Summary
2,4-D affects actin polymerization by post-translational modifications (carbonylation and S-nitrosylation), thereby disturbing the actin cytoskeleton and the dynamics of peroxisomes. Xanthine dehydrogenase is involved in ROS production under these conditions.
Key words: Actin, cytoskeleton, 2, 4-D, nitric oxide, peroxisomes, ROS, S-nitrosylation, xanthine dehydrogenase.
Abstract
2,4-Dichlorophenoxyacetic acid (2,4-D) is a synthetic auxin used as a herbicide to control weeds in agriculture. A high concentration of 2,4-D promotes leaf epinasty and cell death. In this work, the molecular mechanisms involved in the toxicity of this herbicide are studied by analysing in Arabidopsis plants the accumulation of reactive oxygen species (ROS) and nitric oxide (NO), and their effect on cytoskeleton structure and peroxisome dynamics. 2,4-D (23mM) promotes leaf epinasty, whereas this process was prevented by EDTA, which can reduce ·OH accumulation. The analysis of ROS accumulation by confocal microscopy showed a 2,4-D-dependent increase in both H2O2 and O2·–, whereas total NO was not affected by the treatment. The herbicide promotes disturbances on the actin cytoskeleton structure as a result of post-translational modification of actin by oxidation and S-nitrosylation, which could disturb actin polymerization, as suggested by the reduction of the F-actin/G-actin ratio. These effects were reduced by EDTA, and the reduction of ROS production in Arabidopsis mutants deficient in xanthine dehydrogenase (Atxdh) gave rise to a reduction in actin oxidation. Also, 2,4-D alters the dynamics of the peroxisome, slowing the speed and shortening the distances by which these organelles are displaced. It is concluded that 2,4-D promotes oxidative and nitrosative stress, causing disturbances in the actin cytoskeleton, thereby affecting the dynamics of peroxisomes and some other organelles such as the mitochondria, with xanthine dehydrogenase being involved in ROS production under these conditions. These structural changes in turn appear to be responsible for the leaf epinasty.
Introduction
Auxin herbicides have been one of the most successful chemicals used to control weeds in agriculture. 2,4-Dichlorophenoxyacetic acid (2,4-D) was the first synthetic auxin analogue of indole-3-acetic acid (IAA, natural auxin) used in agriculture (Grossmann, 2010). The dose-dependent mode of action of 2,4-D causes different effects on sensitive species, and this marks the difference between its action as a growth promoter or as a herbicide. Thus, at low concentrations, 2,4-D stimulates growth and developmental processes, but at high concentrations upsets normal growth and provokes lethal damage in the plant (Grossmann, 2010). Common visible effects induced by 2,4-D and auxin herbicides are epinastic deformations, stem curvature, senescence, and growth inhibition of roots and shoots (Grossmann et al., 2001; Pazmiño et al., 2012). Pea plants exposed to 2,4-D also develop oxidative stress symptoms characterized by H2O2 overaccumulation, lipid peroxidation, protein oxidation, and the induction of proteolysis (Romero-Puertas et al., 2004a ; Pazmiño et al., 2011, 2012). In young leaves, reactive oxygen species (ROS) accumulation is involved in 2,4-D-induced epinasty, while in adult leaves ROS overproduction triggers senescence (Pazmiño et al., 2011). Peroxisomes have been identified as one of the main sites involved in ROS production in response to 2,4-D by the activation of xanthine oxidase and acyl-CoA oxidase (Romero-Puertas et al., 2004a ; Pazmiño et al., 2011, 2014). Peroxisomes are subcellular organelles delimited by a single membrane that contain, as basic enzymatic constituents, catalase and hydrogen peroxide (H2O2)-producing flavin oxidases, and occur in almost all eukaryotic cells (Sandalio et al., 2013). Peroxisomes can change their enzymatic composition, shape, size, number, and motility depending on the tissue and environmental conditions (Rodríguez-Serrano et al., 2009; Sandalio et al., 2013).
ROS have a double, antagonistic function in the cells depending on their concentration. That is, at low concentrations, ROS, and particularly H2O2, can act as signal molecules and regulate the expression of a large number of genes involved in cell response to different stress conditions and development (Mittler et al., 2011). However, high accumulation of ROS is dangerous because it promotes oxidative damage to proteins, lipids, and nucleic acids. Oxidative damage has been demonstrated to be involved in the toxicity mechanisms of different abiotic factors (Suzuki et al., 2011; Sandalio et al., 2012).
In plants, nitric oxide (NO) is a key signalling molecule involved in several physiological processes from development to defence responses to both biotic and abiotic stress (Delledonne, 2005; Neill et al., 2008; Astier et al., 2011; del Río, 2011; Yemets et al., 2011). NO can regulate diverse biological processes by directly altering proteins through oxidization, nitration, or nitrosylation (Zaninotto et al., 2006; Astier et al. 2011; Vandelle and Delledonne, 2011). S-Nitrosylation refers to the binding of an NO group to a cysteine residue and can play a significant role in NO-mediated signalling (Stamler et al., 2001; Astier et al., 2011; Romero-Puertas et al., 2013).
In vivo visualization of actin filaments in cells has allowed the study of numerous roles of the actin cytoskeleton in different processes in the cell. These studies have been carried out by using specific actin reporters such as the fusion protein between green fluorescent protein (GFP) and the second actin-binding domain (FABD2) of Arabidopsis fimbrin, AtFIM1 (GFP–FABD2; Sheahan et al., 2004). The cytoskeleton governs important cell processes such as cell division and growth, vesicle transport, organelle movement, and the response of the cell to a wide range of stimuli such as light, gravity, phytohormones, pathogens, or wounding (Wasteneys and Yang, 2004; Yemets et al., 2011; Lanza et al., 2012; Sheremet et al., 2012; Song et al., 2012). The cytoskeleton has also been suggested to be one of the major targets of signalling events (Wasteneys and Yang, 2004). Recently, it has been demonstrated that the actin cytoskeleton can acts as a node of convergence in brassinosteroid (BR) and auxin signalling by regulating the bundling of actin filaments (Lanza et al., 2012). A large body of evidence shows that the actin cytoskeleton plays an important role in the regulation and execution of cell expansion (Baluska et al., 2001; Ketelaar et al., 2004; Collings et al., 2006). Dynamic actin cytoskeleton rearrangements are regulated by a pool of actin-binding proteins, which sense environmental changes and modulate the actin cytoskeleton through various biochemical activities (Hussey et al., 2006; Staiger and Blanchoin, 2006; Staiger et al., 2009). A number of drugs and herbicides such as dinitroanilines, benzoic acids, phosphoroamidates, pyridines, and carbamates use the cytoskeleton as a target, affecting microtubules in plant cells (Ovidi et al., 2001; Blume et al., 2003; Délye et al., 2004). Most of these compounds alter polymerization or binding site properties of tubulin heterodimers, although the molecular mechanism is not well known (Délye et al., 2004). Rahman et al. (2007) observed that 2,4-D and naphthylphthalamic acid removed actin and slowed down cytoplasmic streaming, although the mechanism involved was not specified. Proteomic studies have shown that plant cytoskeletal proteins can undergo many post-translational modifications including phosphorylation, S-glutathionylation, nitration, and S-nitrosylation, although their functional role and physiological relevance have yet to be elucidated (Yemets et al., 2011).
For this reason, in this work, the effect of 2,4-D on the actin cytoskeleton structure and the mobility of peroxisomes and mitochondria as well as the effect of post-translational modifications of actin by oxidation and S-nitrosylation were analaysed. The accumulation of ROS (H2O2 and O2·–) and NO induced by 2,4-D is also studied by in vivo confocal imaging. It is reported that 2,4-D considerably affects the actin cytoskeleton by inducing oxidative and S-nitrosylated modifications on the actin, disturbing actin polymerization and compromising the dynamics of peroxisomes and mitochondria.
Materials and methods
Chemicals and plant materials
Arabidopsis thaliana (L.) ecotype Columbia was germinated after 48h incubation at 4 °C, and plants were grown in compost at 22 ºC, 16h light, and 8h darkness for 3 weeks. To study the effect of 2,4-D on Arabidopsis plants, the plants were sprayed once with a 23mM 2,4-D solution [prepared in 1% dimethylsulphoxide (DMSO)], and kept for 72h until analysed. Control plants were sprayed with the same concentration of DMSO used to prepare 2,4-D. The treatment time and 2,4-D concentration used in this work has been previously optimized in pea plants (Romero-Puertas et al., 2004a ). The effect of EDTA (10mM) on Arabidopsis leaves was studied by spraying the chemical 24h before 2,4-D treatment and the application was repeated with 2,4-D spray. To study the effect of 2,4-D on peroxisome movement, Arabidopsis lines expressing the fusion protein between GFP and the peroxisomal targeting signal SKL from the hydroxypyruvate reductase (GFP–SKL) were used (Rodríguez-Serrano et al., 2009). The actin cytoskeleton was imaged by using the Arabidopsis line expressing the fusion protein GFP–FABD2 (Sheahan et al., 2004). Arabidopsis lines simultaneously expressing cyan fluorescent protein (CFP) and yellow fluorescent protein (YFP) associated with peroxisomes and mitochondria, respectively, were obtained by cross-pollinating Arabidopsis marker lines px-ck and mt-yk (Nelson et al., 2007) and selecting homozygous double lines. Arabidopsis Atxdh mutants were supplied by Dr Sagi (Ben-Gurion University, Beer Sheva, Israel) and homozygous lines were selected by analysing xanthine dehydrogenase (XDH) activity by native-PAGE and nitro blue tetrazolium staining (Pazmiño et al., 2014).
Confocal microscopy
Transgenic Arabidopsis leaves were sliced with razor blades and mounted between a slide and a coverslip in phosphate-buffered saline (PBS)/70% glycerol. Sections were examined using a Leica confocal laser scanning microscope, Model TCS SL (Leica Microsystems, Wetzlar, Germany). Digital images were taken across the epidermal cells. The movement of individual peroxisome stacks was analysed using the classification and particle-tracking routine of Volocity version 3.0 (Improvision; Perkin-Elmer, Palo Alto, CA, USA). This software can track the movement of individual fluorescent particles in time-resolved two or three dimensions, and automatically generates the speed and track length. For the speed analysis, the images were acquired in the x, y, z, and t dimensions. Each movie contained 15 z-series each containing 6–9 frames in the z-axis (1 μm interval; 512×512 resolution and bidirectional scan mode). The movies were generated taking 20 frames in the x, y, and t dimension with a 1024×1024 resolution. Quick-time movies of peroxisome movement were generated from sequential images (five frames per second). Arabidopsis plants expressing the fusion protein GFP–FABD2 were used to visualize the actin cytoskeleton. Images of GFP-expressing cells were acquired as a z-series with 1 μm interval using a Leica confocal laser scanning microscope (Exc/Em: 488/508nm) and at different times following 2,4-D (23mM) treatment (1, 24, 47, and 72h). The effect of 25 μM latrunculin B (Lat B, an inhibitor of actin polymerization, prepared in 0.2% DMSO) on the actin cytoskeleton was also studied in GFP–FABD2 Arabidopsis plants treated with these compounds for 45min.
Analysis of H2O2 and NO in plants extracts
The H2O2 concentration was determined in acid extracts from Arabidopsis leaves by spectrofluorimetry as described by Pazmiño et al. (2011). All processes were conducted at 4 ºC. Leaves (0.5g) were extracted with 1.5ml of 1M HClO4, in the presence of insoluble PVP (polyvinylpyrrolidone; 5%) and centrifuged at 12 000 g for 10min (4 ºC); the supernatant was filtered through a 0.45 μm Millipore filter. The pH was adjusted to 7.0 with 5M K2CO3 and the filtrate was finally centrifuged at 12 000 g for 2min to remove KClO4. The supernatant was used to measure the H2O2 by spectrofluorimetry using homovanillinic acid (Ex/Em: 325/425nm) and horseradish peroxidase (HRP).
NO was analysed by fluorimetry using 4,5-diaminofluorescein (DAF-2), as described by Nakatsubo et al. (1998). After treatment with 2,4-D, leaf extracts were made and incubated with DAF-2 in 50mM HEPES buffer, pH 7.5, for 2h at 37 ºC. Afterwards, NO was measured by analysing DAF-2 fluorescence (Ex/Em: 495/515nm).
ROS and NO detection by confocal laser scanning fluorescence microscopy
ROS and NO accumulation were imaged by confocal laser scanning microscopy (CLSM). Superoxide radicals were detected by incubating leaf sections with 10 μM dihydroethidium (DHE; Fluka, Buchs, Switzerland; Ex/Em: 450–490/520nm) in 10mM TRIS-HCl (pH 7.4), for 30min at 37 ºC, as describeed by Sandalio et al. (2008). H2O2 was detected by using 2′,7′-dichlorodihydrofluorescein diacetate (DCF-DA) in 10mM TRIS-HCl (pH 7.4) for 30min at 37 ºC, and NO was detected with 4,5-diaminofluorescein diacetate (DAF-2DA) for 1h at 25 ºC as indicated by Sandalio et al. (2008). As a negative control, 2-(4-carboxyphenyl)-4,4,5,5-tetramethylimidazoline-1-oxyl-3-oxide (cPTIO; 2mM) was used as an NO scavenger. After leaves were embedded in 30% (w/v) polyacrylamide blocks, sections were cut by a vibratome and mounted for examination with a confocal laser scanning microscope (Leica TCS SL; Leica Microsystems). Fluorescence was quantified using LAS AF Leica software and expressed as arbitrary units.
Histochemical analyses
For histochemical analyses of H2O2, leaves from control and 2,4-D-treated plants were excised and immersed in a 1% solution of 3,3′-diaminobenzidine (DAB) in 10mM MES buffer (pH 6.5), vacuum-infiltrated for 5min, and then incubated at room temperature for 8h in the absence of light. Leaves were illuminated until the appearance of brown spots characteristic of the reaction of DAB with H2O2. Leaves were bleached by immersion in boiling ethanol to visualize the brown spots (Romero-Puertas et al., 2004b ). Cell death was evaluated by histochemical analysis using Trypan Blue (Koch and Slusarenko, 1990) at different times during treatment.
Western blot analysis
To analyse the effect of 2,4-D on GFP–fimbrin and actin expression, leaves were homogenized in buffer containing 50mM TRIS-HCl (pH 7.8), 0.1mM EDTA (0.2% v/v) Triton X-100, and protease inhibitor cocktail (Sigma, St. Louis, MO, USA). Homogenates were centrifuged at 16 000 g for 30min at 4 ºC. Equal amounts of proteins were loaded for SDS–PAGE (12% acrylamide) and transferred onto a polyvinylidene fluoride (PVDF) membrane (Millipore Co., Bedford, MA, USA) in a Bio-Rad Semi-Dry Transfer Cell (Bio-Rad, Hercules, CA, USA). GFP was detected using anti-GFP monoclonal antibody (Clontech; 1/10 000 dilution) and goat anti-mouse IgG conjugated with HRP as secondary antibody (Bio-Rad; 1/ 10 000 dilution). Actin was detected using a specific polyclonal antibody (1/1000 dilution, Molecular Probes™) and IgG anti-rabbit–HRP (Bio-Rad; 1/10 000 dilution). To analyse the total amount of filamentous actin (F-actin) versus free globular actin (G-actin), leaves were homogenized in buffer containing 0.1M PIPES (pH 6.9) 30% (v/v) glycerol, 5% (w/v) DMSO, 1mM MgSO4, 1mM EGTA, 1% (v/v) Triton X-100, 1mM ATP, and protease inhibitor cocktail. Homogenates were centrifuged at 16 000 g for 75min at 4 ºC to separate F-actin from G-actin. F-actin from the pellet was depolymerized with cytochalasin and solubilized in an equal volume of supernatant containing 0.1M PIPES (pH 6.9), 1mM MgSO4, 10mM CaCl2, and 5 μM cytochalasin D. After incubation for 1h, equal volumes of both fractions were analysed by western blot using a specific antibody against actin as mentioned above (Rasmussen et al., 2010).
Immunochemical detection of S-nitrosylated actin
S-Nitrosylated proteins were detected following the biotin-switch method that converts –SNO into biotinylated groups (Jaffrey et al., 2001). Arabidopsis leaves were homogenized in MAE buffer (25mM HEPES, 1mM EDTA, 0.1mM neocuproine, 0.2% Triton X-100, pH 7.7) containing complete protease inhibitor cocktail (Sigma). The extract was centrifuged at 4 °C for 30min. Proteins were then assayed with the biotin-switch method. Briefly, an equal amount of protein (300 μg) from control and treated plant leaf extracts was subjected to the biotin-switch assay (Ortega-Galisteo et al., 2012) and biotinylated proteins were purified by immunoprecipitation overnight at 4 ºC with 15 μl of IPA (UltralLink Immobilized Protein A/G Pierce) mg–1 of protein and pre-incubated with 2 μl of anti-biotin antibody (Sigma). Beads were washed three times with PBS, and bound proteins were eluted with 10mM dithiothreitol (DTT) in SDS–PAGE solubilization buffer, loaded on a 12% SDS–polyacrylamide gel, transferred to a PVDF (polyvinylidene fluoride) membrane, and actin was detected with specific antibodies (1/1000 dilution, Molecular Probes™).
Immunochemical detection of oxidative modified actin
The proteins containing carbonyl groups were identified as described by Romero-Puertas et al. (2002). Equal amount of proteins (500 μg) from leaf extracts were derivatized with 10mM 2,4-dinitrophenylhydrazine (DNPH; Sigma-Aldrich Co.) and immunoprecipitated with antibodies against DNP linked to IPA overnight at 4 ºC. Oxidized-purified proteins (10 μl) were subjected to SDS–PAGE (12% acrylamide) and transferred onto PVDF membranes as mentioned above. Actin was detected using specific antibodies (1/1000 dilution, Molecular Probes™).
Protein and statistical analysis
Protein concentration was determined with the Bio-Rad Bradford Protein Assay kit using bovine serum albumin (BSA) as standard. Data were subjected to one-way analysis of variance for each parameter. When the effect was significant (P>0.05), differences among means were evaluated for significance by Duncan’s multiple-range test (P>0.05).
Results
Effect of 2,4-D on Arabidopsis leaf phenotype and oxygen and nitrogen reactive species accumulation
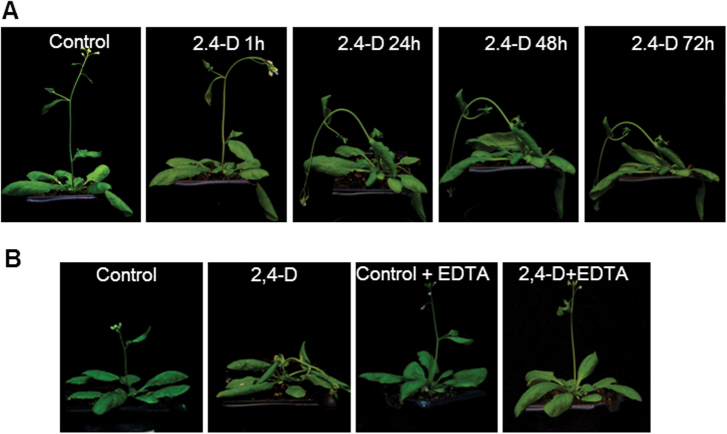
In previous work, the concentration of the herbicide 2,4-D and the time of treatment was optimized in order to visualize its toxic effects on pea plants, with 23mM 2,4-D and 72h of treatment being the experimental conditions selected (Romero-Puertas et al., 2004a ). Therefore, these conditions were used to carry out the experiments in Arabidopsis plants. The supply of 23mM 2,4-D to Arabidopsis plants produced a severe curling or epinasty of rosette leaves, loss of leaf turgidity, and curling of the flower stem which started after 1h, reaching a maximum after 72h of treatment (Fig. 1A; Supplementary Fig. S1A available at JXB online). This effect was reduced by the treatment with EDTA (Fig. 1B), as was shown in previous work on pea leaves (Pazmiño, 2009, 2014).
Fig. 1.
Effect of 2,4-D and EDTA on Arabidopsis phenotype. (A) Plants were treated once by foliar application of 23mM 2,4-D and the effect on phenotype was followed after different periods of treatment (1–72h). Images correspond to the same plant at different treatment times. (B) Effect of EDTA (10mM) on 2,4-D-induced phenotype. Plants were sprayed with EDTA before treatment with 2,4-D and the effect was analysed after 72h of treatment.
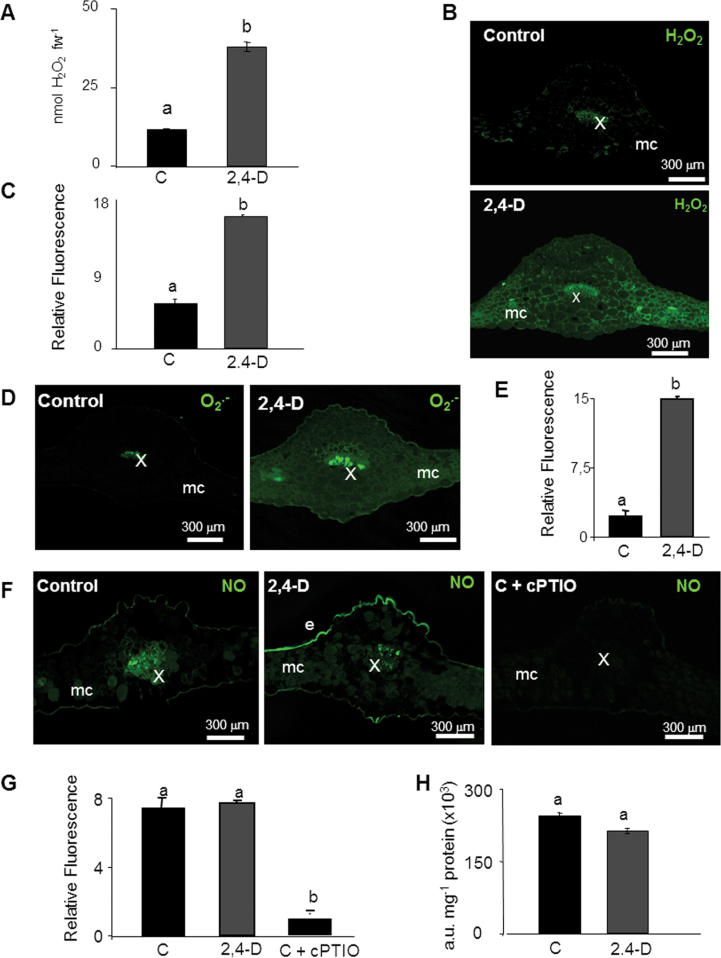
The analysis of total H2O2 in Arabidopsis leaf extracts after treatment with 2,4-D shows a 2-fold increase in H2O2 (Fig. 2A). By using histochemistry with DAB, a strong increase of H2O2 was detected in 2,4-D-treated plants in comparison with untreated plants, the highest accumulation being registered in vascular tissues (Supplementary Fig. S1B at JXB online). The accumulation of H2O2 was also studied in leaf cross-sections using DCF-DA and CLSM. 2,4-D induced an increase in DCF fluorescence, associated mainly with mesophyll cells, although an increase in fluorescence in secondary veins also appeared in 2,4-D-treated leaves (Fig. 2B, C). The analysis of O2·– in cross-sections of Arabidopsis leaves showed an induction of O2·– by the herbicide (Fig. 2D, E) which was reversed by incubation with superoxide dismutase (SOD; data not shown). The O2·– accumulated in the main and secondary veins, but also in mesophyll and epidermal cells (Fig. 2D; Supplementary Fig. S2). A higher magnification of mesophyll cells revealed the O2·–-dependent fluorescence associated mainly in small puncta which could represent localization to small organelles such as mitochondria and peroxisomes, while neither chloroplasts nor plasma membrane show any DHE signal (Supplementary Fig. S2). In turn, the image of NO accumulation displayed by DAF-2D fluorescence showed no apparent differences due to the treatment with 2,4-D in terms of total NO accumulation, although a slight increase in fluorescence was observed in the epidermis (Fig. 2F, G). The NO scavenger cPTIO was used as a negative control, showing a considerable reduction of DAF-2DA fluorescence (Fig. 2F, G). NO production was analysed using spectrofluorimetry in order to quantify changes in NO accumulation by the herbicide, but no changes were found in comparison with the values in untreated plants (Fig. 2H).
Fig. 2.
Imaging and quantification of H2O2, O2·–, and NO production induced by the treatment with 2,4-D in Arabidopsis thaliana leaves. (A) H2O2 content was analysed in acid extracts from Arabidopsis leaves by fluorimetry. Values are means ±SE of four different experiments with three independent extracts each. (B) Imaging of H2O2 accumulation in cross-sections of Arabidopsis leaves by CLSM using DCF-DA (Ex/Em: 485/530nm). DCF-DA fluorescence was quantified in arbitrary units (C). (D, E) Imaging and quantification of O2·– production using DHE (Ex/Em: 450–490/520nm. (F, G) Imaging and quantification of NO production using DAF-2DA (Ex/Em: 495/515nm). cPTIO was used as an NO scavenger. (H) NO content was quantified in arbitrary units (a.u.) in leaf extracts from Arabidopsis leaves by fluorimetry. Images are maximal projections from several optical sections and are representative of at least 15 leaf sections from four different experiments. Different letters indicate a significant difference at P<0.05 as determined by Duncan′s multiple-range test. e, epidermis; mc, mesophyll cells; st, stomata; x, xylem.
2,4-D disturbs the actin cytoskeleton by post-translational changes in actin
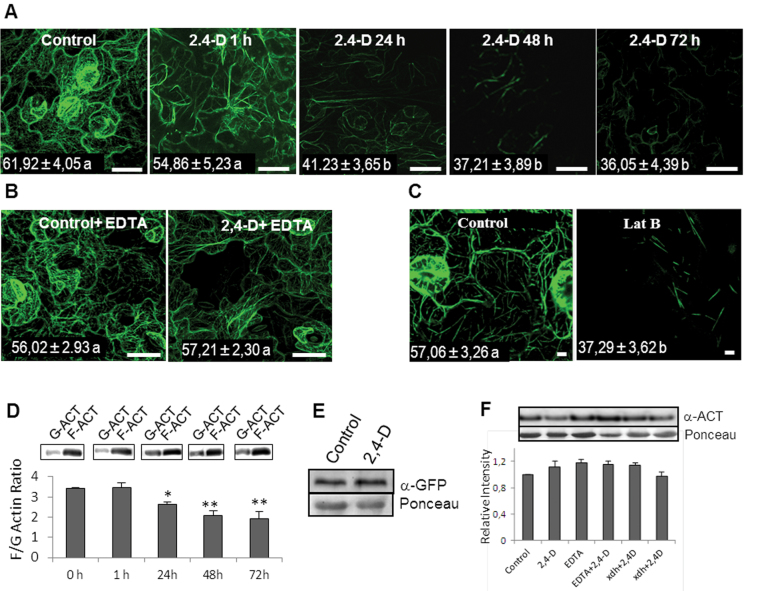
Recently Rahman et al. (2007) have shown that 2,4-D can affect actin cytoskeleton structure, although the mechanism involved has not been established so far. To look at this in more depth, in this study the effect of this chemical on the structure of the actin cytoskeleton over time was analysed by using a transgenic Arabidopsis line expressing GFP associated with an actin-binding protein (GFP–FABD2; Sheahan et al., 2004). The analysis revealed a slight but not significant reduction of GFP associated with actin filaments after 1h of treatment, and after 24h a significant reduction was observed. The maximum effect was observed after 72h, which revealed a reduction in the number and thickness of actin filaments (Fig. 3A). EDTA prevented the disturbances to the organization of the actin cytoskeleton (Fig. 3B) in the same way as it prevented epinasty. To characterize the disturbances of the actin cytoskeleton induced by 2,4-D, GFP–FABD2 plants were treated with Lat B, which is a well known inhibitor of actin polymerization (Sheahan et al., 2004). Lat B produced a severe reduction in most of the filamentous actin after 45min of treatment, showing a similar image to that observed with 2,4-D, which suggests that the changes observed in the actin filament network induced by this herbicide could be due to a reduction in the ability of actin to polymerize (Fig. 3C). This fact was studied by analysing the content of G-actin and F-actin in leaf extracts at different treatment times. A statistically significant reduction in the F-actin/G-actin ratio was observed after 24h of treatment, with a maximum after 72h of treatment (Fig. 3D). To study if the disturbance in the actin cytoskeleton is associated with cell death, leaves of Arabidopsis plants were stained with Trypan Blue, a marker of cell viability, at different treatment times (24, 48, and 72h). The results obtained did not show any cell death due to the treatment with 2,4-D even after 72h (Supplementary Fig. S3 at JXB online). In addition to this, to rule out degradation processes affecting GFP–FABD2 during the treatment with 2,4-D, a western blot analysis was carried out using a monoclonal anti-GFP antibody. The results obtained shown that the content of GFP–FABD2 was not affected by the treatment with 2,4-D for 72h (Fig. 3E). The total actin present in extracts was also analysed by western blot using specific antibodies against actin. No differences were detected in terms of total protein between control and 2,4-D-treated plants after 72h of treatment, demonstrating that actin is not down-regulated or proteolitically degraded by the 2,4-D treatment (Fig. 3F). The same results were obtained in plants treated with EDTA.
Fig. 3.
Effect of 2,4-D on the actin cytoskeleton in epidermal cells of Arabidopsis leaves. (A) Arabidopsis plants expressing the fusion protein GFP–FABD2 were used to visualize the effect of 2,4-D (23mM) on the actin cytoskeleton at different treatment times. (B) Effect of EDTA (10mM) and 2,4-D on the actin cytoskeleton after 72h of treatment. (C) Effect of latrunculin B (Lat B; 25 μM) on the actin cytoskeleton. Fluorescence for each treatment was quantified as indicated in the Materials and methods and expressed in arbitrary units. The mean ±SE of at least 10 leaf sections from three different experiments is shown inside the panels. Data followed by the same latter are not statistically different according to Duncan’s multiple-range test. Bars represent 25 μm in A and B, and 5 μm in C. (D) Effect of 2,4-D on the F-actin/G-actin ratio during the treatment, analysed by western blot of proteins using anti-actin antibodies. An equal volume of proteins was used for each fraction. (E) GFP–FABD2 expression in leaves after 72h of treatment analysed by western blot of proteins using a specific anti-GFP antibody. An equal amount of protein was loaded per well. (F) Variation in total actin protein accumulation in WT plants after 72h of treatment with 2,4-D and EDTA and in Atxdh plants monitored by western blot analysis using a specific anti-actin antibody. An equal amount of protein was loaded per well. (This figure is available in colour at JXB online.)
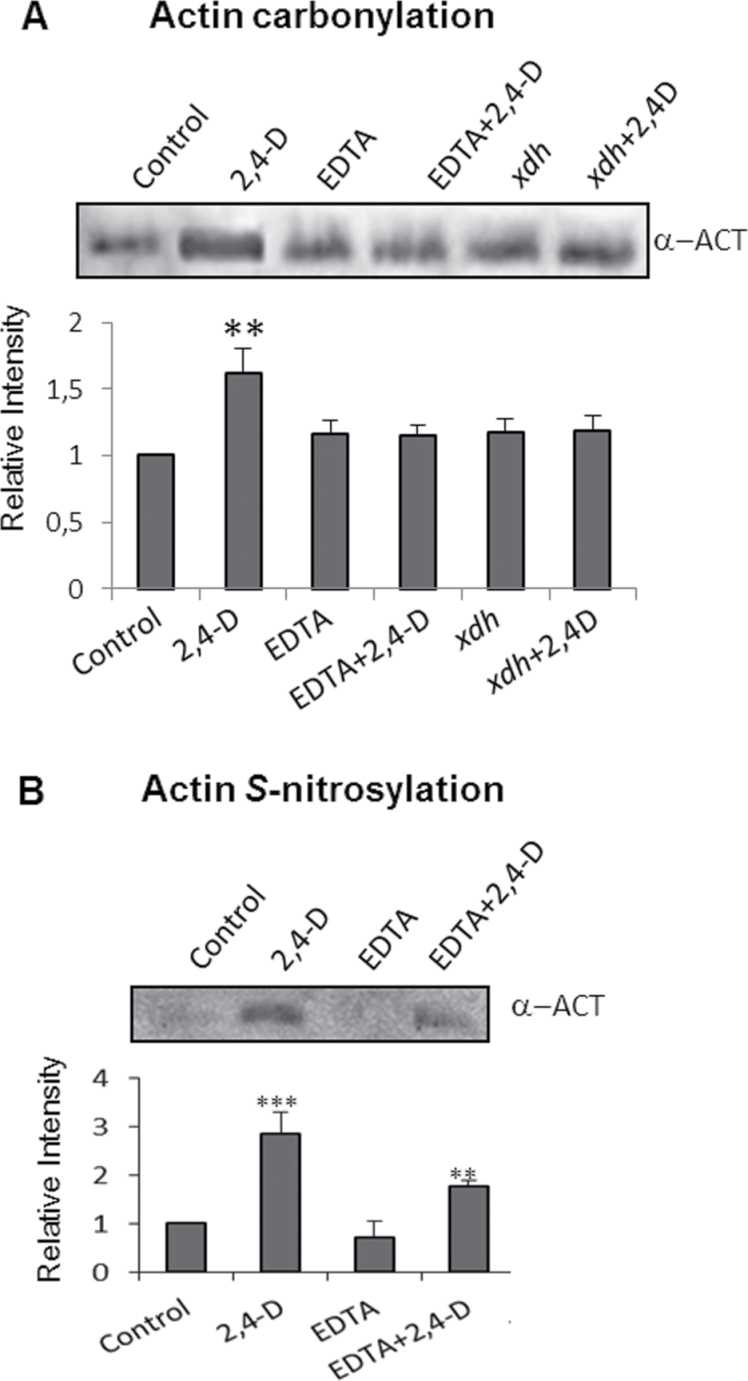
In previous work, it was demonstrated that xanthine oxidoreductase (XOD/XDH) is involved in ROS production induced by 2,4-D (Pazmiño et al., 2014), and Arabidopsis mutants deficient in this protein (Atxdh) show a significant reduction of epinasty induced by 2,4-D; for this reason the effect of 2,4-D on the content of actin in this mutant was analysed. No differences were observed between the wild type (WT) and Atxdh (Fig. 3F). To study the cause of 2,4-D-dependent disturbances in the structure of the actin cytoskeleton, post-translational modifications of actin by oxidation and S-nitrosylation were analysed after 72h of 2,4-D exposure. Leaf extracts from WT and Atxdh plants treated with 2,4-D for 72h were incubated with 10mM DNPH, and oxidized proteins containing carbonyl groups were purified by immunoprecipitation using antibodies against DNP linked to IPA; actin was visualized by western blot using a specific antibody. The results obtained revealed a strong increase in oxidized actin in 2,4-D-treated plants which was considerably reduced in Atxdh lines and the WT treated with EDTA (Fig. 4A). These results indicate that ROS production stimulated by 2,4-D is involved in oxidative alterations of actin, which in turn would promote disturbances in the actin cytoskeleton ultrastructure. Actin reportedly undergoes S-nitrosylation in both animal and plant tissue, and in animal tissue this change has been demonstrated to affect the rate of actin polymerization under oxidative stress (Dalle-Donne et al., 2000). Therefore, S-nitrosylated proteins from Arabidopsis leaf extracts were analysed by the biotin-switch method and purified by immunoprecipitation using anti-biotin antibody–IPA, and actin was identified with specific antibodies. The treatment with 2,4-D boosted the content of S-nitrosylated actin in comparison with untreated control plants, and EDTA significantly reduced it (Fig. 4B).
Fig. 4.
Analysis of post-translational modifications of actin by carbonylation and S-nitrosylation. (A) Detection of carbonylated actin. Proteins from leaf extracts (500 μg) were derivatized with DNPH and immunoprecipitated with anti-DNP-IPA as indicated in the Materials and methods. Oxidized-purified proteins (10 μl) were subjected to SDS–PAGE, transferred onto PVDF membranes, and analysed with an anti-actin antibody. The figure is representative of four independent experiments. (B) Detection of S-nitrosylated actin. S-Nitrosylated proteins were labelled with biotin and immunopurified with anti-biotin–IPA, separated by SDS–PAGE, and the actin was identify by western blot analysis using an anti-actin antibody. The figure is representative of three independent experiments.
Peroxisomal dynamics is affected by 2,4-D
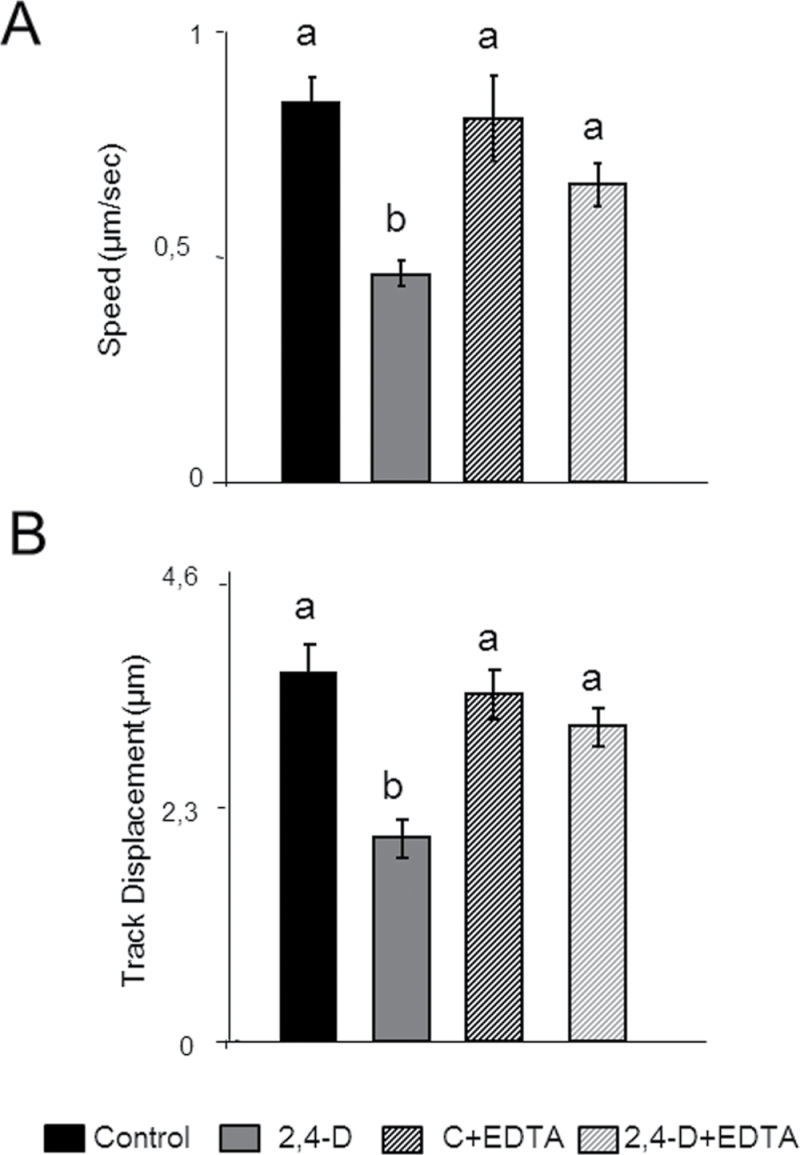
Because peroxisomes move along the actin cytoskeleton (Mano et al., 2002; Van Gestel et al., 2002) the dynamics of these organelle under 2,4-D toxicity was studied. Peroxisome movement in epidermal cells was assessed after 72h of treatment. The herbicide caused a 2-fold reduction of speed (Fig. 5A) and a reduction of the displacement rate of these organelles (Fig. 5B). Movies of control and 2,4-D-treated plants showing differences in the dynamics of peroxisomes are provided in Video S1A, B and S2A, B at JXB online. As mentioned previously, EDTA can reduced the disturbances of the actin cytoskeleton induced by 2,4-D, and therefore the role of EDTA in peroxisomal movement was also investigated. The treatment with EDTA reversed the effect of 2,4-D on both the speed and displacement of peroxisomes, reaching values similar to those of the untreated leaves (Fig. 5A, B). In order to determine if the effect of 2,4-D is specific for peroxisomes, or is a general effect on organelle motility, the effect of 2,4-D on the movement of mitochondria in Arabidopsis lines expressing YFP in mitochondria and CFP associated with peroxisomes was analysed. The dynamics of mitochondria was also disturbed by the herbicide, showing a severe reduction in their motility (Supplementary Video S2A, B).
Fig. 5.
Effect of 2,4-D on peroxisome dynamics in epidermal cells. Seedlings expressing GFP–SKL were treated with 23mM 2,4-D with or without 10mM EDTA for 72h. The speed (A) and displacement (B) were studied by time-lapse analysis using a confocal laser microscope, and the images obtained were processed using Volocity3 software. Results are means ±SE of three different parts of the leaf, and at least 10 different plants from three different experiments were used. Values with different letters are significantly different (P<0.05) as determined by Duncan’s multiple-range test.
Discussion
Auxins regulate a number of processes related to development and growth in plants, being involved in cell elongation, tissue differentiation, tissue polarity, or leaf expansion (Benjamins and Scheres, 2008; Delker et al., 2008). 2,4-D is a synthetic auxin specific for dicotyledons and is considered to be among the most successful herbicides used in agriculture (Grossmann, 2010; Pazmiño et al., 2012). One of the most characteristic effects of 2,4-D on sensitive plants is the development of epinasty and stem curvature, as well as reduction of root and stem growth (Grossmann, 2010; Pazmiño et al., 2011, 2012). Different studies have demonstrated that the toxicity of this herbicide is mediated by uncoupling oxidative phosphorylation, changes in the plasma membrane potential, or oxidative stress (Grossmann et al., 2001; Pazmiño et al., 2012). Recently, it was demonstrated that ROS are involved in the toxicity of 2,4-D, being responsible for both the epinasty and senescence induced by this herbicide (Pazmiño et al., 2011, 2014). The analysis of different sources of ROS under 2,4-D toxicity point to XOD/XDH and acyl-CoA oxidase (ACX) as the main agents responsible for the oxidative stress imposed by 2,4-D (Pazmiño et al., 2011, 2014). Both enzymes are components of peroxisomes, which are characterized by a strong oxidative metabolism (Sandalio et al., 2013). The role of peroxisomes in ROS and NO metabolism has recently been demonstrated, and the importance of these molecules in signalling has been elucidated over the past 10 years (Vandenabeele et al., 2004; del Río 2011; Ortega-Galisteo et al., 2012; Sandalio et al., 2013). The rate of ROS accumulation would define the role of these reactive species as signal molecules (low production) or as dangerous compounds (high accumulation) (Mittler et al., 2011; Sandalio et al., 2013). In turn, NO can interfere with signal transduction pathways or can modify proteins, modulating their activities or properties (Moreau et al., 2010). The analysis of H2O2 accumulation by different methods showed that 2,4-D induced the overaccumulation of this ROS, which in turn caused oxidative alteration of proteins, as was demonstrated recently by carbonyl content analyses (Pazmiño et al., 2014). One of the target proteins of this oxidative modification was actin. Concerning the main sources of ROS under these conditions, the analyses of ROS accumulation by CLSM suggest that peroxisomes and mitochondria may be the main cell compartments involved in ROS production. Recently, the production of H2O2 in peroxisomes induced by 2,4-D in tobacco leaves transiently expressing the biosensor HyperAs-SKL in peroxisomes and pea leaf peroxisomes was reported (Sandalio et al., 2013; Pazmiño et al., 2014). The generation of ROS and particularly ·OH is a prerequisite for cell wall loosening and normal growth (Schopfer et al., 2002; Liszkay et al., 2004) and, under the conditions used in this work, 2,4-D could promote overaccumulation of these species, which in turn could trigger cell malformation, leading to epinasty, a hypothesis supported by the protective role of EDTA which can act as a metal chelator, avoiding Fenton-type reactions, and also reacts directly with ·OH at a rate constant of 2.8×10–9 (Halliwell and Gutteridge, 2007).
The plants treated with 2,4-D showed a strong reduction in actin bundling and polymerization, which increase with the time of treatment and were completely prevented by EDTA. EDTA also prevented epinasty, demonstrating that the cytoskeletal disturbances are involved in the development of this phenotype. The protective effect of EDTA was due mainly to the reduction of actin oxidation. Recently it was observed that EDTA reduces oxidation of proteins in Arabidopsis plants treated with 2,4-D (Pazmiño et al., 2014) and also reduced the accumulation of H2O2 induced by 2,4-D in pea shoots (Pazmiño et al., 2014). These results suggest that ·OH is the main ROS involved in the actin cytoskeleton disturbances, and the results obtained with Atxdh plants suggest that XOD/XDH are partially involved in the production of this ROS. In addition to ROS, NO is also a key molecule involved in signalling and controlling functionality of different proteins by combining with cysteines and giving rise to S-nitrosylation of proteins. Despite the absence of changes in total NO accumulation, an increase of S-nitrosylation of actin was observed in 2,4-D-treated plants when S-nitrosylated proteins were purified. A reduction of S-nitrosylation was observed when plants were pre-treated with EDTA however. It appears that this decrease it is not metal dependent as EDTA would favour and protect S-nitrosylation (Jaffrey et al., 2001). Other molecules, however, could also regulate this post-translational modification, such as ascorbate or glutathione, whose concentration is altered by EDTA and 2,4-D treatment in pea plants (Pazmiño, 2009). Studies carried out in vitro and in animal cells have shown that actin is a major target of different post-translational modifications, such as oxidation and S-nitrosylation, and both processes triggered disturbances in actin polymerization, giving rise to changes in cell morphology through the formation of multiple surface blebbs on the plasma membrane (Dalle-Donne et al., 2001). Similar changes in cell morphology have also been observed in pea leaves treated with 2,4-D (Pazmiño et al., 2011). Actin has several cysteines susceptible to redox modifications and also has several methionines susceptible to oxidation (Terman and Kashina, 2013). However, the role and hierarchical relationship between these post-translational modifications of actin under physiological and stress conditions have not being established so far (Terman and Kashina, 2013). The interplay between post-translational modifications of proteins has emerged as a very important regulatory mechanism (Sun et al., 2006; Lounifi et al., 2013). ROS and NO can compete for the same cysteine residues to regulate proteins, and therefore can exert antagonistic roles. S-Nitrosylation has been suggested to protect proteins against irreversible carbonylation, and, in its turn, irreversible oxidation of thiols can block the physiological modification by S-nitrosylation (Sun et al., 2006; Lounifi et al., 2013). In addition to post-translational modification of actin in animal cells, it has been reported that ROS can also disturb the actin cytoskeleton by activating mitogen-activated protein kinases, which lead to the phosphorylation of F-actin, affecting actin polymerization and cytoskeleton dynamics (Dalle-Donne et al., 2001; Foissner et al., 2002).
There is a cross-talk between auxins and actin, and a self-referring regulatory circuit between polar auxin transport and actin organization has been reported, although the mechanism is not well understood (Dhonukshe et al., 2008; Nick et al., 2009). Auxin transport inhibitors, such as 2,3,5-triiodobenzoic acid (TIBA) or sodium 4-phenylbutyrate (PBA), repress vesicle trafficking by influencing the actin cytoskeleton (Dhonukshe et al., 2008), and exogenous IAA regulates actin bundling, promoting the transformation of massive longitudinal bundles into finer strands (Nick et al., 2009), although the mechanism involved in these changes of the actin cytoskeleton have not been demonstrated. More recently, it was observed that BRs induced a wavy phenotype in Arabidopsis roots which was due to changes in the distribution of actin filaments and their dynamics (Lanza et al., 2012). NO can induce actin depolymerization in sycamore tree cells treated with fusicoccin, and this process has also been associated with the induction of programmed cell death (Malerba et al., 2008). ROS and NO also mediated actin reorganization and the induction of programmed cell death in the pollen self-incompatibility response of Papaver, although the molecular mechanisms have not established so far (Wilkins et al., 2011). In the present work, it has been demonstrated that 2,4-D does not induce degradation or down-regulation of actin and GFP–FABD2 but induces actin modifications by oxidation and S-nitrosylation, which affect the polymerization of F-actin, leading to a strong reduction of actin organization. In animal cells, S-nitrosylation interferes with the normal state of F-actin, resulting in depolymerization (Dalle-Donne et al., 2000). S-Nitrosylated G-actin polymerizes less efficiently than control actin and forms a lower amount of F-actin, compared with unmodified actin, which shortens the actin length distribution (Dalle-Donne et al., 2000). In maize roots, exogenous NO donors reportedly disturbed the actin cytoskeleton and vesicle trafficking by reorganization of F-actin, and this effect was specific for cell type and developmental state (Kasprowicz et al., 2009).
The disturbances observed in the actin cytoskeleton could be responsible for the leaf epinasty induced by 2,4-D, and, in fact, actin has been demonstrated to regulate the growth and the shape of leaf epidermal pavement cells and trichomes (Smith and Oppenheimer, 2005), and has been found to be involved in growth alterations (Ketelaar et al., 2004). Baluska et al. (2001) have also observed that a reduction of the actin cytoskeleton induces cell radial growth, resulting in the cells being shorter and wider, which could explain the epinasty induced by 2,4-D observed in this work. In addition to this, the disturbances in the actin cytoskeleton promote a reduction of the mobility and displacement of peroxisomes in response to 2,4-D. This effect was not specific for peroxisomes, and the motility of mitochondria was also affected by 2,4-D. These disturbances could considerably affect the metabolism of these organelles because they share several metabolites with each other and with chloroplasts, and the disruption of their dynamics could compromise the metabolic pathways where they are involved. Thus, 2,4-D promotes reduction of carbon fixation and starch formation in plants (Grossmann, 2010), and affects mitochondrial respiration and fatty acid β-oxidation in yeast, animal, and plant cells (Teixeira et al., 2007; Romero-Puertas et al., 2004a ; Grossmann, 2010). Peroxisomes contain a large battery of antioxidants and can participate in removing ROS from different parts of the cells, and disturbances in their mobility could limit their role in antioxidative defence.
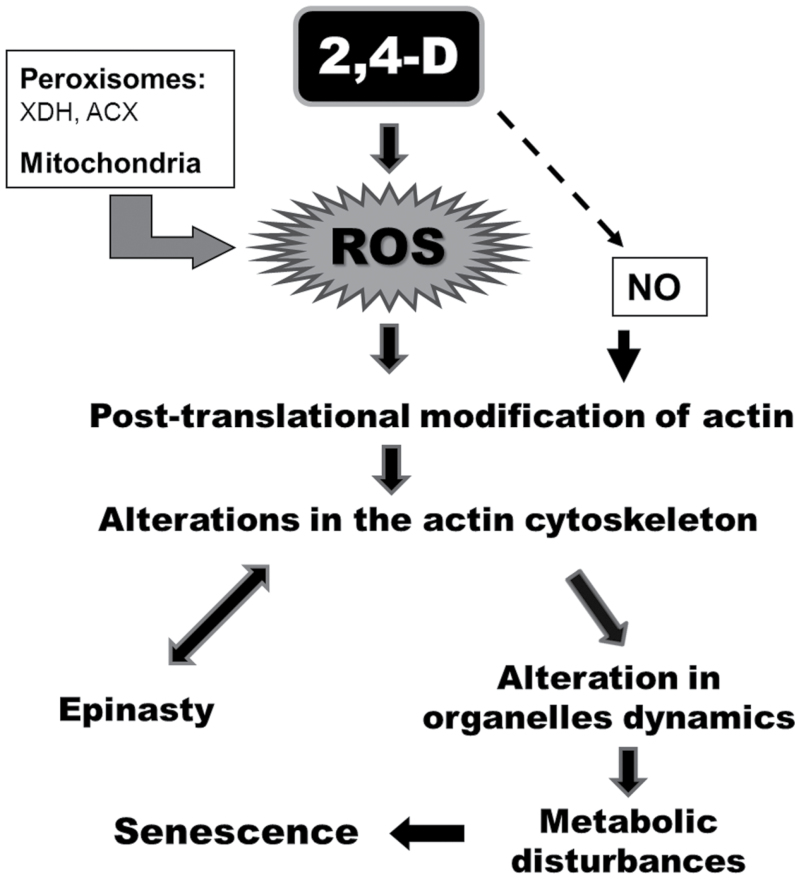
In conclusion, 2,4-D promotes oxidative stress, giving rise to post-translational changes of actin by oxidation and S-nitrosylation, causing disturbances in the actin cytoskeleton and thereby affecting the dynamics and metabolism of peroxisomes and mitochondria. These structural changes in turn appear to be responsible for epinastic deformation of the leaf characteristic of this herbicide. Disturbances in the actin cytoskeleton could also affect vesicle trafficking and, in general, organelle movement, giving rise to the metabolic disturbances and even further cell death after very long exposure to the herbicide (Fig. 6).
Fig. 6.
Schematic showing the possible mechanistic toxicity of 2,4-D in Arabidopsis plants. 2,4-D promotes oxidative stress, where peroxisomes and mitochondria represent the main sources of ROS, giving rise to post-translational changes of actin by oxidation and S-nitrosylation, causing disturbances in the actin cytoskeleton and thereby affecting trafficking of organelles. These structural changes in turn appear to be responsible for leaf epinasty, although processes involved in developing epinasty can also contribute to actin cytoskeleton disturbances. Alteration of the cytoskeleton could also be responsible for metabolic disturbances, signalling disruption, and further senescence. XDH, xanthine dehydrogenase; ACX, acyl-CoA oxidase.
Supplementary data
Supplementary data are available at JXB online.
Figure S1. 2,4-D produces epinasty in Arabidopsis leaves and accumulation of H2O2 mainly in vascular tissue.
Figure S2. Imaging of O2·– production by CLSM using DHE (Ex/Em: 450–490/520nm, green) and chlorophyll autofluorescence (red) showing magnifications of mesophyll cells from 2,4-D-treated plants.
Figure S3. Histochemical staining with Trypan Blue of Arabiodopsis leaves treated for different times with 2,4-D (23mM).
Video S1A. Movies showing peroxisomal dynamics in epidermal cells from control Arabidopsis plants expressing GFP–SKL.
Video S1B. Movies showing the effect of 23mM 2,4-D on peroxisomal dynamics in epidermal cells from control Arabidopsis plants expressing GFP–SKL.
Video S2A. Movies showing peroxisomal and mitochondrial dynamics in epidermal cells from control double marker Arabidopsis px-ck×mt-yk plants.
Video S2B. Movies showing the effect of 2,4-D on peroxisomal and mitochondrial dynamics in epidermal cells from double marker Arabidopsis px-ck×mt-yk plants.
Acknowledgements
DP and MR-S acknowledge fellowships JAE-Pre and JAE-DOC, respectively, from the CSIC, and the European Social Fund (ESF). This work was supported by ERDF-co-financed grants BIO2008-04067 and BIO2012-36742 from MICINN and Junta de Andalucía (BIO-337), Spain. The authors acknowledge Dr D. McCurdy and Dr Sagi for providing the GFP–FABD2 and xdh Arabidopsis lines, respectively. We thank Dr T. Ketelaar for critical reading of the manuscript. The confocal laser fluorescence microscopy analyses were carried out at the Technical Services of the University of Jaén and University of Granada.
Glossary
Abbreviations:
- ACX
acyl-CoA oxidase
- BR
brassinosteroid
- CFP
cyan fluorescent protein
- 2,4-D
2,4-dichlorophenoxyacetic acid
- cPTIO
2-(4-carboxyphenyl)-4,4,5,5-tetramethylimidazoline-1-oxyl-3-oxide
- DAB
3,3′-diaminobenzidine
- DAF-2
4,5-diaminofluorescein
- DCF-DA
2′,7′-dichlorodihydrofluorescein diacetate
- DHE
dihydroethidium
- DMSO
dimethylsulphoxide
- DNPH
2,4-dinitrophenylhydrazine
- FABD2
actin-binding domain 2 of fimbrin
- GFP
green fluorescent protein
- HRP
horseradish peroxidase
- IAA
indole-3-acetic acid (natural auxin)
- IPA
immobilized protein A
- Lat B
latrunculin B
- ROS
reactive oxygen species
- SOD
superoxide dismutase
- TIBA
2,3,5-triiodobenzoic acid
- XDH
xanthine dehydrogenase
- XOD
xanthine oxidase
- YFP
yellow fluorescent protein.
References
- Astier J, Rasul S, Koen E, Manzoor H, Besson-Bard A, Lamotte O, Jendroz S, Durner J, Lindernayr C, Wendehenne D. 2011. S-Nitrosylation: an emerging post-translational protein modification in plants. Plant Science 181, 527–533 [DOI] [PubMed] [Google Scholar]
- Baluska F, Jasik J, Edelmann HG, Salajová T, Volkmann D. 2001. Latrunculin B-induced plant dwarfism: plant cell elongation is F-actin-dependent. Developmental Biology 231, 113–124 [DOI] [PubMed] [Google Scholar]
- Benjamins R, Scheres B. 2008. Auxin: the looping star in plant development. Annual Review of Plant Biology 59, 443–465 [DOI] [PubMed] [Google Scholar]
- Blume YB, Nyporko AY, Yemets AI, Baird WV. 2003. Structural modeling of the interaction of plant alpha-tubulin with dinitroaniline and phosphoroamidate herbicides. Cell Biology International 27, 171–174 [DOI] [PubMed] [Google Scholar]
- Collings DA, Lill AW, Himmelspach R, Wasteneys GO. 2006. Hypersensitivity to cytoskeletal antagonists demonstrates microtubule–microfilament cross-talk in the control of root elongation in Arabidopsis thaliana . New Phytologist 170, 275–290 [DOI] [PubMed] [Google Scholar]
- Dalle-Donne I, Milzani A, Giustarini D, Di Simplicio P, Colombo R, Rossi R. 2000. S-NO-actin: S-nitrosylation kinetics and the effect on isolated vascular smooth muscle. Journal of Muscle Research and Cell Motility 21, 171–181 [DOI] [PubMed] [Google Scholar]
- Dalle-Donne I, Rossi R, Milzani A, Di Simplicio P, Colombo R. 2001. The actin cytoskeleton response to oxidants: from small heat shock protein phosphorylation to changes in the redox state of actin itself. Free Radical Biology and Medicine 31, 1624–1632 [DOI] [PubMed] [Google Scholar]
- Delker C, Raschke A, Quint M. 2008. Auxin dynamics: the dazzling complexity of a small molecule’s message. Planta 227, 929–941 [DOI] [PubMed] [Google Scholar]
- Delledonne M. 2005. NO news is good news for plants. Current Opinion in Plant Biology 8, 390–396 [DOI] [PubMed] [Google Scholar]
- del Río LA. 2011. Peroxisomes as a cellular source of reactive nitrogen species signal molecules. Archives of Biochemistry and Biophysics 506, 1–11 [DOI] [PubMed] [Google Scholar]
- Délye C, Menchari Y, Michel S, Darmency H. 2004. Molecular bases for sensitivity to tubulin-binding herbicides in green foxtail. Plant Physiology 136, 3920–3932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhonukshe P, Grigoriev I, Fischer R, et al. 2008. Auxin transport inhibitors impair vesicle motility and actin cytoskeleton dynamics in diverse eukaryotes. Proceedings of the National Academy of Sciences, USA 105, 4489–4494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foissner I, Grolig F, Obermeyer G. 2002. Reversible protein phosphorylation regulates the dynamic organization of the pollen tube cytoskeleton: effects of calyculin A and okadaic acid. Protoplasma 220, 1–5 [DOI] [PubMed] [Google Scholar]
- Grossmann K. 2010. Auxin herbicides: current status of mechanism and mode of action. Pest Managment Science 66, 113–120 [DOI] [PubMed] [Google Scholar]
- Grossmann K, Kwiatkowski J, Tresch S. 2001. Auxin herbicides induce H2O2 overproduction and tissue damage in cleavers (Galiumaparine L.). Journal of Experimental Botany 52, 1811–1816 [DOI] [PubMed] [Google Scholar]
- Halliwell B, Gutteridge J. 2007. Free radicals in biology and medicine , 4th edn Oxford: Oxford University Press [Google Scholar]
- Hussey PJ, Ketelaar T, Deeks MJ. 2006. Control of the actin cytoskeleton in plant cell growth. Annual Review of Plant Biology 57, 109–125 [DOI] [PubMed] [Google Scholar]
- Jaffrey SR, Erdjument-Bromage H, Ferris CD, Tempst P, Snyder SH. 2001. Protein S-nitrosylation: a physiological signal for neuronal nitric oxide. Nature Cell Biology 3, 193–197 [DOI] [PubMed] [Google Scholar]
- Kasprowicz A, Szuba A, Volkmann D, Baluska F, Wojtaszek P. 2009. Nitric oxide modulates dynamic actin cytoskeleton and vesicle trafficking in a cell type-specific manner in root apices. Journal of Experimental Botany 60, 1605–1617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ketelaar T, Allwood EG, Anthony RG, Voigt B, Menzel D, Hussey PJ. 2004. The actin-interacting protein AIP1 is essential for actin organization and plant development. Current Biology 14, 57–62 [DOI] [PubMed] [Google Scholar]
- Koch E, Slusarenko A. 1990. Arabidopsis is susceptible to infection by a downy mildew fungus. The Plant Cell 2, 437–445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanza M, Garcia-Ponce B, Castrillo G, et al. 2012. Role of actin cytoskeleton in brassinosteroid signaling and in its integration with the auxin response in plants. Developmental Cell 22, 1275–1285 [DOI] [PubMed] [Google Scholar]
- Liszkay A, van der Zalm E, Schopfer P. 2004. Production of reactive oxygen intermediates (O2·–, H2O2, and ·OH) by maize roots and their role in wall loosening and elongation growth. Plant Physiology 136, 3114–3123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lounifi I, Arc E, Molassiotis A, Job D, Rajjou L, Tanou G. 2013. Interplay between protein carbonylation and nitrosylation in plants. Proteomics 13, 568–578 [DOI] [PubMed] [Google Scholar]
- Malerba M, Contran N, Tonelli M, Crosti P, Cerana R. 2008. Role of nitric oxide in actin depolymerization and programmed cell death induced by fucicoccin in sycamore (Acer pseudoplatanus) culture cells. Physiologia Plantarum 133, 449–467 [DOI] [PubMed] [Google Scholar]
- Mano S, Nakamori C, Hayashi M, Kato A, Kondo M, Nishimura M. 2002. Distribution and characterization of peroxisomes in arabidopsis by visualization with GFP: dynamic morphology and actin-dependent movement. Plant and Cell Physiology 43, 331–341 [DOI] [PubMed] [Google Scholar]
- Mittler R, Vanderauwera S, Suzuki N, Miller GAD, Tognetti VB, Vandepoele K, Gollery M, Shulaev V, Van Breusegem F. 2011. ROS signaling: the new wave? Trends in Plant Science 16, 300–309 [DOI] [PubMed] [Google Scholar]
- Moreau M, Lindermayr C, Durner J, Klessig DF. 2010. NO synthesis and signaling in plants—where do we stand? Physiologia Plantarum 138, 372–383 [DOI] [PubMed] [Google Scholar]
- Nakatsubo N, Kojima H, Kikuchi K, Nagoshi H, Hirata Y, Maeda D, Imai Y, Irimura T, Nagano T. 1998. Direct evidence of nitric oxide production from bovine aortic endothelial cells using new fluorescence indicators: diaminofluoresceins. FEBS Letters 427, 263–266 [DOI] [PubMed] [Google Scholar]
- Neill S, Barros R, Bright J, Desikan R, Hancock J, Harrison J, Morris P, Ribeiro D, Wilson I. 2008. Nitric oxide, stomatal closure, and abiotic stress. Journal of Experimental Botany 59, 165–176 [DOI] [PubMed] [Google Scholar]
- Nelson BK, Cai X, Nebenführ A. 2007. A multicolored set of in vivo organelle markers for co-localization studies in Arabidopsis and other plants. The Plant Journal 51, 1126–1136 [DOI] [PubMed] [Google Scholar]
- Nick P, Han MJ, An G. 2009. Auxin stimulates its own transport by shaping actin filaments. Plant Physiology 151, 155–167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortega-Galisteo A, Rodríguez-Serrano M, Pazmino DM, Gupta D, Sandalio LM, Romero-Puertas MC. 2012. S-nitrosylated proteins in pea (Pisum sativum L.) leaf peroxisomes: changes under abiotic stress. Journal of Experimental Botany 63, 2089–2103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ovidi E, Gambellini G, Taddei AR, Cai G, Del Casino C, Ceci M, Rondini S, Tiezzi A. 2001. Herbicides and the microtubular apparatus of Nicotiana tabacum pollen tube: immunofluorescence and immunogold labelling studies. Toxicology in vitro 15, 143–151 [DOI] [PubMed] [Google Scholar]
- Pazmiño DM. 2009. Contribución de las especies de oxígeno y nitrógeno reactivo, y de los peroxisomas a la toxicidad del 2,4-D en plantas. PhD Thesis. University of Granada, Spain. [Google Scholar]
- Pazmiño DM, Rodríguez-Serrano M, Romero-Puertas MC, Archilla-Ruiz A, Del Río LA, Sandalio LM. 2011. Differential response of young and adult leaves to herbicide 2,4-dichlorophenoxyacetic acid in pea plants: role of reactive oxygen species. Plant, Cell and Environment 34, 1874–1889 [DOI] [PubMed] [Google Scholar]
- Pazmiño DM, Rodríguez-Serrano M, Sanz M, Romero-Puertas MC, Sandalio LM. 2014. Regulation of epinasty induced by 2,4-dichlorophenoxyacetic acid in pea and Arabidopsis plants. Plant Biology (in press). 10.1111/plb.12128. [DOI] [PubMed] [Google Scholar]
- Pazmiño DM, Romero-Puertas MC, Sandalio LM. 2012. Insights into the toxicity mechanism of and cell response to the herbicide 2,4-D in plants. Plant Signaling and Behaviour 7, 1–3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahman A, Bannigan A, Sulaman W, Pechter P, Blancaflor EB, Baskin TI. 2007. Auxin, actin and growth of the Arabidopsis thaliana primary root. The Plant Journal 50, 514–528 [DOI] [PubMed] [Google Scholar]
- Rasmussen I, Pedersen LH, Byg L, Suzuki K, Sumimoto H, Vilhardt F. 2010. Effects of F/G-actin ratio and actin turn-over rate on NADPH oxidase activity in microglia. BMC Immunology 11, 44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodríguez-Serrano M, Romero-Puertas MC, Sparkes I, Hawes C, del Río LA, Sandalio LM. 2009. Peroxisome dynamics in Arabidopsis plants under oxidative stress induced by cadmium. Free Radicals in Biology and Medicine 47, 1632–1639 [DOI] [PubMed] [Google Scholar]
- Romero-Puertas MC, Palma JM, Gómez M, del Río LA, Sandalio LM. 2002. Cadmium causes the oxidative modification of proteins in pea plants. Plant, Cell and Environment 25, 677–686 [Google Scholar]
- Romero-Puertas MC, McCarthy I, Gómez M, Sandalio LM, Corpas FJ, del Río LA, Palma JM. 2004a. Reactive oxygen species-mediated enzymatic systems involved in the oxidative action of 2,4-dichlorophenoxyacetic acid. Plant, Cell and Environment 27, 1135–1148 [Google Scholar]
- Romero-Puertas MC, Rodríguez-Serrano M, Corpas FJ, Gómez M, del Río LA, Sandalio LM. 2004b. Cadmium-induced subcellular accumulation of O2·– and H2O2 in pea leaves. Plant, Cell and Environment 27, 1122–1134 [Google Scholar]
- Romero-Puertas MC, Rodríguez-Serrano M, Sandalio LM. 2013. Protein S-nitrosylation in plants under abiotic stress: an overview. Frontiers in Plant Science 4, 373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandalio LM, Rodríguez-Serrano M, Gupta DK, Archilla A, Romero-Puertas MC, del Río LA. 2012. Reactive oxygen species and nitric oxide in plants under cadmium stress. In: Amad P, Prassad MNV, eds. Toxicity to signaling. Environmental adaptations and stress tolerance of plants in the era of climate change. Berlin: Springer, 199–215 [Google Scholar]
- Sandalio LM, Rodríguez-Serrano M, Romero-Puertas MC, del Río LA. 2008. Imaging of reactive oxygen species and nitric oxide in vivo in plant tissues. Methods in Enzymology 440: 397–409 [DOI] [PubMed] [Google Scholar]
- Sandalio LM, Rodríguez-Serrano M, Romero-Puertas MC, del Río LA. 2013. Role of peroxisomes as a source of reactive oxygen species (ROS) signaling molecules. In: del Río LA, ed. Peroxisomes and their key role in cellular signaling and metabolism. Dordrecht, Springer Science+Business, 231–249 [DOI] [PubMed] [Google Scholar]
- Schopfer P, Liszkay A, Bechtold M, Frahry G, Wagner A. 2002. Evidence that hydroxyl radicals mediate auxin-induced extension growth. Planta 214, 821–828 [DOI] [PubMed] [Google Scholar]
- Sheahan MB, Staiger JC, Rose RJ, McCurdy DW. 2004. A green fluorescent protein fusion to actin-binding domain 2 of Arabidopsis fimbrin highlights new features of a dynamic actin cytoskeleton in live plant cells. Plant Physiology 136, 3968–3978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheremet YA, Yemets AI, Blume YB. 2012. Inhibitors of tyrosine kinases and phosphatases as a tool for the investigation of microtubule role in plant cold response. Cytology and Genetics 46, 1–8 [PubMed] [Google Scholar]
- Smith LG, Oppenheimer DG. 2005. Spatial control of cell expansion by the plant cytoskeleton. Annual Review of Cell and Developmental Biology 21, 271–294 [DOI] [PubMed] [Google Scholar]
- Song X, Ma Q, Hao X, Li H. 2012. Roles of the actin cytoskeleton and an actin-binding protein in wheat resistance against Puccinic striiformis f. sp. tritici . Protoplasma 249, 99–106 [DOI] [PubMed] [Google Scholar]
- Staiger CJ, Blanchoin L. 2006. Actin dynamics: old friends with new stories. Current Opinion in Plant Biology 19, 554–562 [DOI] [PubMed] [Google Scholar]
- Staiger CJ, Sheahan MB, Khurana P, Wang X, McCurdy DW, Blanchoin L. 2009. Actin filament dynamics are dominated by rapid growth and severing activity in the Arabidopsis cortical array. Journal of Cell Biology 184, 269–280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamler JS, Lamas S, Fang FC. 2001. Nitrosylation, the prototypic redox-based signaling mechanism. Cell 106, 675–683 [DOI] [PubMed] [Google Scholar]
- Sun J, Steenbergen C, Murphy E. 2006. S-nitrosylation: NO-related redox signaling to protect against oxidative stress. Antioxidants and Redox Signaling 8, 1693–1705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki N, Koussevitzky S, Mittler R, Miller G. 2011. ROS and redox signalling in the response of plants to abiotic stress. Plant, Cell and Environment 35, 259–270 [DOI] [PubMed] [Google Scholar]
- Teixeira MC, Duque P, Sá-Correia I. 2007. Environmental genomics: mechanistic insights into toxicity of and resistance to the herbicide 2,4-D. Trends in Biotechnology 25, 363–370 [DOI] [PubMed] [Google Scholar]
- Terman JR, Kashina A. 2013. Post-translational modification and regulation of actin. Current Opinion in Cell Biology 25, 30–38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandenabeele S, Vanderauwera S, Vuylsteke M, Rombauts S, Langebartels C, Seidlitz HK, Zabeau M, Van Montagu M, Inzé D, Van Breusegem F. 2004. Catalase deficiency drastically affects gene expression induced by high light in Arabidopsis thaliana. The Plant Journal 39, 45–58 [DOI] [PubMed] [Google Scholar]
- Vandelle E, Delledonne M. 2011. Peroxinitrite formation and function in plants. Plant Science 181, 534–539 [DOI] [PubMed] [Google Scholar]
- Van Gestel K, Kohler RH, Verbelen JP. 2002. Plant mitochondria move on F-actin, but their positioning in the cortical cytoplasm depends on both F-actin and microtubules. Journal of Experimental Botany 53, 659–667 [DOI] [PubMed] [Google Scholar]
- Wasteneys GO, Yang Z. 2004. New views on the plant cytoskeleton. Plant Physiology 136, 3884–3891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkins KA, Bancroft J, Bosch M, Ings J, Smirnoff N, Franklin-Tong VE. 2011. Reactive oxygen species and nitric oxide mediate actin reorganization and programmed cell death in the self-incompatibility response of Papaver. Plant Physiology 156, 404–416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yemets AI, Kraslylenko YA, Lytryn DI, Sheremet YA, Blume YB. 2011. Nitric oxide signalling via cytoskeleton in plants. Plant Science 181, 545–554 [DOI] [PubMed] [Google Scholar]
- Zaninotto F, Camera SL, Polverari A, Delledonne M. 2006. Cross talk between reactive nitrogen and oxygen species during the hypersensitive disease resistance response. Plant Physiology 141, 379–383 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.