Abstract
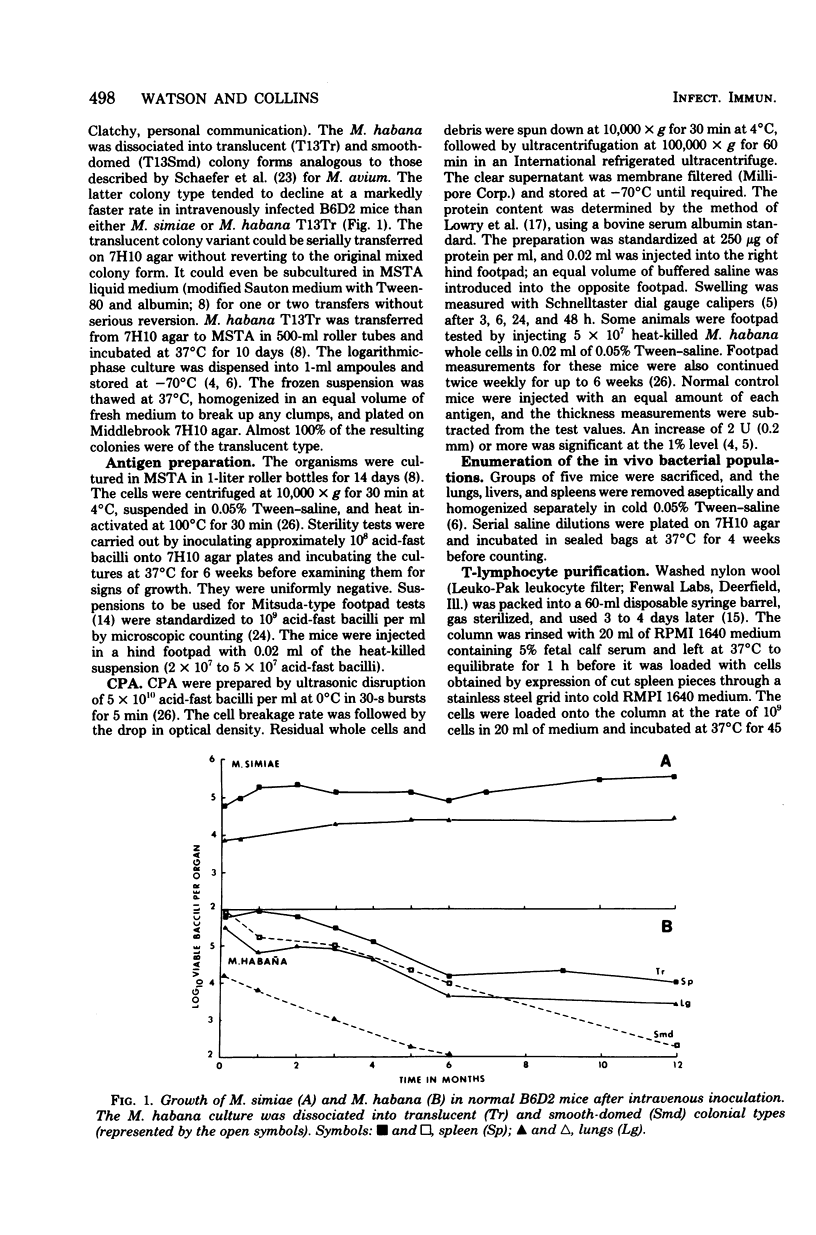
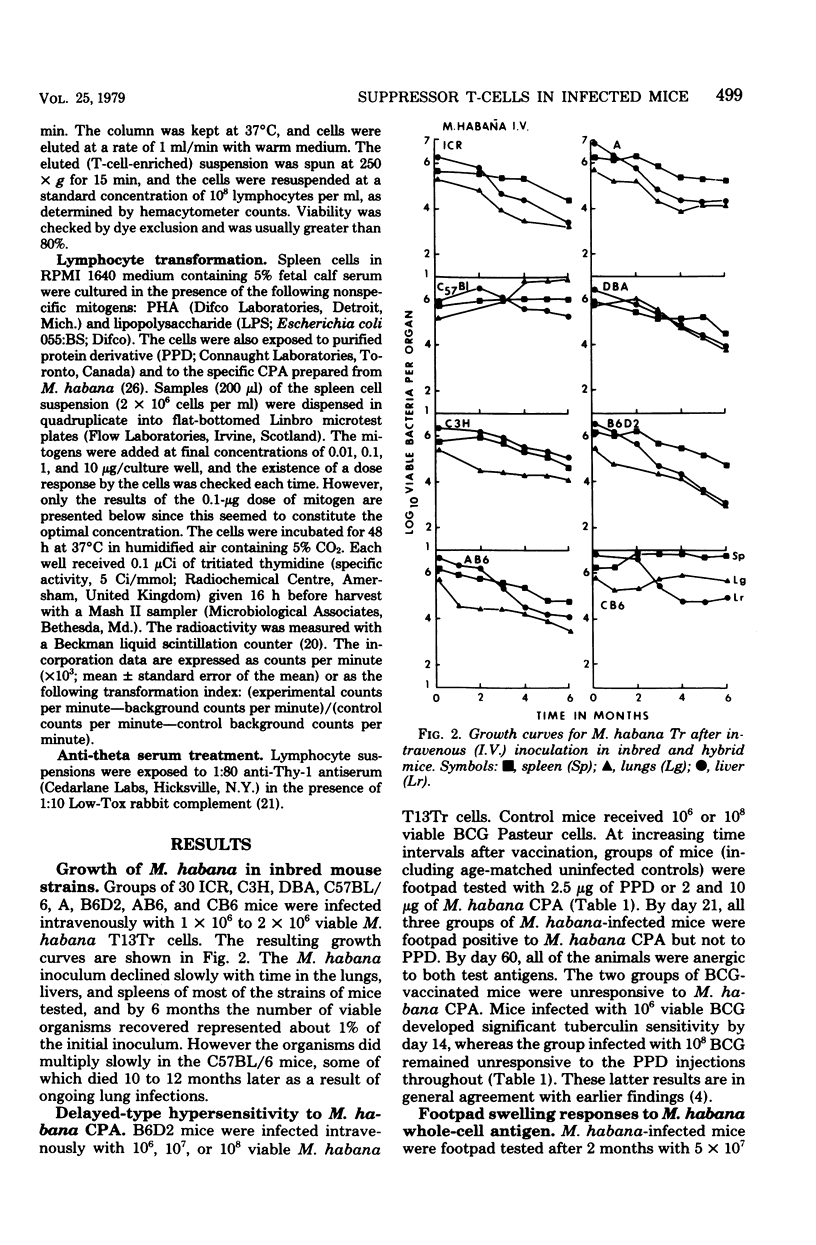
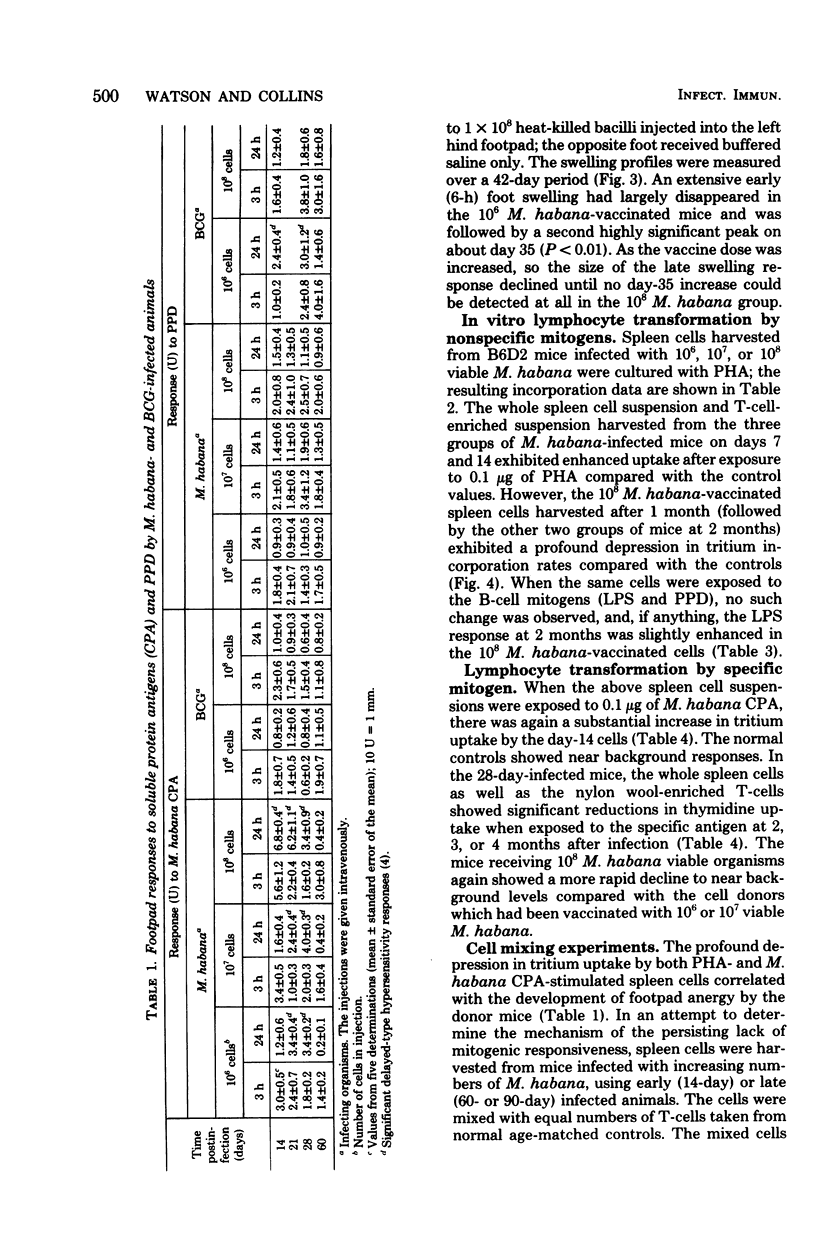
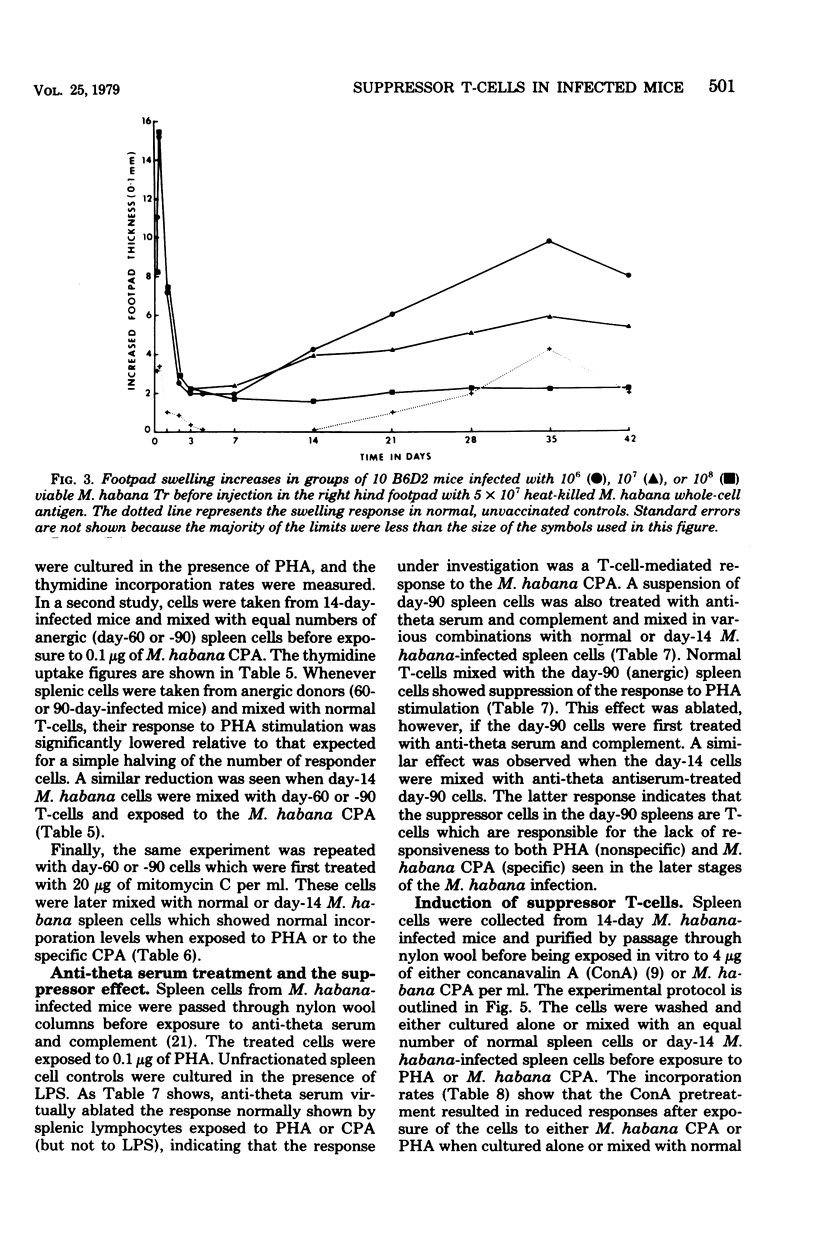
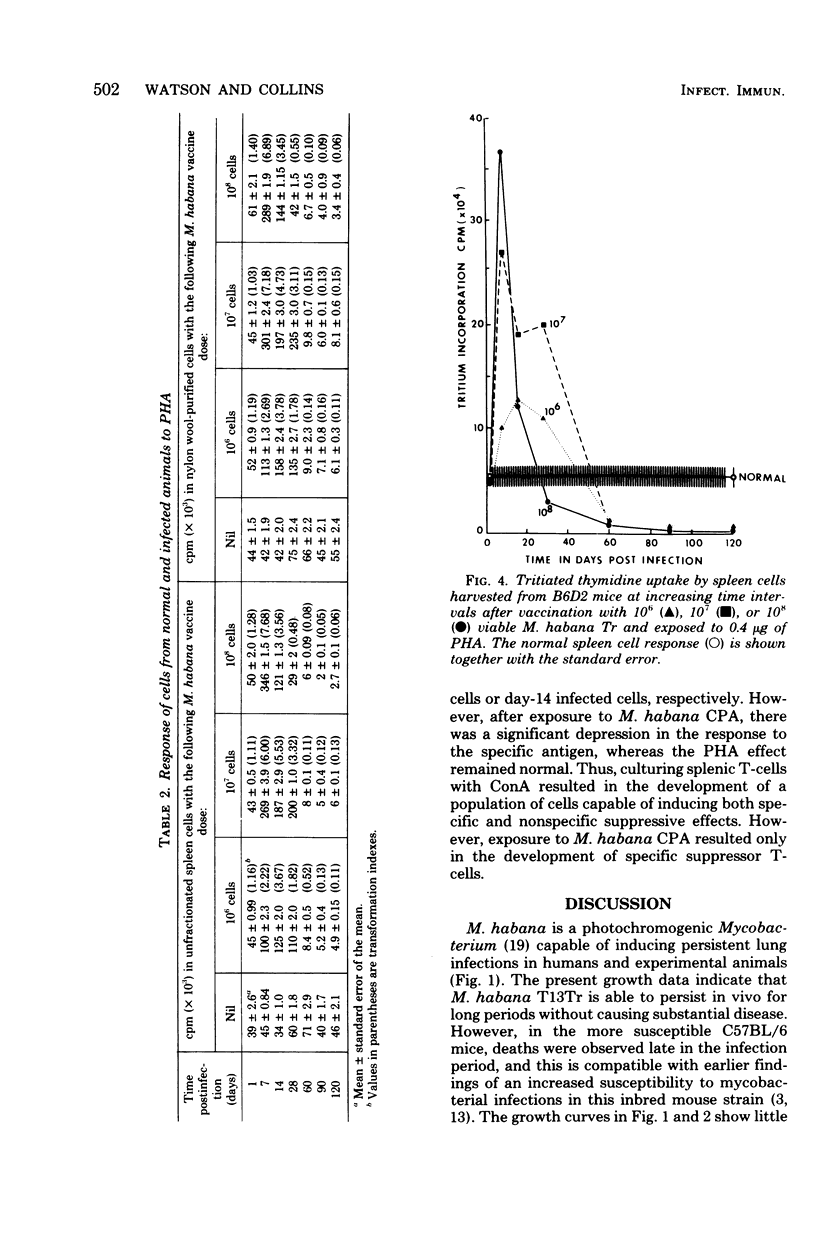
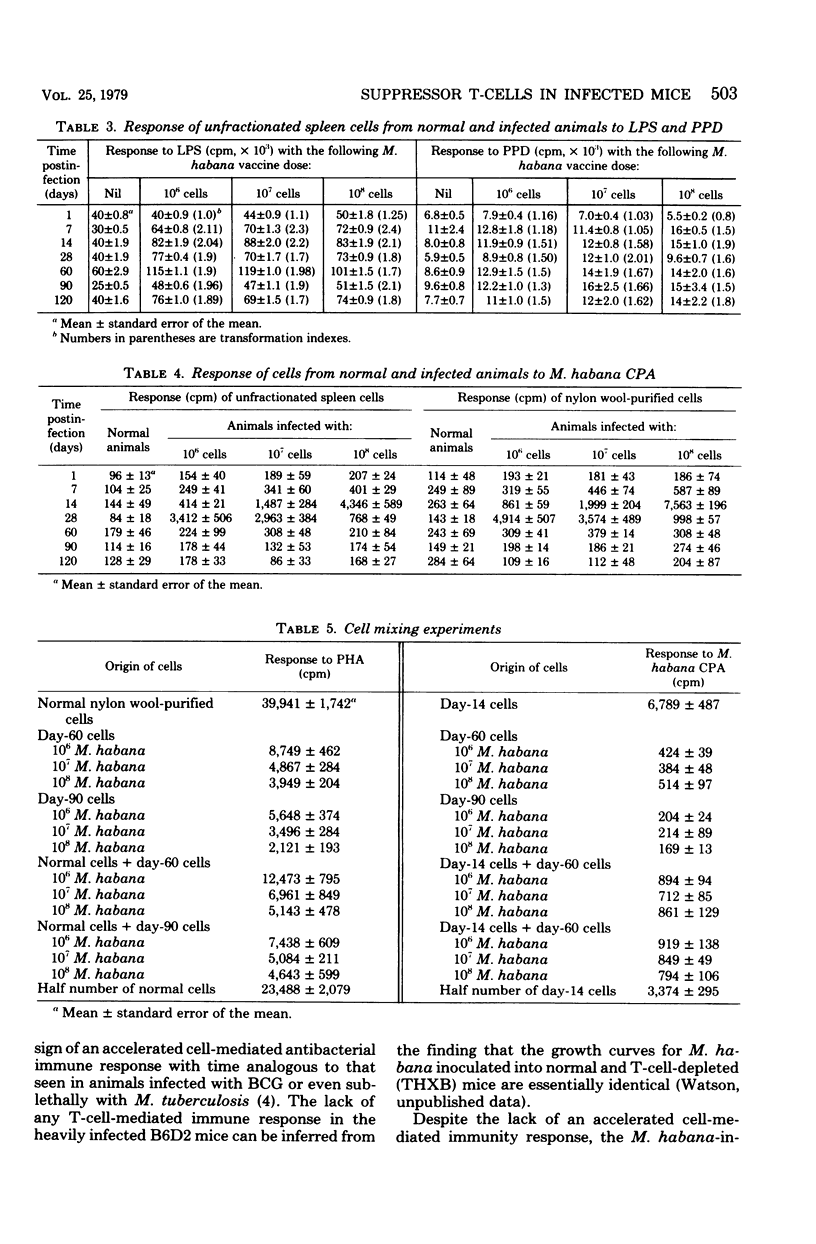
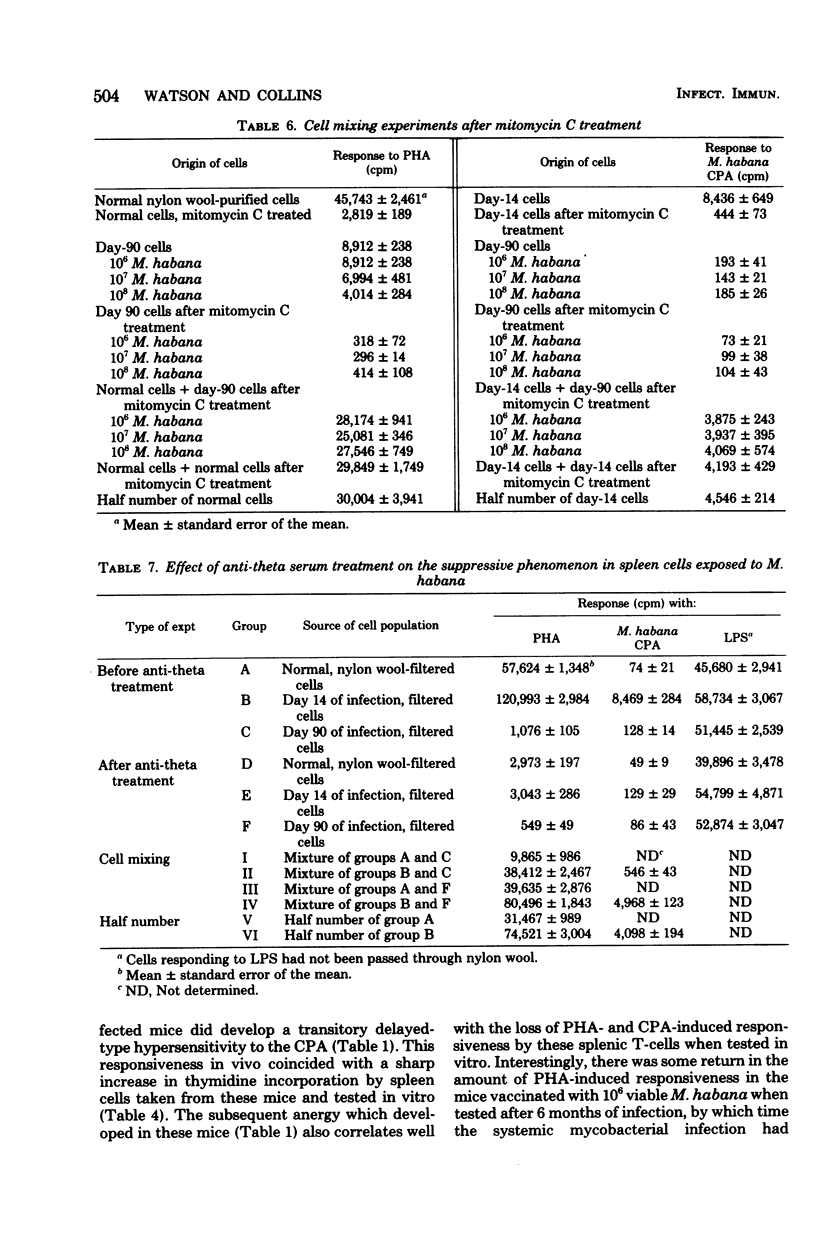
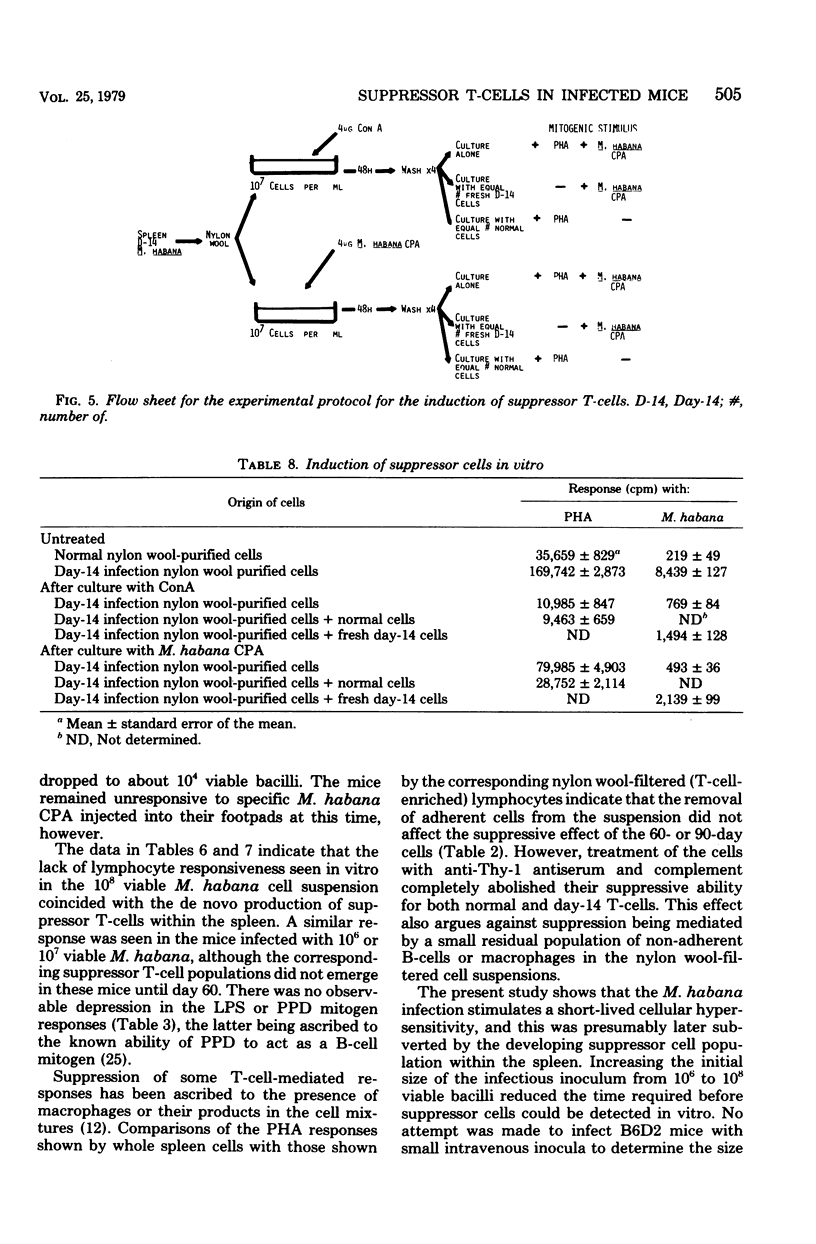
Mice were infected intravenously with increasing numbers of Mycobacterium habana (simiae serotype II), and the levels of delayed-type hypersensitivity to purified protein derivative and M. habana cytoplasmic protein antigen were determined after 14, 30, and 90 days. A footpad delayed-type hypersensitivity response was seen in 14-day-infected mice and was followed by a persisting anergy. T-cell-enriched suspensions collected 30 and 90 days into the infection (anergic donors) showed depressed transformation indexes after phytohemagglutinin and M. habana cytoplasmic protein antigen treatment in vitro. The corresponding B-cell mitogen (lipopolysaccharide) responses were not affected. Mixing experiments with T-cell-enriched suspensions from day-90 M. habana-infected donors adoptively suppressed lymphocyte transformation by normal and day-14 spleen cells. This effect could be ablated by anti-theta serum and complement treatment of the day-90 cells, indicating that the lack of in vitro responsiveness to cytoplasmic protein antigen was mediated by a population of suppressor T-cells present in the heavily infected spleens. There was no evidence that similar cells were present in the spleens of the 14-day-infected animals. Suppressor T-cells could be induced in vitro by exposure of day-14 spleen cells to concanavalin A or M. habana cytoplasmic protein antigen before they were mixed with normal or day-14 indicator splenic lymphocytes. The timing of the appearance of suppressor T-cells in the infected spleens corresponded to a loss of footpad hypersensitivity by the M. habana-infected animals.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bullock W. E. Anergy and infection. Adv Intern Med. 1976;21:149–173. [PubMed] [Google Scholar]
- Closs O., Haugen O. A. Experimental murine leprosy. 2. Further evidence for varying susceptibility of outbred mice and evaluation of the response of 5 inbred mouse strains to infection with Mycobacterium lepraemurium. Acta Pathol Microbiol Scand A. 1974 Jul;82(4):459–474. [PubMed] [Google Scholar]
- Collins F. M., Mackaness G. B. The relationship of delayed hypersensitivity to acquired antituberculous immunity. I. Tuberculin sensitivity and resistance to reinfection in BCG-vaccinated mice. Cell Immunol. 1970 Sep;1(3):253–265. doi: 10.1016/0008-8749(70)90047-x. [DOI] [PubMed] [Google Scholar]
- Collins F. M., Montalbine V., Morrison N. E. Growth and immunogenicity of photochromogenic strains of mycobacteria in the footpads of normal mice. Infect Immun. 1975 May;11(5):1079–1087. doi: 10.1128/iai.11.5.1079-1087.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins F. M., Morrison N. E., Montalbine V. Immune response to persistent mycobacterial infection in mice. Infect Immun. 1978 May;20(2):430–438. doi: 10.1128/iai.20.2.430-438.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins F. M., Volkman A., McGregor D. D. Transfer of delayed and Arthus sensitivity with blood plasma from x-irradiated guinea-pigs. Immunology. 1970 Sep;19(3):501–509. [PMC free article] [PubMed] [Google Scholar]
- Collins F. M., Wayne L. G., v Montalbine The effect of cultural conditions on the distribution of Mycobacterium tuberculosis in the spleens and lungs of specific pathogen-free mice. Am Rev Respir Dis. 1974 Aug;110(2):147–156. doi: 10.1164/arrd.1974.110.2.147. [DOI] [PubMed] [Google Scholar]
- Dutton R. W. Inhibitory and stimulatory effects of concanavalin A on the response of mouse spleen cell suspensions to antigen. I. Characterization of the inhibitory cell activity. J Exp Med. 1972 Dec 1;136(6):1445–1460. doi: 10.1084/jem.136.6.1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GRAY D. F. The relative natural resistance of rats and mice to experimental pulmonary tuberculosis. J Hyg (Lond) 1961 Dec;59:471–477. doi: 10.1017/s0022172400039164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godal T., Myklestad B., Samuel D. R., Myrvang B. Characterization of the cellular immune defect in lepromatous leprosy: a specific lack of circulating Mycobacterium leprae-reactive lymphocytes. Clin Exp Immunol. 1971 Dec;9(6):821–831. [PMC free article] [PubMed] [Google Scholar]
- Godal T., Myrvang B., Froland S. S., Shao J., Melaku G. Evidence that the mechanism of immunological tolerance ("central failure") is operative in the lack of host resistance in lepromatous leprosy. Scand J Immunol. 1972;1(4):311–321. doi: 10.1111/j.1365-3083.1972.tb03296.x. [DOI] [PubMed] [Google Scholar]
- Gorczynski R. M. Control of the immune response: role of macrophages in regulation of antibody-and cell-mediated immune responses. Scand J Immunol. 1976;5(9):1031–1047. doi: 10.1111/j.1365-3083.1976.tb03055.x. [DOI] [PubMed] [Google Scholar]
- Guinto R. S. Biology of the mycobacterioses. Skin tests in leprosy. Ann N Y Acad Sci. 1968 Sep 5;154(1):149–156. doi: 10.1111/j.1749-6632.1968.tb16705.x. [DOI] [PubMed] [Google Scholar]
- Julius M. H., Simpson E., Herzenberg L. A. A rapid method for the isolation of functional thymus-derived murine lymphocytes. Eur J Immunol. 1973 Oct;3(10):645–649. doi: 10.1002/eji.1830031011. [DOI] [PubMed] [Google Scholar]
- Kantor F. S. Infection, anergy and cell-mediated immunity. N Engl J Med. 1975 Mar 20;292(12):629–634. doi: 10.1056/NEJM197503202921210. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Meissner G., Schröder K. H. Relationship between Mycobacterium simiae and Mycobacterium habana. Am Rev Respir Dis. 1975 Feb;111(2):196–200. doi: 10.1164/arrd.1975.111.2.196. [DOI] [PubMed] [Google Scholar]
- Morrison N. E., Collins F. M. Immunogenicity of an aerogenic BCG vaccine in T-cell-depleted and normal mice. Infect Immun. 1975 May;11(5):1110–1121. doi: 10.1128/iai.11.5.1110-1121.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- North R. J. Cellular mediators of anti-Listeria immunity as an enlarged population of short lived, replicating T cells. Kinetics of their production. J Exp Med. 1973 Aug 1;138(2):342–355. doi: 10.1084/jem.138.2.342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orbach-Arbouys S., Poupon M. F. Active suppression of in vitro reactivity of spleen cells after BCG treatment. Immunology. 1978 Mar;34(3):431–437. [PMC free article] [PubMed] [Google Scholar]
- Schaefer W. B., Davis C. L., Cohn M. L. Pathogenicity of transparent, opaque, and rough variants of Mycobacterium avium in chickens and mice. Am Rev Respir Dis. 1970 Oct;102(4):499–506. doi: 10.1164/arrd.1970.102.4.499. [DOI] [PubMed] [Google Scholar]
- Shepard C. C., McRae D. H. A method for counting acid-fast bacteria. Int J Lepr Other Mycobact Dis. 1968 Jan-Mar;36(1):78–82. [PubMed] [Google Scholar]
- Sultzer B. M. Infection with Bacillus Calmette-Guérin activates murine thymus-independent (B) lymphocytes. J Immunol. 1978 Jan;120(1):254–261. [PubMed] [Google Scholar]
- Watson S. R., Morrison N. E., Collins F. M. Delayed hypersensitivity responses in mice and guinea pigs to Mycobacterium leprae, Mycobacterium vaccae, and Mycobacterium nonchromogenicum cytoplasmic proteins. Infect Immun. 1979 Jul;25(1):229–236. doi: 10.1128/iai.25.1.229-236.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]



