Abstract
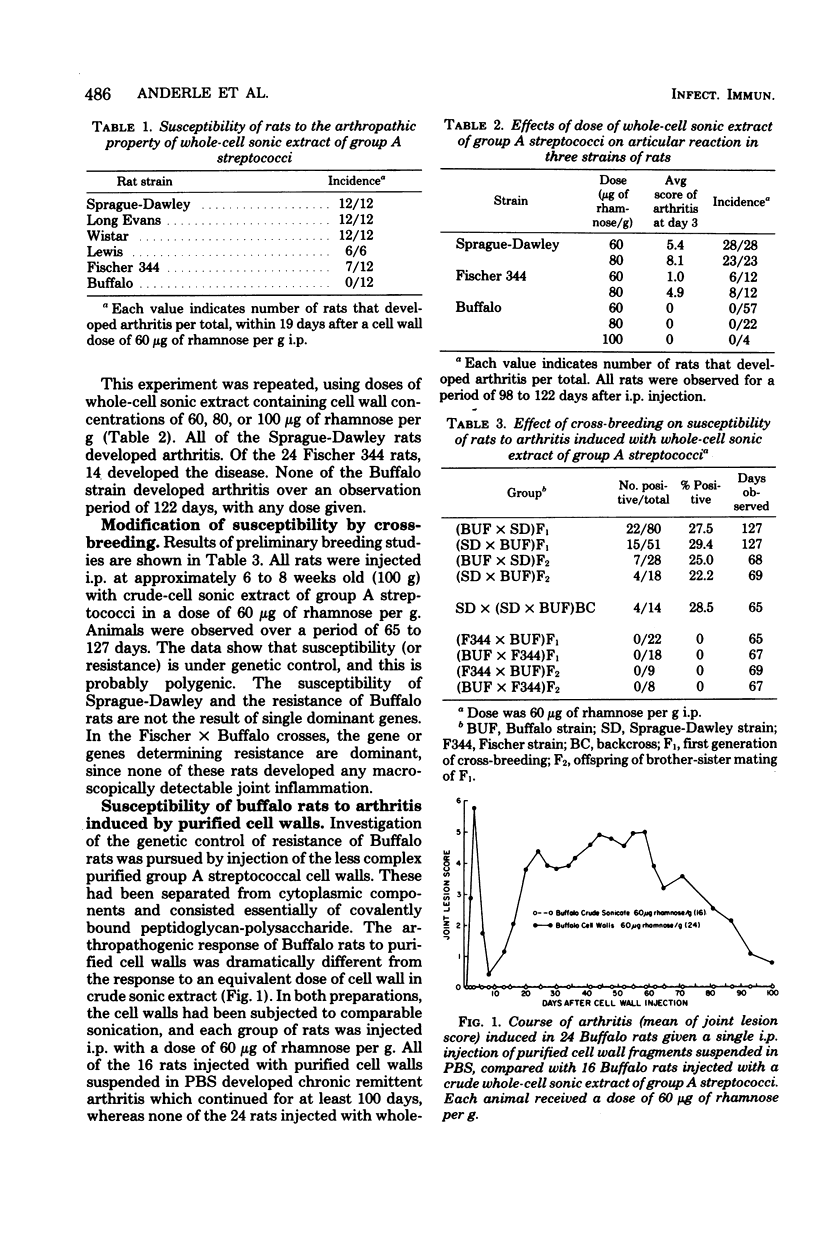
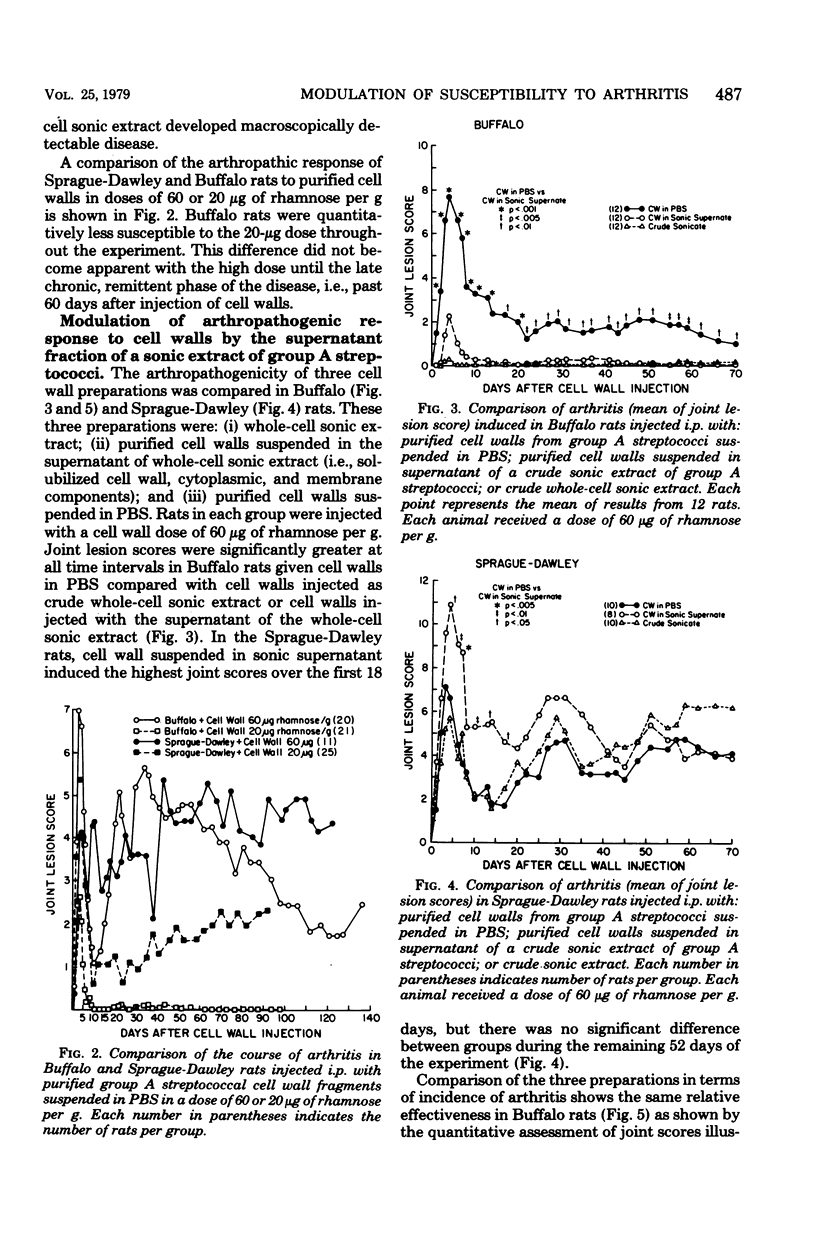
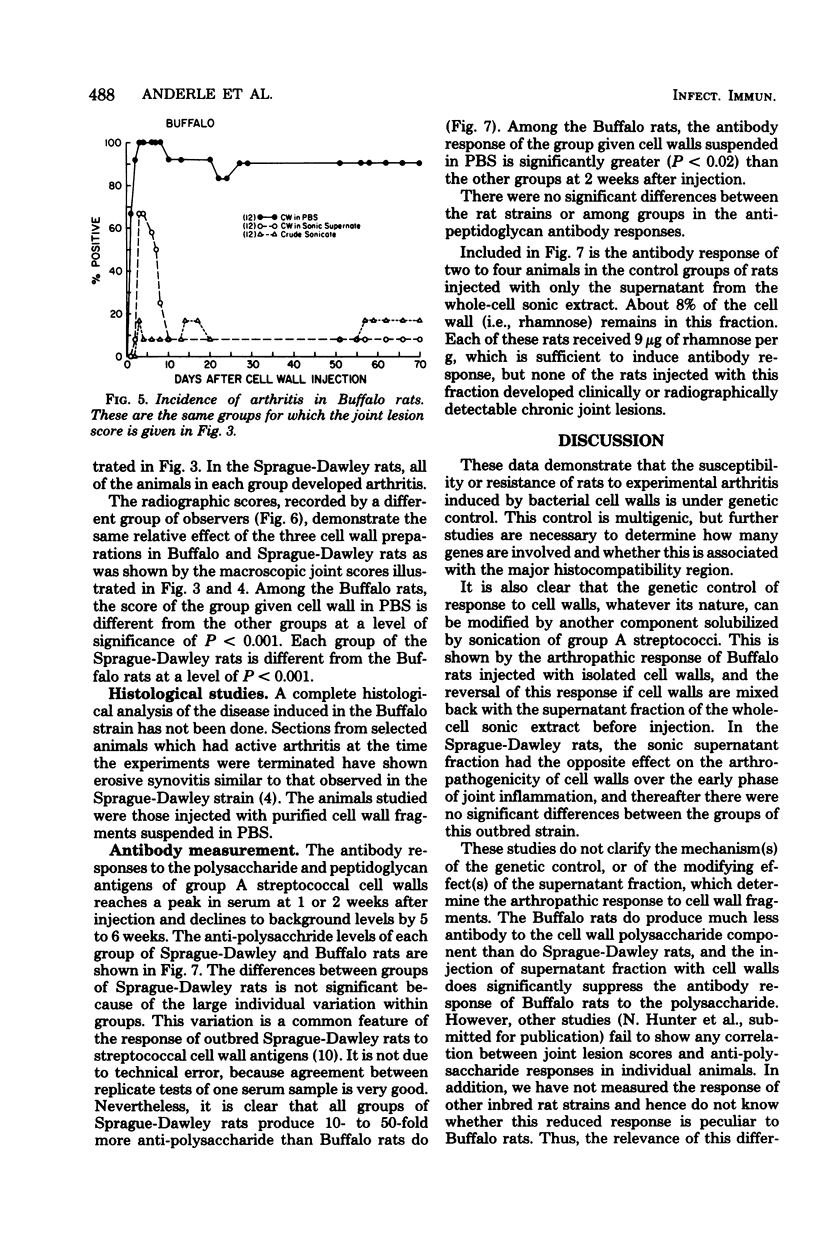
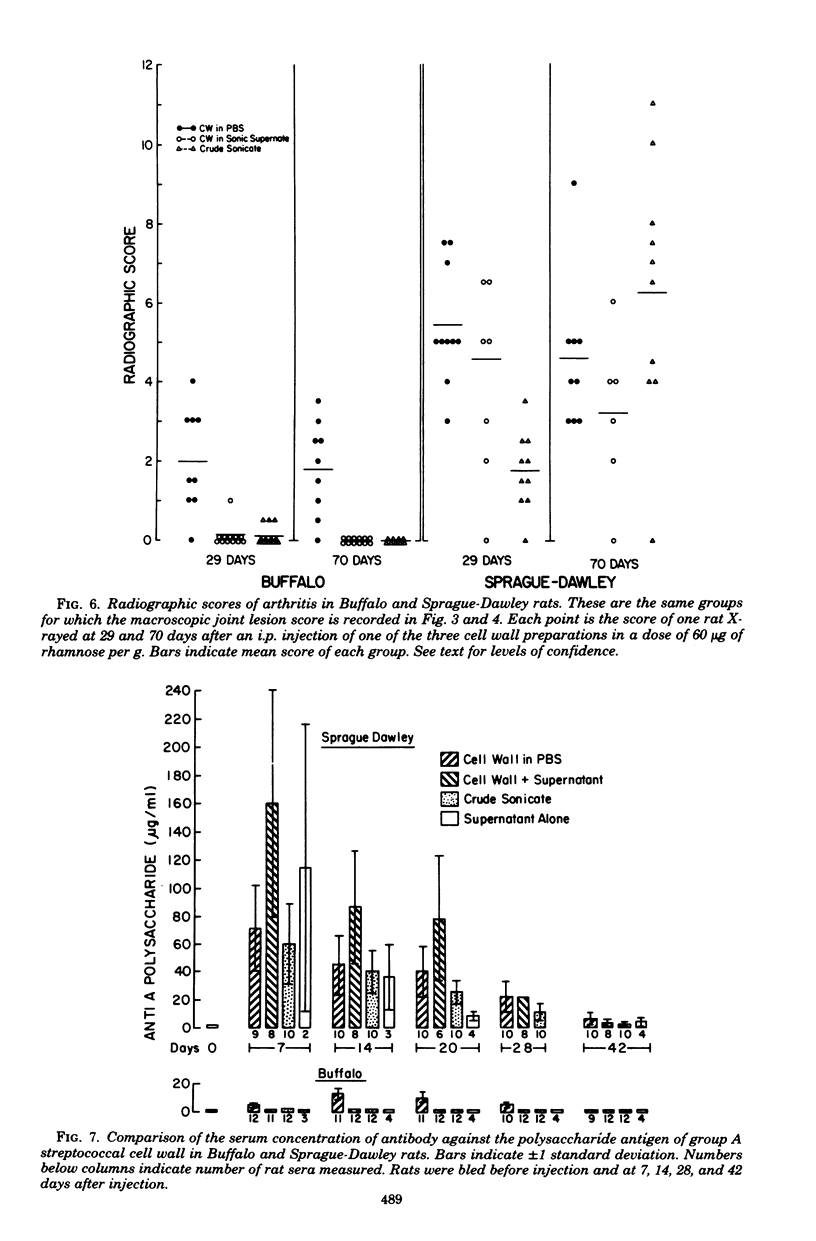
Inbred Buffalo rats were resistant to the induction of experimental arthritis induced by systemic injection of cell wall fragments in a crude whole-cell sonic extract of group A streptococci. This was in contrast to the susceptibility of outbred Sprague-Dawley and certain other inbred strains. Preliminary breeding studies indicated that genetic control of resistance of susceptibility is multigenic. When Buffalo rats were infected with a saline suspension of isolated cell wall fragments, chronic remittent arthritis developed. Suspension of isolated cell wall fragments, chronic remittent arthritis developed. Suspension of the isolated cell walls in the supernatant fraction of group A streptococci solubilized by sonication eliminated the arthropathogenicity in Buffalo rats. Thus, a component separable from the cell wall fraction can modulate the arthropathogenicity of cell walls in rats, but the effect depends upon the genetic background of the rat. The antibody response of Buffalo rats to the polysaccharide antigen of cell walls was also affected by the supernatant fraction of sonicated group A streptococci.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bernstein D., Klapper D. G., Krause R. M. Use of radioimmunoassays to determine the concentration of streptococcal group-specific antibodies in rabbit antisera. J Immunol. 1975 Jan;114(1 Pt 1):59–63. [PubMed] [Google Scholar]
- Clark R. L., Cuttino J. T., Jr, Anderle S. K., Cromartie W. J., Schwab J. H. Radiologic analysis of arthritis in rats after systemic injection of streptococcal cell walls. Arthritis Rheum. 1979 Jan;22(1):25–35. doi: 10.1002/art.1780220105. [DOI] [PubMed] [Google Scholar]
- Cromartie W. J., Craddock J. G. Rheumatic-like cardiac lesions in mice. Science. 1966 Oct 14;154(3746):285–287. doi: 10.1126/science.154.3746.285. [DOI] [PubMed] [Google Scholar]
- Cromartie W. J., Craddock J. G., Schwab J. H., Anderle S. K., Yang C. H. Arthritis in rats after systemic injection of streptococcal cells or cell walls. J Exp Med. 1977 Dec 1;146(6):1585–1602. doi: 10.1084/jem.146.6.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotschlich E. C. A simplification of the radioactive antigen-binding test by a double label technique. J Immunol. 1971 Sep;107(3):910–911. [PubMed] [Google Scholar]
- Hadler N. M., Granovetter D. A. Phlogistic properties of bacterial debris. Semin Arthritis Rheum. 1978 Aug;8(1):1–16. doi: 10.1016/0049-0172(78)90031-8. [DOI] [PubMed] [Google Scholar]
- Heymer B., Bernstein D., Schleifer K. H., Krause R. M. Measurement of peptidoglycan antibodies by a radioimmunoassay. Z Immunitatsforsch Exp Klin Immunol. 1975 Jul;149(2-4):168–178. [PubMed] [Google Scholar]
- Kohashi O., Pearson M., Beck F. J., Alexander M. Effect of oil composition on both adjuvant-induced arthritis and delayed hypersensitivity to purified protein derivative and peptidoglycans in various rat strains. Infect Immun. 1977 Aug;17(2):244–249. doi: 10.1128/iai.17.2.244-249.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leslie G. A., Carwile H. F. Immune response of rats to group A streptococcal vaccine. Infect Immun. 1973 May;7(5):781–785. doi: 10.1128/iai.7.5.781-785.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwab J. H. Biological properties of streptococcal cell-wall particles. I. Determinants of the chronic nodular lesion of connective tissue. J Bacteriol. 1965 Nov;90(5):1405–1411. doi: 10.1128/jb.90.5.1405-1411.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwab J. H., Cromartie W. J., Ohanian S. H., Craddock J. G. Association of experimental chronic arthritis with the persistence of group A streptococcal cell walls in the articular tissue. J Bacteriol. 1967 Nov;94(5):1728–1735. doi: 10.1128/jb.94.5.1728-1735.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverman D. A., Rose N. R. Neonatal thymectomy increases the incidence of spontaneous and methylcholanthrene-enhanced thyroiditis in rats. Science. 1974 Apr 12;184(4133):162–163. doi: 10.1126/science.184.4133.162. [DOI] [PubMed] [Google Scholar]
- Smialowicz R. J., Schwab J. H. Processing of streptococcal cell walls by rat macrophages and human monocytes in vitro. Infect Immun. 1977 Sep;17(3):591–598. doi: 10.1128/iai.17.3.591-598.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toffaletti D. L., Schwab J. H. Modulation of lymphocyte functions by group A streptococcal membrane. Cell Immunol. 1979 Jan;42(1):3–17. doi: 10.1016/0008-8749(79)90216-8. [DOI] [PubMed] [Google Scholar]
- Wood F. D., Pearson C. M., Tanaka A. Capacity of mycobacterial wax D and its subfractions to induce adjuvant arthritis in rats. Int Arch Allergy Appl Immunol. 1969;35(5):456–467. doi: 10.1159/000230198. [DOI] [PubMed] [Google Scholar]



