Introduction
Adrenergic signaling is activated in response to perceived stress, a complex process consisting of environmental and psychosocial factors that initiate a cascade of information-processing events in both the peripheral and central nervous system. Acutely, stress prepares a person to endure and ultimately survive a threat. However, under states of chronic stress, exposures to social stressors can have negative health implications if unrelenting and overwhelming. Until recently, stress was known to increase the risk of cardiac disease, infection and death [1]. Now, there is growing recognition of the role of chronic neuroendocrine stimulation in altering various cellular processes, such as adhesion of tumor cells to the extracellular matrix, dysregulated immune surveillance, cancer cell migration and invasion, angiogenesis, and cell survival, all of which are critical to cancer pathogenesis [2].
Reproduction is a vital process for the survival of the species and an important mechanism of natural selection. Chronic stress leads to a state of disrupted homeostasis, under which reproductive function may be impaired due to the central and peripheral actions of stress hormones. This imbalance has been linked to various reproductive dysfunctions ranging from mild disruption of menstrual cycles and polycystic ovary syndrome, all of which have been associated with an increased risk of gynecological malignancies such as ovarian and uterine cancers [3-9]. The profound effect of stress on human reproductive/endocrine physiology and the putative link to cancer has led scientists to examine the molecular connections between the neuro-endocrine response and the development of gynecological cancers.
In this article, we review the evidence linking the neuroendocrine stress response to disruptions in reproductive physiology, and ultimately to the potential development and progression of gynecologic malignancies with an emphasis on adrenergic signaling.
A-Neuroendocrine effects on the female reproductive system
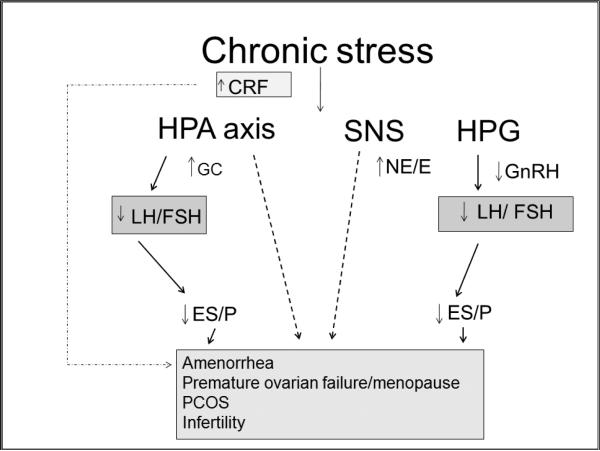
In response to environmental stress, the hypothalamus activates the hypothalamic-pituitary-adrenal (HPA) axis by secreting corticotropin-releasing factor (CRF) and arginine vasopressin. These signals in turn trigger the release of pituitary peptides produced by differential cleavage of pro-opiomelanocortin (POMC), most notably adrenocorticotrophic hormone (ACTH), enkephalins, and endorphins. ACTH then induces downstream release of glucocorticoids (GCs) from the adrenal cortex. The activation of the sympathetic nervous system (SNS) by CRF is mediated by direct innervation of the locus coeruleus in the brainstem, which leads to widespread release of norepinephrine (NE) throughout the brain and peripheral tissues. Activation of the SNS also stimulates the release of CRF by hypothalamic paraventricular nuclei. Thus, the stress-response system functions as a positive, bidirectional feedback loop: activation of one component of the system stimulates the other components at multiple levels [6, 10] . Centrally acting hypothalamic neuropeptide corticotropin-releasing hormone (CRH) inhibits hypothalamic gonadotropin-releasing hormone (GnRH) secretion, which in turn affects pituitary luteinizing hormone (LH) and follicular stimulating hormone (FSH) release and conversely inhibits gonadal estrogen (E) and progesterone (P) secretion. Systemically, CRH and associated receptors have been identified in most female reproductive tissues, including the ovary and uterus where they are involved in reproductive function [10]. In addition, glucocorticoids secreted from the adrenal cortex in response to HPA activation also suppress the gonadal axis via receptors in the female reproductive system [11]. (Figure 1) Chronic stress also activates the SNS which in turn directly innervates human ovaries to release dopamine and NE, both of which have been found to affect ovarian function [12]. Catecholaminergic receptors are expressed in both endovascular and endocrine cells responsible for ovarian follicular development [14, 15]. Thecal and granulosa cells also exhibit α- and β-adrenergic receptors which regulate cyclic AMP and steroid production [13, 14].
Figure 1. Potential effects of chronic stress on the reproductive system.
Chronic stress increases corticotropin-releasing factor (CRF) which in turn activates the hypothalamic-pituitary-adrenal (HPA) axis, sympathetic nervous system (SNS), and suppresses the hypothalamic-pituitary-gonadal (HPG) axis through decreases in gonadotropin-releasing hormone (GnRH). These changes lead to decreases in pituitary luteinizing hormone (LH) and follicular stimulating hormone (FSH) and conversely inhibit ovarian estrogen (ES) and progesterone (P) secretion. Ultimately chronic activation of the stress pathways, lead to potential disruptions in reproductive organ function. Epinephrine (E); Glucocorticoids (GC); Norepinephrine (NE); Polycystic Ovary syndrome (PCOS).
In summary, aside from promotion of cancer via disruption of the hypothalamic-pituitary-gonadal axis, chronic stress and its ensuing adrenergic response may lead to changes at the gonadal cellular level that can affect cancer development and progression.
B- Neuroendocrine signaling pathway and cellular function
The main adrenergic receptors involved in the stress response are the alpha (α–ADR) and beta (β-ADR) adrenergic receptors [15]. In regards to tumor biology and the adrenergic system, the data predominantly implicates the β–ADRs, a family of G-protein coupled receptors, which upon binding with ligands, activate a number of cAMP-dependent and independent phosphorylation signaling cascades. The main β-adrenergic receptors have been characterized according to their physiological functions. β1-ADRs have chronotropic effects (affecting heart rate and thereby increasing cardiac output), release renin, and increase lipolysis. β2-ADRs cause dilation of blood vessels and bronchioles, stimulate insulin release, and increase lipolysis, glycogenolysis, and gluconeogenesis. β3-ADRs are involved in lipolysis and mediate vascular relaxation but are activated by higher concentrations of catecholamines[16]. β-adrenergic signaling can affect a broad range of cancer-related molecular pathways via both direct (e.g. regulation of β-receptor–bearing tumor cells) and indirect (e.g. regulation of other β-receptor–bearing cells present in the tumor microenvironment cells, such as epithelial cells, vascular myocytes, pericytes, adipocytes, fibroblasts, and most lymphoid and myeloid immune cells) mechanisms.
A primary signaling cascade activated by the β-ADRs is the adenylyl cyclase/cAMP/protein kinase A (PKA) pathway [17]. PKA regulates a wide variety of cellular processes ranging from metabolism to cell-specific processes such as differentiation, morphology, motility, secretion, neurotransmission, and gene transcription. PKA induces phosphorylation of multiple transcription factors, including members of the cAMP response element binding protein/activating transcription factor (CREB/ATF) family. PKA can also cross-regulate activity of the pro-inflammatory nuclear factor kappa light chain enhancer of activated B cells (NF-κB) family and signal transducer and activator of transcription (STAT) family of transcription factors, and the growth-promoting Ets transcription factors, providing multiple signaling pathways for functional genomic regulation by catecholamines [18]. This signaling pathway can be inhibited by the neurotransmitter γ-aminobutyric acid (GABA) via the inhibitory G-protein coupled GABA receptor [18].
A second downstream signaling pathway activated by adrenergic signaling is the cAMP target, exchange protein activated by adenylyl cyclase, EPAC (also known as Rap guanine-nucleotide-exchange factor 3 (RAPGEF3)). EPAC was shown to control a number of cellular processes that were previously attributed to PKA, and overlap exists between the two pathways [19]. EPAC activates the Ras-like guanine triphosphatase Rap1A, which in turn stimulates downstream effectors B-Raf, MAP/extracellular signal-regulated kinase 1/ 2 (ERK) [20, 21]. In addition to the well-known effects of the MAPK pathway on cell growth and proliferation, EPAC signaling accounts for many cAMP-induced effects on cell morphology, motility, and secretion.
Neuroendocrine signaling can alter the immune system function. Certain cancers are firmly linked to a weakened immune system, especially virally-mediated cancers. Growth and progression of virally initiated tumors may be seen during times of high stress. Dysregulated cortisol and catecholamine release following chronic stress alters immune function. Described stress induced changes in the immune system include depressed natural killer (NK) cell cytotoxicity, reduced lymphocyte proliferation, secretion of pro-inflammatory cytokines such as interleukin-6 (IL-6) and tumor necrosis factor-alpha (TNF-α), and altered T-cell responses to antigen presentation ultimately lead to decreased immune surveillance [1] [22- 26].
Adrenergic signaling also plays a pivotal role in angiogenesis. The induction of angiogenesis is also an important mechanism by which tumors promote their own continued growth and metastasis [27, 28]. Angiogenesis is important during certain specialized reproductive organ functions including endometrial proliferation and embryo implantation. Activation of PKA by adrenergic receptors leads to triggering of endothelial NO synthase (eNOS) and of nitric oxide which causes vasodilation. Further, activation of β-ADRs results in the synthesis of proangiogenic factors, including vascular endothelial growth factor (VEGF), basic fibroblast growth factor (bFGF), and matrix metalloproteinases (MMP-2 and MMP-9) which activate pro-angiogenic cascades (ERK/MAPK) [27] and play pivotal roles in tumor angiogenesis, migration, and invasive potential [29].
C-Adrenergic signaling and ovarian cancer
Ovarian cancer is the second most common gynecologic malignancy and the most common cause of gynecologic cancer death in the United States [4]. The majority of ovarian malignancies (95 percent) are derived from epithelial cells; the remainder arises from other ovarian cell types (germ cell tumors, sex cord-stromal tumors). High grade serous epithelial ovarian carcinoma (EOC), fallopian tube, and primary peritoneal carcinomas are considered a single clinical entity due to their shared clinical behavior and treatment. There is accumulating evidence of a common pathogenesis for these carcinomas [30]. We will use the term EOC to refer this group of malignancies in the discussion that follows. The majority of women with EOC have advanced disease (Stages III or IV) at the time of diagnosis leading to overall 5 year survival rates of 30- 45%. Recent identification of biobehavioral factors contributing to tumor growth and disease progression provides new targets for therapy design.
Preclinical
There has been extensive pre-clinical data firmly linking activation of adrenergic signaling to the growth and progression of ovarian cancer. Most of the data involves animal models whereby rodents exposed to chronic stress had a significant increase in implanted tumors [10, 20].
In 2003, Lutgendorf and colleagues found that NE and isoproterenol (an adrenergic agonist) significantly enhanced VEGF production, which plays a critical role in angiogenesis [28]. These effects were blocked by the non-specific beta-antagonist propranolol, supporting a role for beta-adrenergic receptors in these in vitro effects. Reverse transcriptase-PCR studies demonstrated constitutive expression of adrenergic receptors in the cell lines studied [30].
NE and epinephrine (E) were later found to increase the in vitro invasive potential of ovarian cancer cells an effect that was completely blocked by propranolol. NE also increased tumor cell expression of MMP-2/MMP-9, and pharmacologic blockade of MMPs abrogated the effects of NE on tumor cell invasive potential [31]. In an orthotopic mouse model, chronic behavioral stress resulted in higher levels of tissue catecholamines, greater tumor burden and a more infiltrative pattern of ovarian cancer. These effects were mediated primarily through β2-ADR activation of PKA signaling pathway. Tumors in stressed animals showed increased vascularization and enhanced expression of VEGF, MMP-2 and MMP-9, and these effects could be abrogated by propranolol [17]. MMP-2/MMP-9 are important in the steps of ovarian cancer invasion and metastasis by mediating degradation of the extracellular matrix (ECM).
STAT factors are involved in inhibition of apoptosis, cell cycle dysregulation, induction of angiogenesis, and evasion of the immune response which are all key pathways in cancer pathogenesis [32]. Exposure of ovarian cancer cell lines to NE and E increases levels of phosphorylated STAT3 in a dose-dependent fashion. Activation of STAT3 can also be inhibited by propranolol. These effects were completely blocked by STAT3-targeting siRNA. In mice, treatment with liposome-incorporated siRNA directed against STAT3 significantly reduced isoproterenol-stimulated tumor growth [33].
Ovarian cancer cells exposed to NE exhibit lower levels of anoikis, a process by which cells enter apoptosis when separated from ECM and neighboring cells, leading to tumor cell survival. In an orthotopic mouse model of human ovarian cancer, restraint stress and the associated increases in NE/E protected cancer cells from anoikis and promoted their growth by activating focal adhesion kinase (FAK) which is a non-receptor tyrosine kinase that mediates physical attachment of cells to their ECM [34].
Interestingly, dopamine, an inhibitory catecholamine, blocks the effects of chronic stress on tumor growth both in vitro and in vivo. Mice subjected to stress by daily restraint had significantly increased tumor growth in both immunodeficient (SKOV3ip1 and HeyA8) and immunocompetent (ID8) ovarian cancer models. This increased growth was completely blocked with dopamine replacement. Dopamine treatment also blocked the stress-induced increase in angiogenesis as exhibited by decreased mean vessel density counts in these tumor samples. Dopamine significantly inhibited cell viability and stimulated apoptosis in vitro. Moreover, dopamine reduced cyclic AMP levels and inhibited NE and VEGF-induced Src kinase activation [35].
Translational/clinical
Motivated by previous indications that adrenergic signaling can promote tumor growth, investigators sought to determine whether similar dynamics occur in primary human ovarian cancer. Building on their preclinical knowledge, Lutgendorf and colleagues analyzed intratumoral NE levels from 68 patients with ovarian carcinoma by comparing patients with high depressive symptoms and low social support prior to surgery to patients with low levels of depressive symptoms. Higher tumor NE levels correlated with advanced stage (p=0.006) and higher grade (p=0.001) tumors. Patients with a perceived lack of social support also had significantly higher tumor NE (p=0.012). A similar trend was seen in human ascites NE levels (p=0.075) [36]. A study examining distress in ovarian cancer patients found that tumor samples from depressed patients with low social support showed significant elevations in MMP-9 in tumor-associated macrophages. Others have shown ovarian cancer patients with higher social support have lower tumor levels of MMP-9 and VEGF and lower serum and ascites VEGF [37, 38].
DNA microarray analyses of ovarian cancer samples identified 266 human transcripts that were differentially expressed in tumors from patients with elevated biobehavioral risk factors (high depressive symptoms and low social support) relative to grade- and stage-matched tumors from low-risk patients. Promoter-based bioinformatic analyses indicated increased activity of several beta-adrenergically-linked transcription control pathways (e.g. CREB/ATF, NF-kappaB/Rel, STAT, and Ets family transcription factors). Consistent with increased beta-adrenergic signaling, high biobehavioral stress correlates with increased intra-tumor concentrations of norepinephrine, but no difference in plasma norepinephrine. These data suggest that genome-wide transcriptional profiles are significantly altered in tumors from patients with adverse behavioral risk profiles, and they identify beta-adrenergic signal transduction as a likely mediator of those effects [18].
A prospective study examining how social support relates to long-term survival among consecutive patients with ovarian cancer showed that social attachment is associated with a survival advantage for patients with ovarian cancer. Patients were prospectively recruited during a pre-surgical clinic visit and completed surveys before surgery. One hundred sixty-eight patients with histologically confirmed epithelial ovarian cancer were observed from the date of surgery until death or December 2010. After adjusting for disease stage, grade, histology, residual disease and age, greater social attachment was associated with a lower likelihood of death (hazard ratio 0.87; p = .018). The median survival time for patients with low social attachment 3.35, in contrast, by study completion, 59% of patients with high social attachment were still alive after 4.70 years. This study highlights the importance of support activities during adjuvant therapy and how social support may act as stress buffer which leads to decreased adrenergic effects on the tumor[39].
Epidemiologic
The epidemiologic data specifically assessing the relationship between gynecological malignancies and adrenergic signaling has been sparse compared to the pre-clinical data available. Until recently, the data was limited to a case-control study conducted in eastern Massachusetts and New Hampshire. A total of 563 women with ovarian cancer diagnosed between 1992 and 1997 were interviewed and compared to 523 controls. The use of several psychotropic medications including amphetamines was assessed. Self-reported use of psychotropic medication for 6 months or longer was associated with a statistically significant increase in risk of invasive ovarian cancer. The association was largely confined to use of medications that act on the adrenergic response to inhibit the reuptake of dopamine and NE (OR, 2.9; CI, 1.3-6.4) [40].
Recently, data linking adrenergic blockade to cancer progression, led to two studies assessing the association between beta-blockers intake and EOC recurrence. A retrospective review of patients with EOC treated at a single institution with cytoreductive surgery followed by platinum-based chemotherapy, found that patients on beta-blocker had a 50% reduced chance of cancer death compared to non-users (p=0.03) [41]. A larger multi-institutional retrospective cohort of 1,425 patients with EOC found an improved survival for patients on beta-blockers compared to non-users and will be presented at the Society of Gynecologic Oncology 2013 Annual Meeting. Currently, a prospective clinical trial is underway to evaluate the use of beta-blockers peri-operatively and during chemotherapy for EOC patients [42].
D. Adrenergic signaling in cervix cancer
The primary cause of cervical cancer is infection with certain types of human papilloma virus (HPV) [43]. Worldwide, cervical cancer is the second most common cause of cancer deaths in women [44]. Virally mediated cancer may be promoted through β-ADR mediated immune suppression and other pathways [24-26]. Psychoneuroimmunological effects in patients with early cervical cancer cell transformation as demonstrated by the regression and disappearance of HPV are mediated via a cell-mediated immune response involving T helper type 1 (TH1) cells, NK cells, and interleukin (IL)-10 [45, 46]. In the setting of inadequate immunity and elevated psychological stress, HPV infection is more likely to persist [46-50]. Studies of other virally mediated cancers support the importance of β-ADRs in cancer progression. Importantly, oral squamous cell carcinoma (potentially HPV-related) cells express high levels of β2-ADR, and its expression is significantly correlated with lymph node metastasis and advanced tumor stages [51, 52].
Cigarette smoking may contribute to HPV infection, and HPV infection has been noted to persist in smokers [53]. Persistence of infection has been associated with presence of serum cotinine concentrations although the actual mechanism is unclear [53, 54]. However, researchers have noted that nicotine promotes gastric, pancreatic, lung, esophageal, and colon tumor and melanoma growth and angiogenesis via β-ADR activation with subsequent stimulation of cyclooxygenase-2, prostaglandin E2, and VEGF [55-60]. The mechanistic links between adrenergic stimulation and cervical cancer require additional work.
Translational/Clinical
Cervical cancer patients are of particular interest regarding the impact of the stress response given the evidence that women diagnosed with cervical cancer may develop greater disruption of quality of life (QOL) than patients with other types of cancer [61]. Further, multiple investigators have suggested an association between stress and dysplasia and that psychoneuroimmunological mechanisms play a role in human papillomavirus (HPV)-mediated cervical neoplasia, the initiation point for development of invasive cancer [46-48]. For example, Pereira showed that in HIV-positive women, increased life stress correlated with progression and persistence of HPV-mediated cervical neoplasia [46]. Further in a small study, cervical dysplasia patients had significant differences in cortisol levels compared with healthy controls [48].
As mentioned previously, in an animal model increased ovarian tumor vascularization and enhanced expression of VEGF and MMP-2 and -9 in tumor cells of stressed animals could be abrogated by the β-adrenergic antagonist propranolol [17]. Supporting a relationship between β-ADR stimulation and cervical cancer neoplasia, VEGF expression and microvessel density counts have been documented to progressively increase during the transition from normal cervical epithelium to squamous cell carcinoma [62-67] . Polymorphisms of VEGF genes may correlate with survival of early-stage cervical cancer by modulating angiogenesis as measured according to microvessel density [68]. Additionally, the HPV-18 E6 oncoprotein is known to induce VEGF transcription in a p53-dependent manner [69].
Decreasing the expression of VEGF in tumor cells by reducing the stress response may be an interesting method of cervical cancer therapy since microvessel density is associated with positive lymph node status, poor prognosis, and increased risk of recurrence in women with cervical cancer [64-67]. Five-year survival rates for cervical tumors with high and low microvessel densities are 50% and 65%, respectively [67]. Finally, serum VEGF levels are significantly elevated in cervical cancer patients with large and/or high-grade tumors or those who smoke [55, 70].
To date, much targeted therapy for recurrent cervical cancer has focused on the use of angiogenesis inhibitors. In heavily pre-treated cervical patients, these trials have demonstrated clinically significant response rates of 10-34%, with stable disease rates of 23-33% and limited response durations. However, agents used in these trials, such as bevacizumab, are expensive and until significant improvement in progression-free survival (PFS) and overall survival (OS) is demonstrated, their use will be relatively restricted to clinical trials [71, 72]. Research is clearly needed for development of effective treatment of this preventable cancer.
Interleukins, stress, and cervical cancer development
IL-10 is an immunosuppressive mediator of stress-induced immunosuppression. IL-10 is extremely important for the initial transformation of cervical dysplasia to premalignant lesions. Investigators have shown that IL-10 expression is directly proportional to the development of HPV-induced cervical cancer [73]. In mouse models, stress-induced increases in IL-10 expression can be prevented by treatment with nadolol, a β1/2 blocker as well as a benzodiazepine, an anxiolytic [45]. In a study of a behavioral (telephone-mediated) intervention targeting cervical cancer survivors, improvement in QOL was seen in the intervention cohort and importantly correlated with an inverse association between modulation of QOL and serum levels of IL-10 [74].
Chronic stress is associated with increased IL-6, IL-8 and IL-10 [75-77]. Although relatively little data on IL-6 and cervical cancer are available, interestingly the stress hormones (norepinephrine and cortisol) can increase IL-6 mRNA production in oral squamous cell carcinoma cells, and this effect is blocked by treatment with propranolol. In a study of patients with cervical dysplasia, IL-6 and IL-8 concentrations in cervicovaginal washings were progressively higher in non-affected patients, cervical carcinoma in situ (non-invasive dysplasia) patients, and cervical cancer patients respectively [45]. IL-8 has a key role in regulation of angiogenesis, chemotaxis, and enhancement of growth in a variety of tumors. IL-8 contributes to the migration of tumor cells toward blood vessels and provides proliferative and anti-apoptotic signals to tumor cells and is produced by tumor cells and macrophages [78, 79]. IL-8 overexpression is associated with advanced disease stage, high tumor grade, and decreased OS in cervical cancer patients [80]. Furthermore, IL-8 is thought to have an effect on tumor growth owing to expression of an angiogenic factor supplied by macrophages within and around cervical tumors. IL-8 expression has correlated with microvessel counts in cervical cancer patients, and microvessel density correlates with radioresistance and decreased OS [80, 81]. Poorly oxygenated cervical tumors have increased microvessel densities, and this has correlated with decreased survival duration [81].
Due to the younger age of cervical cancer patients, it may be difficult to detect beta-blocker effects in an epidemiologic study. However, we have recently completed initial immunohistochemistry work documenting increased β1-ADR expression and β2-ADR expression in a cohort of tissue from cervical cancer patients. β2-ADR was significantly related to survival (p = 0.038) (SGO submission 2013).
Psychological interventions aimed at stress reduction may help cancer patients through a variety of mechanisms, including reducing the severity of side effects of cancer and its treatment, as well as anxiety and distress. More recently, these interventions have been linked with improved immune function which may result in “improved OS durations” [46, 74, 82]. Based on our preliminary observations, we plan to initiate a clinical trial evaluating the effect of β-adrenergic blockade using propranolol and stress reduction to treat advanced incurable cervical patients. This trial will include translational end-points as well as QOL assessments. We believe that targeting the effects of psychological and physiologic stress with pharmacologic, psychological interventions, or both may be extremely effective in this world-wide killer of women.
E- Adrenergic signaling and endometrial cancer
Endometrial cancer is the most common gynecologic malignancy in the United States, and each year the number of patients diagnosed with endometrial cancer increases due to the obesity epidemic fully underway in the Western world and starting even in developing Eastern nations. Risk factors for developing endometrial cancer partially stemming from obesity related insulin resistance include irregular menstrual cycles and polycystic ovarian syndrome [5]. For over a decade, numerous studies and meta-analysis have shown that polycystic ovary syndrome has been linked to the development of endometrial cancers [5, 83]. PCOS patients have been shown to have increased HPA-axis mediators such as ACTH and cortisol as well as IL-6 which are known to affect the tumor environment in other cancers [84, 85].
Preclinical/translational
To date, there is limited information about the importance of adrenergic receptors in endometrial cancer. Hypothetically, β-ADRs may play a more specialized role in development of uterine cancer. In contrast to the other gynecologic cancers where the β2-ADR is important, the limited data on beta adrenergic receptors is focused on the β3-ADR since it is one of the genes important in obesity and insulin resistance [86]. However, this area will require further investigation to understand the full scope of such effects.
The endometrial metabolic syndrome is characterized by abdominal obesity, impaired insulin sensitivity, hypertension, dyslipidemia, hyperglycemia, and a systemic pro-inflammatory state [87, 88]. In an in vivo model, chronic stress combined with a high-fat/high sugar diet led to increase of the sympathetic neurotransmitter, neuropeptide Y (NPY) accelerating diet-induced obesity and insulin resistance are both key risk factors for endometrial cancer [89]. The neuropeptide Y5 receptor has been cloned in the endometrial cancer cell line HEC-1b, but no further investigations have occurred [90]. In summary, the role of adrenergic pathways in endometrial cancer is yet to be elucidated.
F-Future directions
Collectively, there is strong and growing evidence to suggest the importance of adrenergic pathways in gynecologic malignancies especially in ovarian cancer. Despite the significant progress seen in the past decade linking biobehavioral factors and tumor progression, additional research is required to completely understand how stress hormones affect cancer initiation, growth and metastatic cascades. Such research efforts may lead to the development of new behavioral and pharmacological alternatives for the treatment of cancer patients especially in the context of individualized medicine.
References
- 1.Glaser R, Kiecolt-Glaser JK. Stress-induced immune dysfunction: implications for health. Nat Rev Immunol. 2005;5:243–251. doi: 10.1038/nri1571. [DOI] [PubMed] [Google Scholar]
- 2.Thaker PH, Sood AK. Neuroendocrine influences on cancer biology. Semin Cancer Biol. 2008;18:164–170. doi: 10.1016/j.semcancer.2007.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Diamanti-Kandarakis E, Economou F. Stress in women: metabolic syndrome and polycystic ovary syndrome. Ann N Y Acad Sci. 2006;1083:54–62. doi: 10.1196/annals.1367.006. [DOI] [PubMed] [Google Scholar]
- 4.Hunn J, Rodriguez GC. Ovarian cancer: etiology, risk factors, and epidemiology. Clin Obstet Gynecol. 2012;55:3–23. doi: 10.1097/GRF.0b013e31824b4611. [DOI] [PubMed] [Google Scholar]
- 5.Cramer DW. The epidemiology of endometrial and ovarian cancer. Hematol Oncol Clin North Am. 2012;26:1–12. doi: 10.1016/j.hoc.2011.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Breen KM, Karsch FJ. New insights regarding glucocorticoids, stress and gonadotropin suppression. Front Neuroendocrinol. 2006;27:233–245. doi: 10.1016/j.yfrne.2006.03.335. [DOI] [PubMed] [Google Scholar]
- 7.Barria A, Leyton V, Ojeda SR, Lara HE. Ovarian steroidal response to gonadotropins and beta-adrenergic stimulation is enhanced in polycystic ovary syndrome: role of sympathetic innervation. Endocrinology. 1993;133:2696–2703. doi: 10.1210/endo.133.6.8243293. [DOI] [PubMed] [Google Scholar]
- 8.Pal L, Zhang K, Zeitlian G, Santoro N. Characterizing the reproductive hormone milieu in infertile women with diminished ovarian reserve. Fertil Steril. 2010;93:1074–1079. doi: 10.1016/j.fertnstert.2008.10.069. [DOI] [PubMed] [Google Scholar]
- 9.Bleil ME, Adler NE, Pasch LA, et al. Depressive symptomatology, psychological stress, and ovarian reserve: a role for psychological factors in ovarian aging? Menopause. 2012 doi: 10.1097/gme.0b013e31825540d8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wypior G, Jeschke U, Kurpisz M, Szekeres-Bartho J. Expression of CRH, CRH-related peptide and CRH receptor in the ovary and potential CRH signalling pathways. J Reprod Immunol. 2011;90:67–73. doi: 10.1016/j.jri.2011.04.009. [DOI] [PubMed] [Google Scholar]
- 11.Kalantaridou SN, Makrigiannakis A, Zoumakis E, Chrousos GP. Stress and the female reproductive system. J Reprod Immunol. 2004;62:61–68. doi: 10.1016/j.jri.2003.09.004. [DOI] [PubMed] [Google Scholar]
- 12.D'Albora H, Anesetti G, Lombide P, et al. Intrinsic neurons in the mammalian ovary. Microsc Res Tech. 2002;59:484–489. doi: 10.1002/jemt.10231. [DOI] [PubMed] [Google Scholar]
- 13.Mayerhofer A, Smith GD, Danilchik M, et al. Oocytes are a source of catecholamines in the primate ovary: evidence for a cell-cell regulatory loop. Proc Natl Acad Sci U S A. 1998;95:10990–10995. doi: 10.1073/pnas.95.18.10990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Aguado LI, Petrovic SL, Ojeda SR. Ovarian beta-adrenergic receptors during the onset of puberty: characterization, distribution, and coupling to steroidogenic responses. Endocrinology. 1982;110:1124–1132. doi: 10.1210/endo-110-4-1124. [DOI] [PubMed] [Google Scholar]
- 15.Carrasco GA, Van de Kar LD. Neuroendocrine pharmacology of stress. Eur J Pharmacol. 2003;463:235–272. doi: 10.1016/s0014-2999(03)01285-8. [DOI] [PubMed] [Google Scholar]
- 16.Santos IN, Spadari-Bratfisch RC. Stress and cardiac beta adrenoceptors. Stress. 2006;9:69–84. doi: 10.1080/10253890600771858. [DOI] [PubMed] [Google Scholar]
- 17.Thaker PH, Han LY, Kamat AA, et al. Chronic stress promotes tumor growth and angiogenesis in a mouse model of ovarian carcinoma. Nat Med. 2006;12:939–944. doi: 10.1038/nm1447. [DOI] [PubMed] [Google Scholar]
- 18.Lutgendorf SK, DeGeest K, Sung CY, et al. Depression, social support, and beta-adrenergic transcription control in human ovarian cancer. Brain Behav Immun. 2009;23:176–183. doi: 10.1016/j.bbi.2008.04.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.de Rooij J, Zwartkruis FJ, Verheijen MH, et al. Epac is a Rap1 guanine-nucleotide-exchange factor directly activated by cyclic AMP. Nature. 1998;396:474–477. doi: 10.1038/24884. [DOI] [PubMed] [Google Scholar]
- 20.Dugan LL, Kim JS, Zhang Y, et al. Differential effects of cAMP in neurons and astrocytes. Role of B-raf. J Biol Chem. 1999;274:25842–25848. doi: 10.1074/jbc.274.36.25842. [DOI] [PubMed] [Google Scholar]
- 21.Takahashi H, Honma M, Miyauchi Y, et al. Cyclic AMP differentially regulates cell proliferation of normal human keratinocytes through ERK activation depending on the expression pattern of B-Raf. Arch Dermatol Res. 2004;296:74–82. doi: 10.1007/s00403-004-0478-z. [DOI] [PubMed] [Google Scholar]
- 22.Lutgendorf SK, Moore MB, Bradley S, et al. Distress and expression of natural killer receptors on lymphocytes. Brain Behav Immun. 2005;19:185–194. doi: 10.1016/j.bbi.2004.11.003. [DOI] [PubMed] [Google Scholar]
- 23.Lutgendorf SK, Weinrib AZ, Penedo F, et al. Interleukin-6, cortisol, and depressive symptoms in ovarian cancer patients. J Clin Oncol. 2008;26:4820–4827. doi: 10.1200/JCO.2007.14.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lutgendorf SK, Lamkin DM, DeGeest K, et al. Depressed and anxious mood and T-cell cytokine expressing populations in ovarian cancer patients. Brain Behav Immun. 2008;22:890–900. doi: 10.1016/j.bbi.2007.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Glaser R, Padgett DA, Litsky ML, et al. Stress-associated changes in the steady-state expression of latent Epstein-Barr virus: implications for chronic fatigue syndrome and cancer. Brain Behav Immun. 2005;19:91–103. doi: 10.1016/j.bbi.2004.09.001. [DOI] [PubMed] [Google Scholar]
- 26.Chrousos GP. Stress and disorders of the stress system. Nat Rev Endocrinol. 2009;5:374–381. doi: 10.1038/nrendo.2009.106. [DOI] [PubMed] [Google Scholar]
- 27.Storch CH, Hoeger PH. Propranolol for infantile haemangiomas: insights into the molecular mechanisms of action. Br J Dermatol. 2010;163:269–274. doi: 10.1111/j.1365-2133.2010.09848.x. [DOI] [PubMed] [Google Scholar]
- 28.Lutgendorf S, Cole S, Costanzo ES, et al. Stress-related mediators stimulate vascular endothelial growth factor secretaion by two ovarian cancer cell lines. Clin Cancer Res. 2003;12:4514–4521. [PubMed] [Google Scholar]
- 29.Cole SW, Sood AK. Molecular pathways: Beta-adrenergic signaling in cancer. Clin Cancer Res. 2012;18:1201–1206. doi: 10.1158/1078-0432.CCR-11-0641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Salvador S, Gilks B, Kobel M, et al. The fallopian tube: primary site of most pelvic high-grade serous carcinomas. Int J Gynecol Cancer. 2009;19:58–64. doi: 10.1111/IGC.0b013e318199009c. [DOI] [PubMed] [Google Scholar]
- 31.Sood AK, Bhatty R, Kamat AA, et al. Stress hormone-mediated invasion of ovarian cancer cells. Clin Cancer Res. 2006;12:369–375. doi: 10.1158/1078-0432.CCR-05-1698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Turkson J. STAT proteins as novel targets for cancer drug discovery. Expert Opin Ther Targets. 2004;8:409–422. doi: 10.1517/14728222.8.5.409. [DOI] [PubMed] [Google Scholar]
- 33.Landen CN, Lin YG, Armaiz-Pena G, et al. Neuroendocrine modulation of signal transducer and activator of transcription-3 in ovarian cancer. Cancer Res. 2007;67:10389–10396. doi: 10.1158/0008-5472.CAN-07-0858. [DOI] [PubMed] [Google Scholar]
- 34.Sood AK, Armaiz-Pena GN, Halder J, et al. Adrenergic modulation of focal adhesion kinase protects human ovarian cancer cells from anoikis. J Clin Invest. 2010;120:1515–1523. doi: 10.1172/JCI40802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Moreno-Smith M, Lu C, Shahzad MM, et al. Dopamine Blocks Stress-Mediated Ovarian Carcinoma Growth. Clin Cancer Res. 2011;17:3649–3659. doi: 10.1158/1078-0432.CCR-10-2441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lutgendorf SK, DeGeest K, Dahmoush L, et al. Social isolation is associated with elevated tumor norepinephrine in ovarian carcinoma patients. Brain Behav Immun. 2011;25:250–255. doi: 10.1016/j.bbi.2010.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lutgendorf SK, Lamkin DM, Jennings NB, et al. Biobehavioral influences on matrix metalloproteinase expression in ovarian carcinoma. Clin Cancer Res. 2008;14:6839–6846. doi: 10.1158/1078-0432.CCR-08-0230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lutgendorf SK, Johnsen EL, Cooper B, et al. Vascular endothelial growth factor and social support in patients with ovarian carcinoma. Cancer. 2002;95:808–815. doi: 10.1002/cncr.10739. [DOI] [PubMed] [Google Scholar]
- 39.Lutgendorf SK, De Geest K, Bender D, et al. Social influences on clinical outcomes of patients with ovarian cancer. J Clin Oncol. 2012;30:2885–2890. doi: 10.1200/JCO.2011.39.4411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Harlow BL, Cramer DW, Baron JA, et al. Psychotropic medication use and risk of epithelial ovarian cancer. Cancer Epidemiol Biomarkers Prev. 1998;7:697–702. [PubMed] [Google Scholar]
- 41.Diaz E, Karlan B, Cass I, et al. Impact of beta blockers on epithelial ovarian cancer survival. Gynecol Oncol. 2011;120:S2–S133. doi: 10.1016/j.ygyno.2012.07.102. [DOI] [PubMed] [Google Scholar]
- 42.Trials.gov C . Therapeutic targeting of stress factors in ovarian cancer patients. Washington University School of Medicine; 2011. [Google Scholar]
- 43.Walboomers JM, Jacobs MV, Manos MM, et al. Human papillomavirus is a necessary cause of invasive cervical cancer worldwide. J Pathol. 1999;189:12–19. doi: 10.1002/(SICI)1096-9896(199909)189:1<12::AID-PATH431>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 44.Jemal A, Bray F, Center MM, et al. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 45.Bermudez-Morales VH, Peralta-Zaragoza O, Alcocer-Gonzalez JM, et al. IL-10 expression is regulated by HPV E2 protein in cervical cancer cells. Mol Med Report. 2011;4:369–375. doi: 10.3892/mmr.2011.429. [DOI] [PubMed] [Google Scholar]
- 46.Pereira DB, Antoni MH, Fletcher MA, O'Sullivan MJ. Cognitive behavioral stress management (CBSM) effects on regression of cervical dysplasia among HIV+ women. [Abstract]. Psychosom Med. 2005;67:1152. [Google Scholar]
- 47.Jensen SE, Lehman B, Antoni MH, Pereira DB. Virally mediated cervical cancer in the iatrogenically immunocompromised: applications for psychoneuroimmunology. Brain Behav Immun. 2007;21:758–766. doi: 10.1016/j.bbi.2007.01.003. [DOI] [PubMed] [Google Scholar]
- 48.Coker AL, Bond S, Madeleine MM, et al. Psychosocial stress and cervical neoplasia risk. Psychosom Med. 2003;65:644–651. doi: 10.1097/01.psy.0000041471.57895.08. [DOI] [PubMed] [Google Scholar]
- 49.De Punzio C, Salvestroni C, Guazzelli G, et al. Stess and cervical dysplasia. Eur J Gynaecol Oncol. 1998;XIX:287–290. [PubMed] [Google Scholar]
- 50.Eustace D, Han X, Gooding R, et al. Interleukin-6 (IL-6) functions as an autocrine growth factor in cervical carcinomas in vitro. Gynecol Oncol. 1993;50:15–19. doi: 10.1006/gyno.1993.1156. [DOI] [PubMed] [Google Scholar]
- 51.Shang ZJ, Liu K, Liang DF. Expression of beta2-adrenergic receptor in oral squamous cell carcinoma. J Oral Pathol Med. 2009;38:371–376. doi: 10.1111/j.1600-0714.2008.00691.x. [DOI] [PubMed] [Google Scholar]
- 52.Bancroft CC, Chen Z, Dong G, et al. Coexpression of proangiogenic factors IL-8 and VEGF by human head and neck squamous cell carcinoma involves coactivation by MEK-MAPK and IKK-NF-kappaB signal pathways. Clin Cancer Res. 2001;7:435–442. [PubMed] [Google Scholar]
- 53.Jensen KE, Schmiedel S, Frederiksen K, et al. Risk for cervical intraepithelial neoplasia grade 3 or worse in relation to smoking among women with persistent human papillomavirus infection. Cancer Epidemiol Biomarkers Prev. 2012 doi: 10.1158/1055-9965.EPI-12-0663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Samir R, Asplund A, Tot T, et al. Tissue tumor marker expression in smokers, including serum cotinine concentrations, in women with cervical intraepithelial neoplasia or normal squamous cervical epithelium. Am J Obstet Gynecol. 2010;202:579, e571–577. doi: 10.1016/j.ajog.2009.11.034. [DOI] [PubMed] [Google Scholar]
- 55.Mathur RS, Mathur SP, Young RC. Up-regulation of epidermal growth factor-receptors (EGF-R) by nicotine in cervical cancer cell lines: this effect may be mediated by EGF. Amer J of Reproductive Immunol. 2000;44:114–120. doi: 10.1111/j.8755-8920.2000.440207.x. [DOI] [PubMed] [Google Scholar]
- 56.Shin VY, Jin HC, Ng EK, et al. Nicotine and 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone induce cyclooxygenase-2 activity in human gastric cancer cells: Involvement of nicotinic acetylcholine receptor (nAChR) and beta-adrenergic receptor signaling pathways. Toxicol Appl Pharmacol. 2008;233:254–261. doi: 10.1016/j.taap.2008.08.012. [DOI] [PubMed] [Google Scholar]
- 57.Al-Wadei MH, Al-Wadei HA, Schuller HM. Pancreatic Cancer Cells and Normal Pancreatic Duct Epithelial Cells Express an Autocrine Catecholamine Loop that Is Activated by Nicotinic Acetylcholine Receptors alpha3, alpha5, and alpha7. Mol Cancer Res. 2012;10:239–249. doi: 10.1158/1541-7786.MCR-11-0332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Al-Wadei HA, Al-Wadei MH, Schuller HM. Cooperative regulation of non-small cell lung carcinoma by nicotinic and beta-adrenergic receptors: a novel target for intervention. PLoS One. 2012;7:e29915. doi: 10.1371/journal.pone.0029915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Li P, Wu WK, Wong HP, et al. Chloroform extract of cigarette smoke induces proliferation of human esophageal squamous-cell carcinoma cells: modulation by beta-adrenoceptors. Drug Chem Toxicol. 2009;32:175–181. doi: 10.1080/01480540902875253. [DOI] [PubMed] [Google Scholar]
- 60.Liu X, Wu WK, Yu L, et al. Epinephrine stimulates esophageal squamous-cell carcinoma cell proliferation via beta-adrenoceptor-dependent transactivation of extracellular signal-regulated kinase/cyclooxygenase-2 pathway. J Cell Biochem. 2008;105:53–60. doi: 10.1002/jcb.21802. [DOI] [PubMed] [Google Scholar]
- 61.Wenzel L, DeAlba I, Habbal R, et al. Quality of life in long-term cervical cancer survivors. Gynecol Oncol. 2005;97:310–317. doi: 10.1016/j.ygyno.2005.01.010. [DOI] [PubMed] [Google Scholar]
- 62.Lee JS, Kim HS, Park JT, et al. Expression of vascular endothelial growth factor in the progression of cervical neoplasia and its relation to angiogenesis and p53 status. Anal Quant Cytol Histol. 2003;25:303–311. [PubMed] [Google Scholar]
- 63.Gaffney DK, Haslam D, Tsodikov A, et al. Epidermal growth factor receptor (EGFR) and vascular endothelial growth factor (VEGF) negatively affect overall survival in carcinoma of the cervix treated with radiotherapy. International Journal of Radiation Oncology, Biology, Physics. 2003;56:922–928. doi: 10.1016/s0360-3016(03)00209-8. [DOI] [PubMed] [Google Scholar]
- 64.Botting SK, Fouad H, Elwell K, et al. Prognostic significance of peritumoral lymphatic vessel density and vascular endothelial growth factor receptor 3 in invasive squamous cell cervical cancer. Transl Oncol. 2010;2010:170–175. doi: 10.1593/tlo.09292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Dinh TV, Hannigan EV, Smith ER, et al. Tumor angiogenesis as a predictor of recurrence in Stage 1b squamous cell carcinoma of the cervix. OB.GYN. 1996;87:751–754. doi: 10.1016/0029-7844(96)00039-7. [DOI] [PubMed] [Google Scholar]
- 66.Cooper RA, Wilks DP, Logue JP, et al. High tumor angiogenesis is associated with poorer survival in carcinoma of the cervix treated with radiotherapy. Clin Cancer Res. 1998;4:2795–2800. [PubMed] [Google Scholar]
- 67.Srivastava S, Gupta A, Agarwal GG, et al. Correlation of serum vascular endothelial growth factor with clinicopathological parameters in cervical cancer. Biioscience Trends. 2009;3:144–150. [PubMed] [Google Scholar]
- 68.Kim YH, Kim MA, Park IA, et al. VEGF polymorphisms in early cervical cancer susceptibility, angiogenesis, and survival. Gynecol Oncol. 2010;119:232–236. doi: 10.1016/j.ygyno.2010.07.035. [DOI] [PubMed] [Google Scholar]
- 69.Clere N, Bermont L, Fauconnet S, et al. The human papillomavirus type 18 E6 oncoprotein induces Vascular Endothelial Growth Factor 121 (VEGF121) transcription from the promoter through a p53-independent mechanism. Exp Cell Res. 2007;313:3239–3250. doi: 10.1016/j.yexcr.2007.06.029. [DOI] [PubMed] [Google Scholar]
- 70.Lane D, Gray EA, Mathur RS, Mathur SP. Up-regulation of vascular endothelial growth factor-C by nicotine in cervical cancer cell lines. Am J Reproductive Immunol. 2005;53:153–158. doi: 10.1111/j.1600-0897.2005.00259.x. [DOI] [PubMed] [Google Scholar]
- 71.Mackay HJ, Tinker A, Winquist E, et al. A phase II study of sunitinib in patients with locally advanced or metastatic cervical carcinoma: NCIC CTG Trial IND.184. Gynecol Oncol. 2010;116:163–167. doi: 10.1016/j.ygyno.2009.08.012. [DOI] [PubMed] [Google Scholar]
- 72.Wright JD, Viviano D, Powell MA, et al. Bevacizumab combination therapy in heavily pretreated, recurrent cervical cancer. Gynecol Oncol. 2006;103:489–493. doi: 10.1016/j.ygyno.2006.03.023. [DOI] [PubMed] [Google Scholar]
- 73.Curtin NM, Mills KH, Connor TJ. Psychological stress increases expression of IL-10 and its homolog IL-19 via beta-adrenoceptor activation: reversal by the anxiolytic chlordiazepoxide. Brain Behav Immun. 2009;23:371–379. doi: 10.1016/j.bbi.2008.12.010. [DOI] [PubMed] [Google Scholar]
- 74.Nelson EL, Wenzel LB, Osann K, et al. Stress, immunity, and cervical cancer: biobehavioral outcomes of a randomized clinical trial. Cancer Therapy: Clinical. 2008;14:2111–2118. doi: 10.1158/1078-0432.CCR-07-1632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Tjiong MY, van der Vange N, ten Kate FJ, et al. Increased IL-6 and IL-8 levels in cervicovaginal secretions of patients with cervical cancer. Gynecol Oncol. 1999;73:285–291. doi: 10.1006/gyno.1999.5358. [DOI] [PubMed] [Google Scholar]
- 76.Zhou D, Kusnecov AW, Shurin MR, et al. Exposure to physical and psychological stressors elevates plasma interleukin 6: relationship to the activation of hypothalamic-pituitary-adrenal axis. Endocrinology. 1993;133:2523–2530. doi: 10.1210/endo.133.6.8243274. [DOI] [PubMed] [Google Scholar]
- 77.Lutgendorf S, Garad L, Buckwalter K, et al. Life stress, mood disturbance, and elevated IL-6 in healthy older women. J Gerontology. Series A Biol Sci & Med Sci. 1999:M434–439. doi: 10.1093/gerona/54.9.m434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Xu L, Fidler IJ. Interleukin 8: an autocrine growth factor for human ovarian cancer. Oncol Res. 2000;12:97–106. doi: 10.3727/096504001108747567. [DOI] [PubMed] [Google Scholar]
- 79.Chen JJ, Yao PL, Yuan A, et al. Up-regulation of tumor interleukin-8 expression by infiltrating macrophages: its correlation with tumor angiogenesis and patient survival in non-small cell lung cancer. Clin Cancer Res. 2003;9:729–737. [PubMed] [Google Scholar]
- 80.Fujimoto J, Sakaguchi H, Aoki I, Tamaya T. Clinical implications of expression of interleukin 8 related to angiogenesis in uterine cervical cancers. Cancer Res. 2000;60:2632–2635. [PubMed] [Google Scholar]
- 81.Cooper RA, West CML, Wilks DP, et al. Tumour vascularity is a significant prognostic factor for cervic carcinoma treated with radiotherapy: independence from tumour radiosensitivity. Br J Cancer. 1999;81:354–358. doi: 10.1038/sj.bjc.6690700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Lutgendorf SK, Mullen-Houser E, Russell D, et al. Preservation of immune function in cervical cancer patients during chemoradiation using a novel integrative approach. Brain Behav Immun. 2010;24:1231–1240. doi: 10.1016/j.bbi.2010.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Chittenden BG, Fullerton G, Maheshwari A, Bhattacharya S. Polycystic ovary syndrome and the risk of gynaecological cancer: a systematic review. Reprod Biomed Online. 2009;19:398–405. doi: 10.1016/s1472-6483(10)60175-7. [DOI] [PubMed] [Google Scholar]
- 84.Antoni MH, Lutgendorf SK, Cole SW, et al. The influence of bio-behavioural factors on tumour biology: pathways and mechanisms. Nat Rev Cancer. 2006;6:240–248. doi: 10.1038/nrc1820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Benson S, Arck PC, Tan S, et al. Disturbed stress responses in women with polycystic ovary syndrome. Psychoneuroendocrinology. 2009;34:727–735. doi: 10.1016/j.psyneuen.2008.12.001. [DOI] [PubMed] [Google Scholar]
- 86.Iwamoto I, Fujino T, Douchi T, Nagata Y. Association of estrogen receptor alpha and beta3-adrenergic receptor polymorphisms with endometrial cancer. Obstet Gynecol. 2003;102:506–511. doi: 10.1016/s0029-7844(03)00578-7. [DOI] [PubMed] [Google Scholar]
- 87.Friedenreich CM, Biel RK, Lau DC, et al. Case-control study of the metabolic syndrome and metabolic risk factors for endometrial cancer. Cancer Epidemiol Biomarkers Prev. 2011;20:2384–2395. doi: 10.1158/1055-9965.EPI-11-0715. [DOI] [PubMed] [Google Scholar]
- 88.Lambert GW, Straznicky NE, Lambert EA, et al. Sympathetic nervous activation in obesity and the metabolic syndrome--causes, consequences and therapeutic implications. Pharmacol Ther. 2010;126:159–172. doi: 10.1016/j.pharmthera.2010.02.002. [DOI] [PubMed] [Google Scholar]
- 89.Kuo LE, Czarnecka M, Kitlinska JB, et al. Chronic stress, combined with a high-fat/high-sugar diet, shifts sympathetic signaling toward neuropeptide Y and leads to obesity and the metabolic syndrome. Ann N Y Acad Sci. 2008;1148:232–237. doi: 10.1196/annals.1410.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Moser C, Bernhardt G, Michel J, et al. Cloning and functional expression of the hNPY Y5 receptor in human endometrial cancer (HEC-1B) cells. Can J Physiol Pharmacol. 2000;78:134–142. [PubMed] [Google Scholar]