, D.S., Botto M., Bygrave A.E., Hanna S.M., Walport M.J., and Morgan B.P.. 2001. Targeted deletion of the CD59 gene causes spontaneous intravascular hemolysis and hemoglobinuria. Blood. 98:442–449. 10.1182/blood.V98.2.442 [DOI] [PubMed] [Google Scholar]
- Huang, Y., Qiao F., Atkinson C., Holers V.M., and Tomlinson S.. 2008. A novel targeted inhibitor of the alternative pathway of complement and its therapeutic application in ischemia/reperfusion injury. J. Immunol. 181:8068–8076. 10.4049/jimmunol.181.11.8068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang, H., Chen H.W., Evankovich J., Yan W., Rosborough B.R., Nace G.W., Ding Q., Loughran P., Beer-Stolz D., Billiar T.R., et al. 2013. Histones activate the NLRP3 inflammasome in Kupffer cells during sterile inflammatory liver injury. J. Immunol. 191:2665–2679. 10.4049/jimmunol.1202733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilgore, K.S., Schmid E., Shanley T.P., Flory C.M., Maheswari V., Tramontini N.L., Cohen H., Ward P.A., Friedl H.P., and Warren J.S.. 1997. Sublytic concentrations of the membrane attack complex of complement induce endothelial interleukin-8 and monocyte chemoattractant protein-1 through nuclear factor-κB activation. Am. J. Pathol. 150:2019–2031. [PMC free article] [PubMed] [Google Scholar]
- Kilgore, K.S., Park J.L., Tanhehco E.J., Booth E.A., Marks R.M., and Lucchesi B.R.. 1998. Attenuation of interleukin-8 expression in C6-deficient rabbits after myocardial ischemia/reperfusion. J. Mol. Cell. Cardiol. 30:75–85. 10.1006/jmcc.1997.0573 [DOI] [PubMed] [Google Scholar]
- Kim, G.H., Mocco J., Hahn D.K., Kellner C.P., Komotar R.J., Ducruet A.F., Mack W.J., and Connolly E.S. Jr. 2008. Protective effect of C5a receptor inhibition after murine reperfused stroke. Neurosurgery. 63:122–126. 10.1227/01.NEU.0000335079.70222.8D [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimberley, F.C., Sivasankar B., and Paul Morgan B.. 2007. Alternative roles for CD59. Mol. Immunol. 44:73–81. 10.1016/j.molimm.2006.06.019 [DOI] [PubMed] [Google Scholar]
- Kwan, W.H., van der Touw W., Paz-Artal E., Li M.O., and Heeger P.S.. 2013. Signaling through C5a receptor and C3a receptor diminishes function of murine natural regulatory T cells. J. Exp. Med. 210:257–268. 10.1084/jem.20121525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis, A.G., Köhl G., Ma Q., Devarajan P., and Köhl J.. 2008. Pharmacological targeting of C5a receptors during organ preservation improves kidney graft survival. Clin. Exp. Immunol. 153:117–126. 10.1111/j.1365-2249.2008.03678.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, W., Liang X., Kellendonk C., Poli V., and Taub R.. 2002. STAT3 contributes to the mitogenic response of hepatocytes during liver regeneration. J. Biol. Chem. 277:28411–28417. 10.1074/jbc.M202807200 [DOI] [PubMed] [Google Scholar]
- Markiewski, M.M., DeAngelis R.A., Strey C.W., Foukas P.G., Gerard C., Gerard N., Wetsel R.A., and Lambris J.D.. 2009. The regulation of liver cell survival by complement. J. Immunol. 182:5412–5418. 10.4049/jimmunol.0804179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mastellos, D., Papadimitriou J.C., Franchini S., Tsonis P.A., and Lambris J.D.. 2001. A novel role of complement: mice deficient in the fifth component of complement (C5) exhibit impaired liver regeneration. J. Immunol. 166:2479–2486. 10.4049/jimmunol.166.4.2479 [DOI] [PubMed] [Google Scholar]
- Moseley, E.L., Atkinson C., Sharples L.D., Wallwork J., and Goddard M.J.. 2010. Deposition of C4d and C3d in cardiac transplants: a factor in the development of coronary artery vasculopathy. J. Heart Lung Transplant. 29:417–423. 10.1016/j.healun.2009.12.018 [DOI] [PubMed] [Google Scholar]
- Papadimitriou, J.C., Ramm L.E., Drachenberg C.B., Trump B.F., and Shin M.L.. 1991. Quantitative analysis of adenine nucleotides during the prelytic phase of cell death mediated by C5b-9. J. Immunol. 147:212–217. [PubMed] [Google Scholar]
- Rehman, H., Sun J., Shi Y., Ramshesh V.K., Liu Q., Currin R.T., Lemasters J.J., and Zhong Z.. 2011. NIM811 prevents mitochondrial dysfunction, attenuates liver injury, and stimulates liver regeneration after massive hepatectomy. Transplantation. 91:406–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rus, H.G., Niculescu F.I., and Shin M.L.. 2001. Role of the C5b-9 complement complex in cell cycle and apoptosis. Immunol. Rev. 180:49–55. 10.1034/j.1600-065X.2001.1800104.x [DOI] [PubMed] [Google Scholar]
- Scolding, N.J., Houston W.A., Morgan B.P., Campbell A.K., and Compston D.A.. 1989. Reversible injury of cultured rat oligodendrocytes by complement. Immunology. 67:441–446. [PMC free article] [PubMed] [Google Scholar]
- Sekine, H., Kinser T.T., Qiao F., Martinez E., Paulling E., Ruiz P., Gilkeson G.S., and Tomlinson S.. 2011. The benefit of targeted and selective inhibition of the alternative complement pathway for modulating autoimmunity and renal disease in MRL/lpr mice. Arthritis Rheum. 63:1076–1085. 10.1002/art.30222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharkey, R.M., Natale A., Goldenberg D.M., and Mattes M.J.. 1991. Rapid blood clearance of immunoglobulin G2a and immunoglobulin G2b in nude mice. Cancer Res. 51:3102–3107. [PubMed] [Google Scholar]
- Sigala, F., Kostopanagiotou G., Andreadou I., Kavatzas N., Felekouras E., Sigalas P., Bastounis E., and Papalambros E.. 2004. Histological and lipid peroxidation changes after administration of 2-acetylaminofluorene in a rat liver injury model following selective periportal and pericentral damage. Toxicology. 196:155–163. 10.1016/j.tox.2003.12.005 [DOI] [PubMed] [Google Scholar]
- Singhal, R., Ganey P.E., and Roth R.A.. 2012. Complement activation in acetaminophen-induced liver injury in mice. J. Pharmacol. Exp. Ther. 341:377–385. 10.1124/jpet.111.189837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song, H., He C., Knaak C., Guthridge J.M., Holers V.M., and Tomlinson S.. 2003. Complement receptor 2-mediated targeting of complement inhibitors to sites of complement activation. J. Clin. Invest. 111:1875–1885. 10.1172/JCI17348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strey, C.W., Markiewski M., Mastellos D., Tudoran R., Spruce L.A., Greenbaum L.E., and Lambris J.D.. 2003. The proinflammatory mediators C3a and C5a are essential for liver regeneration. J. Exp. Med. 198:913–923. 10.1084/jem.20030374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taub, R. 2004. Liver regeneration: from myth to mechanism. Nat. Rev. Mol. Cell Biol. 5:836–847. 10.1038/nrm1489 [DOI] [PubMed] [Google Scholar]
- Tegla, C.A., Cudrici C., Patel S., Trippe R. III, Rus V., Niculescu F., and Rus H.. 2011. Membrane attack by complement: the assembly and biology of terminal complement complexes. Immunol. Res. 51:45–60. 10.1007/s12026-011-8239-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Topp, S.A., Upadhya G.A., and Strasberg S.M.. 2004. Cold preservation of isolated sinusoidal endothelial cells in MMP 9 knockout mice: effect on morphology and platelet adhesion. Liver Transpl. 10:1041–1048. 10.1002/lt.20216 [DOI] [PubMed] [Google Scholar]
- Tramontini, N.L., Kuipers P.J., Huber C.M., Murphy K., Naylor K.B., Broady A.J., and Kilgore K.S.. 2002. Modulation of leukocyte recruitment and IL-8 expression by the membrane attack complex of complement (C5b-9) in a rabbit model of antigen-induced arthritis. Inflammation. 26:311–319. 10.1023/A:1021420903355 [DOI] [PubMed] [Google Scholar]
- Triantafilou, K., Hughes T.R., Triantafilou M., and Morgan B.P.. 2013. The complement membrane attack complex triggers intracellular Ca2+ fluxes leading to NLRP3 inflammasome activation. J. Cell Sci. 126:2903–2913. 10.1242/jcs.124388 [DOI] [PubMed] [Google Scholar]
- Tuñón, M.J., Alvarez M., Culebras J.M., and González-Gallego J.. 2009. An overview of animal models for investigating the pathogenesis and therapeutic strategies in acute hepatic failure. World J. Gastroenterol. 15:3086–3098. 10.3748/wjg.15.3086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, H., Rollins S.A., Gao Z., Garcia B., Zhang Z., Xing J., Li L., Kellersmann R., Matis L.A., and Zhong R.. 1999. Complement inhibition with an anti-C5 monoclonal antibody prevents hyperacute rejection in a xenograft heart transplantation model. Transplantation. 68:1643–1651. 10.1097/00007890-199912150-00007 [DOI] [PubMed] [Google Scholar]
- Yamada, Y., Kirillova I., Peschon J.J., and Fausto N.. 1997. Initiation of liver growth by tumor necrosis factor: deficient liver regeneration in mice lacking type I tumor necrosis factor receptor. Proc. Natl. Acad. Sci. USA. 94:1441–1446. 10.1073/pnas.94.4.1441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, H.F., Yu J., Bajwa E., Morrison S.L., and Tomlinson S.. 1999. Targeting of functional antibody-CD59 fusion proteins to a cell surface. J. Clin. Invest. 103:55–61. 10.1172/JCI4607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, J., Hu W., Xing W., You T., Xu J., Qin X., and Peng Z.. 2011. The protective role of CD59 and pathogenic role of complement in hepatic ischemia and reperfusion injury. Am. J. Pathol. 179:2876–2884. 10.1016/j.ajpath.2011.08.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng, X., Zhang X., Feng B., Sun H., Suzuki M., Ichim T., Kubo N., Wong A., Min L.R., Budohn M.E., et al. 2008. Gene silencing of complement C5a receptor using siRNA for preventing ischemia/reperfusion injury. Am. J. Pathol. 173:973–980. 10.2353/ajpath.2008.080103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong, Z., Schwabe R.F., Kai Y., He L., Yang L., Bunzendahl H., Brenner D.A., and Lemasters J.J.. 2006. Liver regeneration is suppressed in small-for-size liver grafts after transplantation: involvement of c-Jun N-terminal kinase, cyclin D1, and defective energy supply. Transplantation. 82:241–250. 10.1097/01.tp.0000228867.98158.d2 [DOI] [PubMed] [Google Scholar]
- Zhong, Z., Ramshesh V.K., Rehman H., Currin R.T., Sridharan V., Theruvath T.P., Kim I., Wright G.L., and Lemasters J.J.. 2008. Activation of the oxygen-sensing signal cascade prevents mitochondrial injury after mouse liver ischemia-reperfusion. Am. J. Physiol. Gastrointest. Liver Physiol. 295:G823–G832. 10.1152/ajpgi.90287.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]